Effect of Heat Treatment under Different Atmospheres on the Bonding Properties and Mechanism of Ceramiziable Heat-Resistant Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Testing and Characterization
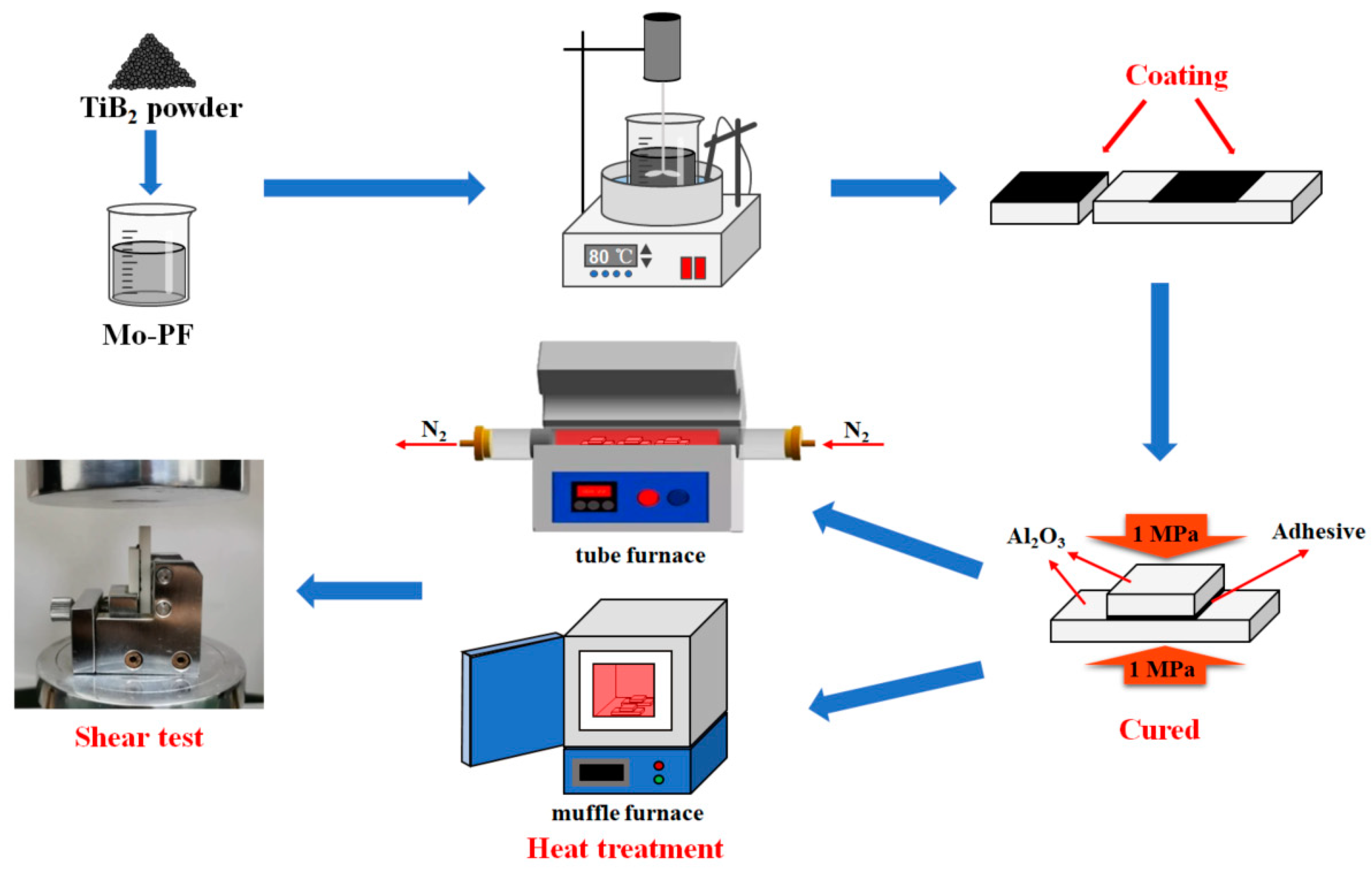
2.3. Synthesis of Molybdenum–Phenolic (Mo-PF) Resin
2.4. Preparation of the Adhesive and Al2O3 Joints
3. Results and Discussion
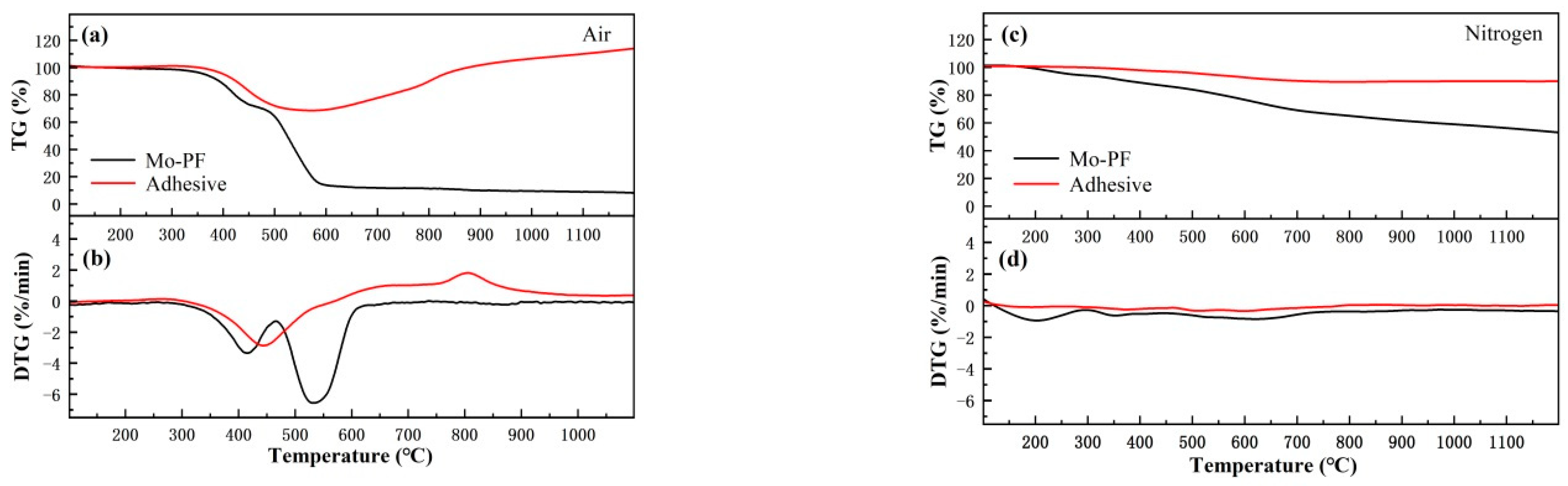
3.1. Thermo Gravimetric Analysis of Mo-PF and the Adhesive
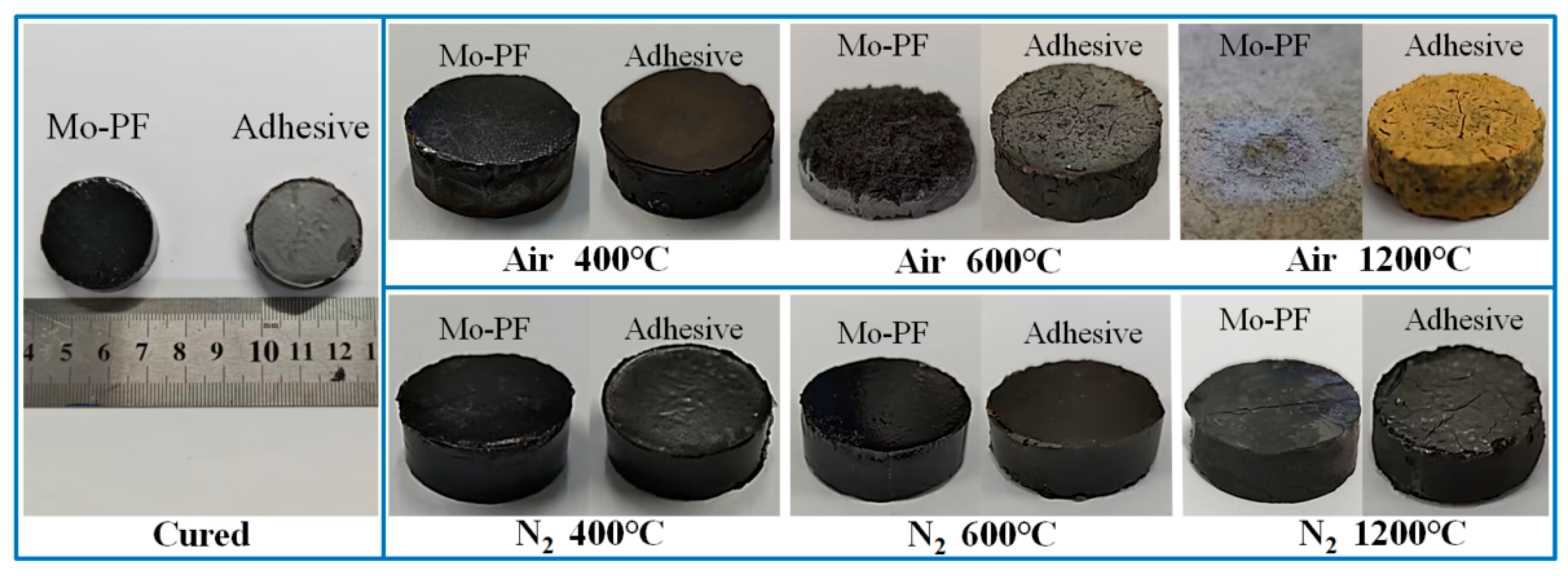
3.2. Bonding Properties of Al2O3 Joints
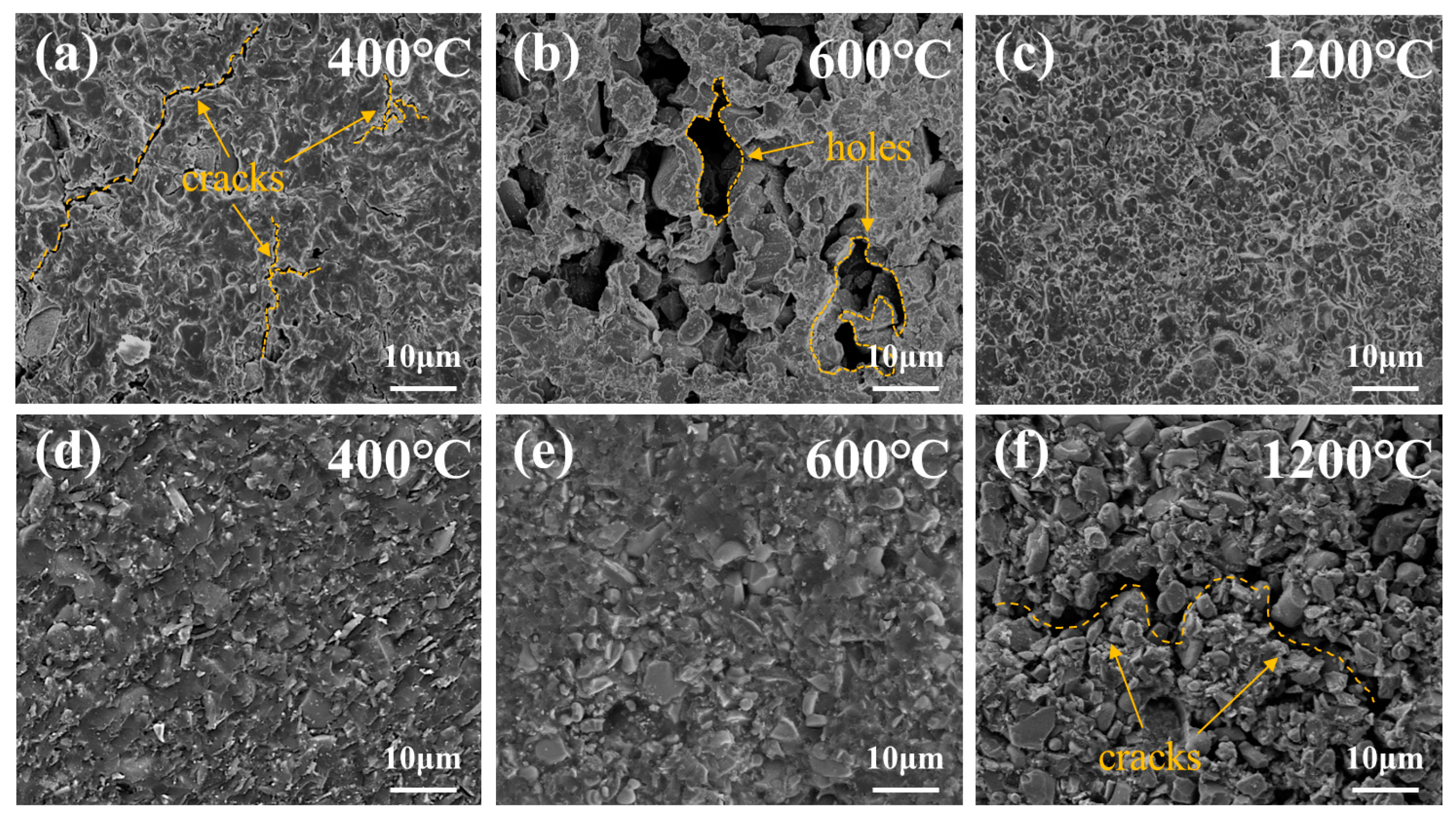
3.3. Fracture Morphology Analysis of Al2O3 Joints
3.4. Compression Strength of the Adhesive
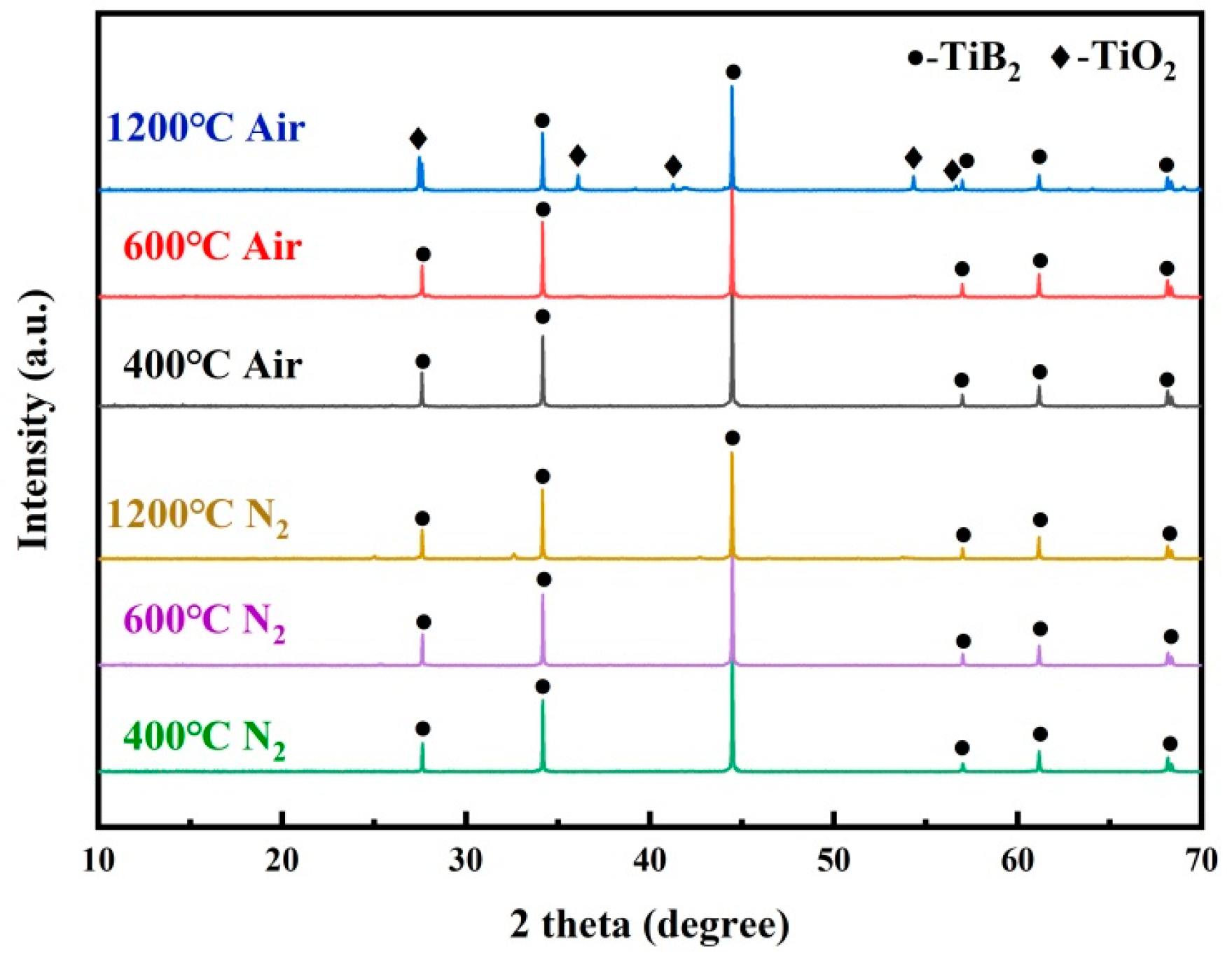
3.5. Compositional Evolution
4. Conclusions
- Before 600 °C, the thermal stability of the adhesive after heat treatment in air and nitrogen was closely related to the pyrolysis behavior of Mo-PF resin. The weight gradually decreased with increasing temperature, but the weight loss rate of the adhesive in nitrogen was lower compared to that in air. After 600 °C, the weight of the adhesive in air started to rise, while the weight change in nitrogen was not significant. In the end, the residual rates of the adhesive at 1200 °C in air and nitrogen were 114.5% and 90.1%, respectively.
- The bonding strength of Al2O3 joints after air heat treatment showed a trend of first decreasing (RT–600 °C) and then increasing (600–1200 °C) with increasing treatment temperature, while the bonding strength after nitrogen heat treatment exhibited a slow decrease with the treatment temperature rise. After heat treatment in air and nitrogen at 1200 °C, the shear strength of the Al2O3 joints was 25.68 MPa and 12.80 MPa, respectively.
