Synthesis of Zinc Oxide Doped Magnesium Hydrate and Its Effect on the Flame Retardant and Mechanical Properties of Polypropylene
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of ZO@MH
2.3. Preparation of KH-570 Functionalized ZO@MH
2.4. Preparation of PP Composites
2.5. Characterization
3. Results and Discussion
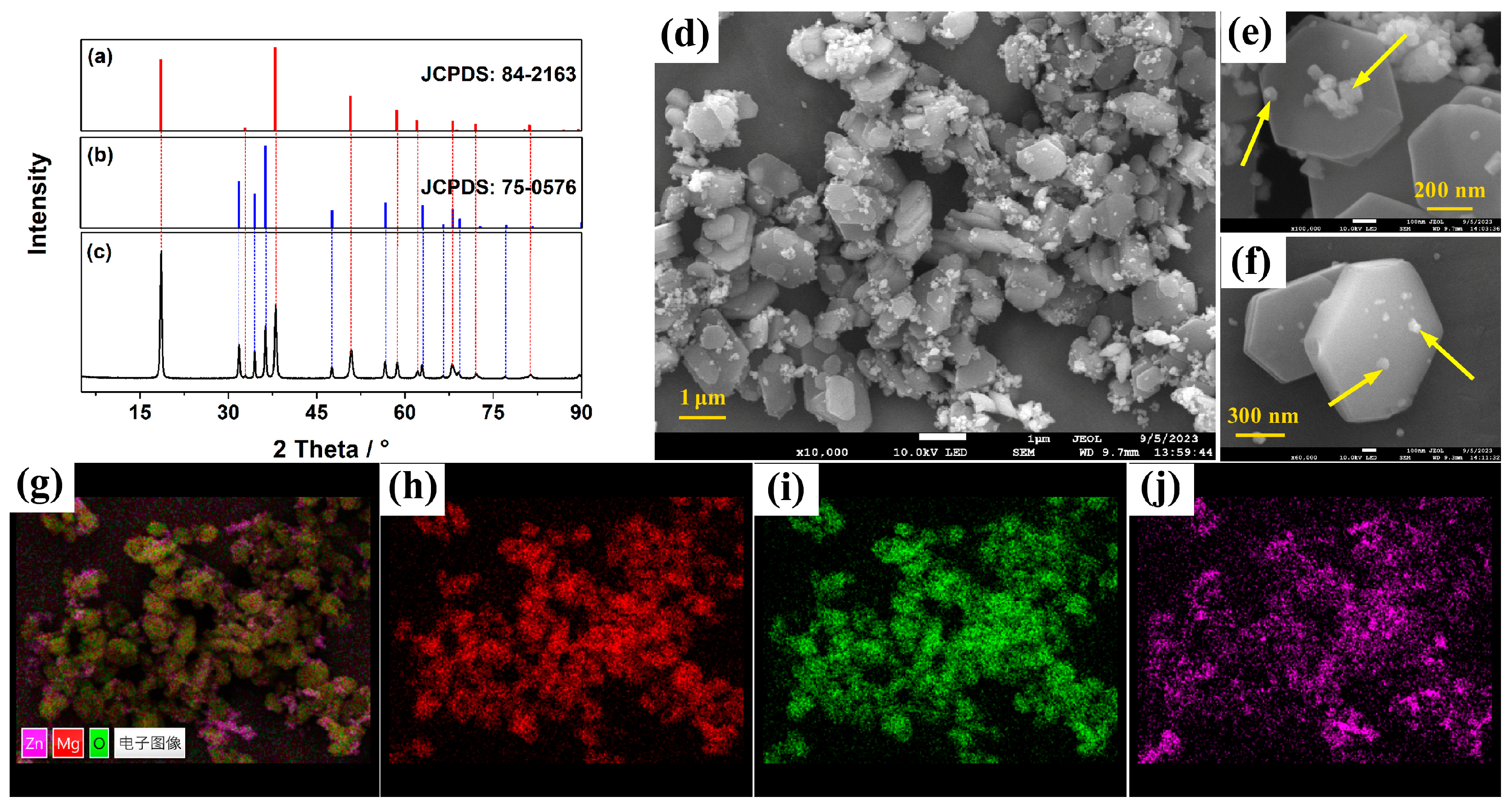
3.1. Characterization of ZO@MH
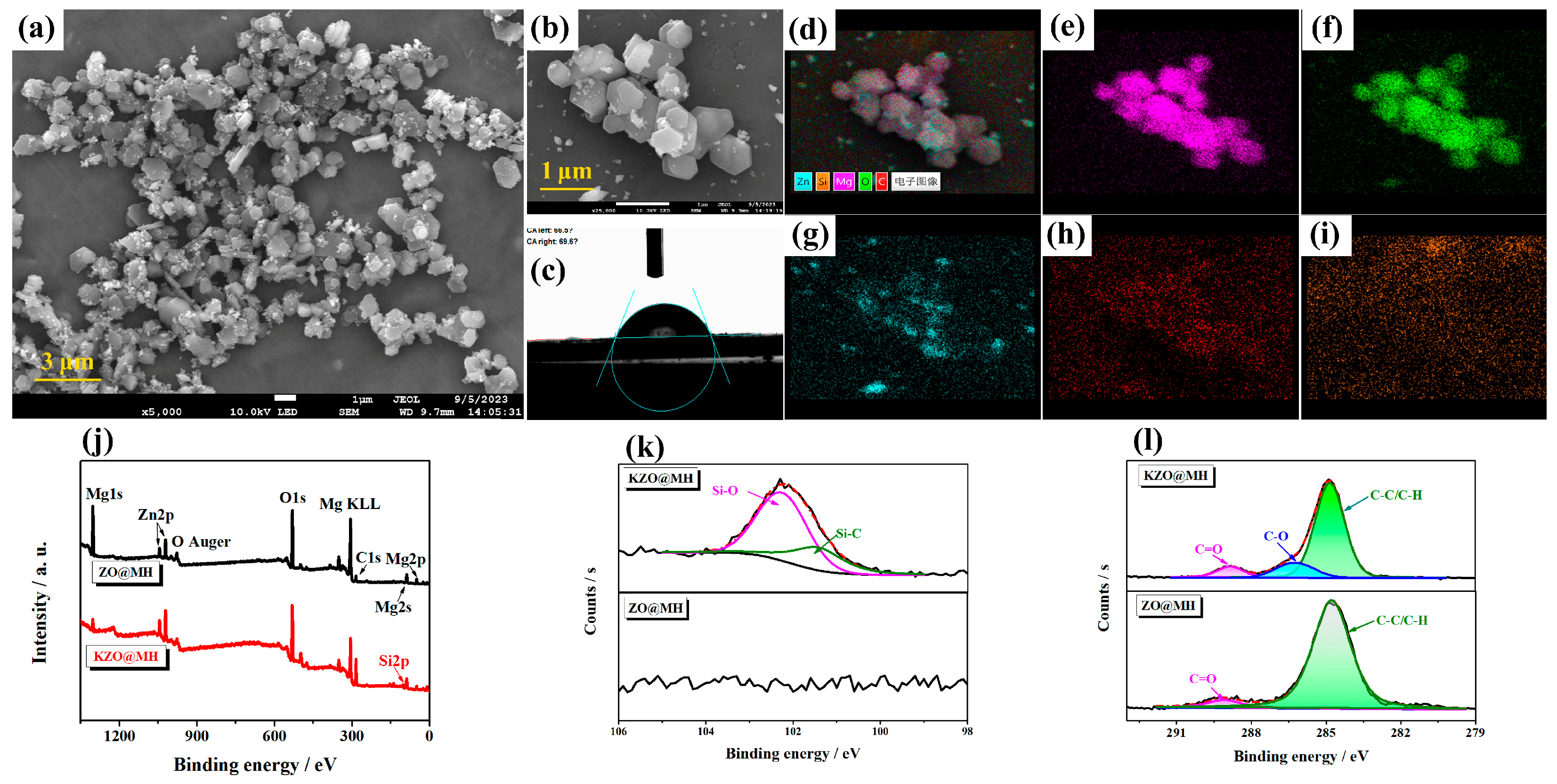
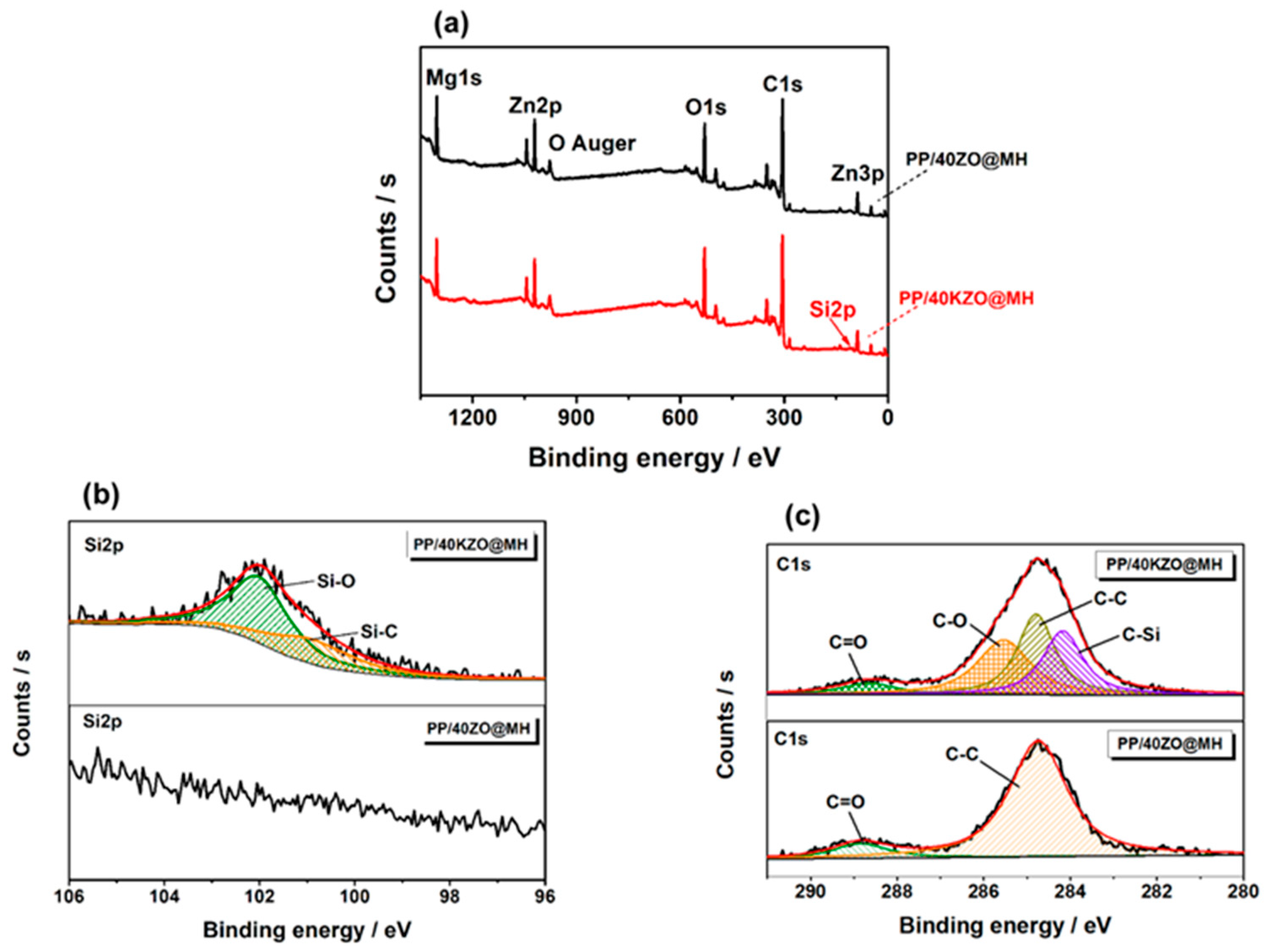
3.2. Characterization of KZO@MH
3.3. Fire Hazards of PP Composites
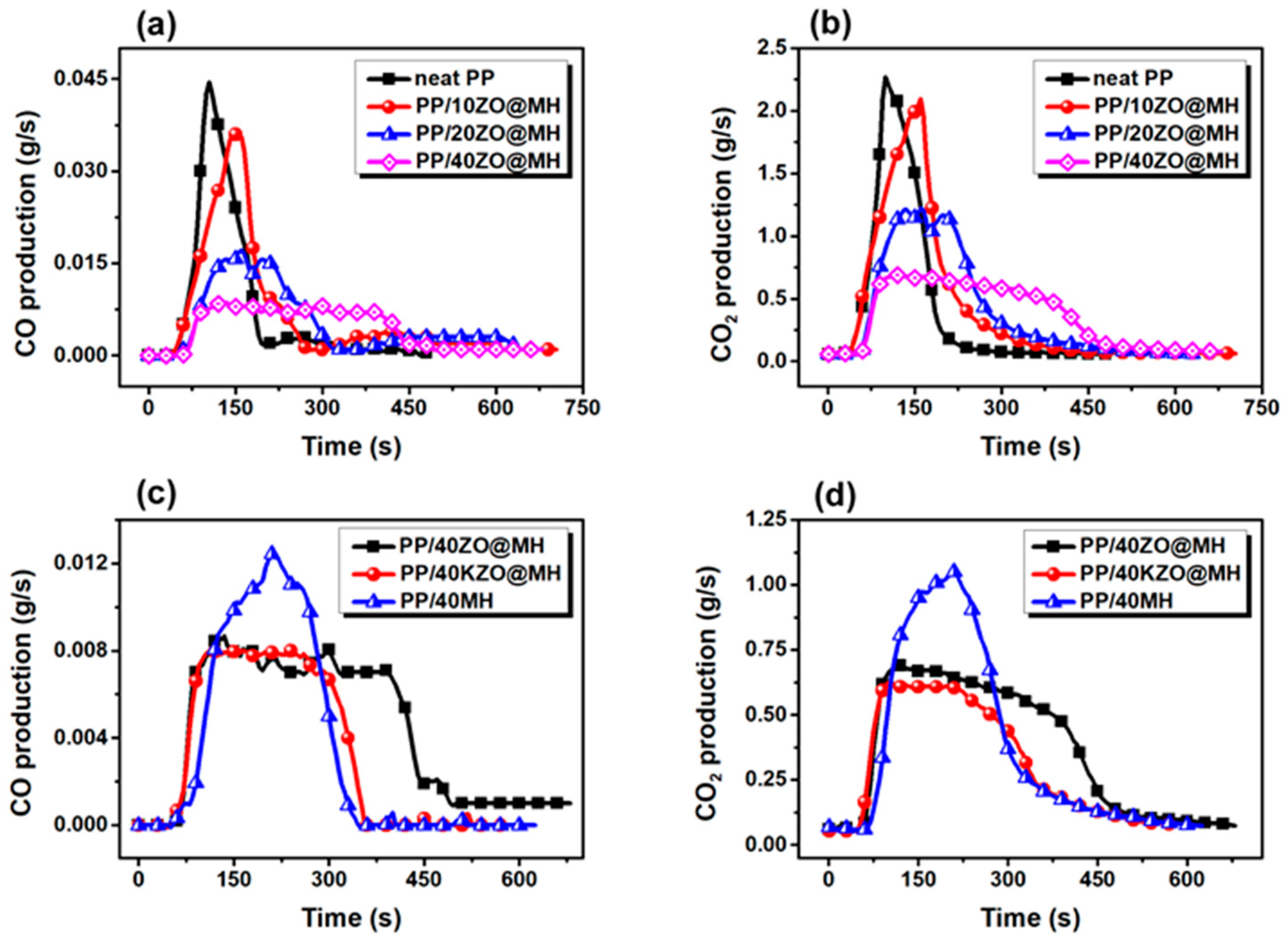
3.3.1. Fire Hazards Assessed by CCT
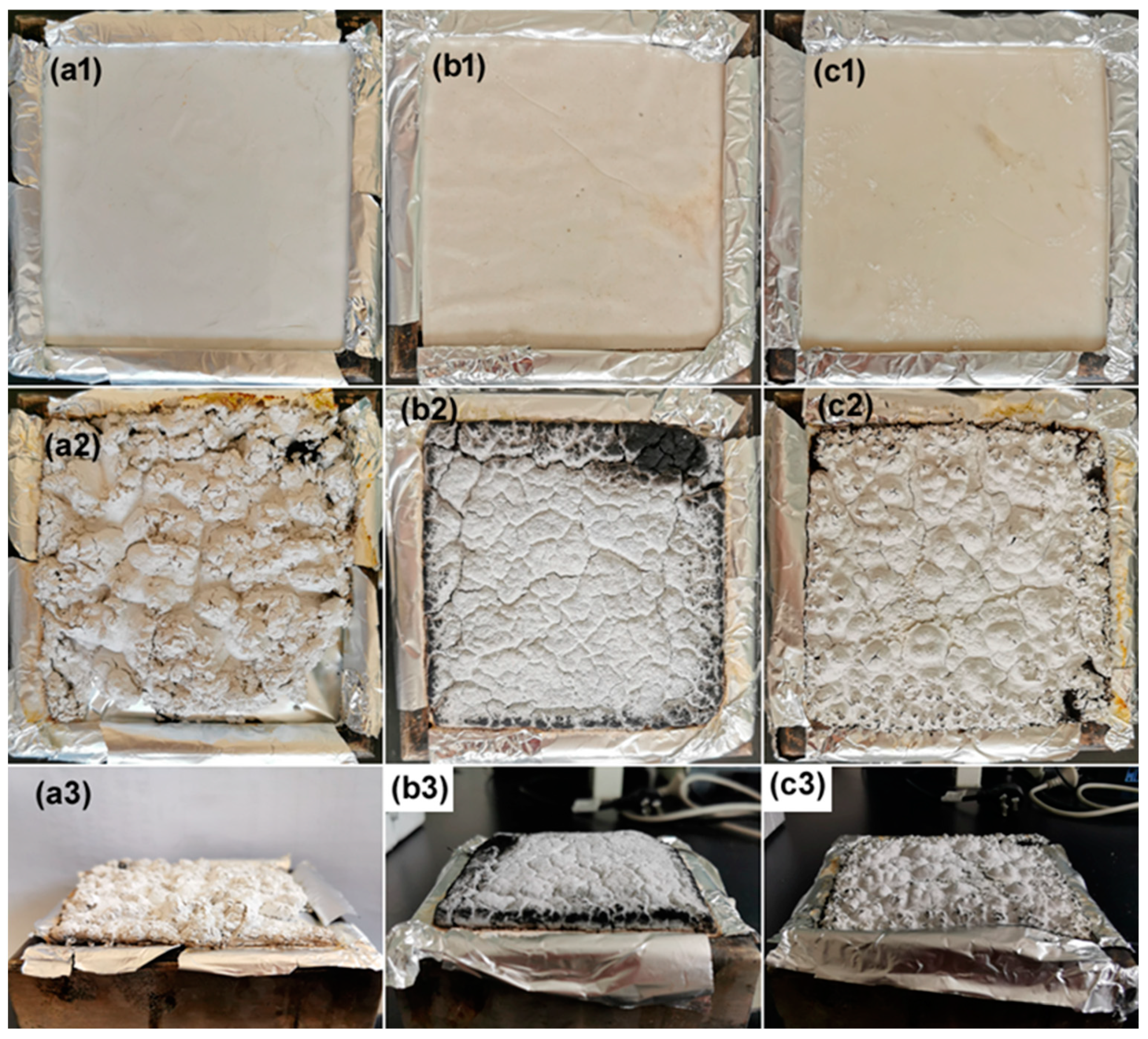
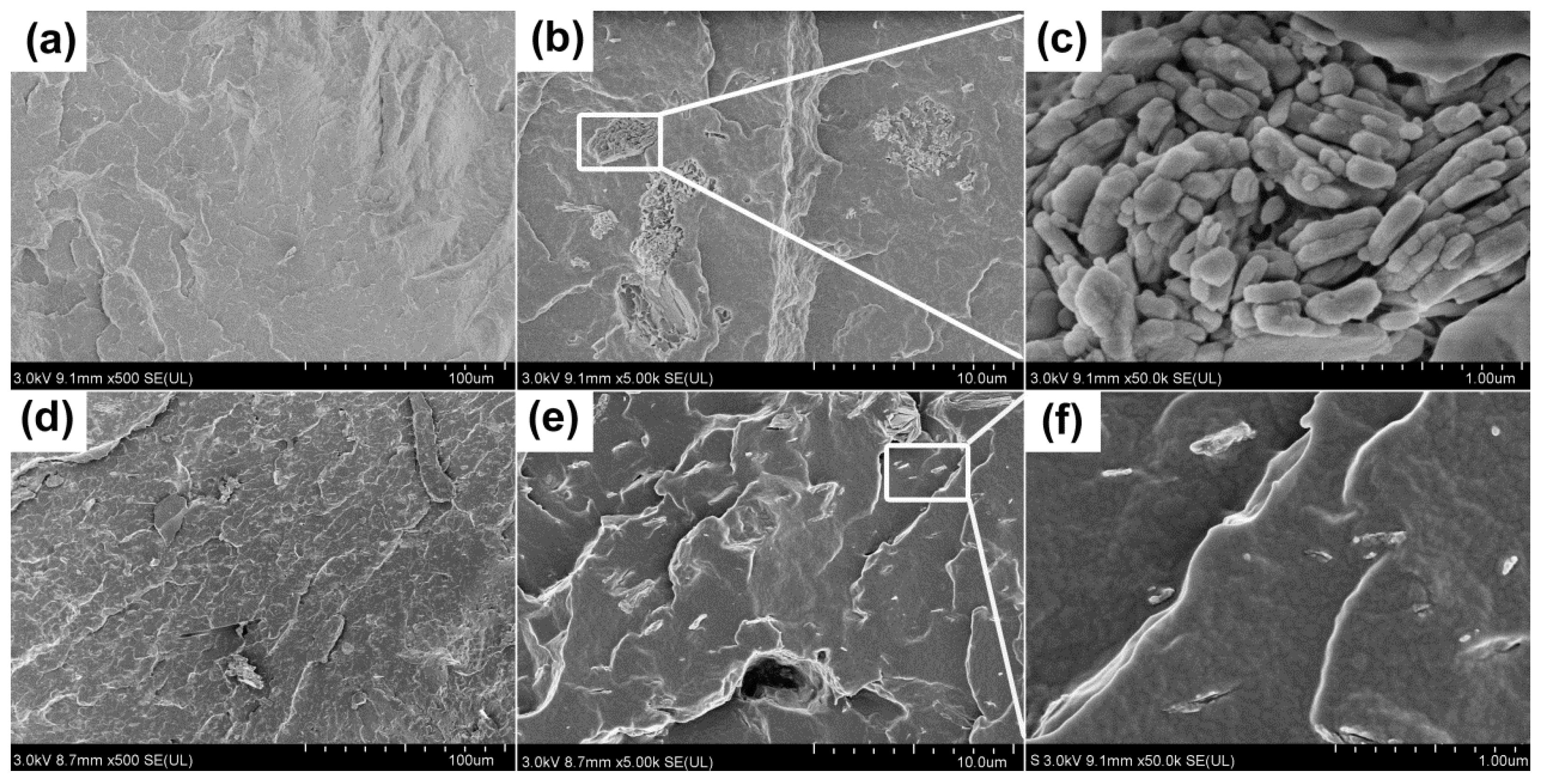
3.3.2. Analysis of Char Residue
3.4. Mechanical Properties of PP Composites
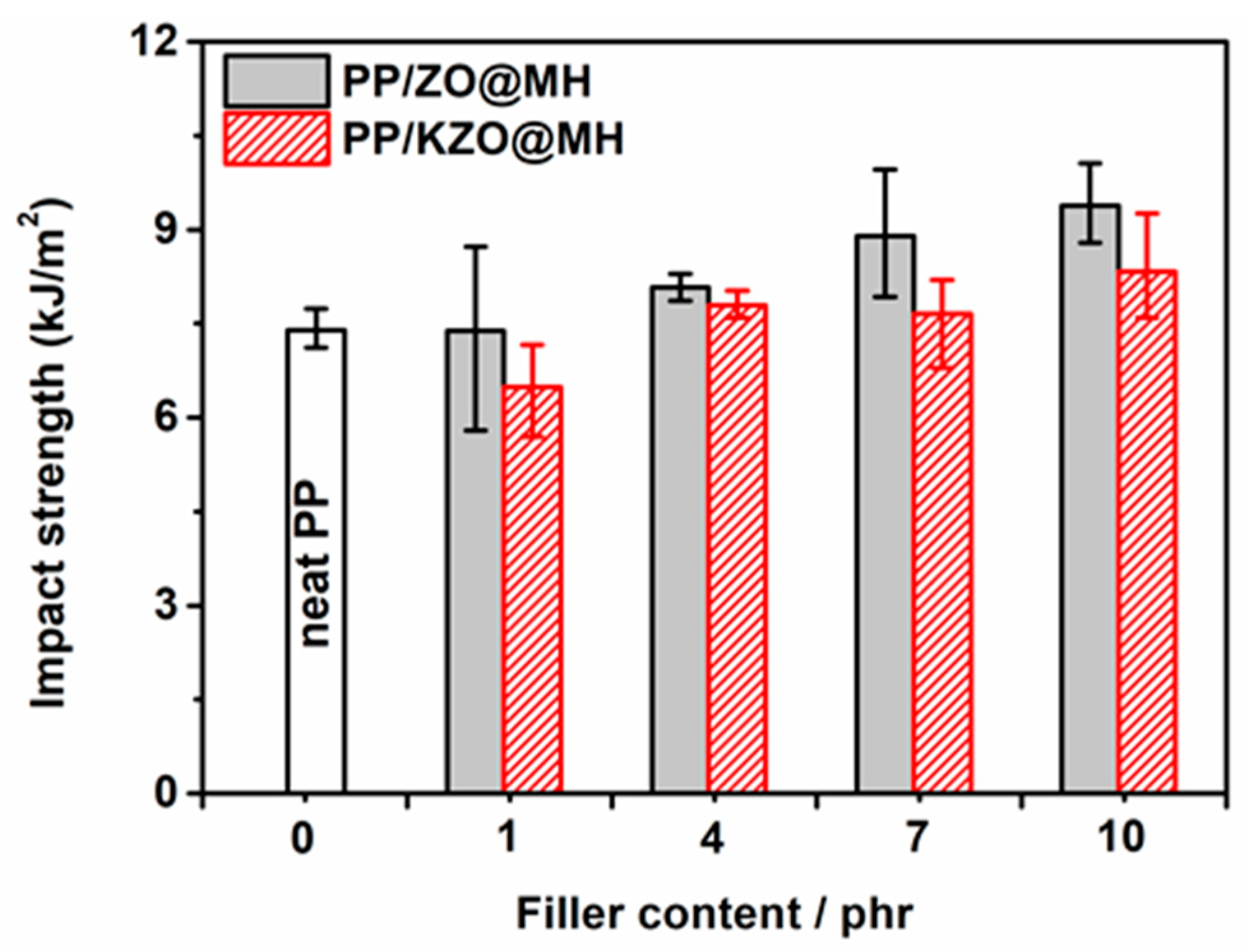
3.4.1. Impact Properties
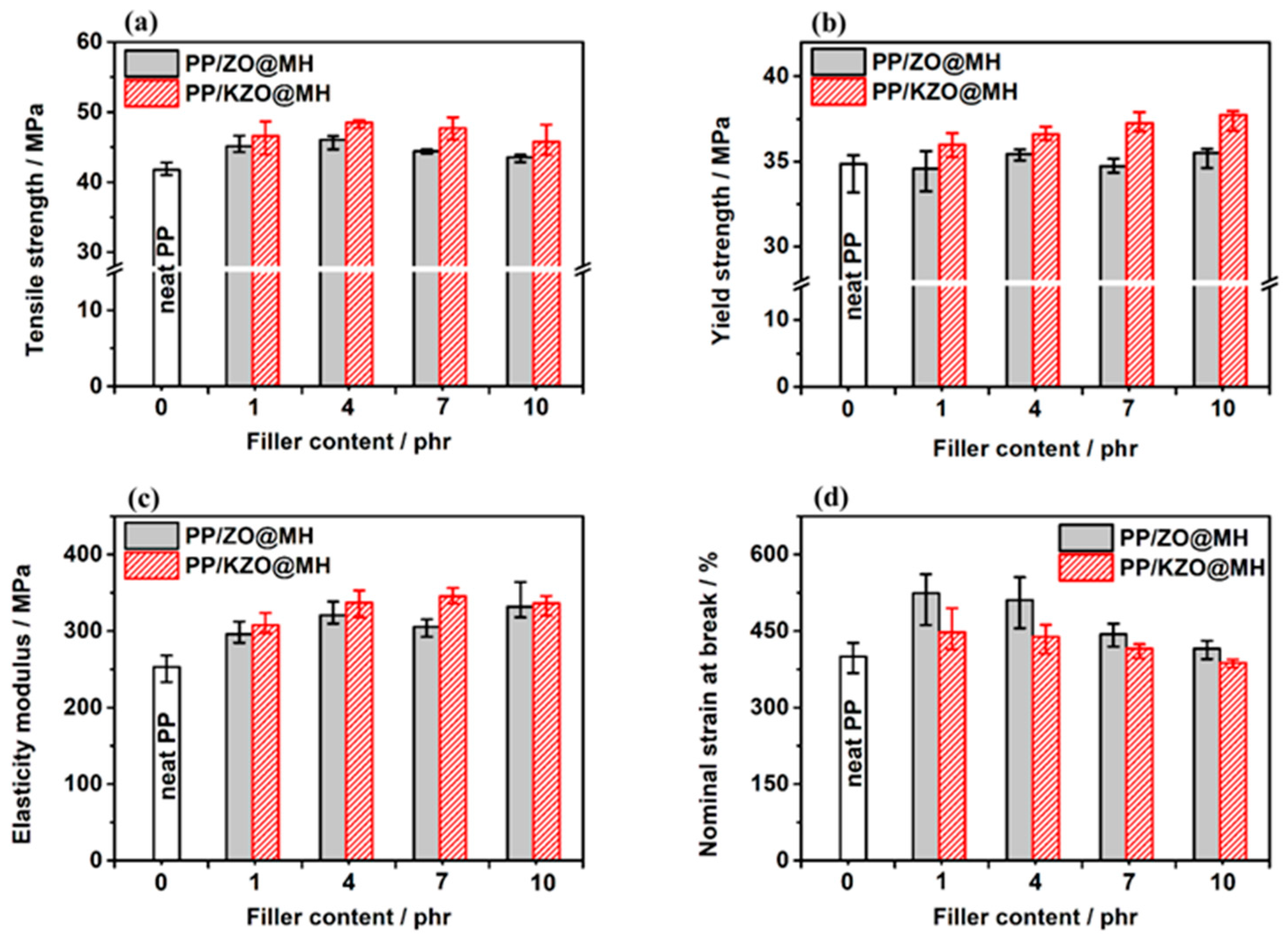
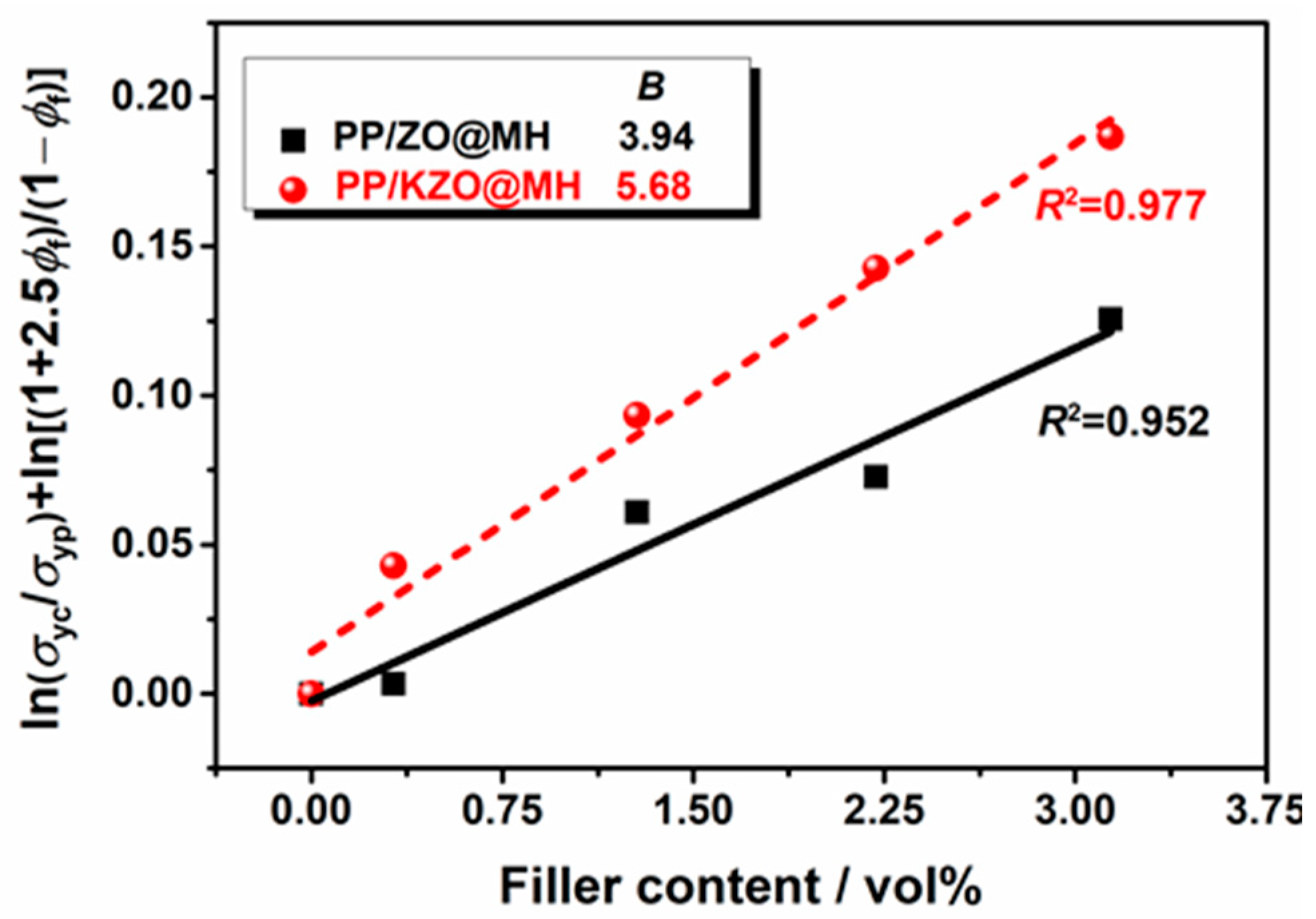
3.4.2. Tensile Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Yu, J.; Guo, S.; Luo, Z.; He, M. Effects of magnesium hydroxide and its surface modification on crystallization and rheological behaviors of polypropylene. Polym. Compos. 2008, 30, 941–947. [Google Scholar] [CrossRef]
- Liang, J.-Z. Tensile and flexural properties of polypropylene composites filled with highly effective flame retardant magnesium hydroxide. Polym. Test. 2017, 60, 110–116. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, C.; Xu, S. Mechanical, thermal and flame retardant properties of magnesium hydroxide filled poly(vinyl chloride) composites: The effect of filler shape. Compos. Part A Appl. Sci. Manuf. 2018, 113, 1–11. [Google Scholar] [CrossRef]
- Dang, L.; Lv, Z.; Du, X.; Tang, D.; Zhao, Y.; Zhu, D.; Xu, S. Flame retardancy and smoke suppression of molybdenum trioxide doped magnesium hydrate in flexible polyvinyl chloride. Polym. Adv. Technol. 2020, 31, 2108–2121. [Google Scholar] [CrossRef]
