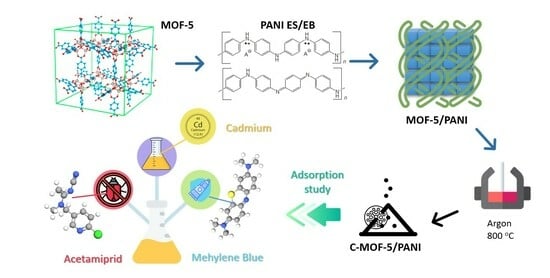
Environmental Potential of Carbonized MOF-5/PANI Composites for Pesticide, Dye, and Metal Cations—Can They Actually Retain Them All?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Syntheses
2.2. Materials Characterization
2.3. Adsorption Study
3. Results and Discussion
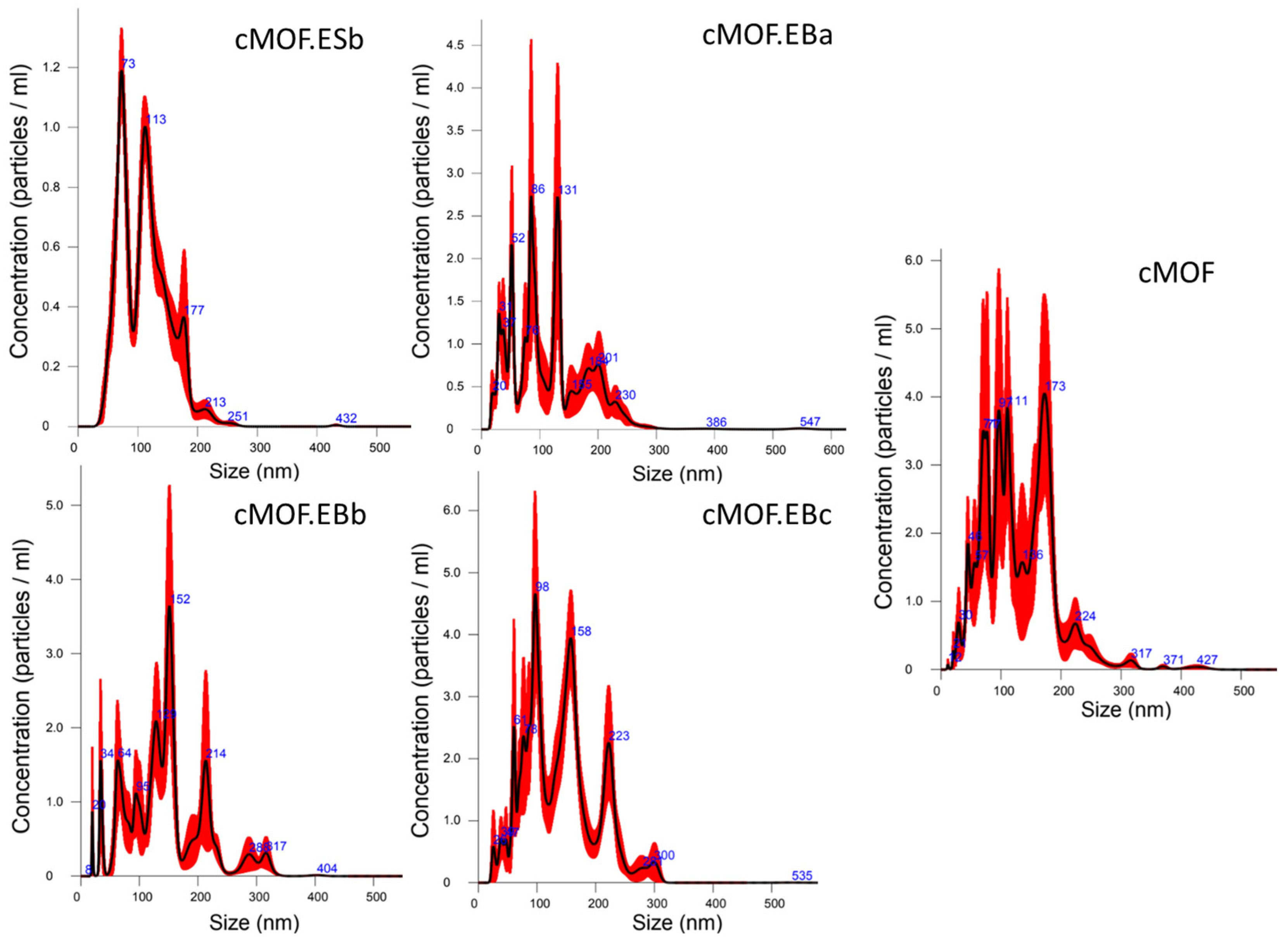
3.1. Particle Size
3.2. Textural Properties
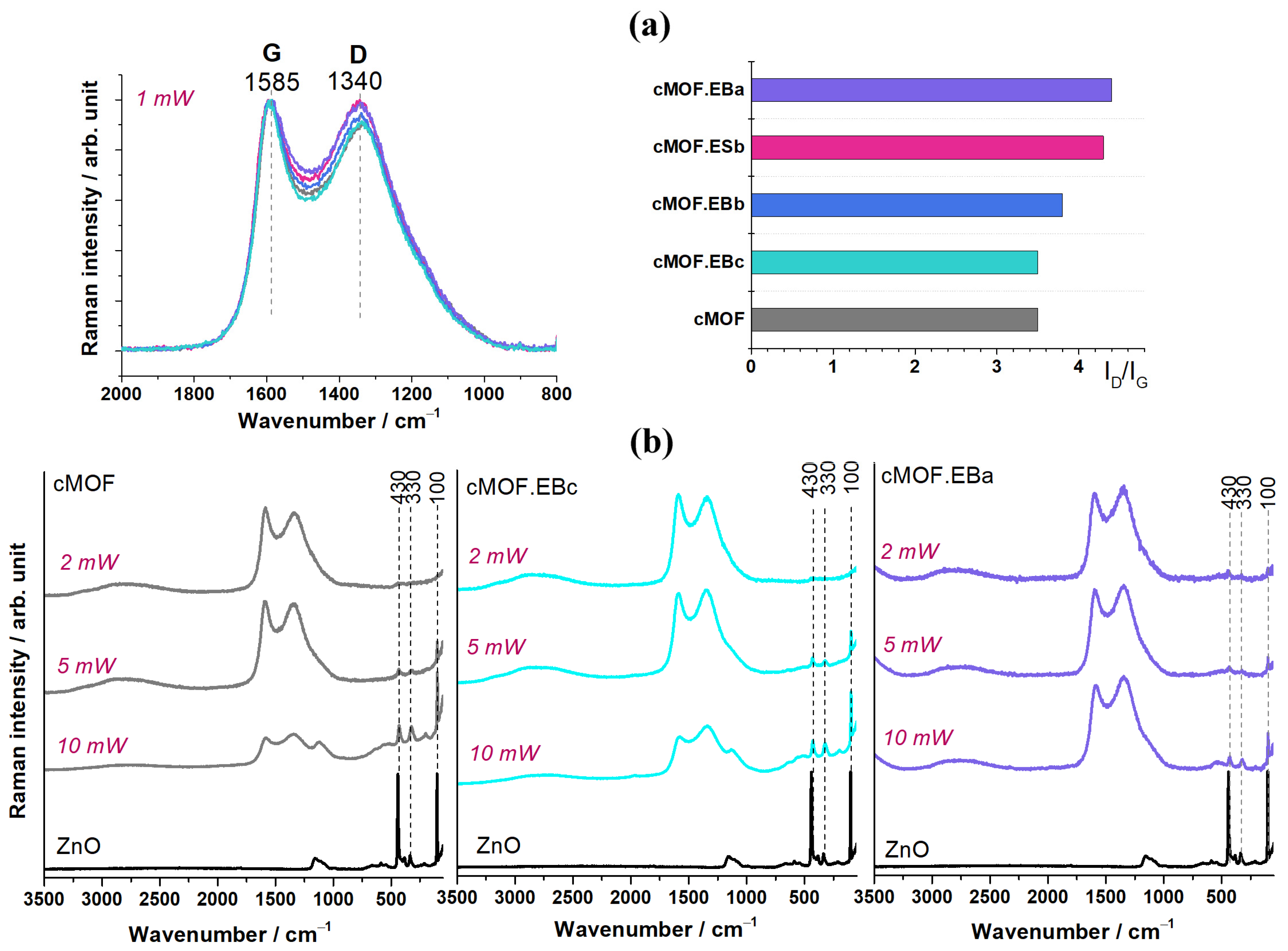
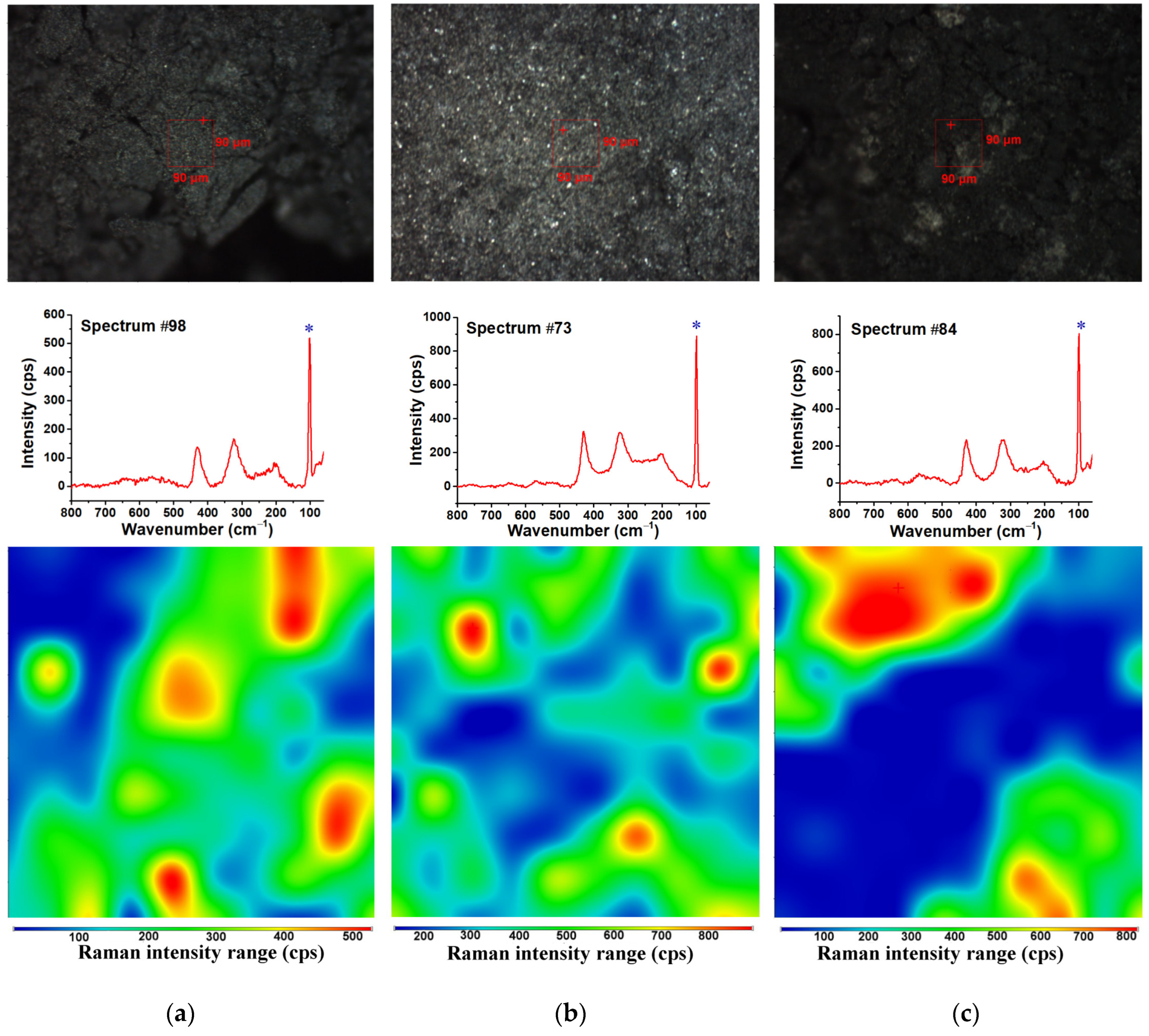
3.3. Raman Spectroscopy
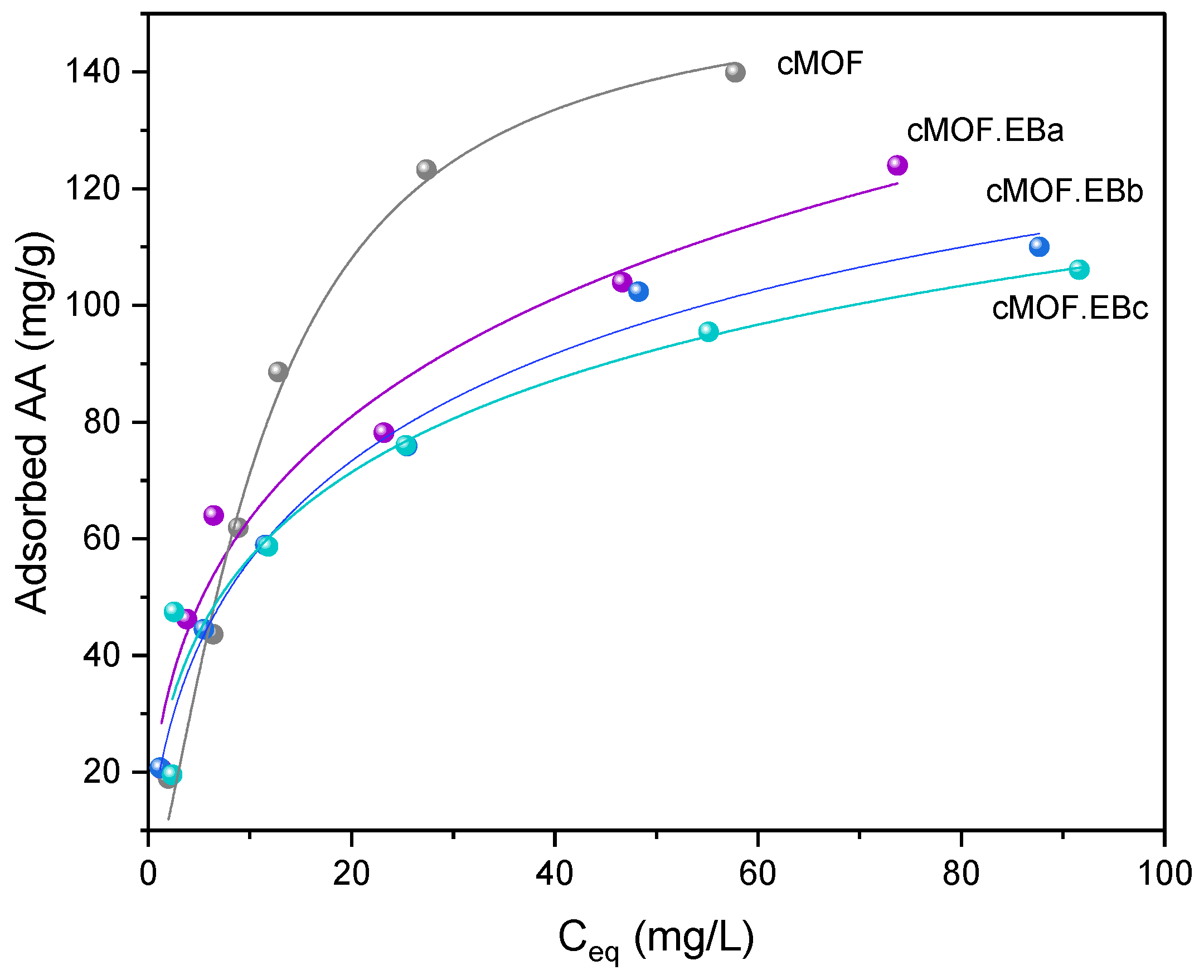
3.4. Pesticide Adsorption
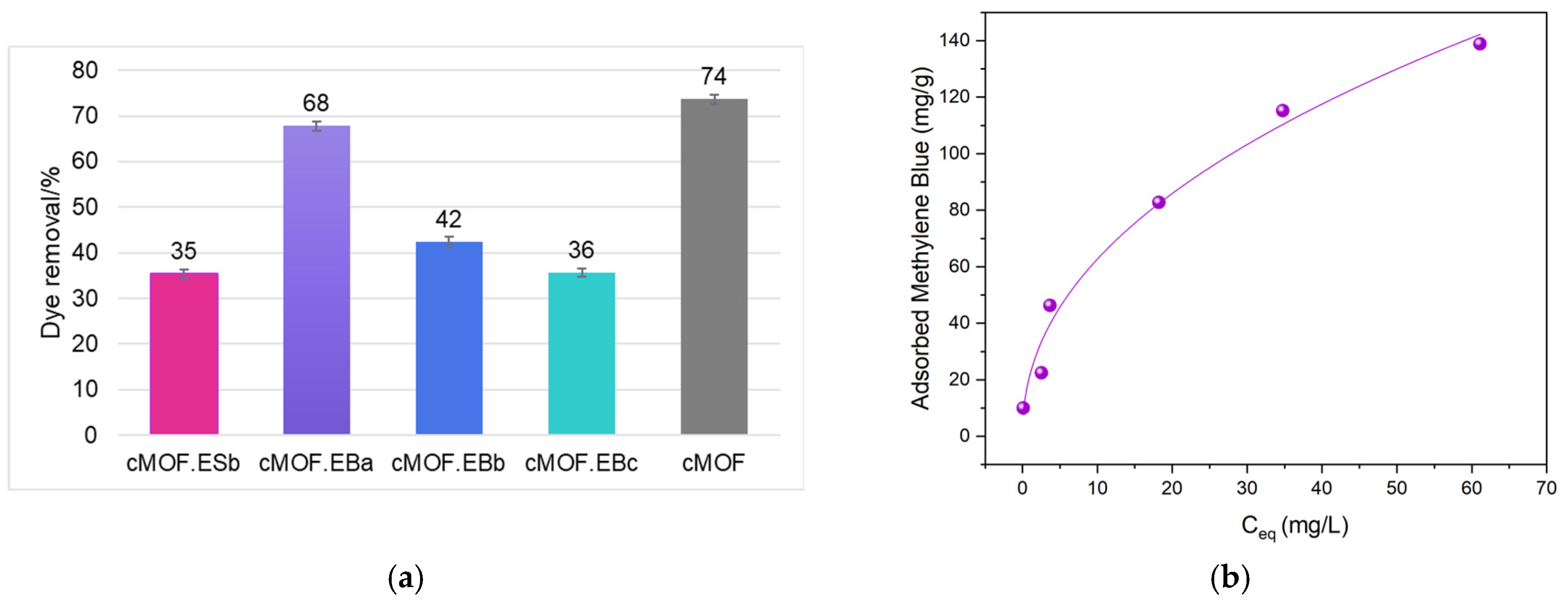
3.5. Dye Adsorption
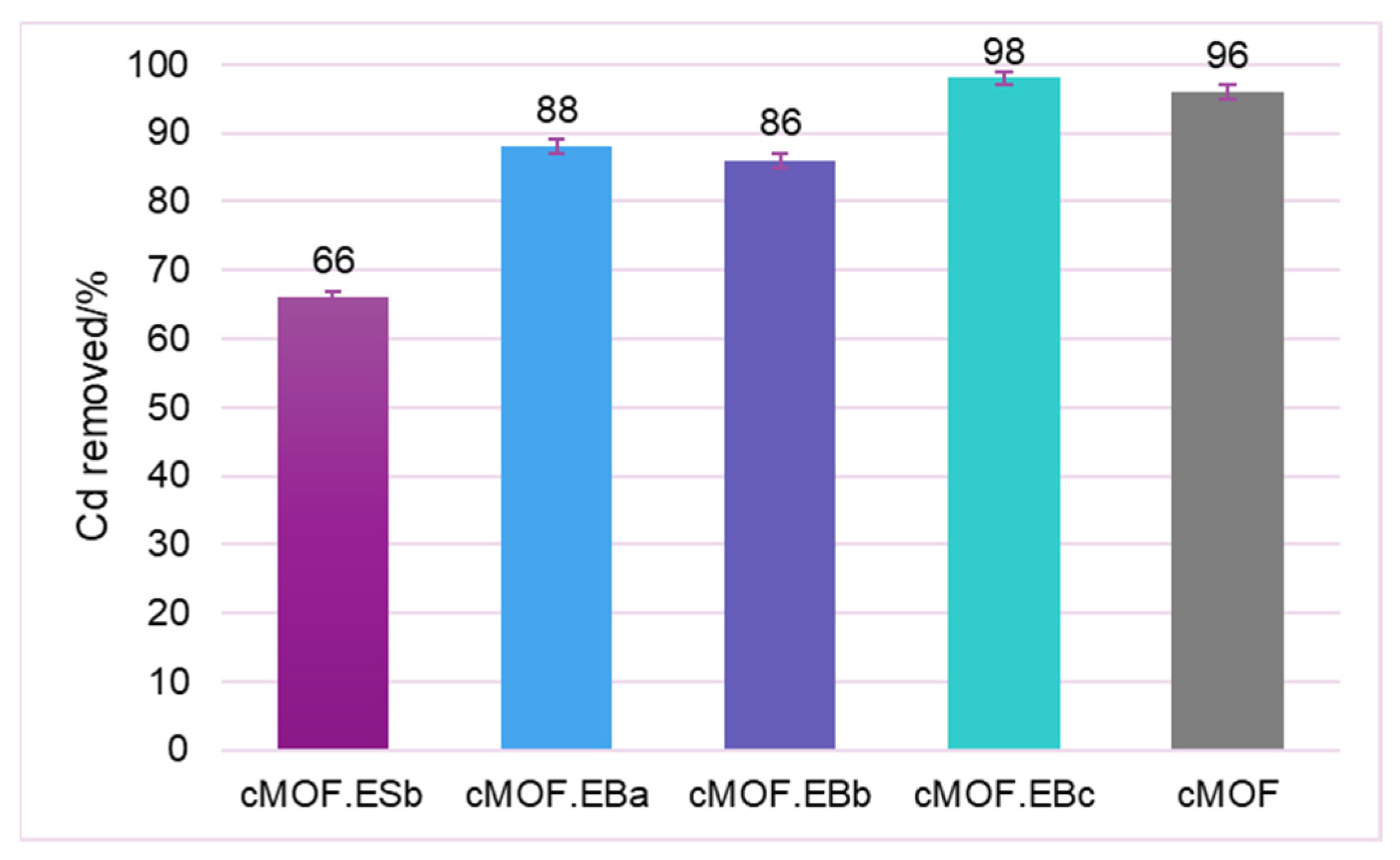
3.6. Metal Ions Adsorption
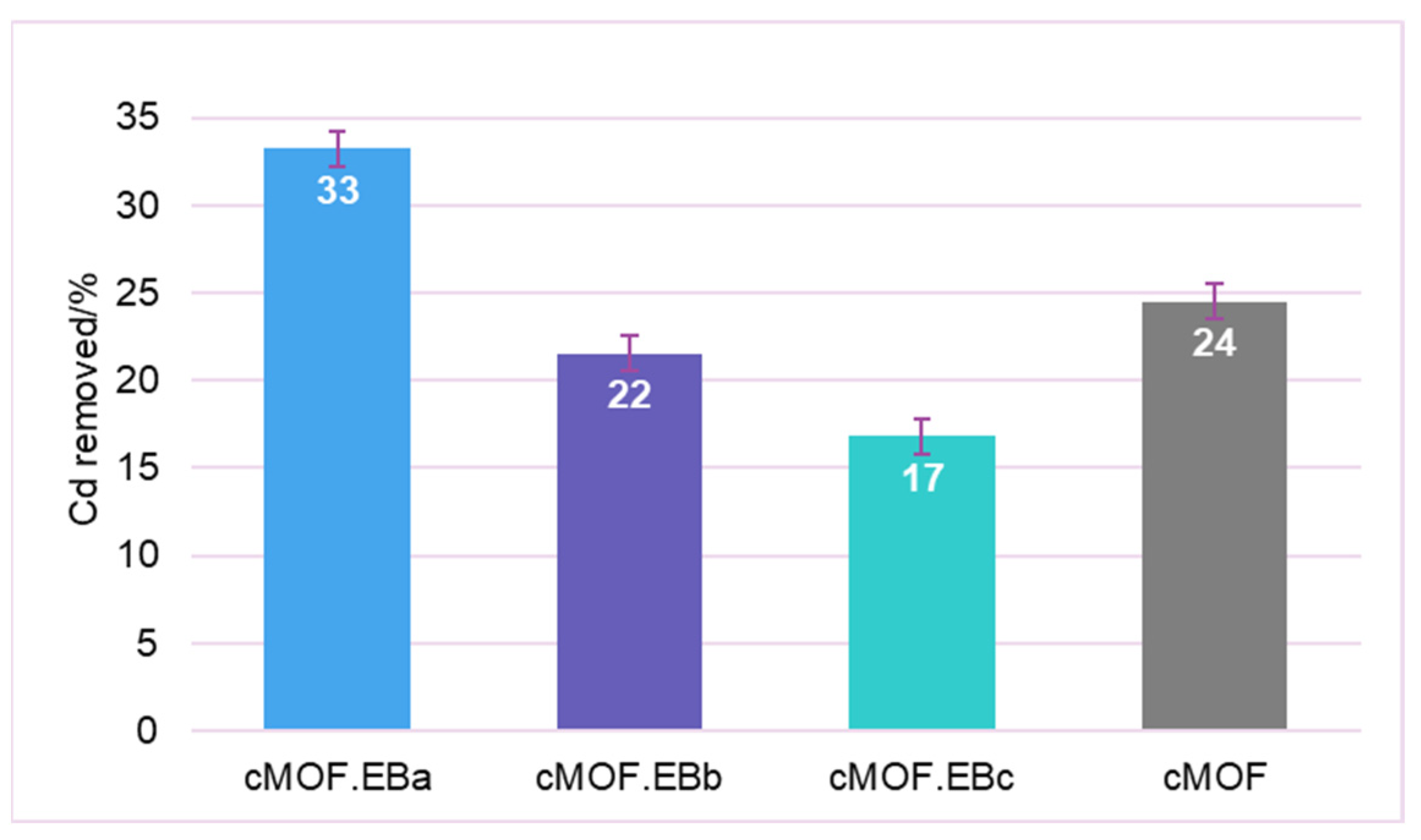
3.7. Materials Efficiency as Adsorbents in Pollutant Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Milojević-Rakić, M.; Bajuk-Bogdanović, D. Recent Advances in Zeolites and Porous Materials Applications in Catalysis and Adsorption Processes. Catalysts 2023, 13, 863. [Google Scholar] [CrossRef]
- Milojević-Rakić, M.; Popadić, D.; Janošević Ležaić, A.; Jevremović, A.; Nedić Vasiljević, B.; Uskoković-Marković, S.; Bajuk-Bogdanović, D. MFI, BEA and FAU Zeolite Scavenging Role in Neonicotinoids and Radical Species Elimination. Environ. Sci. Process Impacts 2022, 24, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Jevremović, A.; Stanojković, A.; Arsenijević, D.; Arsenijević, A.; Arzumanyan, G.; Mamatkulov, K.; Petrović, J.; Nedić Vasiljević, B.; Bajuk-Bogdanović, D.; Milojević-Rakić, M. Mitigating Toxicity of Acetamiprid Removal Techniques—Fe Modified Zeolites in Focus. J. Hazard. Mater. 2022, 436, 129226. [Google Scholar] [CrossRef] [PubMed]
- Popadić, D.; Gavrilov, N.; Krstić, J.; Nedić Vasiljević, B.; Janošević Ležaić, A.; Uskoković-Marković, S.; Milojević-Rakić, M.; Bajuk-Bogdanović, D. Spectral Evidence of Acetamiprid’s Thermal Degradation Products and Mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 301, 122987. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent Advances in Application of Metal-Organic Frameworks (MOFs) as Adsorbent and Catalyst in Removal of Persistent Organic Pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef]
