Alginate Modified Magnetic Polypyrrole Nanocomposite for the Adsorptive Removal of Heavy Metal
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
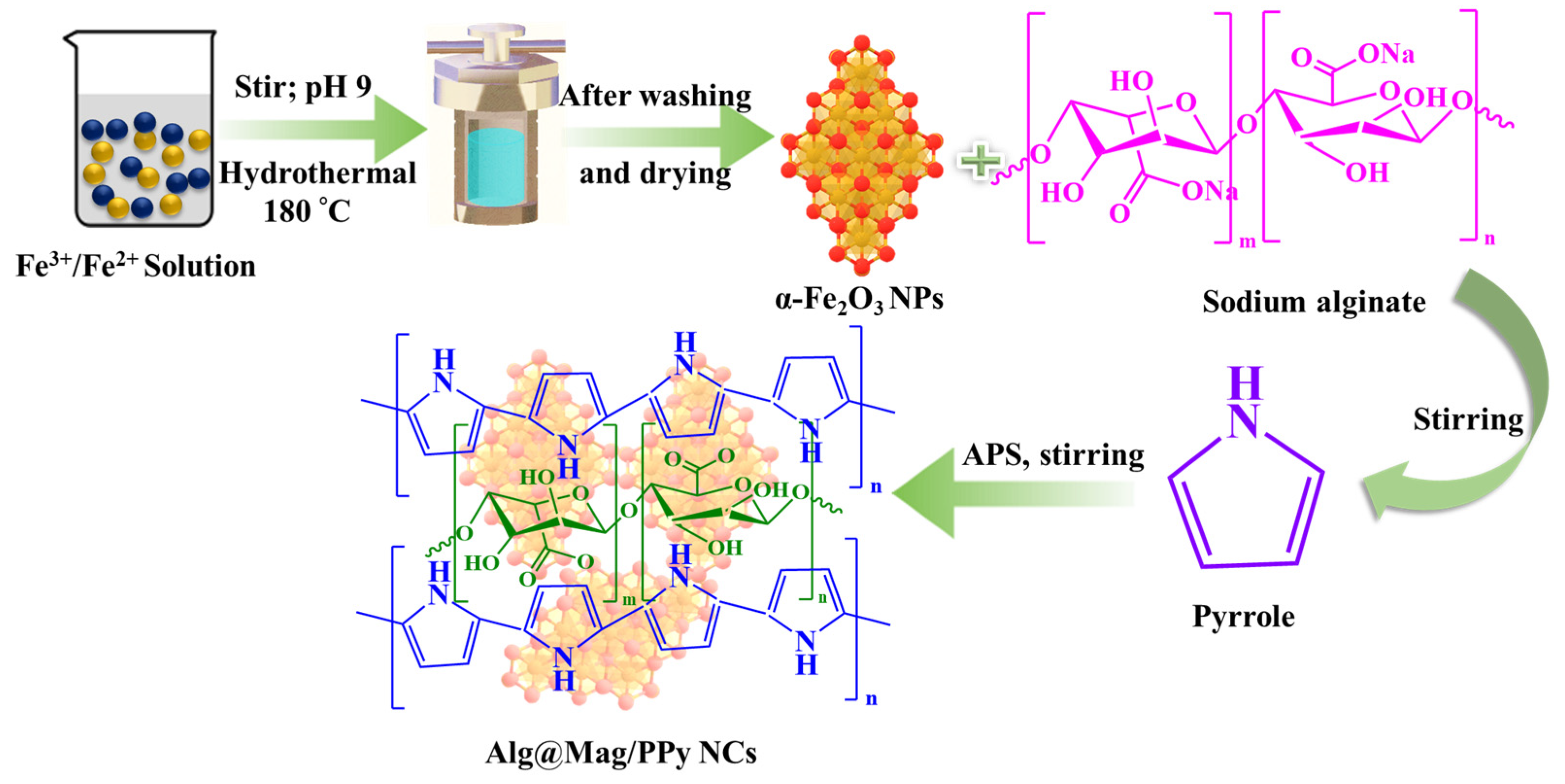
2.2. Synthesis of α-Fe2O3
2.3. Synthesis of Alg@Mag/PPy NCs
2.4. Adsorption Experiments
2.5. Instrumentation Details
3. Results and Discussion
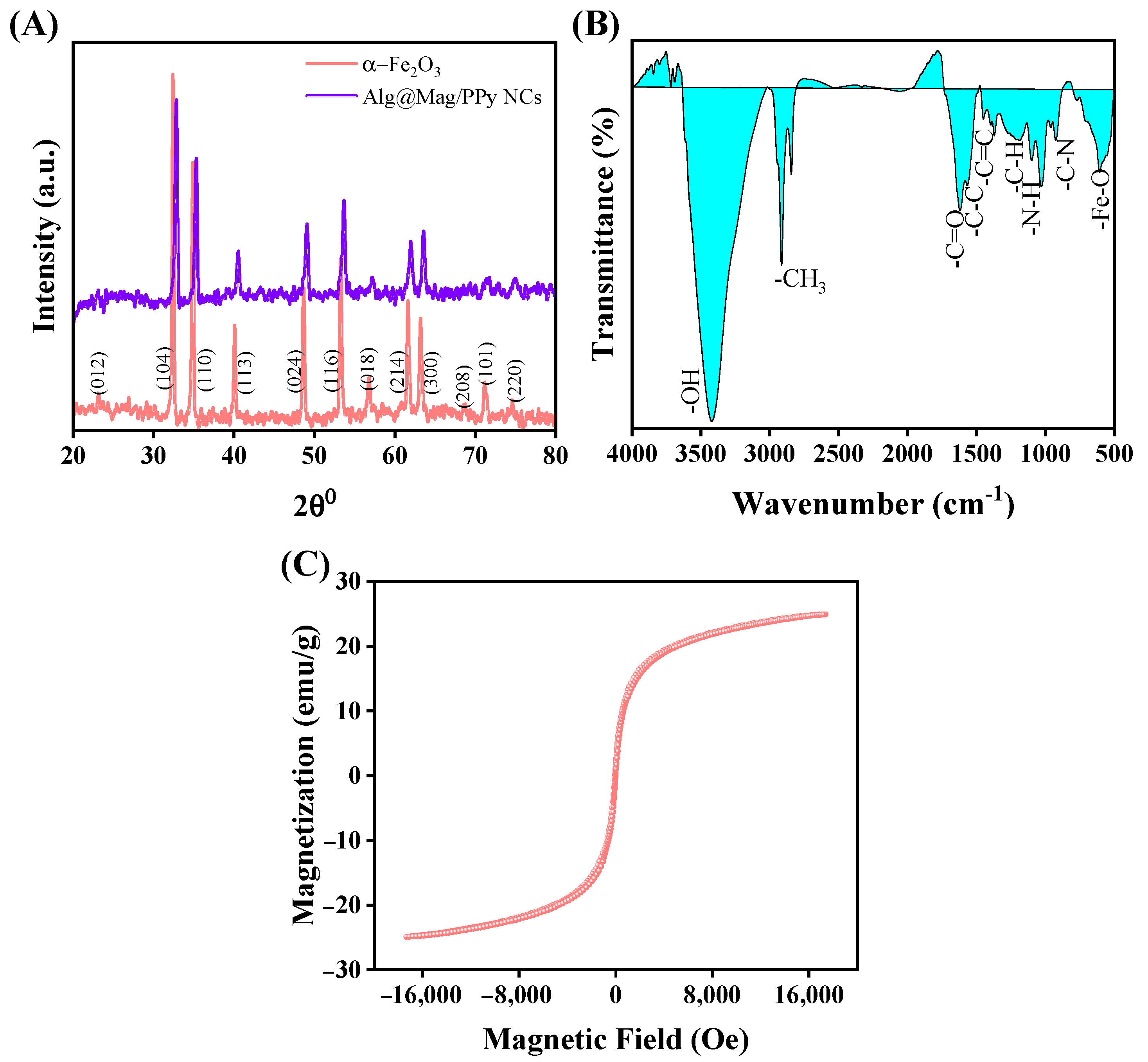
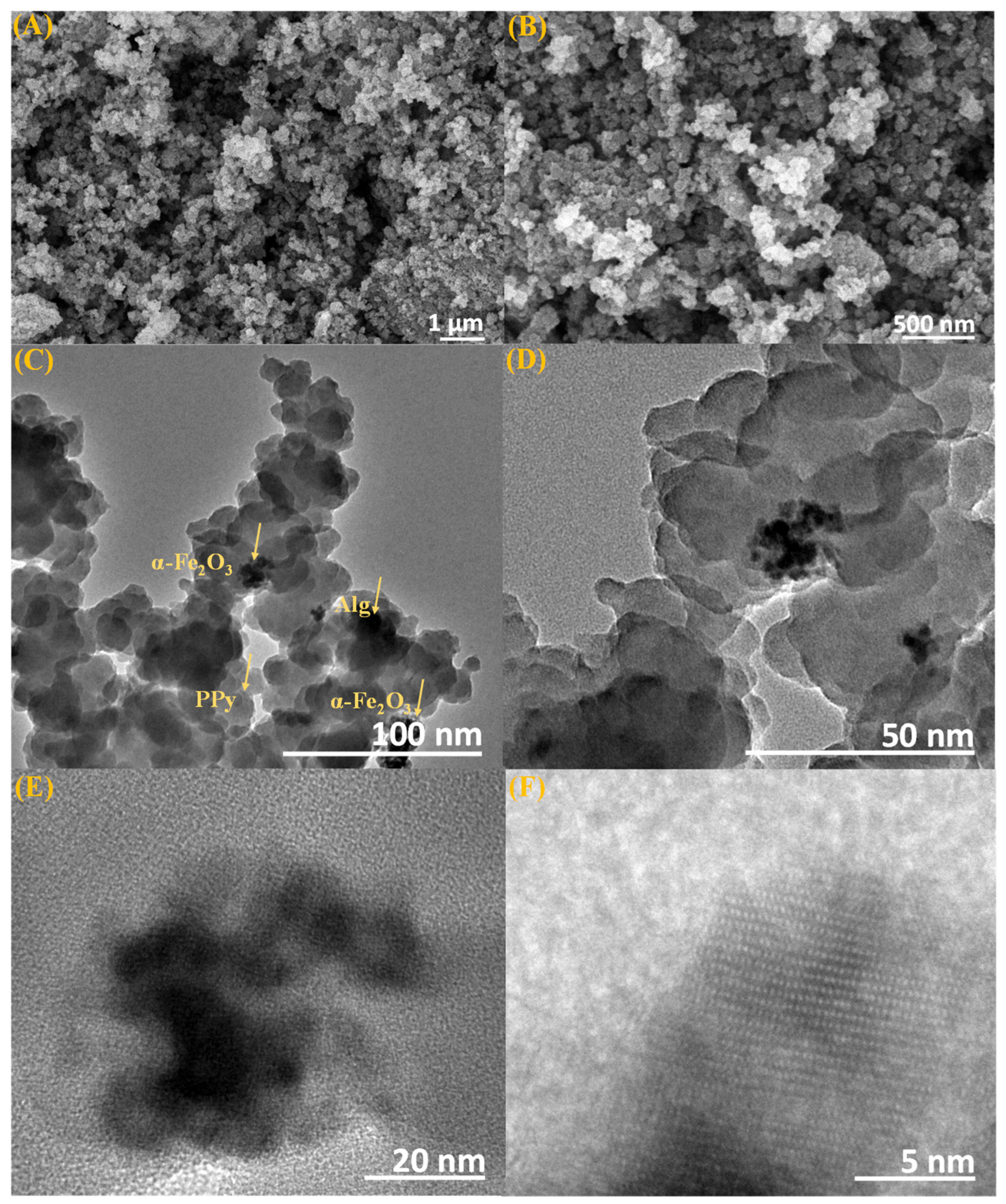
3.1. Characterization
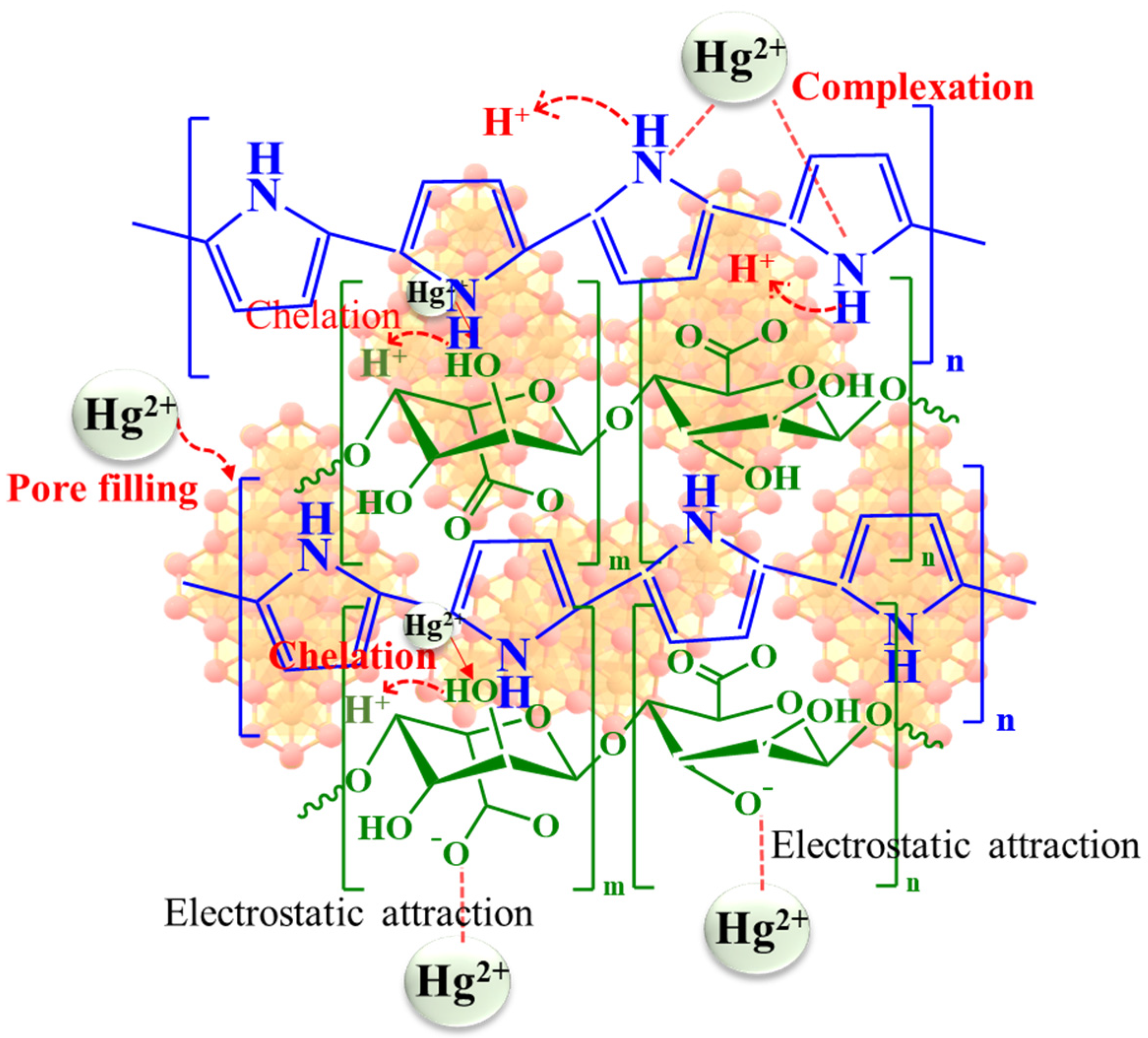
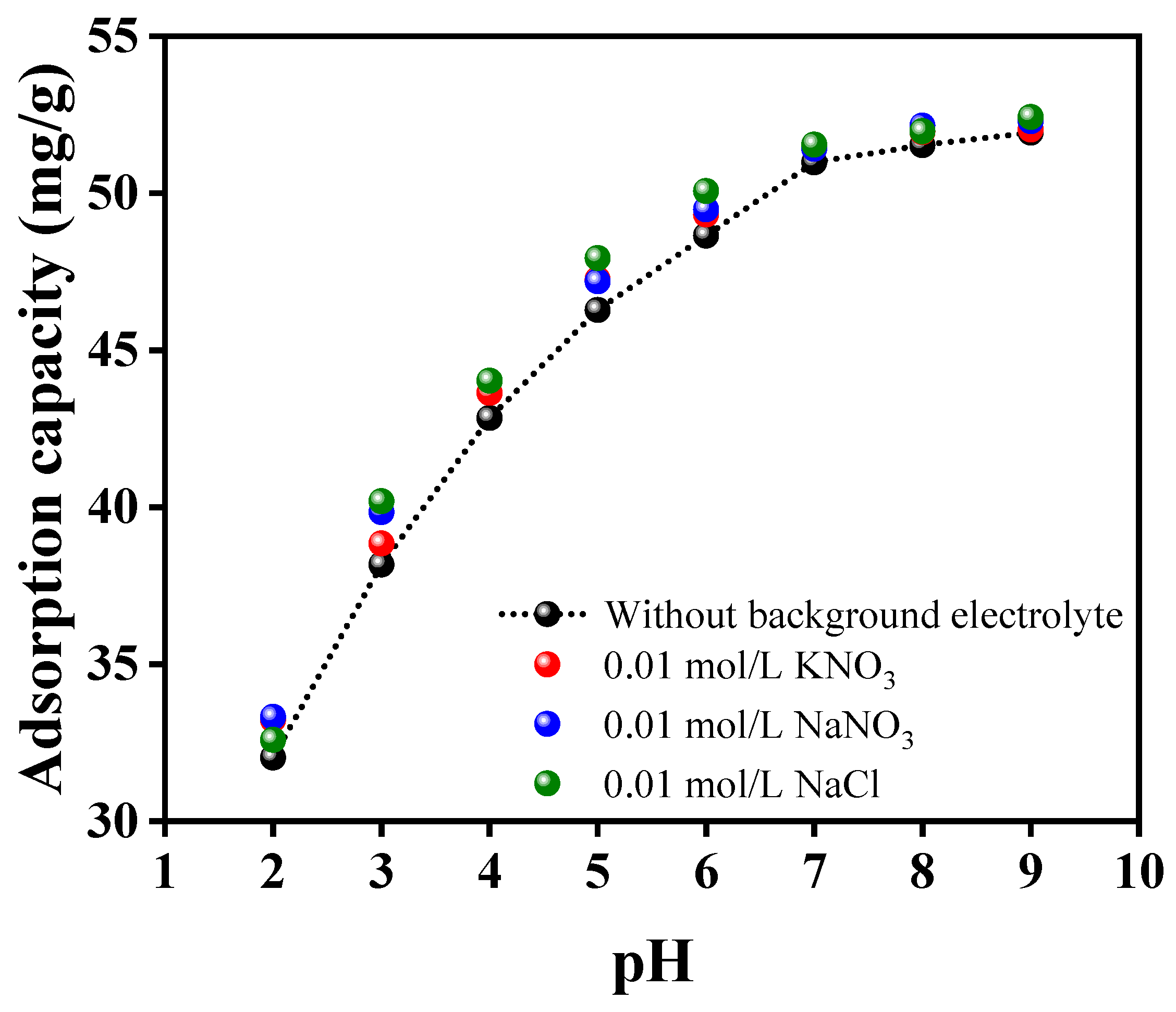
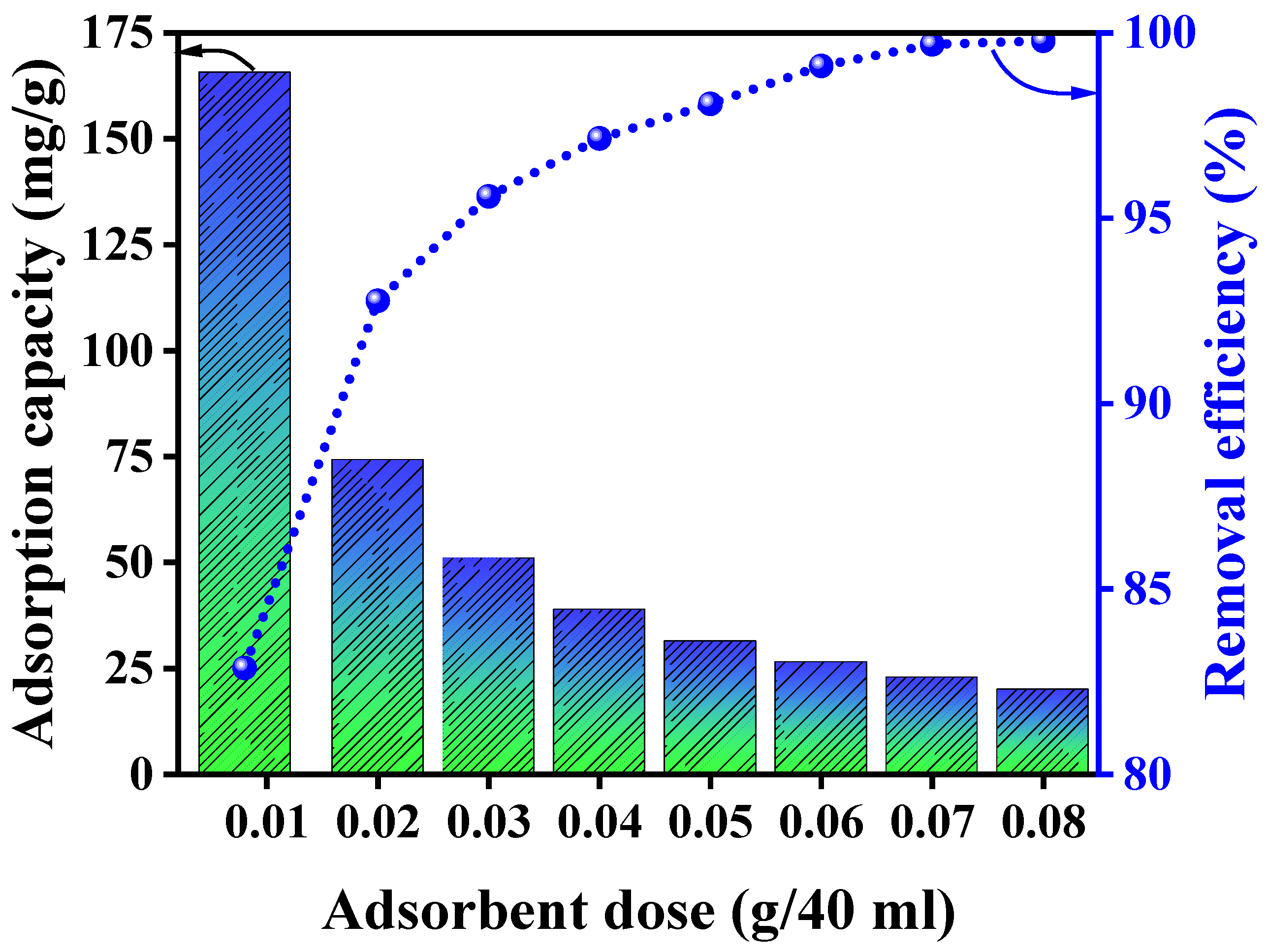
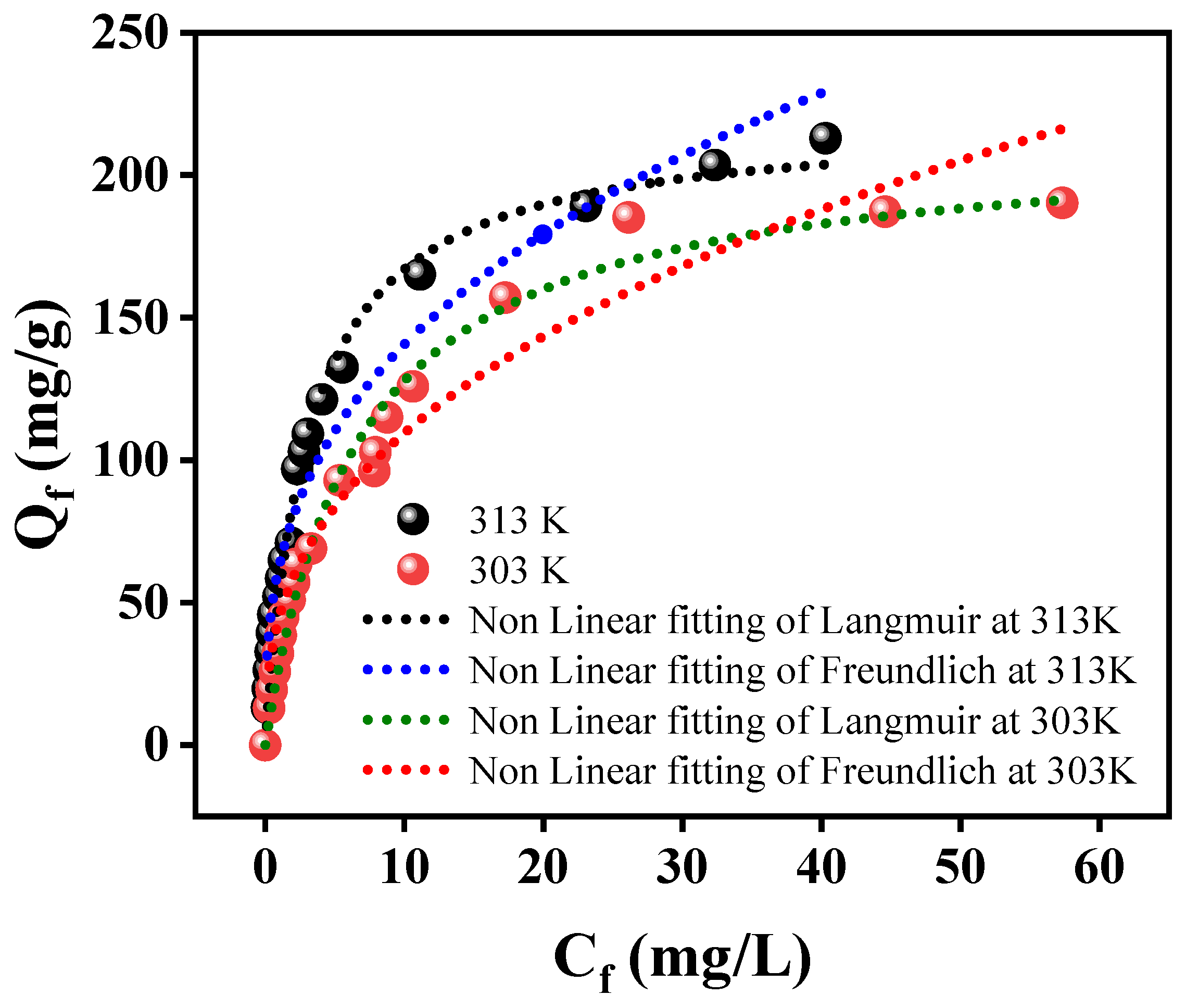
3.2. Adsorption Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mashkoor, F.; Shoeb, M.; Mashkoor, R.; Anwer, A.H.; Zhu, S.; Jeong, H.; Baek, S.-S.; Jung, J.; Jeong, C. Synergistic effects of tungstate trioxide hemihydrate decorated reduced graphene oxide for the adsorption of heavy metals and dyes and postliminary application in supercapacitor device. J. Clean. Prod. 2023, 418, 138067. [Google Scholar] [CrossRef]
- Baig, U.; Uddin, M.K.; Gondal, M.A. Removal of hazardous azo dye from water using synthetic nano adsorbent: Facile synthesis, characterization, adsorption, regeneration and design of experiments. Colloids Surf. Physicochem. Eng. Asp. 2020, 584, 124031. [Google Scholar] [CrossRef]
- Khan Rao, R.A.; Ikram, S.; Uddin, M.K. Removal of Cd(II) from aqueous solution by exploring the biosorption characteristics of gaozaban (Onosma bracteatum). J. Environ. Chem. Eng. 2014, 2, 1155–1164. [Google Scholar] [CrossRef]
- Habila, M.A.; Alothman, Z.A.; Hakami, H.M.; Alanazi, A.G. Influence of Synthesis-Heating Conditions on the Porosity and Performance of a Carbon Nanotube/SDS-Alumina Nanocomposite for Effective Wastewater Treatment: Fabrication, Characterization, and Adsorption Modeling. Ind. Eng. Chem. Res. 2023, 62, 12571–12588. [Google Scholar] [CrossRef]
- Mashkoor, F.; Mashkoor, R.; Shoeb, M.; Anwer, A.H.; Jeong, H.; Jeong, C. Freestanding WS2-MWCNT Nanocomposite for Electrochemical Detection of Contaminants of Emerging Concern—Perfluorooctanoic Acid “A Forever Chemical” and Supercapacitor Applications. ACS Sustain. Chem. Eng. 2023, 11, 13306–13319. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.; Wang, L.; Ji, L.; Li, X.; Qu, Z.; Yan, N. Dual-functional Sites for Selective Adsorption of Mercury and Arsenic ions in [SnS4]4−/MgFe-LDH from Wastewater. J. Hazard. Mater. 2021, 403, 123940. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.R.; Nair, G.B.; Dhoble, S.J. Insights into the extraction of mercury from fluorescent lamps: A review. J. Environ. Chem. Eng. 2019, 7, 103279. [Google Scholar] [CrossRef]
- Gul, S.; Ahmad, Z.; Asma, M.; Ahmad, M.; Rehan, K.; Munir, M.; Bazmi, A.A.; Ali, H.M.; Mazroua, Y.; Salem, M.A.; et al. Effective adsorption of cadmium and lead using SO3H-functionalized Zr-MOFs in aqueous medium. Chemosphere 2022, 307, 135633. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin. Carbon nanotube-based adsorbents for the removal of dyes from waters: A review. Environ. Chem. Lett. 2020, 18, 605–629. [Google Scholar] [CrossRef]
