Abstract
This paper provides evidence and discusses the variability in the thermomechanical behaviour of virgin and recycled polypropylene/high-density polyethylene blends without the addition of other components, which is sparse in the literature. Understanding the performance variability in recycled polymer blends is of critical importance in order to facilitate the re-entering of recycled materials to the consumer market and, thus, contribute towards a circular economy. This is an area that requires further research due to the inhomogeneity of recycled materials. Therefore, the thermal and mechanical properties of virgin and recycled polypropylene/high-density polyethylene blends were investigated systematically. Differential scanning calorimetry concludes that both the recycled and virgin blends are immiscible. Generally, recycled blends have lower overall crystallinity and melting temperatures compared with virgin blends while, remarkably, their crystallisation temperatures are compared favourably. Dynamical mechanical analysis showed little variation in the storage modulus of recycled and virgin blends. However, the alpha and beta relaxation temperatures are lower in recycled blends due to structural deterioration. Deterioration in the thermal and mechanical properties of recycled blends is thought to be caused by the presence of contaminants and structural degradation during reprocessing, resulting in shorter polymeric chains and the formation of imperfect crystallites. The tensile properties of recycled blends are also affected by the recycling process. The Young’s modulus and yield strength of the recycled blends are inferior to those of virgin blends due to the deterioration during the recycling process. However, the elongation at break of the recycled blends is higher compared with the virgin blends, possibly due to the plasticity effect of the low-molecular-weight chain fragments.
1. Introduction
Plastic waste is a major environmental issue, with only 9% of the world’s plastics being recycled [1]. Polyolefins, encompassing polypropylene (PP) and polyethylene (PE), are the main components in municipal waste due to their abundant use in commodity applications as they possess good mechanical properties and processability, in addition to having high availability and low manufacturing costs [2,3,4,5]. The complete separation of PP and PE during mechanical waste recovery is uneconomical due to their close densities and structural similarity; therefore, PP and PE usually remain mixed [6,7,8]. During the mechanical recycling process, PP and PE undergo irreversible thermomechanical degradation processes such as chain scission, which lowers the mechanical properties of the recycled PP (rPP) and recycled PE (rPE) compared with the virgin polymers [9].
Blends of PP and PE are of great commercial interest as they have the potential to reduce the deficient characteristics of PP and PE, such as low impact resistance at low temperatures and poor environmental stress cracking resistance, respectively [6,10,11]. However, blends of PP: High-Density PE (HDPE) and PP: Low-Density PE (LDPE) are thermodynamically immiscible, resulting in poor material performance due to their phase-separated morphology and low interfacial adhesion between the phases [12,13]. The mechanical performance of immiscible blends are dependent upon the blend components’ crystallisation behaviour and final blend morphology [14,15,16]. Several factors are important for morphology development during polymer processing such as composition, viscosity ratio of the components, interfacial properties, crystallinity and processing conditions [6,14,17,18,19,20,21,22]. Several studies have reported the mechanical properties, crystallisation behaviour and morphology of PP:PE blends [6,7,11,22,23,24,25,26,27,28].
Although it is important to determine and understand the mechanical and thermal behaviour of the virgin PP (vPP): virgin polyethylene (vPE) blends in order to optimise the properties of recycled blends, comparative studies are also important. A comparison of the thermomechanical properties of vPP:vHDPE and rPP:rHDPE blends over a wide range of compositions is lacking as the literature mainly focuses on ternary systems (e.g., PP:HDPE and compatibilisers/co-polymers/other polymers/fillers [5,29,30,31,32]). It is important to understand the variability in performance of rPP:rHDPE blends before the addition of further components.
Studies into the crystallinities of rPP, rHDPE and rPP:rHDPE blends have found that crystallinity is affected by degradation mechanisms during the recycling process [33,34,35]. Interestingly, rPP crystallinity has been found to be higher than that of vPP by several authors [33,35,36]. da Costa et al. [35] suggested that the higher value of crystallinity of rPP compared with vPP was caused by a decrease in the molecular weight, which resulted in an increase in chain mobility. Increased chain mobility improved the ability of chains to fold into thicker lamella and, hence, an increased crystallisation rate and crystallinity. Therefore, it is important to understand the variation in crystallinity in rPP:rHDPE blends in order to optimise the mechanical performance of the recyclates.
Research has been undertaken in the literature to understand the effect of recycling cycles on the mechanical properties of PP, PE and their blends. For example, Aurrekoetxea et al. [33] subjected PP to 10 successive injection moulding cycles at 200 °C and found that the degree of crystallinity increased with each cycle. This caused an increase in Young’s modulus and yield stress. On the other hand, Oliveira et al. [37], who subjected PP to seven successive cycles at 175–190 °C, observed a decrease in Young’s modulus and yield stress after the third cycle that was attributed to a reduction in tie molecules between the crystalline and amorphous phases. Conflicting observations by Aurrekoetxea et al. [33] and Oliveira et al. [37] for the Young’s modulus and yield stress of rPP could be due to differences in the processing methodology. Aurrekoetxea et al. [33] used injection moulding, whereas Oliveira et al. [37] opted for a single screw extruder followed by compression moulding. This highlights the importance of the reprocessing methodology but also demonstrates the difficulty of comparing the performance of recycled materials in the literature. Furthermore, chemical analysis studies of recycled PE and PP have revealed their variability, degradation and the presence of impurities and contaminants [38,39,40].
