Cellulose-Based Conductive Materials for Energy and Sensing Applications
Abstract
:1. Introduction
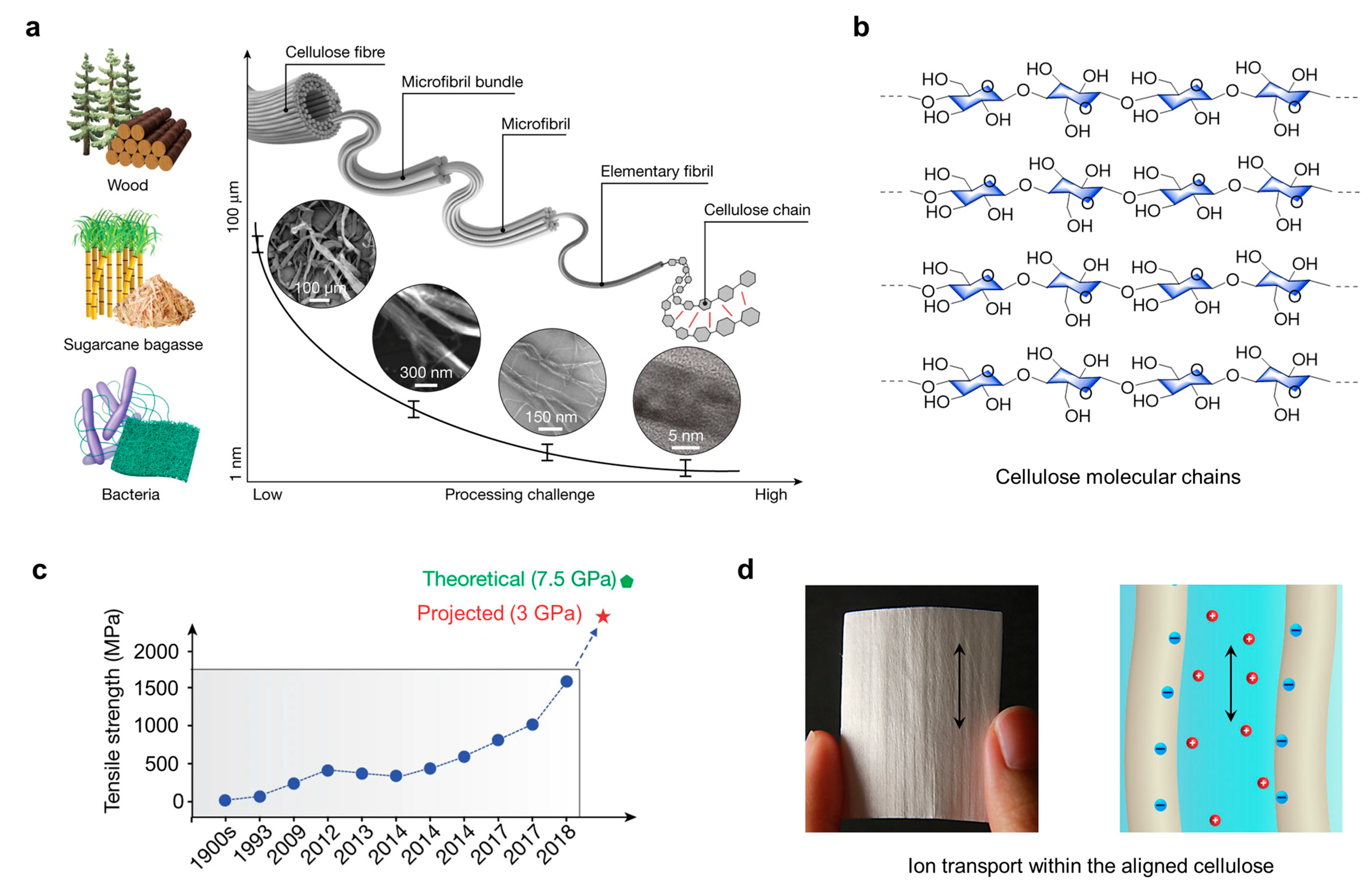
2. Cellulose and Nanocellulose

3. Preparation of Cellulose-Based Conductive Materials (CCMs)
3.1. Carbonized Cellulose Materials

3.2. Cellulose Composite Carbon Materials
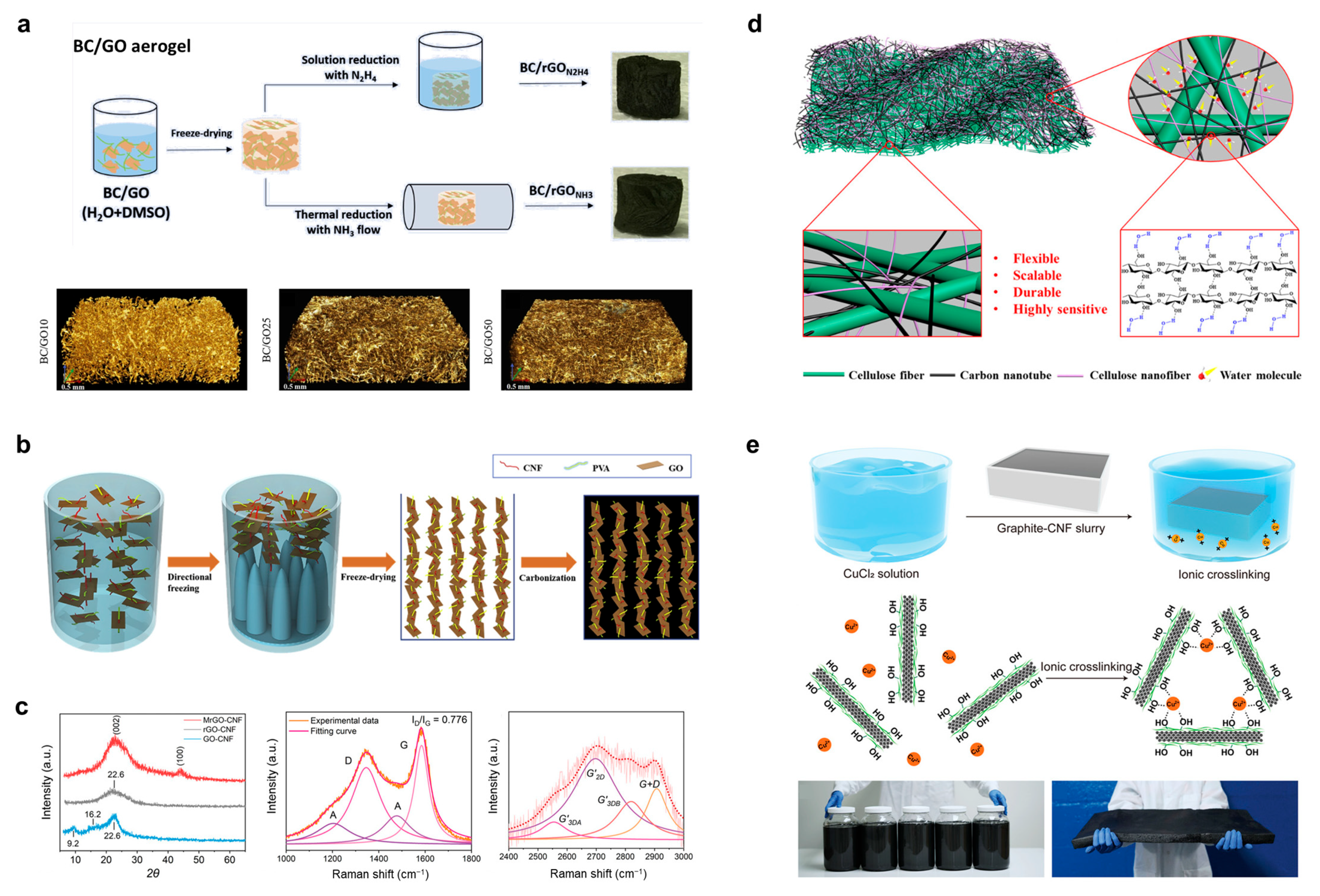
3.3. Cellulose Composite Metal Particles and Inorganic Compounds
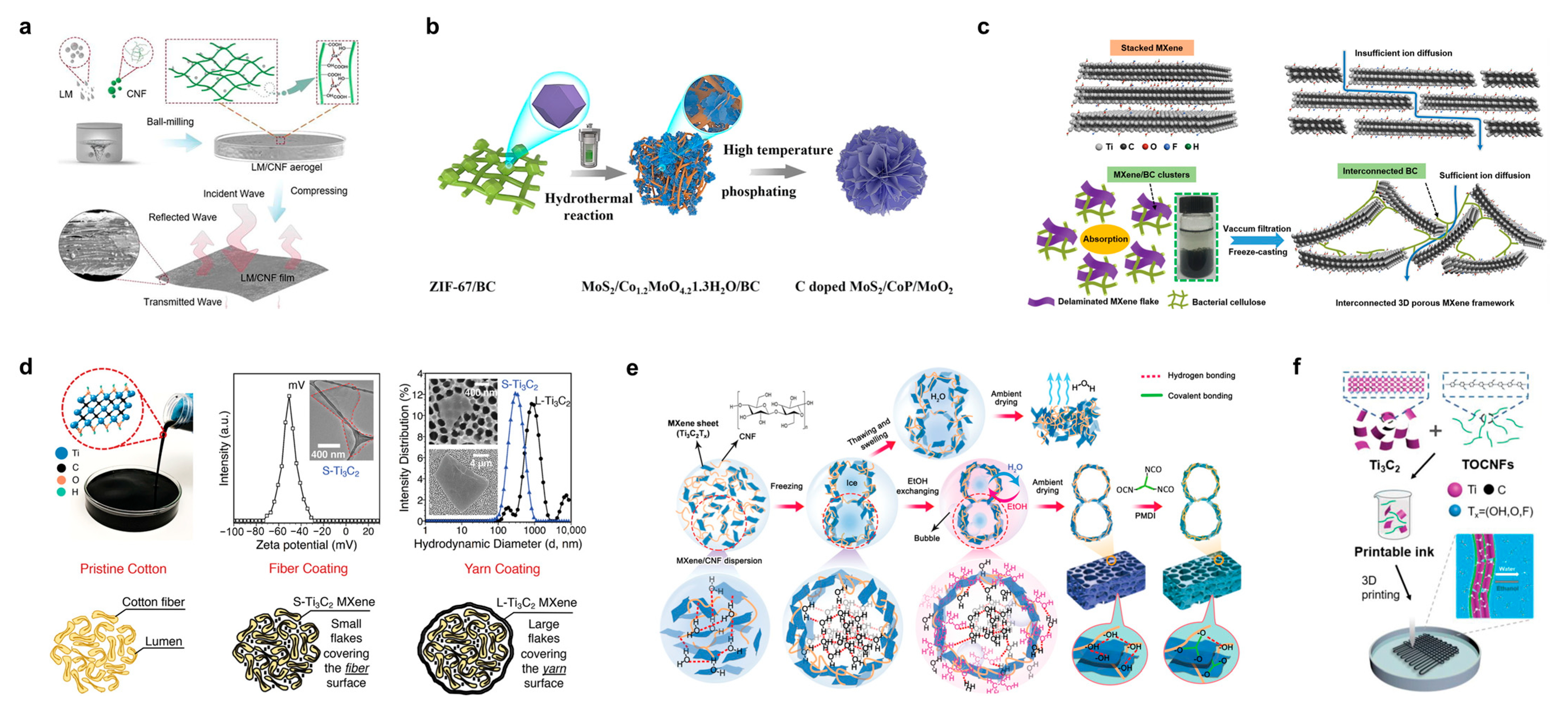
3.4. Cellulose Composite Conductive Polymers

4. Energy Applications
4.1. Batteries
4.2. Supercapacitors
5. Sensing Applications
5.1. Chemical and Biological Sensors
5.2. Mechanical Sensor
6. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1D | One dimensional |
| 2D | Two dimensional |
| 3D | Three dimensional |
| BC | Bacterial cellulose |
| BET | Brunauer–Emmett–Teller |
| CCMs | Cellulose-based conductive materials |
| c-MOF | Conductive metal–organic framework |
| CNCs | Cellulose nanocrystals |
| CNFs | Cellulose nanofibers |
| CNFene | CNF-graphene |
| CNTs | Carbon nanotubes |
| CP | Cellulose-based separator |
| DMSO | Dimethyl sulfoxide |
| ECG | Electrocardiogram |
| EEG | Electroencephalogram |
| EMG | Electromyography |
| EOG | Electrooculogram |
| FTIR | Fourier-transform infrared |
| GO | Graphene oxide |
| MOF | Metal–organic framework |
| MWCNT | Multi-walled CNT |
| MXene | Carbides and nitrides of transition metals |
| NPs | Nanoparticles |
| PA | Polyacetylene |
| PAA | Polyacrylic acid |
| PANI | Polyaniline |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PPC | Polycarbonate acrylic |
| PPy | Polypyrrole |
| PSS | Polystyrene sulfonate |
| PT | Polythiophene |
| PU | Polyurethane |
| PVA | Polyvinyl alcohol |
| R&D | Research and development |
| RGO | Reduced graphene oxide |
| SEM | Scanning electron microscope |
| TEMPO | 2,2,6,6-tetramethylpiperidine-1-oxylradi-cal |
| TENG | Triboelectric nanogenerator |
| TOS | Tosylate |
| TPU | Thermoplastic polyurethane |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Li, Y.; Zhang, J.; Chen, Q.; Xia, X.; Chen, M. Emerging of Heterostructure Materials in Energy Storage: A Review. Adv. Mater. 2021, 33, 2100855. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Jiang, G.; Zhou, J.; Zhu, Y.; Cheng, W.; Zhao, D.; Wang, K.; Xu, G.; Yu, H. Molecular-Scale Design of Cellulose-Based Functional Materials for Flexible Electronic Devices. Adv. Electron. Mater. 2021, 7, 2000944. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.M.; Spinks, G.M.; Kim, S.J. Carbon Nanotube Yarn for Fiber-Shaped Electrical Sensors, Actuators, and Energy Storage for Smart Systems. Adv. Mater. 2020, 32, 1902670. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhuo, H.; Hu, Y.; Lai, H.; Liu, L.; Zhong, L.; Peng, X. Wood-Derived Lightweight and Elastic Carbon Aerogel for Pressure Sensing and Energy Storage. Adv. Funct. Mater. 2020, 30, 1910292. [Google Scholar] [CrossRef]
- Mitchell, S.; Qin, R.; Zheng, N.; Pérez-Ramírez, J. Nanoscale Engineering of Catalytic Materials for Sustainable Technologies. Nat. Nanotechnol. 2021, 16, 129–139. [Google Scholar] [CrossRef]
- Varma, R.S. Biomass-Derived Renewable Carbonaceous Materials for Sustainable Chemical and Environmental Applications. ACS Sustain. Chem. Eng. 2019, 7, 6458–6470. [Google Scholar] [CrossRef]
- Singh, N.; Tang, Y.; Ogunseitan, O.A. Environmentally Sustainable Management of Used Personal Protective Equipment. Environ. Sci. Technol. 2020, 54, 8500–8502. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing Fibrillated Cellulose as a Sustainable Technological Material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Du, H.; Zheng, T.; Liu, H.; Zhang, M.; Zhang, R.; Li, H.; Xie, H.; Zhang, X.; Ma, M.; et al. Recent Advances in Cellulose and Its Derivatives for Oilfield Applications. Carbohydr. Polym. 2021, 259, 117740. [Google Scholar] [CrossRef]