- Wu, R.; Abdulhameed, A.S.; Yong, S.K.; Li, H.; Alothman, Z.A.; Wilson, L.D.; Jawad, A.H. Functionalization of chitosan biopolymer with SiO2 nanoparticles and benzaldehyde via hydrothermal process for acid red 88 dye adsorption: Box-Behnken design optimization. Int. J. Biol. Macromol. 2023, 247, 125806. [Google Scholar] [CrossRef]
- Reghioua, A.; Jawad, A.H.; Selvasembian, R.; ALOthman, Z.A.; Wilson, L.D. Box–Behnken design with desirability function for methylene blue dye adsorption by microporous activated carbon from pomegranate peel using microwave assisted K2CO3 activation. Int. J. Phytoremed. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Işık, B.; Uğraşkan, V. Adsorption of methylene blue on sodium alginate–flax seed ash beads: Isotherm, kinetic and thermodynamic studies. Int. J. Biol. Macromol. 2021, 167, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- El Rhazi, M.; Majid, S.; Elbasri, M.; Salih, F.E.; Oularbi, L.; Lafdi, K. Recent progress in nanocomposites based on conducting polymer: Application as electrochemical sensors. Int. Nano Lett. 2018, 8, 79–99. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R. Recent trends and developments in conducting polymer nanocomposites for multifunctional applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.M.; Shoeb, M.; Ansari, M.Z.; Mashkoor, F.; Mobin, M.; Zaidi, S.; Jeong, C. Fabrication of NiO–CuO decorated polyaniline (PANI/NiO–CuO) nanocomposite based symmetric supercapacitor device for high-energy density performance with wide potential window in aqueous electrolyte. Inorg. Chem. Commun. 2023, 157, 111265. [Google Scholar] [CrossRef]
- Xin, Q.; Fu, J.; Chen, Z.; Liu, S.; Yan, Y.; Zhang, J.; Xu, Q. Polypyrrole nanofibers as a high-efficient adsorbent for the removal of methyl orange from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 1637–1647. [Google Scholar] [CrossRef]
- Ahmad, Z.; Kumar, S.; Kim Trinh, C.; Shim, J.-J.; Lee, J.-S. Decoupling electrochemical parameters of molecular-level-controlled polypyrrole and graphene oxide nanocomposite. Appl. Surf. Sci. 2023, 610, 155464. [Google Scholar] [CrossRef]
- Ahmad, Z.; Kim, W.; Kumar, S.; Yoon, T.-H.; Lee, J.-S. Nanocomposite Supercapacitor Electrode from Sulfonated Graphene Oxide and Poly(pyrrole-(biphenyldisulfonic acid)-pyrrole). ACS Appl. Energy Mater. 2020, 3, 6743–6751. [Google Scholar] [CrossRef]
- Bhaumik, M.; McCrindle, R.; Maity, A. Efficient removal of Congo red from aqueous solutions by adsorption onto interconnected polypyrrole–polyaniline nanofibres. Chem. Eng. J. 2013, 228, 506–515. [Google Scholar] [CrossRef]
- Mohanty, N.; Patra, B.N. Polypyrrole-sodium alginate nanocomposites for enhanced removal of toxic organic and metal pollutants from wastewater. Mater. Today Commun. 2023, 34, 105325. [Google Scholar] [CrossRef]