- Au, V.K.-M. Recent Advances in the Use of Metal-Organic Frameworks for Dye Adsorption. Front. Chem. 2020, 8, 708. [Google Scholar] [CrossRef]
- Beydaghdari, M.; Hooriabad Saboor, F.; Babapoor, A.; Karve, V.; Asgari, M. Recent Advances in MOF-Based Adsorbents for Dye Removal from the Aquatic Environment. Energies 2022, 15, 2023. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Nabipour, H.; Pahlevani, F.; Zhao, Y.; Hussain, Z.; Hojjati-Najafabadi, A.; Hoang, H.Y.; Pei, R. Recent Progress on Adsorption of Cadmium Ions from Water Systems Using Metal-Organic Frameworks (MOFs) as an Efficient Class of Porous Materials. Environ. Res. 2022, 214, 114113. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; El Dafrawy, S.M.; Ahmed, A.I. Fe/Co-MOF Nanocatalysts: Greener Chemistry Approach for the Removal of Toxic Metals and Catalytic Applications. ACS Omega 2022, 7, 23421–23444. [Google Scholar] [CrossRef]
- Mohan, B.; Kamboj, A.; Virender; Singh, K.; Priyanka; Singh, G.; Pombeiro, A.J.L.; Ren, P. Metal-Organic Frameworks (MOFs) Materials for Pesticides, Heavy Metals, and Drugs Removal: Environmental Safety. Sep. Purif. Technol. 2023, 310, 123175. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Bandosz, T.J. Activated Carbon versus Metal-Organic Frameworks: A Review of Their PFAS Adsorption Performance. J. Hazard. Mater. 2022, 425, 127810. [Google Scholar] [CrossRef] [PubMed]
- Nabipour, H.; Rohani, S.; Batool, S.; Yusuff, A.S. An Overview of the Use of Water-Stable Metal-Organic Frameworks in the Removal of Cadmium Ion. J. Environ. Chem. Eng. 2023, 11, 109131. [Google Scholar] [CrossRef]
- Fang, Q.-R.; Yuan, D.-Q.; Sculley, J.; Li, J.-R.; Han, Z.-B.; Zhou, H.-C. Functional Mesoporous Metal−Organic Frameworks for the Capture of Heavy Metal Ions and Size-Selective Catalysis. Inorg. Chem. 2010, 49, 11637–11642. [Google Scholar] [CrossRef]
- Wang, K.; Gu, J.; Yin, N. Efficient Removal of Pb(II) and Cd(II) Using NH 2 -Functionalized Zr-MOFs via Rapid Microwave-Promoted Synthesis. Ind. Eng. Chem. Res. 2017, 56, 1880–1887. [Google Scholar] [CrossRef]