- In air, the pyrolysis of the adhesive matrix resulted in numerous cracks and holes, which were eventually compensated by the ceramic phase formed by the oxidation of TiB2 at a high temperature, improving the mechanical properties of the adhesive. In nitrogen, the pyrolysis of the adhesive was slower, and TiB2 consistently acted as inert filler, with no apparent oxidation occurring.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Fuertes, V.; Reinosa, J.J.; Fernandez, J.F.; Enriquez, E. Engineered feldspar-based ceramics: A review of their potential in ceramic industry. J. Eur. Ceram. Soc. 2022, 42, 307–326. [Google Scholar] [CrossRef]
- Rakshit, R.; Das, A.K. A review on cutting of industrial ceramic materials. Precis. Eng.-J. Int. Soc. Precis. Eng. Nanotechnol. 2019, 59, 90–109. [Google Scholar] [CrossRef]
- Choudhary, A.; Paul, S. Surface generation in high-speed grinding of brittle and tough ceramics. Ceram. Int. 2021, 47, 30546–30562. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, C.; Zhang, X. Notch radius effect on fracture toughness of ceramics pertinent to grain size. J. Eur. Ceram. Soc. 2020, 40, 4217–4223. [Google Scholar] [CrossRef]
- Chen, X.; Xie, R.; Lai, Z.; Liu, L.; Yan, J.; Zou, G. Interfacial Structure and Formation Mechanism of Ultrasonic-assisted Brazed Joint of SiC Ceramics with Al-12Si Filler Metals in Air. J. Mater. Sci. Technol. 2017, 33, 492–498. [Google Scholar] [CrossRef]
- Fernie, J.A.; Drew, R.A.L.; Knowles, K.M. Joining of engineering ceramics. Int. Mater. Rev. 2009, 54, 283–331. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Yang, J.; Qiao, G. Recent advances in joining of SiC-based materials (monolithic SiC and SiCf/SiC composites): Joining processes, joint strength, and interfacial behavior. J. Adv. Ceram. 