- Nezhadali, A.; Koushali, S.E.; Divsar, F. Synthesis of polypyrrole—Chitosan magnetic nanocomposite for the removal of carbamazepine from wastewater: Adsorption isotherm and kinetic study. J. Environ. Chem. Eng. 2021, 9, 105648. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magsorbents: Potential candidates in wastewater treatment technology—A review on the removal of methylene blue dye. J. Magn. Magn. Mater. 2020, 500, 166408. [Google Scholar] [CrossRef]
- Arni, L.A.; Hapiz, A.; Abdulhameed, A.S.; Khadiran, T.; Alothman, Z.A.; Wilson, L.D.; Jawad, A.H. Design of separable magnetic chitosan grafted-benzaldehyde for azo dye removal via a response surface methodology: Characterization and adsorption mechanism. Int. J. Biol. Macromol. 2023, 242, 125086. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Li, X.; Gao, C.; Li, X.; Qiu, H. Adsorption performance of CuFe2O4/rGO nanocomposites towards organic dye. Mater. Chem. Phys. 2017, 185, 114–121. [Google Scholar] [CrossRef]
- Sadeghnezhad, M.; Ghorbani, M.; Nikzad, M. Synthesis of magnetic polypyrrole modified sodium alginate nanocomposite with excellent antibacterial properties and optimization of dye removal performance using RSM. Ind. Crops Prod. 2022, 186, 115192. [Google Scholar] [CrossRef]
- Bayat, M.; Javanbakht, V.; Esmaili, J. Synthesis of zeolite/nickel ferrite/sodium alginate bionanocomposite via a co-precipitation technique for efficient removal of water-soluble methylene blue dye. Int. J. Biol. Macromol. 2018, 116, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Finotelli, P.; Morales, M.; Rocha-Leão, M.; Baggio-Saitovitch, E.; Rossi, A. Magnetic studies of iron (III) nanoparticles in alginate polymer for drug delivery applications. Mater. Sci. Eng. C 2004, 24, 625–629. [Google Scholar] [CrossRef]
- Mashkoor, F.; Mashkoor, R.; Shoeb, M.; Anwer, A.H.; Ansari, M.Z.; Jeong, C. A smart recycling solution: WS2-halloysite nanocomposite for heavy metals remediation from wastewater and postliminar application in electrochemical supercapacitor for energy storage. Appl. Clay Sci. 2023, 245, 107149. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, S.; Zhang, L. Selective recovery mechanism of Au(III) from an aqueous solution by trimethyl phosphate modified poly(glycidyl methacrylate). J. Taiwan Inst. Chem. Eng. 2019, 95, 55–64. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, S.; Hu, P.; Huang, L.; Xue, C.; Yang, Z.; Zhou, X.; Wang, Y.; Ji, H. Efficient selective removal of Pb (II) by using 6-aminothiouracil-modified Zr-based organic frameworks: From experiments to mechanisms. ACS Appl. Mater. Interfaces 2020, 12, 7162–7178. [Google Scholar] [CrossRef]
- Ahamad, Z.; Ahmed, M.; Mashkoor, F.; Nasar, A. Chemically modified Azadirachta indica sawdust for adsorption of methylene blue from aqueous solutions. Biomass Convers. Biorefin. 2023, 1–18. [Google Scholar] [CrossRef]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Al Syouf, M.Q. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Shoeb, M.; Ahmad, S.; Mashkoor, F.; Khan, M.N.; Hasan, I.; Singh, B.R.; Jeong, C. Investigating the size-dependent structural, optical, dielectric, and photocatalytic properties of benign-synthesized ZnO nanoparticles. J. Phys. Chem. Solids 2024, 184, 111707. [Google Scholar] [CrossRef]