- Han, Y.; Xu, S.; Wang, A.; Cheng, P.; Li, J.; Shen, L.; Liu, H. Remarkable effects of silicone rubber on flame retardant property of high-density polyethylene/magnesium hydroxide composites. Polym. Degrad. Stab. 2022, 203, 110061. [Google Scholar] [CrossRef]
- ECabrera-Álvarez, N.; Ramos-deValle, L.F.; Sánchez-Valdes, S.; Candia-García, A.; Soriano-Corral, F.; Ramírez-Vargas, E.; Ibarra-Alonso, M.C.; Patiño-Soto, P. Study of the silane modification of magnesium hydroxide and their effects on the flame retardant and tensile properties of high density polyethylene nanocomposites. Polym. Compos. 2013, 35, 1060–1069. [Google Scholar]
- Li, R.; Sun, B.; Dang, L.; Pan, T.; Xu, J.; Xu, S. Effect of the melt flow index of compatibilizer on the melt processing and properties of highly filled magnesium hydroxide/linear low density polyethylene composites. J. Appl. Polym. Sci. 2023, 140, e54371. [Google Scholar] [CrossRef]
- Cao, B.; Zhou, Y.; Wu, Y.; Cai, J.; Guan, X.; Liu, S.; Zhao, J.; Zhang, M. Simultaneous improvement of processability and toughness of highly filled MH/LLDPE composites by using fluorine-containing flow modifiers. Compos. Part A Appl. Sci. Manuf. 2020, 134, 105900. [Google Scholar] [CrossRef]
- Yao, M.; Wu, H.; Liu, H.; Zhou, Z.; Wang, T.; Jiao, Y.; Qu, H. In-situ growth of boron nitride for the effect of layer-by-layer assembly modified magnesium hydroxide on flame retardancy, smoke suppression, toxicity and char formation in EVA. Polym. Degrad. Stab. 2021, 183, 109417. [Google Scholar] [CrossRef]
- Wang, T.; Yao, D.-W.; Yin, G.-Z.; Jiang, Y.; Wang, N.; Wang, D.-Y. Gallic acid-iron complex modified magnesium hydroxide and its effect on flame retardancy of EVA. Adv. Ind. Eng. Polym. Res. 2022, 6, 172–180. [Google Scholar] [CrossRef]