- Tu, H.; Zhu, M.; Duan, B.; Zhang, L. Recent Progress in High-Strength and Robust Regenerated Cellulose Materials. Adv. Mater. 2021, 33, 2000682. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, L.; Lizundia, E.; Zhu, Y.; Chen, C.; Jiang, F. Cellulose-Based Ionic Conductor: An Emerging Material toward Sustainable Devices. Chem. Rev. 2023, 123, 9204–9264. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, K.; Du, H.; Zheng, T.; Zhang, N.; Xu, T.; Pang, B.; Zhang, X.; Si, C.; Zhang, K. Cellulose Nanopaper: Fabrication, Functionalization, and Applications. Nano-Micro Lett. 2022, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, Y.-H.; Kim, S.-W.; Seo, J.-Y.; Lee, S.-Y.; Nyholm, L. Why Cellulose-Based Electrochemical Energy Storage Devices? Adv. Mater. 2021, 33, 2000892. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, L.; D’Orsi, R.; Operamolla, A. Nanocellulose for Paper and Textile Coating: The Importance of Surface Chemistry. ChemPlusChem 2022, 87, e202200204. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xia, Z.; Mi, Q.; Zhang, J.; Zheng, X.; He, Z.; Wu, J.; Zhang, J. Cellulose-Based Conductive Films with Superior Joule Heating Performance, Electromagnetic Shielding Efficiency, and High Stability by In Situ Welding to Construct a Segregated MWCNT Conductive Network. Ind. Eng. Chem. Res. 2022, 61, 1773–1785. [Google Scholar] [CrossRef]
- Zha, F.; Chen, W.; Hao, L.; Wu, C.; Lu, M.; Zhang, L.; Yu, D. Electrospun Cellulose-Based Conductive Polymer Nanofibrous Mats: Composite Scaffolds and Their Influence on Cell Behavior with Electrical Stimulation for Nerve Tissue Engineering. Soft Matter 2020, 16, 6591–6598. [Google Scholar] [CrossRef]
- Sun, Z.; Qu, K.; You, Y.; Huang, Z.; Liu, S.; Li, J.; Hu, Q.; Guo, Z. Overview of Cellulose-Based Flexible Materials for Supercapacitors. J. Mater. Chem. A 2021, 9, 7278–7300. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, D.; Wang, D.; Luan, H.; Chen, X.; Yan, W. In Situ Polymerized MXene/Polypyrrole/Hydroxyethyl Cellulose-Based Flexible Strain Sensor Enabled by Machine Learning for Handwriting Recognition. ACS Appl. Mater. Interfaces 2023, 15, 5811–5821. [Google Scholar] [CrossRef]
- Wang, D.-C.; Yu, H.-Y.; Qi, D.; Wu, Y.; Chen, L.; Li, Z. Confined Chemical Transitions for Direct Extraction of Conductive Cellulose Nanofibers with Graphitized Carbon Shell at Low Temperature and Pressure. J. Am. Chem. Soc. 2021, 143, 11620–11630. [Google Scholar] [CrossRef]
- Yu, S.; Dong, X.; Zhao, P.; Luo, Z.; Sun, Z.; Yang, X.; Li, Q.; Wang, L.; Zhang, Y.; Zhou, H. Decoupled Temperature and Pressure Hydrothermal Synthesis of Carbon Sub-Micron Spheres from Cellulose. Nat. Commun. 2022, 13, 3616. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Son, S.M.; Kim, Y.E.; Lee, J.-E.; Hwang, S.-H.; Chae, H.G. Structure Evolution Mechanism of Highly Ordered Graphite during Carbonization of Cellulose Nanocrystals. Carbon 2019, 150, 142–152. [Google Scholar] [CrossRef]
- Pan, D.; Dong, J.; Yang, G.; Su, F.; Chang, B.; Liu, C.; Zhu, Y.-C.; Guo, Z. Ice Template Method Assists in Obtaining Carbonized Cellulose/Boron Nitride Aerogel with 3D Spatial Network Structure to Enhance the Thermal Conductivity and Flame Retardancy of Epoxy-Based Composites. Adv. Compos. Hybrid. Mater. 2022, 5, 58–70. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Li, D.; Xu, Y.; Xu, F. Flexible and Sensitivity-Adjustable Pressure Sensors Based on Carbonized Bacterial Nanocellulose/Wood-Derived Cellulose Nanofibril Composite Aerogels. ACS Appl. Mater. Interfaces 2021, 13, 8754–8763. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, T.; Han, J.; Zhang, Z.; Ni, Y. Recent Research Progress of Paper-Based Supercapacitors Based on Cellulose. Energy Environ. Mater. 2023, e12651. [Google Scholar] [CrossRef]
- Zhang, Z.; Abidi, N.; Lucia, L.; Chabi, S.; Denny, C.T.; Parajuli, P.; Rumi, S.S. Cellulose/Nanocellulose Superabsorbent Hydrogels as a Sustainable Platform for Materials Applications: A Mini-Review and Perspective. Carbohydr. Polym. 2023, 299, 120140. [Google Scholar] [CrossRef]
- Isogai, A. Emerging Nanocellulose Technologies: Recent Developments. Adv. Mater. 2021, 33, 2000630. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Amico, S.C. Recent Studies on Modified Cellulose/Nanocellulose Epoxy Composites: A Systematic Review. Carbohydr. Polym. 2021, 255, 117366. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable Isolation of Nanocellulose from Cellulose and Lignocellulosic Feedstocks: Recent Progress and Perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, Y.; Wu, W.; Xiao, H. Dispersion Properties of Nanocellulose: A Review. Carbohydr. Polym. 2020, 250, 116892. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Nonappa; Ikkala, O. Nanocellulose: Recent Fundamental Advances and Emerging Biological and Biomimicking Applications. Adv. Mater. 2021, 33, 2004349. [Google Scholar] [CrossRef] [PubMed]