- Salimi, M.; Behbahani, M.; Sobhi, H.R.; Ghambarian, M.; Esrafili, A. Trace Measurement of Lead and Cadmium Ions in Wastewater Samples Using a Novel Dithizone Immobilized Metal–Organic Framework-based Μ-dispersive Solid-phase Extraction. Appl. Organomet. Chem. 2020, 34, e5715. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, Á.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Synthesis, Characterization and Dye Removal Capacities of N-Doped Mesoporous Carbons. J. Colloid Interface Sci. 2015, 450, 91–100. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Bergna, D.; Grimm, A.; Lima, E.C.; Hu, T.; Naushad, M.; Lassi, U. Preparation of Highly Porous Nitrogen-Doped Biochar Derived from Birch Tree Wastes with Superior Dye Removal Performance. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 669, 131493. [Google Scholar] [CrossRef]
- Fujisaki, T.; Kashima, K.; Serrano-Luginbühl, S.; Kissner, R.; Bajuk-Bogdanović, D.; Milojević-Rakić, M.; Ćirić-Marjanović, G.; Busato, S.; Lizundia, E.; Walde, P. Effect of Template Type on the Preparation of the Emeraldine Salt Form of Polyaniline (PANI-ES) with Horseradish Peroxidase Isoenzyme C (HRPC) and Hydrogen Peroxide. RSC Adv. 2019, 9, 33080–33095. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Milojević-Rakić, M.; Janošević-Ležaić, A.; Luginbühl, S.; Walde, P. Enzymatic Oligomerization and Polymerization of Arylamines: State of the Art and Perspectives. Chem. Pap. 2017, 71, 199–242. [Google Scholar] [CrossRef]
- Pašti, I.; Milojević-Rakić, M.; Junker, K.; Bajuk-Bogdanović, D.; Walde, P.; Ćirić-Marjanović, G. Superior Capacitive Properties of Polyaniline Produced by a One-Pot Peroxidase/H2O2-Triggered Polymerization of Aniline in the Presence of AOT Vesicles. Electrochim. Acta 2017, 258, 834–841. [Google Scholar] [CrossRef]
- Radoičić, M.; Ćirić-Marjanović, G.; Spasojević, V.; Ahrenkiel, P.; Mitrić, M.; Novaković, T.; Šaponjić, Z. Superior Photocatalytic Properties of Carbonized PANI/TiO2 Nanocomposites. Appl. Catal. B Environ. 2017, 213, 155–166. [Google Scholar] [CrossRef]