- Pan, Y.; Zhan, J.; Pan, H.; Wang, W.; Ge, H.; Song, L.; Hu, Y. A novel and effective method to fabricate flame retardant and smoke suppressed flexible polyurethane foam. RSC Adv. 2015, 5, 67878–67885. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Dong, Q.; Yuan, W.; Liu, P.; Ding, Y.; Zhang, S.; Yang, M.; Zheng, G. Expandable graphite encapsulated by magnesium hydroxide nanosheets as an intumescent flame retardant for rigid polyurethane foams. J. Appl. Polym. Sci. 2018, 135, 46749. [Google Scholar] [CrossRef]
- Liu, H.; Yi, J. Polystyrene/magnesium hydroxide nanocomposite particles prepared by surface-initiated in-situ polymerization. Appl. Surf. Sci. 2009, 255, 5714–5720. [Google Scholar] [CrossRef]
- Liu, S.; Ying, J.; Zhou, X.; Xie, X. Core-shell magnesium hydroxide/polystyrene hybrid nanoparticles prepared by ultrasonic wave-assisted in-situ copolymerization. Mater. Lett. 2009, 63, 911–913. [Google Scholar] [CrossRef]
- Xue, B.; Niu, M.; Yang, Y.; Bai, J.; Song, Y.; Peng, Y.; Liu, X. Coating magnesium hydroxide on surface of carbon microspheres and interface binding with poly (ethylene terephthalate) matrix. Appl. Surf. Sci. 2017, 412, 545–553. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, M.; Dai, J.; Bai, J.; Xue, B.; Song, Y.; Peng, Y. Flame-retarded polyethylene terephthalate with carbon microspheres/magnesium hydroxide compound flame retardant. Fire Mater. 2018, 42, 794–804. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Shan, Z.; Wang, S.; Xiao, Y. Surface modification of magnesium hydroxide by wet process and effect on the thermal stability of silicone rubber. Appl. Surf. Sci. 2018, 465, 740–746. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, W.; Zhang, M.; Qu, J.; Shi, L.; Qu, H.; Xu, J. Hydrothermal synthesis of 4ZnO·B2O3·H2O/RGO hybrid material and its flame retardant behavior in flexible PVC and magnesium hydroxide composites. Appl. Surf. Sci. 2017, 425, 896–904. [Google Scholar] [CrossRef]