2019, 8, 19–38. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Hu, H.; Zhang, Y. Preparation and mechanical properties of C/SiC nuts and bolts. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2012, 547, 1–5. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Wang, J. Vacuum diffusion bonding TC4 to Ni80Cr20: Interfacial microstructure, segregation, cracking and properties. Vacuum 2018, 158, 218–222. [Google Scholar] [CrossRef]
- Sushko, G.B.; Verkhovtsev, A.V.; Yakubovich, A.V.; Schramm, S.; Soov’yov, A.V. Molecular Dynamics Simulation of Self-Diffusion Processes in Titanium in Bulk Material, on Grain Junctions and on Surface. J. Phys. Chem. A 2014, 118, 6685–6691. [Google Scholar] [CrossRef]
- Sun, L.; Fang, J.; Liu, C.; Guo, S.; Zhang, J. Crystallization kinetic of Li2O-MgO-Al2O3-SiO2 glass-ceramic and its application for joining of porous Si3N4 ceramic. Mater. Charact. 2021, 172, 110889. [Google Scholar] [CrossRef]
- Wang, N.; Wang, D.P.; Yang, Z.W.; Wang, Y. Interfacial microstructure and mechanical properties of zirconia ceramic and niobium joints vacuum brazed with two Ag-based active filler metals. Ceram. Int. 2016, 42, 12815–12824. [Google Scholar] [CrossRef]
- Wang, M.; Tao, X.; Xu, X.; Miao, R.; Du, H.; Liu, J.; Guo, A. High-temperature bonding performance of modified heat-resistant adhesive for ceramic connection. J. Alloys Compd. 2016, 663, 82–85. [Google Scholar] [CrossRef]
- Lin, C.-T.; Lee, H.-T.; Chen, J.-K. Preparation of Vanadium-Modified Phenolic Resin/Modified Zirconia Composites and Its Applied Properties in Cubic Boron Nitride (cBN) Grinding Wheels. Polym. Compos. 2016, 37, 3354–3364. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, N.; Zhang, T.; Li, T. Thermal stability and thermal degradation study of phenolic resin modified by cardanol. Emerg. Mater. Res. 2020, 9, 180–185. [Google Scholar] [CrossRef]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, K.; Zheng, S. Organic-in organic hybrid nanocomposites involving novolac resin and polyhedral oligomeric silsesquioxane. React. Funct. Polym. 2007, 67, 627–635. [Google Scholar] [CrossRef]
- Marliana, M.M.; Hassan, A.; Yuziah, M.Y.N.; Khalil, H.P.S.A.; Inuwa, I.M.; Syakir, M.I.; Haafiz, M.K.M. Flame Retardancy, Thermal and Mechanical Properties of Kenaf Fiber Reinforced Unsaturated Polyester/Phenolic Composite. Fibers Polym. 2016, 17, 902–909. [Google Scholar] [CrossRef]
- Sandomierski, M.; Buchwald, T.; Strzemiecka, B.; Voelkel, A. Carbon black modified with 4-hydroxymethylbenzenediazonium salt as filler for phenol-formaldehyde resins and abrasive tools. J. Appl. Polym. Sci. 2020, 137, 48160. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, L.; Jia, P.; Zhang, B.; Zhou, Y. Enhancement of thermal stability and chemical reactivity of phenolic resin ameliorated by nanoSiO2. Korean J. Chem. Eng. 2018, 35, 298–302. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Jiang, H.; Zhou, D. Improving the heat-resistance and toughness performance of phenolic resins by adding a rigid aromatic hyperbranched polyester. J. Appl. Polym. Sci. 2016, 133, 42734. [Google Scholar] [CrossRef]
- Zhou, R.; Li, W.; Mu, J.; Ding, Y.; Jiang, J. Synergistic Effects of Aluminum Diethylphosphinate and Melamine on Improving the Flame Retardancy of Phenolic Resin. Materials 2020, 13, 158. [Google Scholar] [CrossRef]
- Li, C.; Ma, Z.; Zhang, X.; Fan, H.; Wan, J. Silicone-modified phenolic resin: Relationships between molecular structure and curing behavior. Thermochim. Acta 2016, 639, 53–65. [Google Scholar] [CrossRef]