- Ali, H.; Ismail, A. Fabrication of magnetic Fe3O4/Polypyrrole/carbon black nanocomposite for effective uptake of Congo red and methylene blue dye: Adsorption investigation and mechanism. J. Polym. Environ. 2023, 31, 976–998. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, H.; Wu, F.; Wang, R.; Pan, M. One-step self-assembly synthesis α-Fe2O3 with carbon-coated nanoparticles for stabilized and enhanced supercapacitors electrode. Energies 2017, 10, 1296. [Google Scholar] [CrossRef]
- Bai, L.; Li, Z.; Zhang, Y.; Wang, T.; Lu, R.; Zhou, W.; Gao, H.; Zhang, S. Synthesis of water-dispersible graphene-modified magnetic polypyrrole nanocomposite and its ability to efficiently adsorb methylene blue from aqueous solution. Chem. Eng. J. 2015, 279, 757–766. [Google Scholar] [CrossRef]
- Soni, A.; Tiwari, A.; Bajpai, A. Removal of malachite green from aqueous solution using nano-iron oxide-loaded alginate microspheres: Batch and column studies. Res. Chem. Intermed. 2014, 40, 913–930. [Google Scholar] [CrossRef]
- Talbot, D.; Abramson, S.; Griffete, N.; Bee, A. pH-sensitive magnetic alginate/γ-Fe2O3 nanoparticles for adsorption/desorption of a cationic dye from water. J. Water Process Eng. 2018, 25, 301–308. [Google Scholar] [CrossRef]
- Huang, W.; Pan, S.; Li, Y.; Yu, L.; Liu, R. Immobilization and characterization of cellulase on hydroxy and aldehyde functionalized magnetic Fe2O3/Fe3O4 nanocomposites prepared via a novel rapid combustion process. Int. J. Biol. Macromol. 2020, 162, 845–852. [Google Scholar] [CrossRef]
- Feng, X.; Sun, S.; Cheng, G.; Shi, L.; Yang, X.; Zhang, Y. Removal of uranyl ion from wastewater by magnetic adsorption material of polyaniline combined with CuFe2O4. Adsorpt. Sci. Technol. 2021, 2021, 5584158. [Google Scholar] [CrossRef]
- Majumdar, S.; Moral, R.; Mahanta, D. Rapid mixing polymerization: A simple method for preparation of free standing polypyrrole film and powder for the removal of anionic pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124643. [Google Scholar] [CrossRef]
- Gai, L.; Zhao, Y.; Song, G.; An, Q.; Xiao, Z.; Zhai, S.; Li, Z. Construction of core-shell PPy@MoS2 with nanotube-like heterostructures for electromagnetic wave absorption: Assembly and enhanced mechanism. Compos. Part A Appl. Sci. Manuf. 2020, 136, 105965. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Q.; Xu, S.; Zheng, Q.; Cao, X. Preparation and properties of 3D printed alginate–chitosan polyion complex hydrogels for tissue engineering. Polymers 2018, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.F.; Herrasti, P.; Furtado, R.F.; Melo, A.M.A.; Alves, C.R. Polymeric Composite including Magnetite Nanoparticles for Hydrogen Peroxide Detection. Chemosensors 2023, 11, 323. [Google Scholar] [CrossRef]