- Żelaziński, T. Properties of Biocomposites from Rapeseed Meal, Fruit Pomace and Microcrystalline Cellulose Made by Press Pressing: Mechanical and Physicochemical Characteristics. Materials 2021, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, S.X.; Kong, W.; Chen, C.; Hitz, E.; Jia, C.; Dai, J.; Zhang, X.; Briber, R.; Siwy, Z.; et al. A Nanofluidic Ion Regulation Membrane with Aligned Cellulose Nanofibers. Sci. Adv. 2019, 5, eaau4238. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, A.; Chen, M.; Couillard, M.; Jakubek, Z.J.; Leng, T.; Johnston, L.J. Correlating Cellulose Nanocrystal Particle Size and Surface Area. Langmuir 2016, 32, 6105–6114. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Rezende, C.A.; Cranston, E.D. Fundamentals of Cellulose Lightweight Materials: Bio-Based Assemblies with Tailored Properties. Green. Chem. 2021, 23, 3542–3568. [Google Scholar] [CrossRef]
- Han, J.; Ding, Q.; Mei, C.; Wu, Q.; Yue, Y.; Xu, X. An Intrinsically Self-Healing and Biocompatible Electroconductive Hydrogel Based on Nanostructured Nanocellulose-Polyaniline Complexes Embedded in a Viscoelastic Polymer Network towards Flexible Conductors and Electrodes. Electrochim. Acta 2019, 318, 660–672. [Google Scholar] [CrossRef]
- Li, K.; Clarkson, C.M.; Wang, L.; Liu, Y.; Lamm, M.; Pang, Z.; Zhou, Y.; Qian, J.; Tajvidi, M.; Gardner, D.J.; et al. Alignment of Cellulose Nanofibers: Harnessing Nanoscale Properties to Macroscale Benefits. ACS Nano 2021, 15, 3646–3673. [Google Scholar] [CrossRef]
- Dizge, N.; Shaulsky, E.; Karanikola, V. Electrospun Cellulose Nanofibers for Superhydrophobic and Oleophobic Membranes. J. Membr. Sci. 2019, 590, 117271. [Google Scholar] [CrossRef]
- Lamm, M.E.; Li, K.; Qian, J.; Wang, L.; Lavoine, N.; Newman, R.; Gardner, D.J.; Li, T.; Hu, L.; Ragauskas, A.J.; et al. Recent Advances in Functional Materials through Cellulose Nanofiber Templating. Adv. Mater. 2021, 33, 2005538. [Google Scholar] [CrossRef]
- Xu, Y.; Song, Y.; Xu, F. TEMPO Oxidized Cellulose Nanofibers-Based Heterogenous Membrane Employed for Concentration-Gradient-Driven Energy Harvesting. Nano Energy 2021, 79, 105468. [Google Scholar] [CrossRef]
- Lee, K.; Jeon, Y.; Kim, D.; Kwon, G.; Kim, U.-J.; Hong, C.; Choung, J.W.; You, J. Double-Crosslinked Cellulose Nanofiber Based Bioplastic Films for Practical Applications. Carbohydr. Polym. 2021, 260, 117817. [Google Scholar] [CrossRef] [PubMed]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2020, 10, 65. [Google Scholar] [CrossRef]
- Yin, Z.; Li, M.; Chen, Z.; Chen, X.; Liu, K.; Zhou, D.; Xue, M.; Ou, J.; Xie, Y.; Lei, S.; et al. A Superhydrophobic Pulp/Cellulose Nanofiber (CNF) Membrane via Coating ZnO Suspensions for Multifunctional Applications. Ind. Crops Prod. 2022, 187, 115526. [Google Scholar] [CrossRef]
- Jiang, J.; Carrillo-Enríquez, N.C.; Oguzlu, H.; Han, X.; Bi, R.; Song, M.; Saddler, J.N.; Sun, R.-C.; Jiang, F. High Production Yield and More Thermally Stable Lignin-Containing Cellulose Nanocrystals Isolated Using a Ternary Acidic Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2020, 8, 7182–7191. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Cranston, E.D. Production Routes to Tailor the Performance of Cellulose Nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Droguet, B.E.; Liang, H.-L.; Frka-Petesic, B.; Parker, R.M.; De Volder, M.F.L.; Baumberg, J.J.; Vignolini, S. Large-Scale Fabrication of Structurally Coloured Cellulose Nanocrystal Films and Effect Pigments. Nat. Mater. 2022, 21, 352–358. [Google Scholar] [CrossRef]