- Liu, L.; Ye, Z. Effects of modified multi-walled carbon nanotubes on the curing behavior and thermal stability of boron phenolic resin. Polym. Degrad. Stab. 2009, 94, 1972–1978. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Z.; Liu, Y.; Li, Y. Novel cardanol-containing boron-modified phenolic resin composites: Non-isothermal curing kinetics, thermal properties, and ablation mechanism. High Perform. Polym. 2017, 29, 279–288. [Google Scholar] [CrossRef]
- Yang, M.-M.; Zhang, Z.-Z.; Zhu, X.-T.; Men, X.-H.; Ren, G.-N.; Liu, W.-M. Tribological Behaviors of Molybdic Acid-Modified Phenolic/Polyfluo Wax Composite Coating. Tribol. Trans. 2016, 59, 428–434. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Shiao, S.J.; Wei, H.F.; Kuo, W.J.; Sha, Y.A. Novel phosphorus-containing dicyclopentadiene-modified phenolic resins for flame-retardancy applications. J. Appl. Polym. Sci. 2001, 79, 342–349. [Google Scholar] [CrossRef]
- Wang, S.; Jing, X.; Wang, Y.; Si, J. High char yield of aryl boron-containing phenolic resins: The effect of phenylboronic acid on the thermal stability and carbonization of phenolic resins. Polym. Degrad. Stab. 2014, 99, 1–11. [Google Scholar] [CrossRef]
- Lin, C.-T.; Lee, H.-T.; Chen, J.-K. Synthesis and characterization of molybdenum/phenolic resin composites binding with aluminum nitride particles for diamond cutters. Appl. Surf. Sci. 2013, 284, 297–307. [Google Scholar] [CrossRef]