- Li, X.; Han, B.; Xu, Y.; Liu, X.; Zhao, C.; Xu, J. Conjugated polymer coating enabled light-resistant black phosphorus with enhanced stability. Nanoscale Adv. 2021, 3, 5650–5655. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Lu, W.; Zhou, Y.; Zhen, R.; He, H.; Wang, Y.; Li, C. Synthesis of polypyrrole/nitrogen-doped porous carbon matrix composite as the electrode material for supercapacitors. Sci. Rep. 2020, 10, 15370. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Yang, Z.; Shi, D.; Chen, M.; Dong, W. Nitrogen-doped carbon boosting Fe2O3 anode performance for supercapacitors. J. Mater. Sci. Mater. Electron. 2022, 33, 13547–13557. [Google Scholar] [CrossRef]
- Han, T.; Wei, Y.; Jin, X.; Jiu, H.; Zhang, L.; Sun, Y.; Tian, J.; Shang, R.; Hang, D.; Zhao, R. Hydrothermal self-assembly of α-Fe2O3 nanorings@ graphene aerogel composites for enhanced Li storage performance. J. Mater. Sci. 2019, 54, 7119–7130. [Google Scholar] [CrossRef]
- Sharma, A.K.; Priya; Kaith, B.S.; Isha; Singh, A.; Chandel, K.; Vipula. Riboflavin Functionalized Dextrin-Sodium Alginate Based Fluorescent Sensor: Detoxification of Cu2+ and Ni2+ Ions. ACS Appl. Polym. Mater. 2019, 1, 3084–3094. [Google Scholar] [CrossRef]
- Long, W.; Yang, C.; Wang, G.; Hu, J. Effective adsorption of Hg(II) ions by new ethylene imine polymer/β-cyclodextrin crosslinked functionalized magnetic composite. Arab. J. Chem. 2023, 16, 104439. [Google Scholar] [CrossRef]
- Saravanakumar, R.; Muthukumaran, K.; Selvaraju, N. Enhanced Pb (II) ions removal by using magnetic NiO/Biochar composite. Mater. Res. Express 2019, 6, 105504. [Google Scholar] [CrossRef]
- Fu, R.; Liu, Y.; Lou, Z.; Wang, Z.; Baig, S.A.; Xu, X. Adsorptive removal of Pb (II) by magnetic activated carbon incorporated with amino groups from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2016, 62, 247–258. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magnetized Tectona grandis sawdust as a novel adsorbent: Preparation, characterization, and utilization for the removal of methylene blue from aqueous solution. Cellulose 2020, 27, 2613–2635. [Google Scholar] [CrossRef]
- Ghasemi, S.S.; Hadavifar, M.; Maleki, B.; Mohammadnia, E. Adsorption of mercury ions from synthetic aqueous solution using polydopamine decorated SWCNTs. J. Water Process Eng. 2019, 32, 100965. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, H.; Wang, Q.; Wang, J.; Cheng, J.; Guo, Y.; Zhou, X.; Bai, R. Adsorption of mercury(ii) with an Fe3O4 magnetic polypyrrole–graphene oxide nanocomposite. RSC Adv. 2017, 7, 18466–18479. [Google Scholar] [CrossRef]
- Duan, W.; Wang, J.; Chang, L.; Zhao, L.; Tian, Z.; Huang, Z.; Huang, W. Adsorption of mercury(ii) from water by a novel sPAN fiber containing sulfhydryl, carboxyl and amino groups. RSC Adv. 2018, 8, 38259–38269. [Google Scholar] [CrossRef] [PubMed]
- Arias Arias, F.E.; Beneduci, A.; Chidichimo, F.; Furia, E.; Straface, S. Study of the adsorption of mercury (II) on lignocellulosic materials under static and dynamic conditions. Chemosphere 2017, 180, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mashkoor, F.; Kim, D.; Zahid Ansari, M.; Hakeem Anwer, A.; Shoeb, M.; Jeong, C. Synergistic effects of multifunctional nanostructured WO3-WS2 decorated on polypyrrole (WO3-WS2/PPy) for the removal of toxic heavy metals from wastewaters and high supercapacitor performance. J. Mol. Liq. 2023, 375, 121312. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Faur-Brasquet, C.; Cloirec, P.L. Removal of Cu(II), Pb(II), and Ni(II) by Adsorption onto Activated Carbon Cloths. Langmuir 2000, 16, 8404–8409. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Vennilamani, N.; Pattabhi, S. Mercury (II) adsorption by activated carbon made from sago waste. Carbon 2004, 42, 745–752. [Google Scholar] [CrossRef]