- Jović, A.; Milikić, J.; Bajuk-Bogdanović, D.; Milojević-Rakić, M.; Nedić Vasiljević, B.; Krstić, J.; Cvjetićanin, N.; Šljukić, B. 12-Phosphotungstic Acid Supported on BEA Zeolite Composite with Carbonized Polyaniline for Electroanalytical Sensing of Phenols in Environmental Samples. J. Electrochem. Soc. 2018, 165, H1013–H1020. [Google Scholar] [CrossRef]
- Jevremović, A.; Bober, P.; Mičušík, M.; Kuliček, J.; Acharya, U.; Pfleger, J.; Milojević-Rakić, M.; Krajišnik, D.; Trchová, M.; Stejskal, J.; et al. Synthesis and Characterization of Polyaniline/BEA Zeolite Composites and Their Application in Nicosulfuron Adsorption. Microporous Mesoporous Mater. 2019, 287, 234–245. [Google Scholar] [CrossRef]
- Milojević-Rakić, M.; Janošević, A.; Krstić, J.; Nedić Vasiljević, B.; Dondur, V.; Ćirić-Marjanović, G. Polyaniline and Its Composites with Zeolite ZSM-5 for Efficient Removal of Glyphosate from Aqueous Solution. Microporous Mesoporous Mater. 2013, 180, 141–155. [Google Scholar] [CrossRef]
- Shanahan, J.; Kissel, D.S.; Sullivan, E. PANI@UiO-66 and PANI@UiO-66-NH 2 Polymer-MOF Hybrid Composites as Tunable Semiconducting Materials. ACS Omega 2020, 5, 6395–6404. [Google Scholar] [CrossRef]
- Ramohlola, K.E.; Monana, G.R.; Hato, M.J.; Modibane, K.D.; Molapo, K.M.; Masikini, M.; Mduli, S.B.; Iwuoha, E.I. Polyaniline-Metal Organic Framework Nanocomposite as an Efficient Electrocatalyst for Hydrogen Evolution Reaction. Compos. Part B Eng. 2018, 137, 129–139. [Google Scholar] [CrossRef]
- Biserčić, M.S.; Marjanović, B.; Zasońska, B.A.; Stojadinović, S.; Ćirić-Marjanović, G. Novel Microporous Composites of MOF-5 and Polyaniline with High Specific Surface Area. Synth. Met. 2020, 262, 116348. [Google Scholar] [CrossRef]
- Savić, M.; Janošević Ležaić, A.; Gavrilov, N.; Pašti, I.; Nedić Vasiljević, B.; Krstić, J.; Ćirić-Marjanović, G. Carbonization of MOF-5/Polyaniline Composites to N,O-Doped Carbon/ZnO/ZnS and N,O-Doped Carbon/ZnO Composites with High Specific Capacitance, Specific Surface Area and Electrical Conductivity. Materials 2023, 16, 1018. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Hu, H.; Yang, Q.; Cai, J. From Metal–Organic Frameworks to Porous Carbon Materials: Recent Progress and Prospects from Energy and Environmental Perspectives. Nanoscale 2020, 12, 4238–4268. [Google Scholar] [CrossRef]