PE can be subjected to a higher number of extrusion cycles before any deterioration in the mechanical properties is observed. Jin et al. [41] found no significant change in crystallinity and, hence, in the mechanical properties of LDPE up to the 40th extrusion cycle. However, a decrease in crystallinity was observed between the 40 and 50th cycles, either caused by short side branches in the backbone chain or side groups, or by crosslinking. Oblak et al. [42] subjected HDPE to 100 consecutive extrusion cycles at 220–270 °C. They found that chain branching and chain scission, which occurred up to the 60th cycle, resulted in a decrease in crystallinity and Young’s modulus. However, crystallinity and Young’s modulus remained stable after the 60th extrusion cycle due to crosslinking. After the 100th cycle, the Young’s modulus of the rHDPE had only reduced by 20% compared with that of the vHDPE.
Studies have been carried out to understand the mechanical properties of PP:PE blends subjected to recycling cycles [9,26,43,44,45,46,47]. Saikrishnan et al. [43] reported that recycling affected the melt flow behaviour of PP:LDPE blends but found that the tensile properties were not substantially affected (subjected to up to five recycling cycles). Interestingly, PP underwent chain scission on each recycling cycle but the overall properties of the blend were maintained. However, they only investigated the PP:LDPE up to 10 wt% of LDPE. The literature is typically limited in the blend composition range investigated. However, due to the variability in the waste streams, it is important to understand the mechanical properties for all blend compositions without the addition of a third component initially. This would enable the recycling industry to be reactive to changes in waste stream composition in different locations and batches and enable more recyclate to re-enter the market. Therefore, this paper aims to understand the variability in thermomechanical properties for virgin and recycled PP:HDPE blends.
This study reports the thermal and mechanical properties of vPP:vHDPE and rPP:rHDPE blends through differential scanning calorimetry (DSC), dynamical mechanical analysis (DMA) and tensile testing. This comparative study enables the mapping of not only the challenges but also the potentially unique opportunities of the recycled systems. The recycling industry is looking to improve the plastic circular economy by obtaining recycled commingled waste blends with desirable end-use properties acceptable for commercial applications but at low cost.
2. Experimental Section
2.1. Materials
PP (Moplen EP440G), supplied by LyondellBasell (London, UK), had a melt flow index (MFI) of 1.3 g 10 min−1 and density of 900 kg m−3. HDPE (HDPE, K46-06-185), supplied by Ineos (Grangemouth, UK), had an MFI of 4.2 g 10 min−1 and a density of 946 kg m−3. Post-consumer rPP and rPE were supplied by Impact Solutions Recycled (Livingston, UK). The rPE was mainly composed of HDPE, but small quantities of LDPE were present. rPP and rHDPE had MFIs of 15 and 1.5 g 10 min−1, respectively. As shown by the MFI values, the grades of virgin and recycled PP and HDPE used are quite different; therefore, the properties are not directly comparable. The comparisons made through the study are more general between virgin and recycled grades.
Virgin and recycled blends of different compositions of PP and HDPE (P10, P20, P25, P40, P50, P60, P75, P80 and P90) were prepared, where P denotes PP and the number corresponds to the percentage composition by weight of PP in the blend PP:HDPE. The pure 100 weight percentage (wt%) PP and HDPE will be denoted as PP and HDPE, respectively. To denote virgin or recycled, the symbols of v and r will be used, respectively, before the blend composition, e.g., virgin P10 would be represented as vP10.
2.2. Preparation
Extrusion and Injection Moulding
vPP and vHDPE were in the form of pellets, whereas rPP and rPE were in the form of flakes. Blends were prepared using a lab scale Haake MiniCTW twin screw extruder (Karlsruhe, Germany) for 5 min with feeder and mixing speeds of 50 rpm and 100 rpm, respectively. The conical screws were 4–15 mm in diameter, 109.4 mm in length and co-rotate. The barrel temperature was 180–185 °C. Molten blends were transferred to the Haake MiniJet injection moulder (Karlsruhe, Germany), where the cylinder temperature was 210 °C, mould temperature was 35 °C, injection pressure was 50 MPa and hold-on pressure time was 10 s. The ISO 527-2-1BA [48] and 557–2296 moulds were used for the dog-bone-shaped and DMA rectangular samples, respectively.
2.3. Characterisation
2.3.1. DSC
The melting and crystallisation behaviour of the vPP:vHDPE and rPP:rHDPE blends were evaluated using a Perkin Elmer DSC 8000 (Waltham, MA, USA). The instrument was calibrated using an indium sample. Approximately 5–6 mg of the sample was scanned under a nitrogen atmosphere. Samples were exposed to the following thermal cycle: heated from 25 to 200 °C at 10 °C min−1, isothermal at 200 °C for 5 min, cooled from 200 to 25 °C at 10 °C min−1, isothermal at 25 °C for 2 min and heated from 25 to 200 °C at 10 °C min−1. The melting temperature Tm and enthalpy of fusion were obtained from the first heating ramp. The crystallisation temperature Tc was taken from the cooling ramp.
The degree of crystallinity was calculated by Equation (1),
where is the observed enthalpy of fusions for the individual PP and HDPE peaks, and is the 100% crystalline HDPE or PP, which are 287 and 207 J g−1, respectively [6]. and were taken from the first heating ramp to calculate the crystallinity. The thermal history of the sample was erased after the first heating ramp [49]. However, very little difference was found when comparing the crystallinity obtained from the first and second heating ramps for the virgin and recycled blends (Tables S1 and S2).
2.3.2. DMA
DMA was used to determine the viscoelastic properties of the virgin and recycled blends. A Triton DMA (Leicestershire, UK) in dual cantilever mode at a frequency of 1 Hz was used. A temperature sweep from −50 to 150 °C at a heating rate of 5 °C min−1 was implemented. Sample dimensions were approximately 45 mm (l) × 10 mm (w) × 2.7 mm (d). A minimum of three samples were tested, and the average and standard deviation were calculated for each blend ratio.