- Thach-Nguyen, R.; Lam, H.-H.; Phan, H.-P.; Dang-Bao, T. Cellulose Nanocrystals Isolated from Corn Leaf: Straightforward Immobilization of Silver Nanoparticles as a Reduction Catalyst. RSC Adv. 2022, 12, 35436–35444. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, C.; Jiang, H.; Liu, F.; Cheng, G.J. Direct Ink Writing of Hierarchically Porous Cellulose/Alginate Monolithic Hydrogel as a Highly Effective Adsorbent for Environmental Applications. ACS Appl. Polym. Mater. 2021, 3, 699–709. [Google Scholar] [CrossRef]
- Yang, L.; Cui, J.; Zhang, L.; Xu, X.; Chen, X.; Sun, D. A Moisture-Driven Actuator Based on Polydopamine-Modified MXene/Bacterial Cellulose Nanofiber Composite Film. Adv. Funct. Mater. 2021, 31, 2101378. [Google Scholar] [CrossRef]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The Optimization of Bacterial Cellulose Production and Its Applications: A Review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial Cellulose-Based Composite Scaffolds for Biomedical Applications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Peter, Z. Order in Cellulosics: Historical Review of Crystal Structure Research on Cellulose. Carbohydr. Polym. 2021, 254, 117417. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Tian, D.; Liang, S.; Jiang, P.; Zhang, L.; Fu, F. Research Progress on the Preparation of Flexible and Green Cellulose-Based Electrothermal Composites for Joule Heating Applications. ACS Appl. Energy Mater. 2022, 5, 13096–13112. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, T.; Zhou, L.; Zhang, Y.; Dai, L.; Zhou, Q.; Ni, Y. Fabrication of Dual-Function Conductive Cellulose-Based Composites with Layered Conductive Network Structures for Supercapacitors and Electromagnetic Shielding. Chem. Eng. J. 2023, 472, 144958. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, K.; Yuan, F.; Zhang, W.; Li, B.; Seidi, F.; Xiao, H.; Sun, D. N-Doped Porous Carbon Nanofibers Fabricated by Bacterial Cellulose-Directed Templating Growth of MOF Crystals for Efficient Oxygen Reduction Reaction and Sodium-Ion Storage. Carbon 2020, 168, 12–21. [Google Scholar] [CrossRef]
- Luo, W.; Guo, N.; Wang, L.; Jia, D.; Xu, M.; Zhang, S.; Ai, L.; Sheng, R.; Feng, S.; Gong, X.; et al. Homogeneous Activation Induced by Bacterial Cellulose Nanofibers to Construct Interconnected Microporous Carbons for Enhanced Capacitive Storage. J. Colloid Interface Sci. 2023, 636, 33–41. [Google Scholar] [CrossRef]
- Chen, C.; Song, J.; Zhu, S.; Li, Y.; Kuang, Y.; Wan, J.; Kirsch, D.; Xu, L.; Wang, Y.; Gao, T.; et al. Scalable and Sustainable Approach toward Highly Compressible, Anisotropic, Lamellar Carbon Sponge. Chem 2018, 4, 544–554. [Google Scholar] [CrossRef]
- Cao, M.-S.; Wang, X.-X.; Zhang, M.; Shu, J.-C.; Cao, W.-Q.; Yang, H.-J.; Fang, X.-Y.; Yuan, J. Electromagnetic Response and Energy Conversion for Functions and Devices in Low-Dimensional Materials. Adv. Funct. Mater. 2019, 29, 1807398. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, P.; Fu, L.; Hu, Y.; Yang, L.; Wu, W.; Kong, X.-Y.; Jiang, L.; Wen, L. Engineered Cellulose Nanofiber Membranes with Ultrathin Low-Dimensional Carbon Material Layers for Photothermal-Enhanced Osmotic Energy Conversion. ACS Appl. Mater. Interfaces 2022, 14, 13223–13230. [Google Scholar] [CrossRef]
- Pinto, S.C.; Gonçalves, G.; Sandoval, S.; López-Periago, A.M.; Borras, A.; Domingo, C.; Tobias, G.; Duarte, I.; Vicente, R.; Marques, P.A.A.P. Bacterial Cellulose/Graphene Oxide Aerogels with Enhanced Dimensional and Thermal Stability. Carbohydr. Polym. 2020, 230, 115598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, Z. Ultralight, Highly Compressible, Hydrophobic and Anisotropic Lamellar Carbon Aerogels from Graphene/Polyvinyl Alcohol/Cellulose Nanofiber Aerogel as Oil Removing Absorbents. J. Hazard. Mater. 2020, 388, 121804. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Liu, D.; Wang, Y.; Zhao, Y.; Yang, X.; Huang, J. High-Performance Sodium-Ion Battery Anode via Rapid Microwave Carbonization of Natural Cellulose Nanofibers with Graphene Initiator. Small 2019, 15, 1901724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Ou, H.; Kuang, Y.; Hao, L.; Diao, J.; Chen, G. Cellulose Nanofiber/Carbon Nanotube Dual Network-Enabled Humidity Sensor with High Sensitivity and Durability. ACS Appl. Mater. Interfaces 2020, 12, 33229–33238. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, Y.; Xie, W.; Meng, T.; Zhao, X.; Pang, Z.; Chen, Q.; Liu, D.; Wang, R.; Yang, V.; et al. Lightweight, Thermally Insulating, Fire-Proof Graphite-Cellulose Foam. Adv. Funct. Mater. 2023, 33, 2204219. [Google Scholar] [CrossRef]