- Trukawka, M.; Cendrowski, K.; Peruzynska, M.; Augustyniak, A.; Nawrotek, P.; Drozdzik, M.; Mijowska, E. Carbonized Metal–Organic Frameworks with Trapped Cobalt Nanoparticles as Biocompatible and Efficient Azo-Dye Adsorbent. Environ. Sci. Eur. 2019, 31, 56. [Google Scholar] [CrossRef]
- Biserčić, M.S.; Marjanović, B.; Vasiljević, B.N.; Mentus, S.; Zasońska, B.A.; Ćirić-Marjanović, G. The Quest for Optimal Water Quantity in the Synthesis of Metal-Organic Framework MOF-5. Microporous Mesoporous Mater. 2019, 278, 23–29. [Google Scholar] [CrossRef]
- Lecloux, A.; Pirard, J.P. The Importance of Standard Isotherms in the Analysis of Adsorption Isotherms for Determining the Porous Texture of Solids. J. Colloid Interface Sci. 1979, 70, 265–281. [Google Scholar] [CrossRef]
- Horváth, G.; Kawazoe, K. Method for the Calculation of Effective Pore Size Distribution in Molecular Sieve Carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef]
- Drozdov, V.A.; Fenelonov, V.B.; Okkel, L.G.; Gulyaeva, T.I.; Antonicheva, N.V.; Sludkina, N.S. Investigation of Reference Catalysts in Boreskov Institute of Catalysis: Texture of Reference Platinum Catalysts. Appl. Catal. A Gen. 1998, 172, 7–13. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Malloy, A. Count, Size and Visualize Nanoparticles. Mater. Today 2011, 14, 170–173. [Google Scholar] [CrossRef]
- Gong, Y.-T.; Li, B.-H.; Pei, T.; Lin, C.-H.; Lee, S. Raman Investigation on Carbonization Process of Metal-Organic Frameworks. J. Raman Spectrosc. 2016, 47, 1271–1275. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Gavrilov, N.; Pašti, I.A.; Mitrić, M.; Travas-Sejdić, J.; Ćirić-Marjanović, G.; Mentus, S.V. Electrocatalysis of Oxygen Reduction Reaction on Polyaniline-Derived Nitrogen-Doped Carbon Nanoparticle Surfaces in Alkaline Media. J. Power Sources 2012, 220, 306–316. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature Dependence of Raman Scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Najib, S.; Bakan, F.; Abdullayeva, N.; Bahariqushchi, R.; Kasap, S.; Franzò, G.; Sankir, M.; Demirci Sankir, N.; Mirabella, S.; Erdem, E. Tailoring Morphology to Control Defect Structures in ZnO Electrodes for High-Performance Supercapacitor Devices. Nanoscale 2020, 12, 16162–16172. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Naidu, H.; Ahmadzadeh, S. A Green Approach to Remove Acetamiprid Insecticide Using Pistachio Shell-Based Modified Activated Carbon; Economical Groundwater Treatment. J. Clean. Prod. 2021, 316, 128226. [Google Scholar] [CrossRef]
- Mohammad, S.G.; Ahmed, S.M.; Amr, A.E.-G.E.; Kamel, A.H. Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions. Molecules 2020, 25, 2339. [Google Scholar] [CrossRef]
- Choumane, F.Z.; Benguella, B. Removal of Acetamiprid from Aqueous Solutions with Low-Cost Sorbents. Desalin. Water Treat. 2014, 57, 419–430. [Google Scholar] [CrossRef]
- Popadić, D.; Gavrilov, N.; Ignjatović, L.; Krajišnik, D.; Mentus, S.; Milojević-Rakić, M.; Bajuk-Bogdanović, D. How to Obtain Maximum Environmental Applicability from Natural Silicates. Catalysts 2022, 12, 519. [Google Scholar] [CrossRef]
- Li, Y.; Gao, C.; Jiao, J.; Cui, J.; Li, Z.; Song, Q. Selective Adsorption of Metal–Organic Framework toward Methylene Blue: Behavior and Mechanism. ACS Omega 2021, 6, 33961–33968. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution with a Metal-Organic Framework Material, Iron Terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef]
- EL-Mekkawi, D.M.; Ibrahim, F.A.; Selim, M.M. Removal of Methylene Blue from Water Using Zeolites Prepared from Egyptian Kaolins Collected from Different Sources. J. Environ. Chem. Eng. 2016, 4, 1417–1422. [Google Scholar] [CrossRef]
- Ullah, A.; Zahoor, M.; Din, W.U.; Muhammad, M.; Khan, F.A.; Sohail, A.; Ullah, R.; Ali, E.A.; Murthy, H.C.A. Removal of Methylene Blue from Aqueous Solution Using Black Tea Wastes: Used as Efficient Adsorbent. Adsorpt. Sci. Technol. 2022, 2022, 5713077. [Google Scholar] [CrossRef]