The dynamic response was given as the elastic (storage modulus, E′), viscous (loss modulus, E″) and damping (tan delta, tanδ) components. The glass transition (Tg) and transition relaxation processes can be seen as changes in the E″ or tanδ traces [50]. The tanδ trace was used to quote the Tg and other relaxation peaks present [51].
2.3.3. Tensile Testing
Tensile properties were determined using an Instron Tensile Machine (Buckinghamshire, UK) with a crosshead speed of 5 mm min−1 and a 10 kN load cell. Tensile properties were carried out at ambient temperature in accordance with the ISO 527-2 standard. Young’s modulus was determined using a Zwick Roell Tensile Machine with a video-extensometer. A crosshead speed of 1 mm min−1, gauge length of 25 mm and a 10 kN load cell were used. A minimum of five samples were tested, and the average and standard deviation were calculated.
The “rule of mixtures” was used to predict the Young’s modulus of the virgin and recycled PP:HDPE blend samples compared to the experimental data. The rule of mixtures was calculated by Equation (2),
where EBlend is the Young’s modulus of the polymer blend; WPP and EPP are the weight fraction and Young’s modulus of PP, respectively; and WHDPE and EHDPE are the weight fraction and Young’s modulus of component HDPE, respectively [52]. The experimental Young’s modulus of the virgin and recycled homogenous PP and HDPE systems was used for the virgin and recycled EPP and EHDPE, respectively.
3. Results and Discussion
3.1. Thermal Properties of Virgin and Recycled PP:HDPE Blends
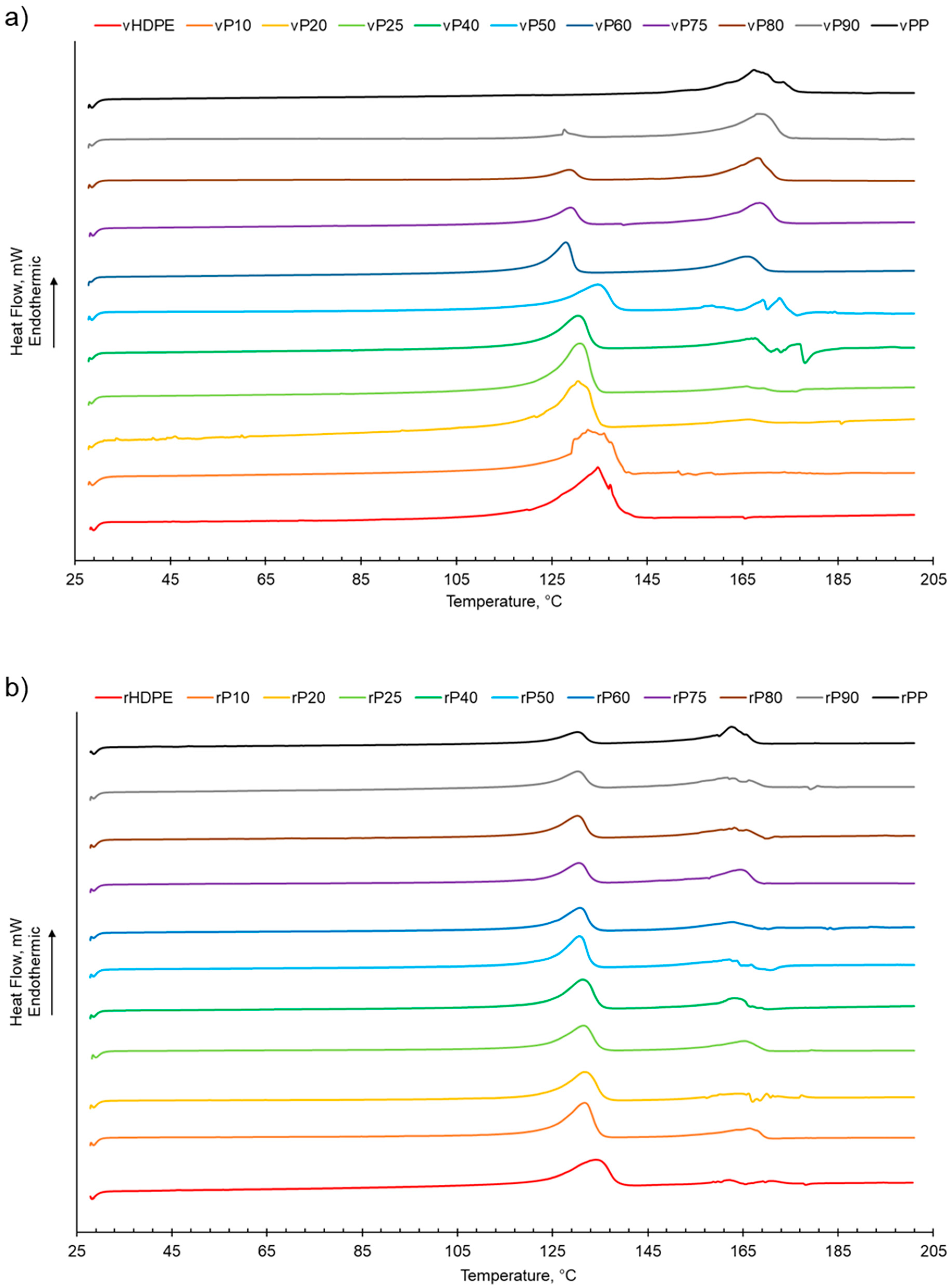
The melting behaviour of both rPP:rHDPE and vPP:vHDPE blends presented two separate peaks assigned to PP and HDPE, suggesting that the blends were immiscible (Table 1 and Figure 1). The two melting peaks present in the rPP and rPE indicated that contaminants were present due to the challenge of complete separation of PP and PE during the recycling process [26,29,53]. Little variation in the Tm of virgin and recycled PP, HDPE and their blends suggested that the blending of PP and HDPE did not significantly alter the Tm of PP and HDPE (Table 1, Figure S1) [6,29]. In some cases, the rPP:rHDPE blends had lower Tm than the respective vPP:vHDPE blends, indicating structural deteriorations of the polymeric components during the mechanical recycling process [54], which could imply that less-perfect crystallites formed [55].