- Zou, Z.; Zhou, W.; Zhang, Y.; Yu, H.; Hu, C.; Xiao, W. High-Performance Flexible All-Solid-State Supercapacitor Constructed by Free-Standing Cellulose/Reduced Graphene Oxide/Silver Nanoparticles Composite Film. Chem. Eng. J. 2019, 357, 45–55. [Google Scholar] [CrossRef]
- Fei, Y.; Liang, M.; Yan, L.; Chen, Y.; Zou, H. Co/C@cellulose Nanofiber Aerogel Derived from Metal-Organic Frameworks for Highly Efficient Electromagnetic Interference Shielding. Chem. Eng. J. 2020, 392, 124815. [Google Scholar] [CrossRef]
- Garino, N.; Lamberti, A.; Stassi, S.; Castellino, M.; Fontana, M.; Roppolo, I.; Sacco, A.; Pirri, C.F.; Chiappone, A. Multifunctional Flexible Membranes Based on Reduced Graphene Oxide/Tin Dioxide Nanocomposite and Cellulose Fibers. Electrochim. Acta 2019, 306, 420–426. [Google Scholar] [CrossRef]
- Liao, S.-Y.; Wang, X.-Y.; Li, X.-M.; Wan, Y.-J.; Zhao, T.; Hu, Y.-G.; Zhu, P.-L.; Sun, R.; Wong, C.-P. Flexible Liquid Metal/Cellulose Nanofiber Composites Film with Excellent Thermal Reliability for Highly Efficient and Broadband EMI Shielding. Chem. Eng. J. 2021, 422, 129962. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Jiang, Y.; Song, X.; Tang, G.; Zhang, X. A Novel C Doped MoS2/CoP/MoO2 Ternary Heterostructure Nanoflower for Hydrogen Evolution Reaction at Wide pH Range and Efficient Overall Water Splitting in Alkaline Media. Chem. A Eur. J. 2023, 29, e202300629. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene Chemistry, Electrochemistry and Energy Storage Applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Li, X.; Bai, Y.; Xiao, H.; Liu, Y.; Liu, R.; Yuan, G. Engineering 3D Ion Transport Channels for Flexible MXene Films with Superior Capacitive Performance. Adv. Funct. Mater. 2019, 29, 1900326. [Google Scholar] [CrossRef]
- Uzun, S.; Seyedin, S.; Stoltzfus, A.L.; Levitt, A.S.; Alhabeb, M.; Anayee, M.; Strobel, C.J.; Razal, J.M.; Dion, G.; Gogotsi, Y. Knittable and Washable Multifunctional MXene-Coated Cellulose Yarns. Adv. Funct. Mater. 2019, 29, 1905015. [Google Scholar] [CrossRef]
- Wu, N.; Yang, Y.; Wang, C.; Wu, Q.; Pan, F.; Zhang, R.; Liu, J.; Zeng, Z. Ultrathin Cellulose Nanofiber Assisted Ambient-Pressure-Dried, Ultralight, Mechanically Robust, Multifunctional MXene Aerogels. Adv. Mater. 2023, 35, 2207969. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.-T.; Ma, C.; Mao, D.-S.; Zhang, J.; Ma, M.-G.; Chen, F. MXene-Reinforced Cellulose Nanofibril Inks for 3D-Printed Smart Fibres and Textiles. Adv. Funct. Mater. 2019, 29, 1905898. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Zhao, Z.; Liang, D.; Zhong, W.-H.; Zhang, J. A Novel Structural Design of Cellulose-Based Conductive Composite Fibers for Wearable e-Textiles. Carbohydr. Polym. 2023, 321, 121308. [Google Scholar] [CrossRef]
- Huang, Y.; Kormakov, S.; He, X.; Gao, X.; Zheng, X.; Liu, Y.; Sun, J.; Wu, D. Conductive Polymer Composites from Renewable Resources: An Overview of Preparation, Properties, and Applications. Polymers 2019, 11, 187. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhu, S.; Huang, C.; Yue, Y.; Mei, C.; Xu, X.; Xia, C. Electrospun Core–Shell Nanofibrous Membranes with Nanocellulose-Stabilized Carbon Nanotubes for Use as High-Performance Flexible Supercapacitor Electrodes with Enhanced Water Resistance, Thermal Stability, and Mechanical Toughness. ACS Appl. Mater. Interfaces 2019, 11, 44624–44635. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Park, J.-O.; Zheng, S.; Choi, E. Ultralow Voltage High-Performance Bioartificial Muscles Based on Ionically Crosslinked Polypyrrole-Coated Functional Carboxylated Bacterial Cellulose for Soft Robots. Adv. Funct. Mater. 2021, 31, 2007749. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chen, Z.-Y.; Mao, M.; Wu, Y.-Y.; Yang, F.; Gong, L.-X.; Zhao, L.; Cao, C.-F.; Song, P.; Gao, J.-F.; et al. Self-Adhesive Polydimethylsiloxane Foam Materials Decorated with MXene/Cellulose Nanofiber Interconnected Network for Versatile Functionalities. Adv. Funct. Mater. 2023, 2304927. [Google Scholar] [CrossRef]