- Zhu, Y.; Yi, B.; Yuan, Q.; Wu, Y.; Wang, M.; Yan, S. Removal of Methylene Blue from Aqueous Solution by Cattle Manure-Derived Low Temperature Biochar. RSC Adv. 2018, 8, 19917–19929. [Google Scholar] [CrossRef] [PubMed]
- Sheela, T.; Nayaka, Y.A.; Viswanatha, R.; Basavanna, S.; Venkatesha, T.G. Kinetics and Thermodynamics Studies on the Adsorption of Zn(II), Cd(II) and Hg(II) from Aqueous Solution Using Zinc Oxide Nanoparticles. Powder Technol. 2012, 217, 163–170. [Google Scholar] [CrossRef]
- Tang, L.; Qiu, R.; Tang, Y.; Wang, S. Cadmium–Zinc Exchange and Their Binary Relationship in the Structure of Zn-Related Proteins: A Mini Review. Metallomics 2014, 6, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Atar, N.; Olgun, A.; Wang, S. Adsorption of Cadmium (II) and Zinc (II) on Boron Enrichment Process Waste in Aqueous Solutions: Batch and Fixed-Bed System Studies. Chem. Eng. J. 2012, 192, 1–7. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and Multi-Component Adsorption of Cadmium and Zinc Using Activated Carbon Derived from Bagasse—An Agricultural Waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Kudo, N.; Yamashina, S.; Waku, K. Protection against Cadmium Toxicity by Zinc: Decrease in the Cd-High Molecular Weight Protein Fraction in Rat Liver and Kidney on Zn Pretreatment. Toxicology 1986, 40, 267–277. [Google Scholar] [CrossRef]
- Cardoso, D.; Narcy, A.; Durosoy, S.; Bordes, C.; Chevalier, Y. Dissolution Kinetics of Zinc Oxide and Its Relationship with Physicochemical Characteristics. Powder Technol. 2021, 378, 746–759. [Google Scholar] [CrossRef]
- De Carvalho Izidoro, J.; Fungaro, D.A.; Wang, S. Bin Zeolite Synthesis from Brazilian Coal Fly Ash for Removal of Zn2+ and Cd2+ from Water. Adv. Mater. Res. 2011, 356–360, 1900–1908. [Google Scholar] [CrossRef]
- Motaghi, H.; Arabkhani, P.; Parvinnia, M.; Asfaram, A. Simultaneous Adsorption of Cobalt Ions, Azo Dye, and Imidacloprid Pesticide on the Magnetic Chitosan/Activated Carbon@UiO-66 Bio-Nanocomposite: Optimization, Mechanisms, Regeneration, and Application. Sep. Purif. Technol. 2022, 284, 120258. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Emam, H.E. Modulation of Metal Organic Framework Hybrid Cotton for Efficient Sweeping of Dyes and Pesticides from Wastewater. Sustain. Mater. Technol. 2022, 31, e00366. [Google Scholar] [CrossRef]