Table 1.
Melting and crystallisation behaviour of vPP:vHDPE and rPP:rHDPE blends obtained from the first heating and cooling cycles using DSC. Bold values are for vPP:vHDPE blends and un-bolded values are for the rPP:rHDPE blends. Related graphs are presented in the Supporting Information (Figures S1–S5).
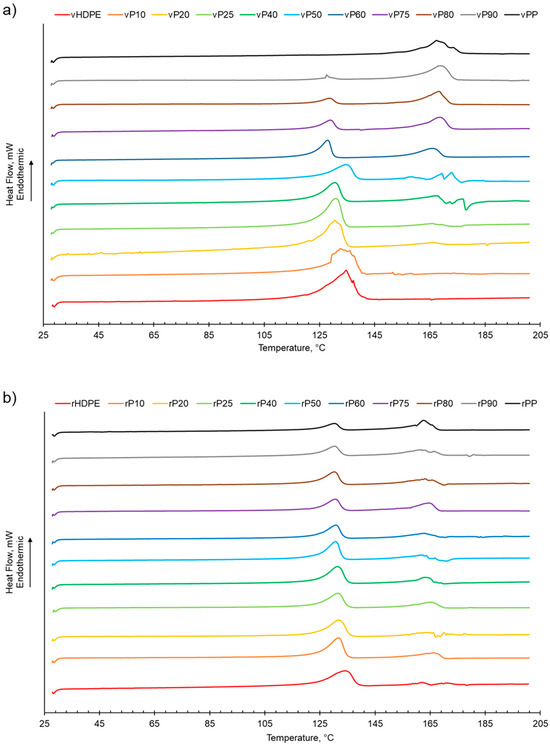
Figure 1.
The melting behaviour of PP:HDPE blends obtained from DSC: (a) vPP:vHDPE blends and (b) rPP:rHDPE blends.
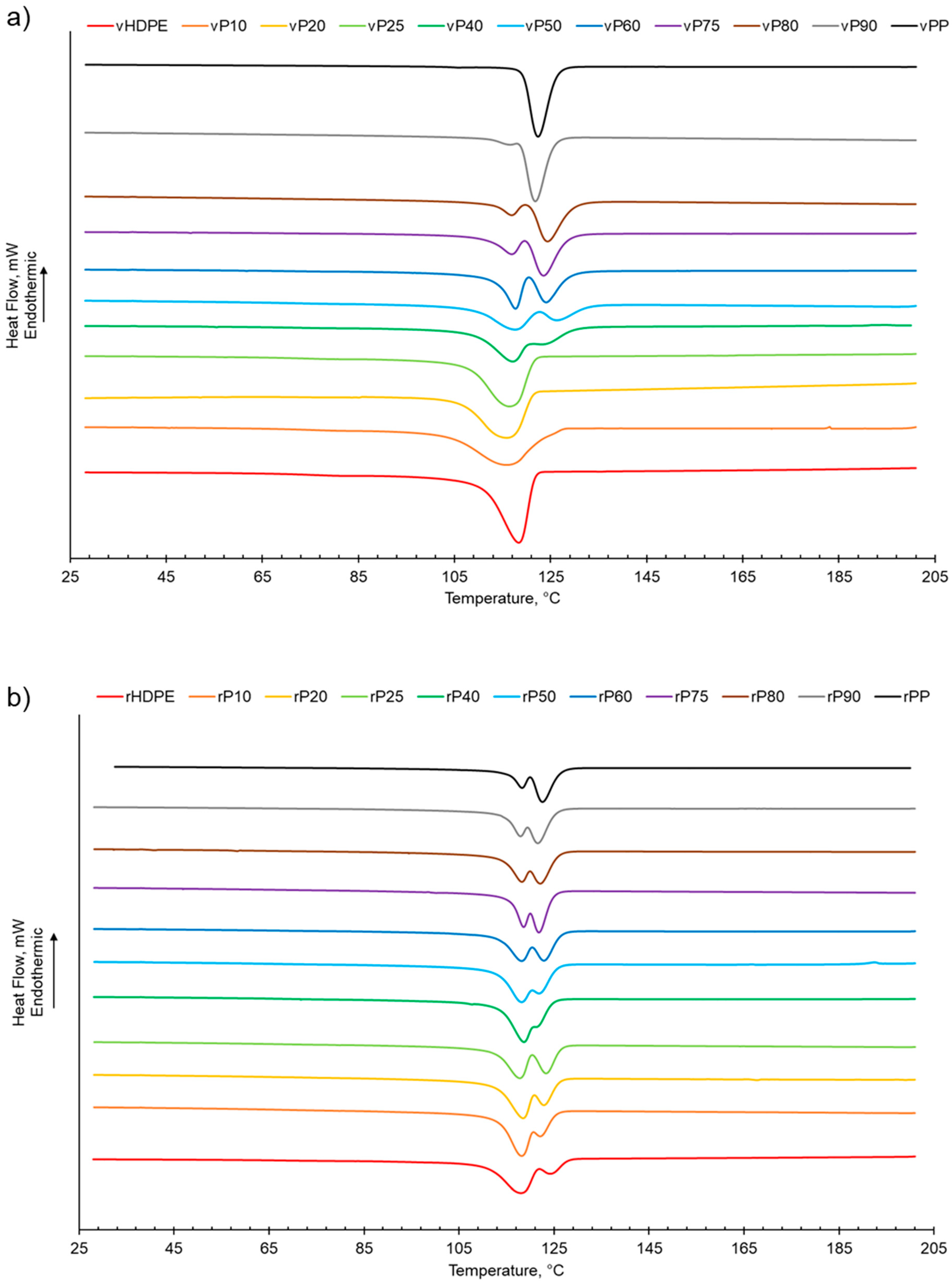
As shown in Table 1 and Figure S2, increasing the PP wt% in the virgin or recycled PP:HDPE blends results a decrease in the enthalpy of crystallisation of HDPE while PP generally presents an increasing trend. This is in agreement with Jose et al. [6], who suggested that the decrease in enthalpy of crystallisation could be attributed to the differing rates of crystallisation for PP and HDPE and the resulting size of crystallites. PP crystallises at a slower rate compared with HDPE, which enables the formation of large spherulites. The large spherulites in PP liberate less energy during crystallisation compared with the smaller crystallites in HDPE [6]. The crystallisation behaviour of semi-crystalline polymers is more complex compared with their melting behaviour due to the numerous factors that can affect the phase structure, such as polymer composition and distribution, intra- and inter-molecular interactions, and processing conditions [56]. The presence of a second semi-crystalline material also complicates the crystallisation behaviour [8,57]. Typically, PP and HDPE crystallise separately and at different rates. HDPE has a quicker nucleation and growth rate compared with PP due to the HDPE’s flexible chain and limited intermolecular interactions [6,26,56]. In