- Li, S.; Chen, F.; Zhang, B.; Luo, Z.; Li, H.; Zhao, T. Structure and improved thermal stability of phenolic resin containing silicon and boron elements. Polym. Degrad. Stab. 2016, 133, 321–329. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Z.; Zhang, G.; Li, Y. Preparation and thermal stability of heat-resistant phenolic resin system constructed by multiple heat-resistant compositions containing boron and silicon. High Perform. Polym. 2017, 29, 493–498. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, S.; Liu, Y. The effect of titanium incorporation on the thermal stability of phenol-formaldehyde resin and its carbonization microstructure. Polym. Degrad. Stab. 2013, 98, 514–518. [Google Scholar] [CrossRef]
- Seong, M.; Jeong, C.; Yi, H.; Park, H.-H.; Bae, W.-G.; Park, Y.-B.; Jeong, H.E. Adhesion of bioinspired nanocomposite microstructure at high temperatures. Appl. Surf. Sci. 2017, 413, 275–283. [Google Scholar] [CrossRef]
- Wang, C.; Ding, J.; Huang, Z.; Zhuang, Y.; Li, Y.; Shi, M.; Qin, Y. Ceramizable phenolic adhesive modified by different inorganic particles: A comparative study of thermal stability, bonding strength and bonding mechanism. J. Eur. Ceram. Soc. 2023, 43, 64–72. [Google Scholar] [CrossRef]
- Ouyang, Z.H.; Wu, L.; Yi, D.L.; Qin, X.R.; Cao, S.C.; Wang, Y.; Lan, L. Study on Mo PF used as bonding agent. Chem. Ind. Eng. Prog. 2005, 8, 901–904. [Google Scholar]
- Ho, L.-N.; Ikegawa, T.; Nishiguchi, H.; Nagaoka, K.; Takita, Y. Synthesis and characterization of molybdenum incorporated mesoporous aluminophosphate. Appl. Surf. Sci. 2006, 252, 6260–6268. [Google Scholar] [CrossRef]
- Qiang, M.; Zhang, S.; Lin, H.; Chen, L. Analysis and application of thermosetting molybdic acid-modified phenolic resin. Naihuo Cailiao/Refract. 2005, 39, 119–122. [Google Scholar]
- Pilato, L. Phenolic resins: 100Years and still going strong. React. Funct. Polym. 2013, 73, 270–277. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Synthesis and Mechanism of Metal-Mediated Polymerization of Phenolic Resins. Polymers 2016, 8, 159. [Google Scholar] [CrossRef]