- Ye, L.; Wu, Q.; Qu, B. Synergistic effects and mechanism of multiwalled carbon nanotubes with magnesium hydroxide in halogen-free flame retardant EVA/MH/MWNT nanocomposites. Polym. Degrad. Stab. 2009, 94, 751–756. [Google Scholar] [CrossRef]
- Wang, S.; Liang, S.; Wang, K.; Liu, J.; Luo, J.; Peng, S. Enhanced flame retardancy, smoke suppression, and acid resistance of polypropylene/magnesium hydroxide composite by expandable graphite and microencapsulated red phosphorus. J. Vinyl Addit. Technol. 2023, 29, 395–409. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, J.; Luo, J.; Liu, H.; Peng, S. Enhancement of thermal stability and flame retardancy of ethylene vinyl acetate/magnesium hydroxide composite by carbon black. Fire Mater. 2022, 47, 251–261. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Chang, H.; Li, H.; Xu, A.; Pan, B. Interaction between magnesium hydroxide and microencapsulated red phosphorus in flame-retarded high-impact polystyrene composite. Fire Mater. 2018, 42, 958–966. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Wang, Q. Effects of magnesium hydroxide and its synergistic systems on the flame retardance of polyformaldehyde. J. Appl. Polym. Sci. 2011, 125, 968–974. [Google Scholar] [CrossRef]
- Liu, T.; Wang, F.; Li, G.; Liu, P.; Gao, C.; Ding, Y.; Zhang, S.; Kong, X.; Yang, M. Magnesium hydroxide nanoparticles grafted by DOPO and its flame retardancy in ethylene-vinyl acetate copolymers. J. Appl. Polym. Sci. 2021, 138, 49607. [Google Scholar] [CrossRef]