PP, crystallisation is hindered by the bulky methyl groups on the polymer chain backbone [56]. There was little variation observed in the crystallisation temperature (Tc) for the recycled and virgin blends (Figure 2, Table 1 and Figure S3). Two crystallisation peaks were observed for rPP:rHDPE blends at the approximate individual PP and HDPE crystallisation temperatures. Phase separation is caused by the PP and HDPE crystals growing at different rates. Crystallisation peaks were observed in the rPP and rPE due to the presence of PE and PP contaminants, respectively. One crystallisation peak was observed for vPP:vHDPE blends up to vP25, suggesting co-crystallisation and/or partial miscibility. However, upon increasing the PP wt% further, two peaks were observed at the approximate individual PP and HDPE crystallisation temperatures, suggesting an onset of independent crystallisation and incompatibility. There is literature reporting a single crystallisation peak for vPP:vHDPE blends over a wide composition range. Lin et al. [27] and Sutar et al. [30] suggested that the addition of HDPE affected the PP crystallisation rate, resulting in one crystallisation peak. Jose et al. [6], who studied a range of PP:HDPE blends, reported only one crystallisation temperature, which possessed an intermediary Tc value between the Tc values of PP and HDPE. Aumunate et al. [26] found a single crystallisation peak for vPP:vHDPE blends caused by the merging of the vPP and vHDPE peaks due to their close Tc. However, they suggested that bimodal behaviour was present at higher vHDPE contents due to the presence of a slight shoulder peak.
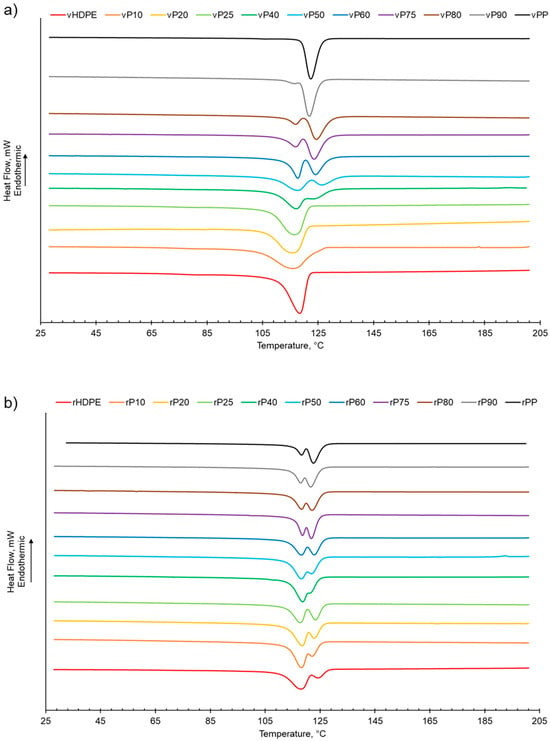
Figure 2.
The crystallisation behaviour of PP:HDPE blends obtained from DSC: (a) vPP:vHDPE blends and (b) rPP:rHDPE blends.