- Shi, K.; Zou, H.; Sun, B.; Jiang, P.; He, J.; Huang, X. Dielectric Modulated Cellulose Paper/PDMS-Based Triboelectric Nanogenerators for Wireless Transmission and Electropolymerization Applications. Adv. Funct. Mater. 2020, 30, 1904536. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Zhu, Y.; Cheng, W.; Zhao, D.; Xu, G.; Yu, H. Assembly of Silver Nanowires and PEDOT:PSS with Hydrocellulose toward Highly Flexible, Transparent and Conductivity-Stable Conductors. Chem. Eng. J. 2020, 392, 123644. [Google Scholar] [CrossRef]
- Françon, H.; Wang, Z.; Marais, A.; Mystek, K.; Piper, A.; Granberg, H.; Malti, A.; Gatenholm, P.; Larsson, P.A.; Wågberg, L. Ambient-Dried, 3D-Printable and Electrically Conducting Cellulose Nanofiber Aerogels by Inclusion of Functional Polymers. Adv. Funct. Mater. 2020, 30, 1909383. [Google Scholar] [CrossRef]
- Liang, X.; Tian, Y.; Yuan, Y.; Kim, Y. Ionic Covalent Organic Frameworks for Energy Devices. Adv. Mater. 2021, 33, 2105647. [Google Scholar] [CrossRef]
- Xu, T.; Du, H.; Liu, H.; Liu, W.; Zhang, X.; Si, C.; Liu, P.; Zhang, K. Advanced Nanocellulose-Based Composites for Flexible Functional Energy Storage Devices. Adv. Mater. 2021, 33, 2101368. [Google Scholar] [CrossRef]
- Liu, H.; Du, H.; Zheng, T.; Liu, K.; Ji, X.; Xu, T.; Zhang, X.; Si, C. Cellulose Based Composite Foams and Aerogels for Advanced Energy Storage Devices. Chem. Eng. J. 2021, 426, 130817. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, F.; Wang, J.; Au, H.; Tebyetekerwa, M.; Guo, Z.; Yang, S.; Hu, Y.-S.; Titirici, M.-M. All-Cellulose-Based Quasi-Solid-State Sodium-Ion Hybrid Capacitors Enabled by Structural Hierarchy. Adv. Funct. Mater. 2019, 29, 1903895. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, W.; Zhang, M.; Liu, H.; Zhang, X.; Si, C. Lignin-Containing Cellulose Nanomaterials: Preparation and Applications. Green Chem. 2021, 23, 9723–9746. [Google Scholar] [CrossRef]
- Deng, J.; Cao, D.; Yang, X.; Zhang, G. Cross-Linked Cellulose/Carboxylated Polyimide Nanofiber Separator for Lithium-Ion Battery Application. Chem. Eng. J. 2022, 433, 133934. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zeng, Q.; Liu, X.; Lai, W.-Y.; Zhang, L. Cellulose Microcrystals with Brush-Like Architectures as Flexible All-Solid-State Polymer Electrolyte for Lithium-Ion Battery. ACS Sustain. Chem. Eng. 2020, 8, 3200–3207. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Acidic Deep Eutectic Solvents as Hydrolytic Media for Cellulose Nanocrystal Production. Biomacromolecules 2016, 17, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, Q.; Xie, W.; Zhang, X.; Brozena, A.; Zheng, J.; Garaga, M.N.; Ko, B.H.; Mao, Y.; He, S.; et al. Copper-Coordinated Cellulose Ion Conductors for Solid-State Batteries. Nature 2021, 598, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Zhao, X.-X.; Zhang, W.; Ren, K.; Luo, X.-X.; Cao, J.-M.; Zheng, S.-H.; Li, W.-L.; Wu, X.-L. “Pore-Hopping” Ion Transport in Cellulose-Based Separator Towards High-Performance Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202300258. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Q.; Zhou, J.; Wang, X.; Lu, B. Natural Supra-Molecular Structure Engineering for High-Performance Potassium Batteries Separator. Adv. Energy Mater. 2022, 12, 2202357. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Liu, R.; Chen, W.; Zhang, H.; Zhang, Z. Gel Polymer-Based Composite Solid-State Electrolyte for Long-Cycle-Life Rechargeable Zinc–Air Batteries. ACS Sustain. Chem. Eng. 2023, 11, 3732–3739. [Google Scholar] [CrossRef]