| cMOF.ESb | cMOF.EBa | cMOF.EBb | cMOF.EBc | cMOF | |
|---|---|---|---|---|---|
| Total pore volume (Gurvich at 0.98 p/p0) Vtot (cm3/g) | 0.315 | 1.106 | 0.464 | 0.359 | 0.715 |
| Specific surface area (B.E.T. method) SBET (m2/g) | 393 | 611 | 471 | 413 | 550 |
| Mesopore volume (B.J.H. Desorption) Vmeso (cm3/g) | 0.169 | 1.047 | 0.259 | 0.201 | 0.584 |
| Median mesopore diameter (B.J.H. Desorption) Dmeso (nm) | 5.8 | 10.7 | 12.3 | 12.5 | 9.4 |
| Micropore volume (Horvath and Kawazoe) Vmic (cm3/g) | 0.164 | 0.251 | 0.194 | 0.170 | 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jevremović, A.; Savić, M.; Janošević Ležaić, A.; Krstić, J.; Gavrilov, N.; Bajuk-Bogdanović, D.; Milojević-Rakić, M.; Ćirić-Marjanović, G. Environmental Potential of Carbonized MOF-5/PANI Composites for Pesticide, Dye, and Metal Cations—Can They Actually Retain Them All? Polymers 2023, 15, 4349. https://doi.org/10.3390/polym15224349
Jevremović A, Savić M, Janošević Ležaić A, Krstić J, Gavrilov N, Bajuk-Bogdanović D, Milojević-Rakić M, Ćirić-Marjanović G. Environmental Potential of Carbonized MOF-5/PANI Composites for Pesticide, Dye, and Metal Cations—Can They Actually Retain Them All? Polymers. 2023; 15(22):4349. https://doi.org/10.3390/polym15224349
Chicago/Turabian StyleJevremović, Anka, Marjetka Savić, Aleksandra Janošević Ležaić, Jugoslav Krstić, Nemanja Gavrilov, Danica Bajuk-Bogdanović, Maja Milojević-Rakić, and Gordana Ćirić-Marjanović. 2023. "Environmental Potential of Carbonized MOF-5/PANI Composites for Pesticide, Dye, and Metal Cations—Can They Actually Retain Them All?" Polymers 15, no. 22: 4349. https://doi.org/10.3390/polym15224349
APA StyleJevremović, A., Savić, M., Janošević Ležaić, A., Krstić, J., Gavrilov, N., Bajuk-Bogdanović, D., Milojević-Rakić, M., & Ćirić-Marjanović, G. (2023). Environmental Potential of Carbonized MOF-5/PANI Composites for Pesticide, Dye, and Metal Cations—Can They Actually Retain Them All? Polymers, 15(22), 4349. https://doi.org/10.3390/polym15224349