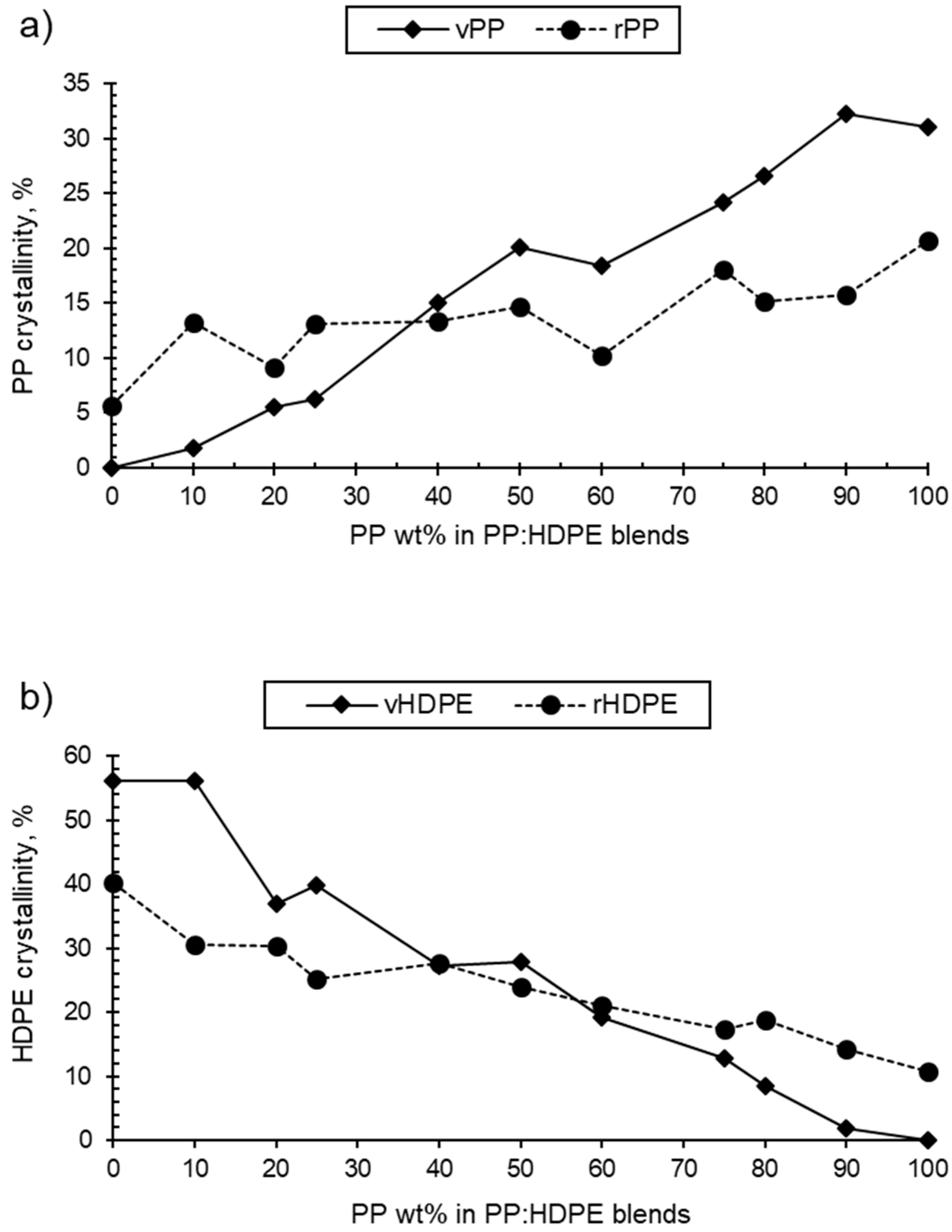
As the PP wt% increased for both the rPP:rHDPE and vPP:vHDPE blends, the PP crystallinity in the blend increased and the HDPE crystallinity decreased (Table 1 and Figure 3). rPP crystallinity was higher than vPP crystallinity in HDPE up to the P25 blend. rHDPE crystallinity was higher than vHDPE crystallinity from P60 to PP. The crystallinity is affected by the presence of PP and HDPE contaminants in the recycled materials. The quantity of other plastic contaminants will be dependent on the waste stream composition and the quality of sorting at the material recycling facility [58,59]. Thermomechanical degradation, which occurs during recycling, results in an increase in polydispersity caused by the presence of shorter polymeric chains [60]. Shorter polymeric chains form crystals more easily compared with long chains due to their low degree of entanglement, which may lead to an increase in crystallinity [61]. On the other hand, the crystallinities of rPP and rHDPE were found to be lower than the vPP and vHDPE crystallinities for blends between P40 and PP, and HDPE and P50, respectively. The presence of other plastics, varying chains lengths and branching, and impurities such as oxidative moieties and additives can lead to the formation of imperfect crystallites and a heterogeneous crystalline morphology, hence reducing crystallinity [58,62]. Therefore, determining the exact cause of (enhanced or reduced) crystallinity in recycled blends is a challenge. It has to be noted that the Tg values of PP and HDPE were not observed in the thermograms as they are located below the starting temperature of the DSC thermograms.
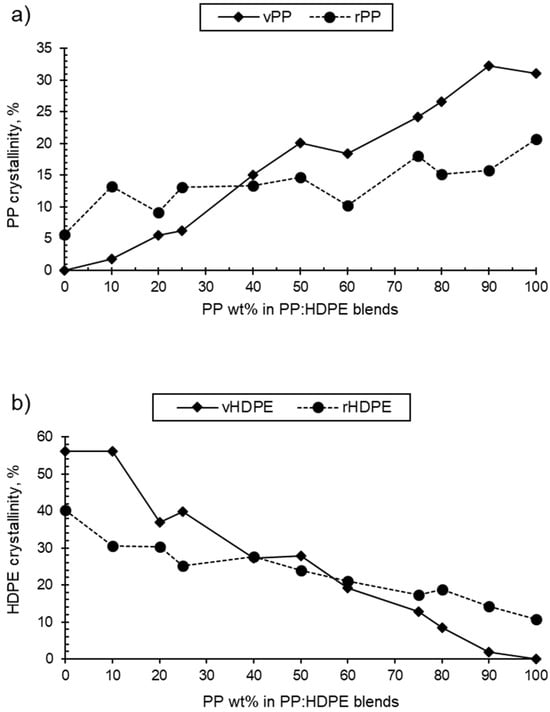
Figure 3.