- Zou, H.; Wang, Y.; Li, X.; Ding, P.; Guo, H.; Li, F. Multilayered Amidated Polymer of Intrinsic Microporosity for Gel Polymer Electrolyte in Lithium Metal Batteries. J. Membr. Sci. 2023, 685, 121939. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, M.; Wang, W.; Liu, S.; Huang, W.; Zhao, Q. Flexible Transparent Supercapacitors: Materials and Devices. Adv. Funct. Mater. 2021, 31, 2009136. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing Flexible, Smart and Self-Sustainable Supercapacitors for Portable/Wearable Electronics: From Conductive Polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A Review on the Recent Advances in Hybrid Supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Zhao, X.; Tao, K.; Han, L. Self-Supported Metal–Organic Framework-Based Nanostructures as Binder-Free Electrodes for Supercapacitors. Nanoscale 2022, 14, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-M.; Yu, H.-Y.; Wang, D.-C.; Yang, T.; Yao, J.-M.; Tam, K.C. Simple Synthesis of Flower-like Manganese Dioxide Nanostructures on Cellulose Nanocrystals for High-Performance Supercapacitors and Wearable Electrodes. ACS Sustain. Chem. Eng. 2019, 7, 11823–11831. [Google Scholar] [CrossRef]
- Meng, X.; Jia, S.; Mo, L.; Wei, J.; Wang, F.; Shao, Z. O/N-Co-Doped Hierarchically Porous Carbon from Carboxymethyl Cellulose Ammonium for High-Performance Supercapacitors. J. Mater. Sci. 2020, 55, 7417–7431. [Google Scholar] [CrossRef]
- Bai, Q.; Shen, Y.; Asoh, T.-A.; Li, C.; Dan, Y.; Uyama, H. Controlled Preparation of Interconnected 3D Hierarchical Porous Carbons from Bacterial Cellulose-Based Composite Monoliths for Supercapacitors. Nanoscale 2020, 12, 15261–15274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Kong, X.; Zheng, B.; Huo, F.; Strømme, M.; Xu, C. Cellulose Nanofiber @ Conductive Metal–Organic Frameworks for High-Performance Flexible Supercapacitors. ACS Nano 2019, 13, 9578–9586. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, L.; Liu, R.; He, H.; Hao, J.; Lu, Y.; Wang, Y.; Liang, G.; Yuan, G.; Guo, Z. Engineering Textile Electrode and Bacterial Cellulose Nanofiber Reinforced Hydrogel Electrolyte to Enable High-Performance Flexible All-Solid-State Supercapacitors. Adv. Energy Mater. 2021, 11, 2003010. [Google Scholar] [CrossRef]
- Zhou, G.; Li, M.-C.; Liu, C.; Wu, Q.; Mei, C. 3D Printed Ti3C2Tx MXene/Cellulose Nanofiber Architectures for Solid-State Supercapacitors: Ink Rheology, 3D Printability, and Electrochemical Performance. Adv. Funct. Mater. 2022, 32, 2109593. [Google Scholar] [CrossRef]
- Wang, D.-C.; Yu, H.-Y.; Qi, D.; Ramasamy, M.; Yao, J.; Tang, F.; Tam, K.C.; Ni, Q. Supramolecular Self-Assembly of 3D Conductive Cellulose Nanofiber Aerogels for Flexible Supercapacitors and Ultrasensitive Sensors. ACS Appl. Mater. Interfaces 2019, 11, 24435–24446. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Qin, C.; Qian, X.; Zhang, L.; Zhou, J.; Lu, A. Transparent, Conductive Cellulose Hydrogel for Flexible Sensor and Triboelectric Nanogenerator at Subzero Temperature. Carbohydr. Polym. 2021, 265, 118078. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhou, J.; Lu, A. Flexible and Transparent Cellulose-Based Ionic Film as a Humidity Sensor. ACS Appl. Mater. Interfaces 2020, 12, 7631–7638. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Sanfelice, R.C.; Migliorini, F.L.; Pavinatto, A.; Facure, M.H.M.; Correa, D.S. A Review on the Role and Performance of Cellulose Nanomaterials in Sensors. ACS Sens. 2021, 6, 2473–2496. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Li, Q.; Wang, S.; Luo, X.; Deng, H.; Liu, S. Porous Structured Cellulose Microsphere Acts as Biosensor for Glucose Detection with “Signal-and-Color” Output. Carbohydr. Polym. 2019, 205, 295–301. [Google Scholar] [CrossRef]
- Palanisamy, S.; Velusamy, V.; Chen, S.-W.; Yang, T.C.K.; Balu, S.; Banks, C.E. Enhanced Reversible Redox Activity of Hemin on Cellulose Microfiber Integrated Reduced Graphene Oxide for H2O2 Biosensor Applications. Carbohydr. Polym. 2019, 204, 152–160. [Google Scholar] [CrossRef]
- Khattab, T.A.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Development of Microporous Cellulose-Based Smart Xerogel Reversible Sensor via Freeze Drying for Naked-Eye Detection of Ammonia Gas. Carbohydr. Polym. 2019, 210, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Choi, J. Hybrid Integration of Carbon Nanotubes and Transition Metal Dichalcogenides on Cellulose Paper for Highly Sensitive and Extremely Deformable Chemical Sensors. ACS Appl. Mater. Interfaces 2019, 11, 19363–19371. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Dong, X.; Li, D.; Bai, L.; Wang, X.; Wang, Y.; Song, F. Photonic Cellulose Films with Vivid Structural Colors: Fabrication and Selectively Chemical Response. Biomacromolecules 2022, 23, 1662–1671. [Google Scholar] [CrossRef]