- Ozcelik, G.; Elcin, O.; Guney, S.; Erdem, A.; Hacioglu, F.; Dogan, M. Flame-retardant features of various boron compounds in thermoplastic polyurethane and performance comparison with aluminum trihydroxide and magnesium hydroxide. Fire Mater. 2022, 46, 1020–1033. [Google Scholar] [CrossRef]
- Lenża, J.; Merkel, K.; Rydarowski, H. Comparison of the effect of montmorillonite, magnesium hydroxide and a mixture of both on the flammability properties and mechanism of char formation of HDPE composites. Polym. Degrad. Stab. 2012, 97, 2581–2593. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yu, Z.; Yang, W.; Luo, J.; Pan, B.; Lu, C. Enhancement of organoclay on thermal and flame retardant properties of polystyrene/magnesium hydroxide composite. Polym. Compos. 2014, 37, 746–755. [Google Scholar] [CrossRef]
- Gul, R.; Islam, A.; Yasin, T.; Mir, S. Flame-retardant synergism of sepiolite and magnesium hydroxide in a linear low-density polyethylene composite. J. Appl. Polym. Sci. 2011, 121, 2772–2777. [Google Scholar] [CrossRef]
- Yin, H.; Dai, H.; Liang, G. Inhibition evaluation of magnesium hydroxide, aluminum hydroxide, and hydrotalcite on the flame propagation of coal dust. Process. Saf. Environ. Prot. 2022, 157, 443–457. [Google Scholar] [CrossRef]
- Yücesoy, A.; Tamer, Y.B.; Berber, H. Improvement of flame retardancy and thermal stability of highly loaded low density polyethylene/magnesium hydroxide composites. J. Appl. Polym. Sci. 2023, 140, e54107. [Google Scholar] [CrossRef]
- Han, L.; Wu, W.; Qu, H.; Han, X.; Wang, A.; Jiao, Y.; Xu, J. Metallic ferrites as flame retardants and smoke suppressants in flexible poly(vinyl chloride). J. Therm. Anal. Calorim. 2016, 123, 293–300. [Google Scholar] [CrossRef]
- Lum, R.M. MoO3 additives for PVC: A study of the molecular interactions. J. Appl. Polym. Sci. 1979, 23, 1247–1263. [Google Scholar] [CrossRef]