Variation of (a) virgin and recycled PP crystallinity in PP:HDPE blends and (b) virgin and recycled HDPE crystallinity in PP:HDPE blends.
3.2. Mechanical Properties of Virgin and Recycled PP:HDPE Blends
3.2.1. DMA Measurements of Virgin and Recycled PP:HDPE Blends
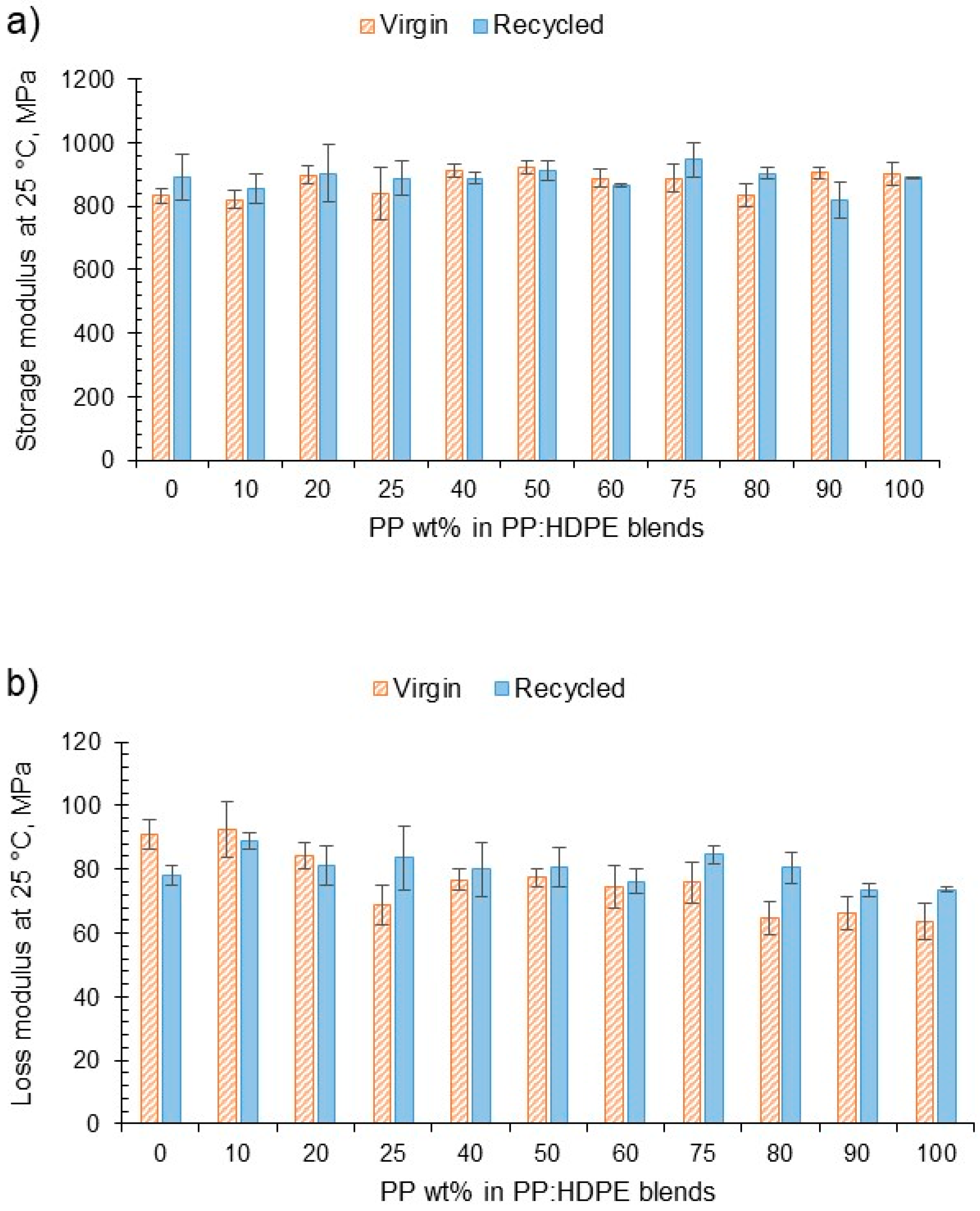
DMA was used to determine the viscoelastic response of the blends as a function of temperature. E′ indicates the relative dynamic stiffness of the material and E″ indicates the ability to dissipate energy (Figure 4). No large variation was observed in E′ at different blend compositions for both the virgin and recycled blends. However, there was a decreasing trend for the loss modulus for the virgin blends. As the PP wt% increased, the vPP:vHDPE blend’s E″ decreased, whereas the rPP:rHDPE blends did not show such an obvious decrease in E″ with variation in composition. Interestingly, Fang et al. [47], who investigated the storage and loss moduli of rPP:rPE blends without the addition of a compatibiliser or filler, found an increase in moduli with rPP content. For example, the rP60 blend presented an E′ at 40 °C, which was twice that of the rP45 blend. They concluded that an increase in stiffness occurs with an increase in PP wt%. The difference in the temperature at which the moduli were taken, different manufacturing processes and MFI of the rPE and rPP could account for the differences observed. Structural deteriorations caused by the recycling process can introduce flexibility and mobility due to the shorter chains. However, impurities can act as fillers in the recycled materials, imposing a mechanical restraint that increases the stiffness [63].