- Rahman, A.; Kang, S.; Wang, W.; Huang, Q.; Kim, I.; Vikesland, P.J. Lectin-Modified Bacterial Cellulose Nanocrystals Decorated with Au Nanoparticles for Selective Detection of Bacteria Using Surface-Enhanced Raman Scattering Coupled with Machine Learning. ACS Appl. Nano Mater. 2022, 5, 259–268. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Yun, Y.J.; Jeong, J.; Kim, C.-Y.; Müller, K.-R.; Lee, S.-W. Leaf-Inspired Homeostatic Cellulose Biosensors. Sci. Adv. 2021, 7, 16. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Wei, X.; Zhang, J.; Wang, D.; Lu, H.; Jia, P. Ultrastretchable, Tough, Antifreezing, and Conductive Cellulose Hydrogel for Wearable Strain Sensor. ACS Appl. Mater. Interfaces 2020, 12, 53247–53256. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, T.; Pan, Z. Review of Flexible Strain Sensors Based on Cellulose Composites for Multi-Faceted Applications. Cellulose 2021, 28, 615–645. [Google Scholar] [CrossRef]
- Wu, G.; Gu, Y.; Hou, X.; Li, R.; Ke, H.; Xiao, X. Hybrid Nanocomposites of Cellulose/Carbon-Nanotubes/Polyurethane with Rapidly Water Sensitive Shape Memory Effect and Strain Sensing Performance. Polymers 2019, 11, 1586. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Shen, Y.; Wang, C.; Wang, J.; Yong, Q.; Chu, F. Liquid-Free, Anti-Freezing, Solvent-Resistant, Cellulose-Derived Ionic Conductive Elastomer for Stretchable Wearable Electronics and Triboelectric Nanogenerators. Adv. Funct. Mater. 2022, 32, 2207714. [Google Scholar] [CrossRef]
- Sheng, N.; Ji, P.; Zhang, M.; Wu, Z.; Liang, Q.; Chen, S.; Wang, H. High Sensitivity Polyurethane-Based Fiber Strain Sensor with Porous Structure via Incorporation of Bacterial Cellulose Nanofibers. Adv. Electron. Mater. 2021, 7, 2001235. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Y.; Zhu, J.; Le, K.; Servati, P.; Jiang, F. Tough and Ultrastretchable Liquid-Free Ion Conductor Strengthened by Deep Eutectic Solvent Hydrolyzed Cellulose Microfibers. Adv. Funct. Mater. 2022, 32, 2202533. [Google Scholar] [CrossRef]
- Wang, D.-C.; Yu, H.-Y.; Ouyang, Z.; Qi, D.; Zhou, Y.; Ju, A.; Li, Z.; Cao, Y. Chain-Ring Covalently Interconnected Cellulose Nanofiber/MWCNT Aerogel for Supercapacitors and Sensors. Nanoscale 2022, 14, 5163–5173. [Google Scholar] [CrossRef]
- Sun, J.; Xiu, K.; Wang, Z.; Hu, N.; Zhao, L.; Zhu, H.; Kong, F.; Xiao, J.; Cheng, L.; Bi, X. Multifunctional Wearable Humidity and Pressure Sensors Based on Biocompatible Graphene/Bacterial Cellulose Bioaerogel for Wireless Monitoring and Early Warning of Sleep Apnea Syndrome. Nano Energy 2023, 108, 108215. [Google Scholar] [CrossRef]
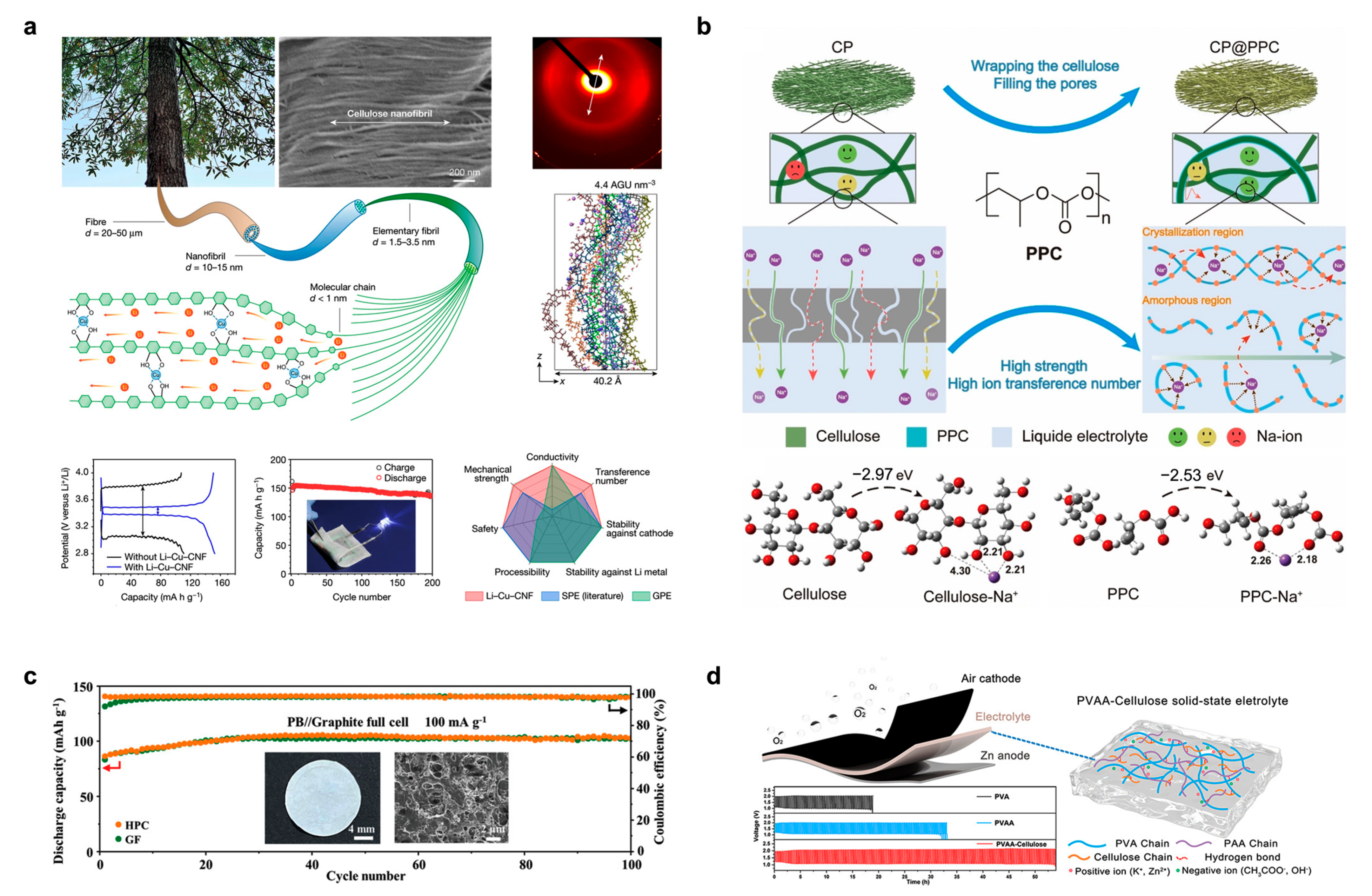

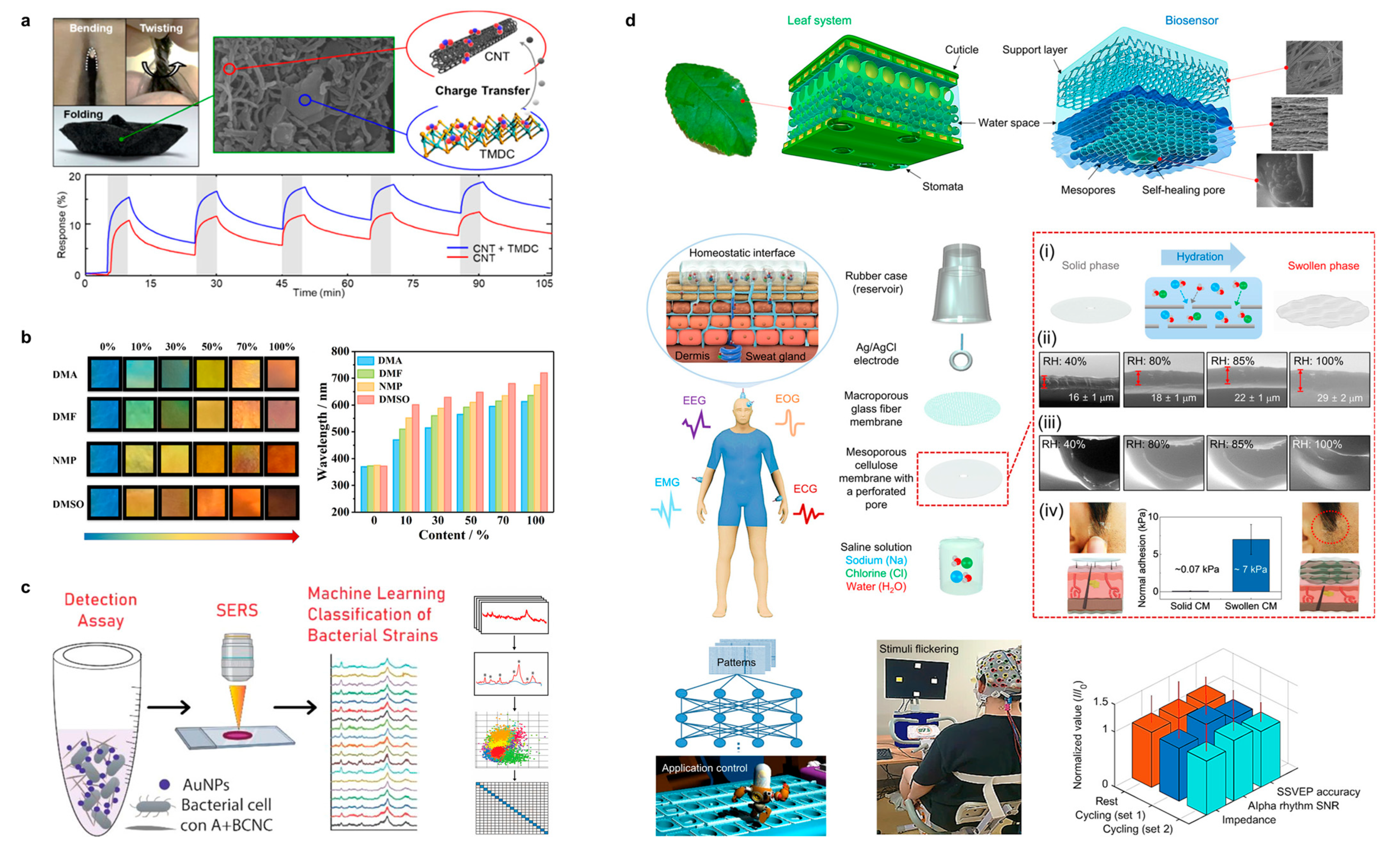
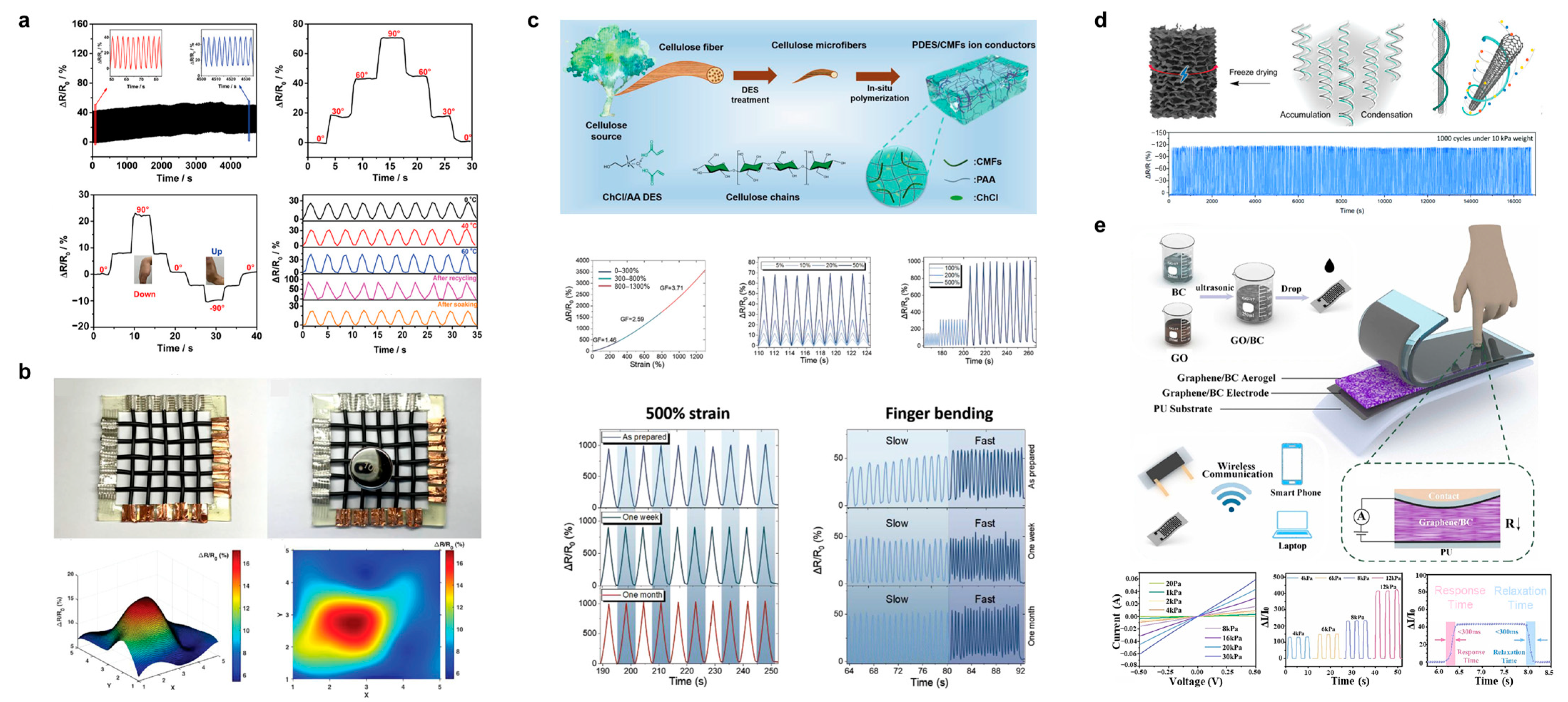
| Sample | Conductivity | Capacitance | Capacitance Retention | Energy Density | Power Density | Ref. |
|---|---|---|---|---|---|---|
| CNF/c-MOF | 100 S/m | 103 F/g | >99% after 10,000 cycles | 6.5 mWh/cm2 | 0.013 mW/cm2 | [105] |
| BC-RGO-PANI | 125 mS/cm | 564 mF/cm2 | 94.4% after 10,000 cycles | 50.1 μWh/cm2 | 20 mW/cm2 | [106] |
| CNF/MXene | - | 2.02 F/cm2 | 85% after 5000 cycles | 101 μWh/cm2 | 0.299 mW/cm2 | [107] |
| PANI/CNF | 0.372 mS/cm | 291.01 F/g | 75.6% after 2000 cycles | - | - | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.-C.; Lei, S.-N.; Zhong, S.; Xiao, X.; Guo, Q.-H. Cellulose-Based Conductive Materials for Energy and Sensing Applications. Polymers 2023, 15, 4159. https://doi.org/10.3390/polym15204159
Wang D-C, Lei S-N, Zhong S, Xiao X, Guo Q-H. Cellulose-Based Conductive Materials for Energy and Sensing Applications. Polymers. 2023; 15(20):4159. https://doi.org/10.3390/polym15204159
Chicago/Turabian StyleWang, Duan-Chao, Sheng-Nan Lei, Shenjie Zhong, Xuedong Xiao, and Qing-Hui Guo. 2023. "Cellulose-Based Conductive Materials for Energy and Sensing Applications" Polymers 15, no. 20: 4159. https://doi.org/10.3390/polym15204159
APA StyleWang, D.-C., Lei, S.-N., Zhong, S., Xiao, X., & Guo, Q.-H. (2023). Cellulose-Based Conductive Materials for Energy and Sensing Applications. Polymers, 15(20), 4159. https://doi.org/10.3390/polym15204159