- Ozaki, J.; Ohizumi, W.; Oya, A. A TG-MS study of poly(vinyl butyral)/phenol-formaldehyde resin blend fiber. Carbon 2000, 38, 1515–1519. [Google Scholar] [CrossRef]
- Trick, K.A.; Saliba, T.E. Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite. Carbon 1995, 33, 1509–1515. [Google Scholar] [CrossRef]
- Xing, X.; Niu, X.; Liu, Y.; Yang, C.; Wang, S.; Li, Y.; Jing, X. In-depth understanding on the early stage of phenolic resin thermal pyrolysis through ReaxFF-molecular dynamics simulation. Polym. Degrad. Stab. 2021, 186, 109534. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, H. Performance and structural evolution of high-temperature organic adhesive for joining Al2O3 ceramics. Int. J. Adhes. Adhes. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- Rallini, M.; Natali, M.; Kenny, J.M.; Torre, L. Effect of boron carbide nanoparticles on the fire reaction and fire resistance of carbon fiber/epoxy composites. Polymer 2013, 54, 5154–5165. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Q.; Liu, L.; Song, J. The preparation and performance of high-temperature adhesives for graphite bonding. Int. J. Adhes. Adhes. 2005, 25, 495–501. [Google Scholar] [CrossRef]
- Guo, Q.; Song, J.; Liu, L.; Zhang, B. Relationship between oxidation resistance and structure of B4C–SiC/C composites with self-healing properties. Carbon 1999, 37, 33–40. [Google Scholar] [CrossRef]
- Luan, X.g.; Chang, S.; Yu, R.; Zou, Y.; Riedel, R.; Cheng, L. Effect of PSO and TiB2 content on the high temperature adhesion strength of SiBCNO ceramic. Ceram. Int. 2019, 45, 9515–9521. [Google Scholar] [CrossRef]