- Xiu, F.-R.; Weng, H.; Qi, Y.; Yu, G.; Zhang, Z.; Zhang, F.-S. A novel reutilization method for waste printed circuit boards as flame retardant and smoke suppressant for poly (vinyl chloride). J. Hazard. Mater. 2016, 315, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Wei, J.; Xu, W.; Wang, B.; Luo, S.; Yu, Q. Effect of organic polymers on mechanical property and toughening mechanism of slag geopolymer matrix. Polymers 2022, 14, 4214. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Jiang, J.; Meng, C.; Hu, X.; Xie, H.; Wu, M.; Guo, Q. Polypropylene composites reinforced by nonmetallic from waste printed circuit boards using spout-fluid bed coating with PP particles enhance fluidization. Polymers 2021, 13, 3106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Yu, Q.; Huang, C.; Chen, S.; Wu, Y.; Lin, J.; Chen, J.; Zhu, P. Preparation of mesoporous spherical magnesium hydroxide particles via the static self-assembled method. J. Mol. Struct. 2019, 1175, 858–864. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, Z.; Liu, X.; Gu, Z.; Li, H.; Chen, Q. Preparation of zinc oxide with core–shell structure and its application in rubber products. Polymers 2023, 15, 2353. [Google Scholar] [CrossRef]
- Lan, S.; Li, L.; Xu, D.; Zhu, D.; Liu, Z.; Nie, F. Surface modification of magnesium hydroxide using vinyltriethoxysilane by dry process. Appl. Surf. Sci. 2016, 382, 56–62. [Google Scholar] [CrossRef]
- Dang, L.; Nai, X.; Zhu, D.; Jing, Y.; Liu, X.; Dong, Y.; Li, W. Study on the mechanism of surface modification of magnesium oxysulfate whisker. Appl. Surf. Sci. 2014, 317, 325–331. [Google Scholar] [CrossRef]
- Rao, N.; Naidu, T.T.M.; Kim, M.S.; Parvatamma, B.; Prashanthi, Y.; Koo, B.H. Influence of zinc oxide nanoparticles and char forming agent polymer on flame retardancy of intumescent flame retardant coatings. Nanomaterials 2020, 10, 42. [Google Scholar]