- Hu, X.-J.; Liu, Y.-G.; Wang, H.; Zeng, G.-M.; Hu, X.; Guo, Y.-M.; Li, T.-T.; Chen, A.-W.; Jiang, L.-H.; Guo, F.-Y. Adsorption of copper by magnetic graphene oxide-supported β-cyclodextrin: Effects of pH, ionic strength, background electrolytes, and citric acid. Chem. Eng. Res. Des. 2015, 93, 675–683. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Chen, C.; Zhao, G.; Yang, X.; Li, J.; Wang, X. Removal of Cu (II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 4991–5000. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-B.; Fugetsu, B.; Terui, N.; Tanaka, S. Removal of organic compounds by alginate gel beads with entrapped activated carbon. J. Hazard. Mater. 2005, 120, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Taher, M.M.; Bajpai, A.K.; Heibati, B.; Tyagi, I.; Asif, M.; Agarwal, S.; Gupta, V.K. Removal of noxious Cr (VI) ions using single-walled carbon nanotubes and multi-walled carbon nanotubes. Chem. Eng. J. 2015, 279, 344–352. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sarı, A.; Tuzen, M. Optimization of parameters with experimental design for the adsorption of mercury using polyethylenimine modified-activated carbon. J. Environ. Chem. Eng. 2017, 5, 1079–1088. [Google Scholar] [CrossRef]
- Heibati, B.; Ghoochani, M.; Albadarin, A.B.; Mesdaghinia, A.; Makhlouf, A.S.H.; Asif, M.; Maity, A.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Removal of linear alkyl benzene sulfonate from aqueous solutions by functionalized multi-walled carbon nanotubes. J. Mol. Liq. 2016, 213, 339–344. [Google Scholar] [CrossRef]
- Robati, D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostruct. Chem. 2013, 3, 55. [Google Scholar] [CrossRef]
- Momenzadeh, H.; Khosravi, A.; Tehrani-Bagha, A.; Gharanjig, K. Investigation of the effective parameters on reactive dye removal from aqueous solution using chitosan nanoparticles emulsion. J. Color Sci. Technol. 2011, 5, 1–10. [Google Scholar]
- Wekoye, J.N.; Wanyonyi, W.C.; Wangila, P.T.; Tonui, M.K. Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environ. Chem. Ecotoxicol. 2020, 2, 24–31. [Google Scholar] [CrossRef]
- Netpradit, S.; Thiravetyan, P.; Towprayoon, S. Adsorption of three azo reactive dyes by metal hydroxide sludge: Effect of temperature, pH, and electrolytes. J. Colloid Interface Sci. 2004, 270, 255–261. [Google Scholar] [CrossRef]
- Wong, Y.; Szeto, Y.; Cheung, W.; McKay, G. Effect of temperature, particle size and percentage deacetylation on the adsorption of acid dyes on chitosan. Adsorption 2008, 14, 11–20. [Google Scholar] [CrossRef]