| Sample | Mass (g) | Atmosphere | Temperature (°C) | Mass (Heat-Treated) (g) | Compressive Strength (MPa) |
|---|---|---|---|---|---|
| Mo-PF | 8.53 | \ | \ | \ | 158.16 |
| 8.14 | Air | 400 | 6.91 | 60.98 | |
| 8.71 | 600 | 2.47 | 10.42 | ||
| 8.12 | 1200 | 0.70 | 0 | ||
| 8.77 | N2 | 400 | 8.40 | 154.04 | |
| 8.04 | 600 | 7.01 | 151.05 | ||
| 7.81 | 1200 | 5.11 | 34.33 | ||
| Adhesive | 12.24 | \ | \ | \ | 171.28 |
| 11.12 | Air | 400 | 10.34 | 74.59 | |
| 12.32 | 600 | 8.54 | 59.32 | ||
| 11.23 | 1200 | 12.24 | 102.41 | ||
| 13.19 | N2 | 400 | 13.03 | 166.01 | |
| 11.76 | 600 | 11.66 | 164.62 | ||
| 11.80 | 1200 | 9.97 | 56.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Tao, J.; Shan, H.; Cui, T.; Ding, J.; Wang, J. Effect of Heat Treatment under Different Atmospheres on the Bonding Properties and Mechanism of Ceramiziable Heat-Resistant Adhesive. Polymers 2024, 16, 557. https://doi.org/10.3390/polym16040557
Wang Q, Tao J, Shan H, Cui T, Ding J, Wang J. Effect of Heat Treatment under Different Atmospheres on the Bonding Properties and Mechanism of Ceramiziable Heat-Resistant Adhesive. Polymers. 2024; 16(4):557. https://doi.org/10.3390/polym16040557
Chicago/Turabian StyleWang, Qingke, Jiadong Tao, Huawei Shan, Tangyin Cui, Jie Ding, and Jianghang Wang. 2024. "Effect of Heat Treatment under Different Atmospheres on the Bonding Properties and Mechanism of Ceramiziable Heat-Resistant Adhesive" Polymers 16, no. 4: 557. https://doi.org/10.3390/polym16040557
APA StyleWang, Q., Tao, J., Shan, H., Cui, T., Ding, J., & Wang, J. (2024). Effect of Heat Treatment under Different Atmospheres on the Bonding Properties and Mechanism of Ceramiziable Heat-Resistant Adhesive. Polymers, 16(4), 557. https://doi.org/10.3390/polym16040557