- Dang, L.; Lv, Z.; Liu, X. Influences of 4ZnO·B2O3·H2O whisker based intumescent flame retardant on the mechanical, flame retardant and smoke suppression properties of polypropylene composites. J. Appl. Polym. Sci. 2021, 138, 51016. [Google Scholar] [CrossRef]
- Dang, L.; Nai, X.-Y.; Liu, X.; Zhu, D.-H.; Dong, Y.-P.; Li, W. Crystallization, mechanical, thermal and rheological properties of polypropylene composites reinforced by magnesium oxysulfate whisker. Chin. J. Polym. Sci. 2017, 35, 659–671. [Google Scholar] [CrossRef]
- Li, R.; Xu, S.; Xu, J.; Pan, T.; Sun, B.; Dang, L. Effect of functionalized polyethylene wax on the melt processing and properties of highly filled magnesium hydroxide/linear low-density polyethylene composites. Polymers 2023, 15, 2575. [Google Scholar] [CrossRef] [PubMed]
- Alasfar, R.H.; Ahzi, S.; Barth, N.; Kochkodan, V.; Khraisheh, M.; Koç, M. A review on the modeling of the elastic modulus and yield stress of polymers and polymer nanocomposites: Effect of temperature, loading rate and porosity. Polymers 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]


| Label | Filler Type | phr a of Filler |
|---|---|---|
| neat PP | / | I = 1, 4, 7, 10, 20, 40 |
| PP/iMH | MH | |
| PP/iZO@MH | ZO@MH | |
| PP/iKZO@MH | KZO@MH |
| Sample | pHRR (kW/m2) | avHRR (kW/m2) | pFIGRA (W/(m2 × s)) | pCOP (g/s) | pCO2P (g/s) | Residue (wt%) |
|---|---|---|---|---|---|---|
| neat PP | 932.7 | 174.5 | 7208.3 | 0.044 | 2.27 | 0.79 |
| PP/10ZO@MH | 862.3 | 183.8 | 5489.5 | 0.036 | 2.10 | 16.14 |
| PP/20ZO@MH | 577.5 | 182.3 | 3966.0 | 0.017 | 1.22 | 26.69 |
| PP/40ZO@MH | 348.4 | 163.9 | 2928.7 | 0.009 | 0.69 | 31.25 |
| PP/40KZO@MH | 327.0 | 137.2 | 2621.3 | 0.008 | 0.62 | 37.23 |
| PP/40MH | 475.1 | 153.7 | 2883.6 | 0.012 | 1.05 | 34.93 |