- Aksu, Z. Equilibrium and kinetic modelling of cadmium(II) biosorption by C. vulgaris in a batch system: Effect of temperature. Sep. Purif. Technol. 2001, 21, 285–294. [Google Scholar] [CrossRef]
- Singh, D.K.; Mohan, S.; Kumar, V.; Hasan, S.H. Kinetic, isotherm and thermodynamic studies of adsorption behaviour of CNT/CuO nanocomposite for the removal of As(iii) and As(v) from water. RSC Adv. 2016, 6, 1218–1230. [Google Scholar] [CrossRef]
- Lin, Z.; Pan, Z.; Zhao, Y.; Qian, L.; Shen, J.; Xia, K.; Guo, Y.; Qu, Z. Removal of Hg2+ with polypyrrole-functionalized Fe3O4/kaolin: Synthesis, performance and optimization with response surface methodology. Nanomaterials 2020, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, G.; Zhu, J. Selective adsorption of heavy metals from water by a hyper-branched magnetic composite material: Characterization, performance, and mechanism. J. Environ. Manag. 2022, 314, 114979. [Google Scholar] [CrossRef] [PubMed]
- Benettayeb, A.; Morsli, A.; Guibal, E.; Kessas, R. New derivatives of urea-grafted alginate for improving the sorption of mercury ions in aqueous solutions. Mater. Res. Express 2021, 8, 035303. [Google Scholar]
- Hanif, Z.; Lee, S.; Hussain Qasim, G.; Ardiningsih, I.; Kim, J.-A.; Seon, J.; Han, S.; Hong, S.; Yoon, M.-H. Polypyrrole multilayer-laminated cellulose for large-scale repeatable mercury ion removal. J. Mater. Chem. A 2016, 4, 12425–12433. [Google Scholar] [CrossRef]
- Azari, A.; Gharibi, H.; Kakavandi, B.; Ghanizadeh, G.; Javid, A.; Mahvi, A.H.; Sharafi, K.; Khosravia, T. Magnetic adsorption separation process: An alternative method of mercury extracting from aqueous solution using modified chitosan coated Fe3O4 nanocomposites. J. Chem. Technol. Biotechnol. 2017, 92, 188–200. [Google Scholar] [CrossRef]
- Monier, M. Adsorption of Hg2+, Cu2+ and Zn2+ ions from aqueous solution using formaldehyde cross-linked modified chitosan–thioglyceraldehyde Schiff’s base. Int. J. Biol. Macromol. 2012, 50, 773–781. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhao, Y.; Xia, K.; Guo, Y.; Qu, Z.; Bai, R. A mild and facile synthesis of amino functionalized CoFe2O4@ SiO2 for Hg (II) removal. Nanomaterials 2018, 8, 673. [Google Scholar] [CrossRef]
- Shi, T.; Xie, Z.; Zhu, Z.; Shi, W.; Liu, Y.; Liu, M. Highly efficient and selective adsorption of heavy metal ions by hydrazide-modified sodium alginate. Carbohydr. Polym. 2022, 276, 118797. [Google Scholar] [CrossRef]

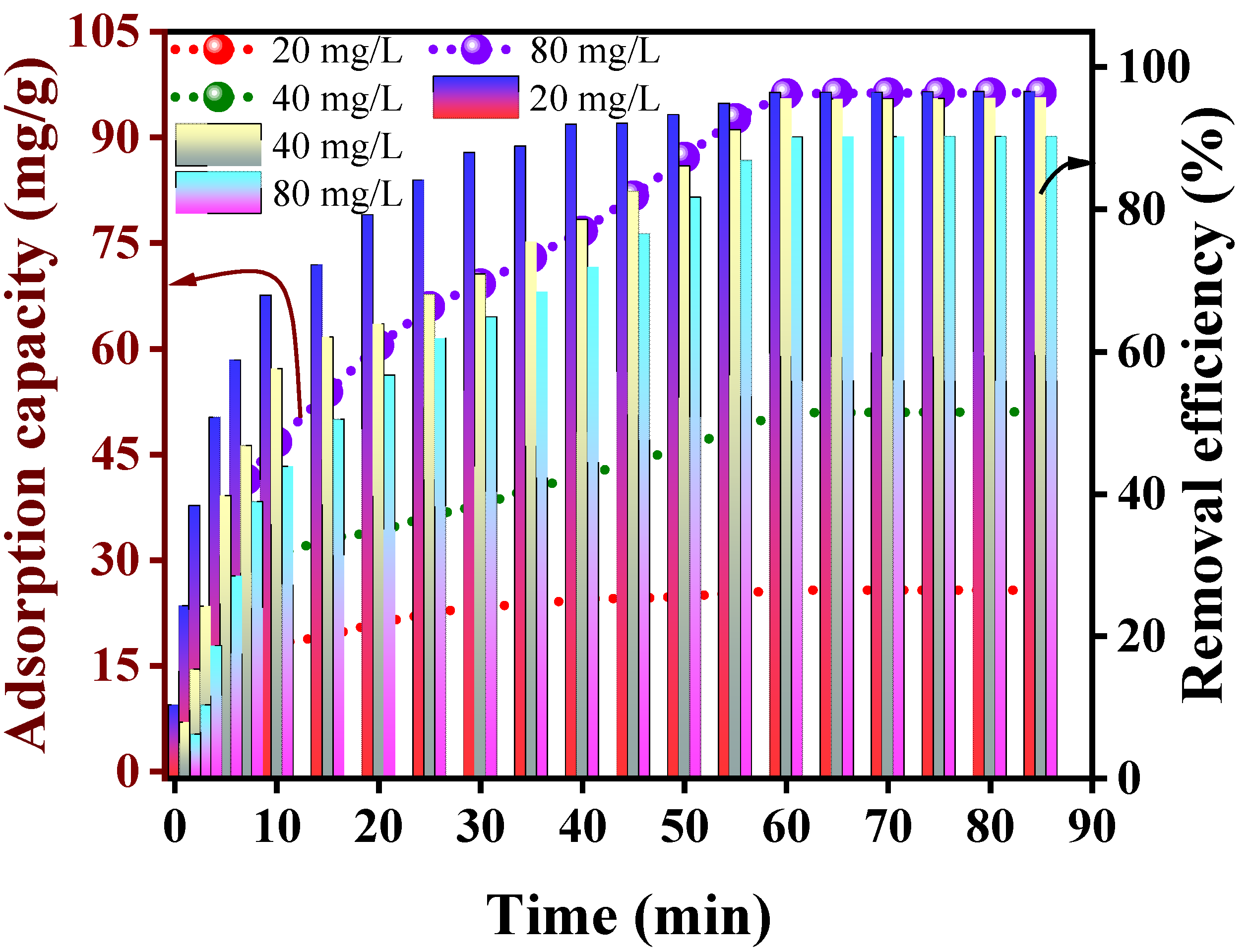

| Kinetics | Parameters | 20 mg/L | 40 mg/L | 80 mg/L | Kinetics | Parameters | 20 mg/L | 40 mg/L | 80 mg/L |
|---|---|---|---|---|---|---|---|---|---|
| P-fo | Qexp (mg/g) | 25.7209 | 50.9800 | 96.1867 | I-DI * | KI (mg/min1/2/g) | 6.2558 | 10.4559 | 15.7822 |
| Qfo (mg/g) | 18.368 | 41.264 | 90.477 | CI | −1.3585 | −3.6302 | −5.5511 | ||
| K1 (1/min) | 0.066 | 0.0435 | 0.0465 | Radj2 | 0.9597 | 0.9439 | 0.8868 | ||
| Radj2 | 0.9827 | 0.9368 | 0.9218 | R2-linear | 0.9664 | 0.9532 | 0.9094 | ||
| R2-linear | 0.9839 | 0.9413 | 0.9274 | I-DII * | KII (mg/min1/2/g) | 1.7178 | 4.1280 | 10.2239 | |
| CII | 13.220 | 16.3937 | 14.1056 | ||||||