| Sample | Impact Strength (kJ/m2) | Tensile Strength (MPa) | Yield Strength (MPa) | Elasticity Modulus (MPa) | Nominal Strains at Break (%) |
|---|---|---|---|---|---|
| neat PP | 7.39 | 41.76 | 34.85 | 252.99 | 400.15 |
| PP/1ZO@MH | 7.39 | 45.13 | 34.57 | 295.86 | 524.20 |
| PP/4ZO@MH | 8.08 | 46.04 | 35.43 | 320.39 | 510.80 |
| PP/7ZO@MH | 8.90 | 44.43 | 34.72 | 305.62 | 443.74 |
| PP/10ZO@MH | 9.38 | 43.54 | 35.49 | 331.88 | 415.14 |
| PP/1KZO@MH | 6.49 | 46.58 | 35.97 | 307.50 | 447.44 |
| PP/4KZO@MH | 7.80 | 48.52 | 36.60 | 336.79 | 438.37 |
| PP/7KZO@MH | 7.67 | 47.71 | 37.24 | 345.56 | 415.56 |
| PP/10KZO@MH | 8.33 | 45.75 | 37.73 | 336.09 | 387.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, H.; Liu, X.; Lv, Z.; Jin, Y.; Zhu, D.; Dang, L. Synthesis of Zinc Oxide Doped Magnesium Hydrate and Its Effect on the Flame Retardant and Mechanical Properties of Polypropylene. Polymers 2023, 15, 4248. https://doi.org/10.3390/polym15214248
Li X, Zhang H, Liu X, Lv Z, Jin Y, Zhu D, Dang L. Synthesis of Zinc Oxide Doped Magnesium Hydrate and Its Effect on the Flame Retardant and Mechanical Properties of Polypropylene. Polymers. 2023; 15(21):4248. https://doi.org/10.3390/polym15214248
Chicago/Turabian StyleLi, Xue, Hongbo Zhang, Xiaoyuan Liu, Zhihui Lv, Yankui Jin, Donghai Zhu, and Li Dang. 2023. "Synthesis of Zinc Oxide Doped Magnesium Hydrate and Its Effect on the Flame Retardant and Mechanical Properties of Polypropylene" Polymers 15, no. 21: 4248. https://doi.org/10.3390/polym15214248
APA StyleLi, X., Zhang, H., Liu, X., Lv, Z., Jin, Y., Zhu, D., & Dang, L. (2023). Synthesis of Zinc Oxide Doped Magnesium Hydrate and Its Effect on the Flame Retardant and Mechanical Properties of Polypropylene. Polymers, 15(21), 4248. https://doi.org/10.3390/polym15214248