| P-so | Qexp (mg/g) | 25.7209 | 50.9800 | 96.1867 | Radj2 | 0.9428 | 0.9702 | 0.9925 | |
| Qso (mg/g) | 28.249 | 54.348 | 111.111 | R2-linear | 0.9491 | 0.9736 | 0.9933 | ||
| K2 (g/min/mg) | 5.48 × 10−3 | 1.78 × 10−3 | 5.82 × 10−5 | I-DIII * | KIII (mg/min1/2/g) | 0.0277 | 0.0628 | 0.0731 | |
| Radj2 | 0.9972 | 0.9869 | 0.9822 | CIII | 25.5047 | 50.4595 | 95.9451 | ||
| R2-linear | 0.9974 | 0.9869 | 0.9834 | Radj2 | 0.9428 | 0.5587 | 0.8075 | ||
| R2-linear | 0.9524 | 0.6470 | 0.8460 |
| Isotherm | Parameters | 303 K | 313 K |
|---|---|---|---|
| Langmuir | Qm (mg/g) | 213.719 | 219.750 |
| KL (L/mg) | 0.1484 | 0.3160 | |
| R2 | 0.9812 | 0.9910 | |
| Radj2 | 0.9801 | 0.9905 | |
| Freundlich | KF (mg1−1/n L1/n g−1) | 44.5933 | 62.5156 |
| 1/n | 2.6316 | 2.8425 | |
| R2 | 0.9499 | 0.9547 | |
| Radj2 | 0.9469 | 0.9521 | |
| Dubinin–Radushkevich | Qm (mg/g) | 99.484 | 120.313 |
| KDR (mol2/K2J2) | 2 × 10−7 | 9 × 10−8 | |
| E (KJ/mol) | 2.236 | 3.333 | |
| R2 | 0.7163 | 0.7951 | |
| Radj2 | 0.6998 | 0.7849 |
| Adsorbent | Isotherm Model | Kinetic Model | Percent Removal (%R) | Adsorption Capacity (mg/g) | pH | Conc. (mg/L) | Ref. |
|---|---|---|---|---|---|---|---|
| Magnetic PPy–Graphene oxide | Langmuir | P-so | 99.0 | 400 | 7 | 100 | [55] |
| Hydrazide-modified sodium alginate | Freundlich | P-fo | 96.1 | 7.833 * | 6 | 0.499 ** | [81] |
| Polypyrrole-Fe3O4/Kaolin | Langmuir | P-so | - | 317.1 | 7.2 | 50 | [74] |
| Fe3O4@SiO2-S4 | Langmuir | P-so | - | 2.42 * | 6 | 2 ** | [75] |
| Urea-grafted alginate | Langmuir | P-so | - | 200 | 5.5 | 0.25 | [76] |
| Polypyrrole multilayer-laminated cellulose | Langmuir | P-so | 96 | 31.689 | 6 | 100 | [77] |
| Modified chitosan coated Fe3O4 | Langmuir | P-so | 97.3 | 96 | 5 | 25 | [78] |
| Magnetic chitosan-thioglyceraldehyde | Langmuir | P-so | 85 | 98 | 5 | 100 | [79] |
| CoFe2O4@SiO2 | Langmuir | P-so | - | 149.3 | 7 | 20 | [80] |
| Alg@Mag/PPy NCs | Langmuir | P-so | 95.58 | 213.72 | 7 | 40 | Our work |
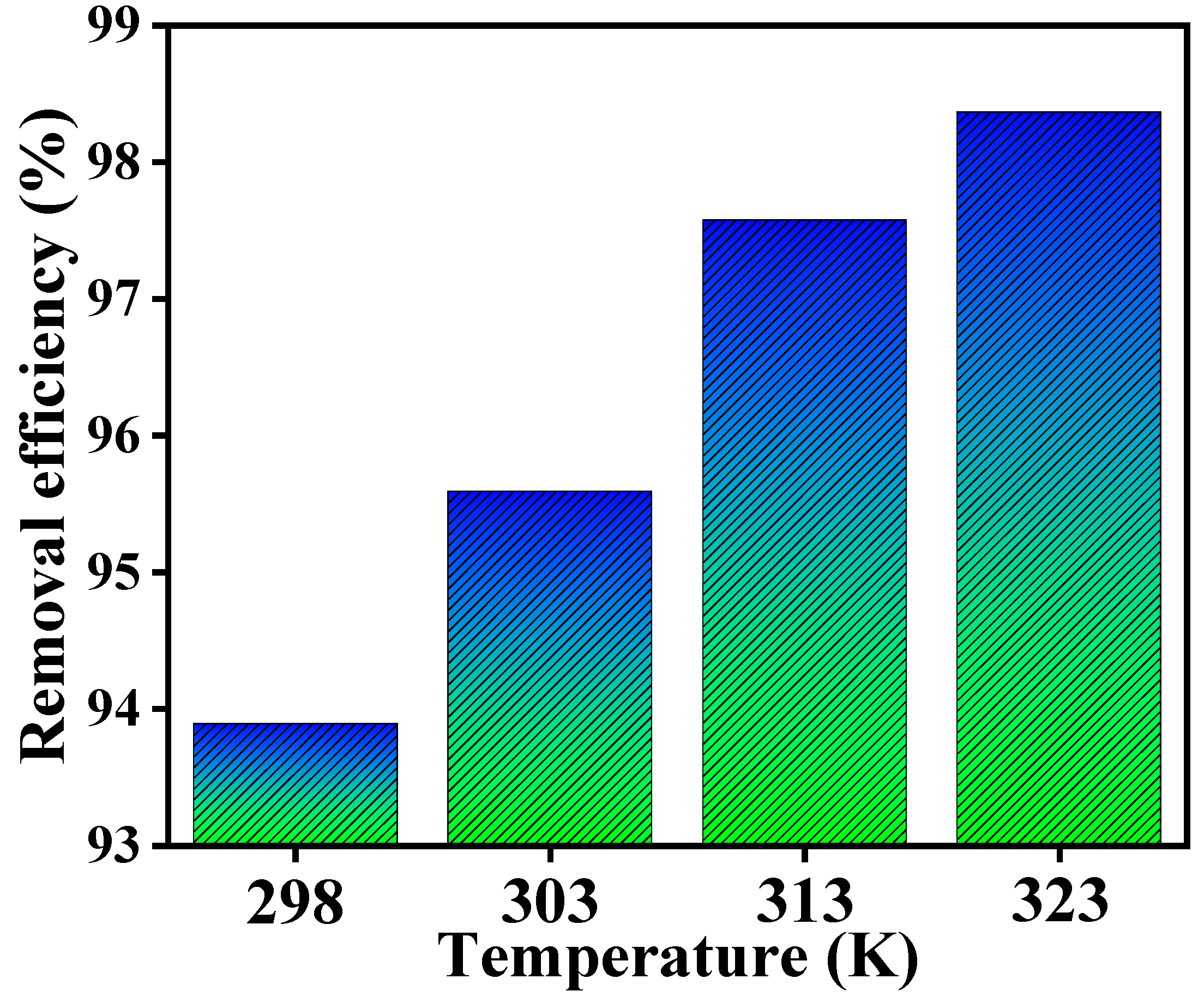
| T (K) | ΔS° (J/K/mol) | ΔH° (kJ/mol) | −ΔG° (kJ/mol) | R2 |
|---|---|---|---|---|
| 298 | 175.649 | 43.123 | 9.134 | 0.9923 |
| 303 | 10.125 | |||
| 313 | 12.037 | |||
| 323 | 13.489 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashkoor, F.; Shoeb, M.; Jeong, C. Alginate Modified Magnetic Polypyrrole Nanocomposite for the Adsorptive Removal of Heavy Metal. Polymers 2023, 15, 4285. https://doi.org/10.3390/polym15214285
Mashkoor F, Shoeb M, Jeong C. Alginate Modified Magnetic Polypyrrole Nanocomposite for the Adsorptive Removal of Heavy Metal. Polymers. 2023; 15(21):4285. https://doi.org/10.3390/polym15214285
Chicago/Turabian StyleMashkoor, Fouzia, Mohd Shoeb, and Changyoon Jeong. 2023. "Alginate Modified Magnetic Polypyrrole Nanocomposite for the Adsorptive Removal of Heavy Metal" Polymers 15, no. 21: 4285. https://doi.org/10.3390/polym15214285
APA StyleMashkoor, F., Shoeb, M., & Jeong, C. (2023). Alginate Modified Magnetic Polypyrrole Nanocomposite for the Adsorptive Removal of Heavy Metal. Polymers, 15(21), 4285. https://doi.org/10.3390/polym15214285