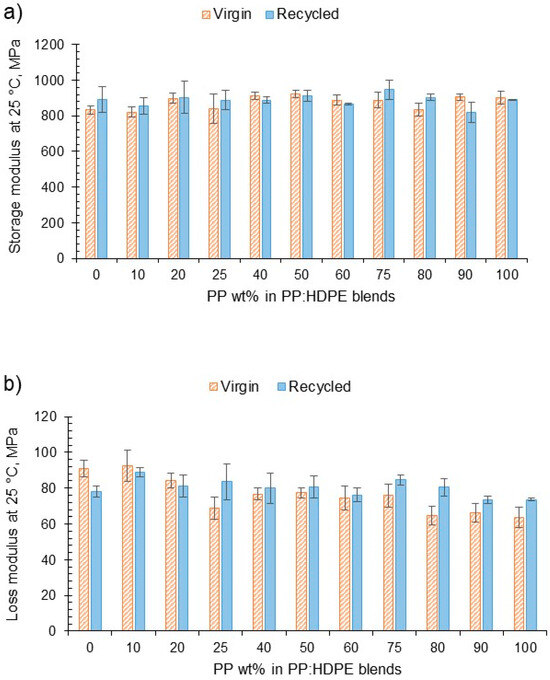
Figure 4.
(a) The variation in storage modulus at 25 °C for vPP:vHDPE and rPP:rHDPE blends and (b) the variation in loss modulus at 25 °C for vPP:vHDPE and rPP:rHDPE blends.
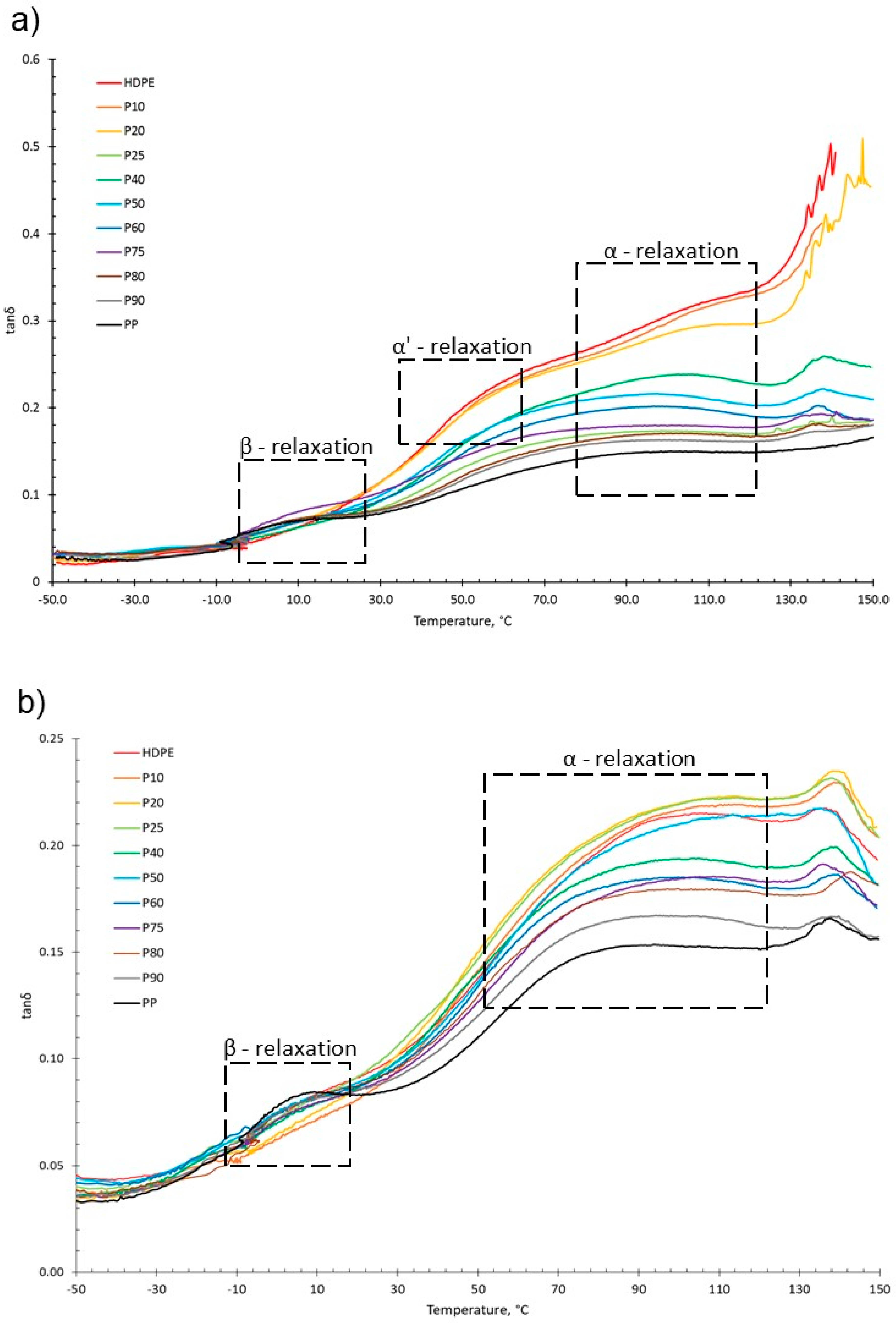
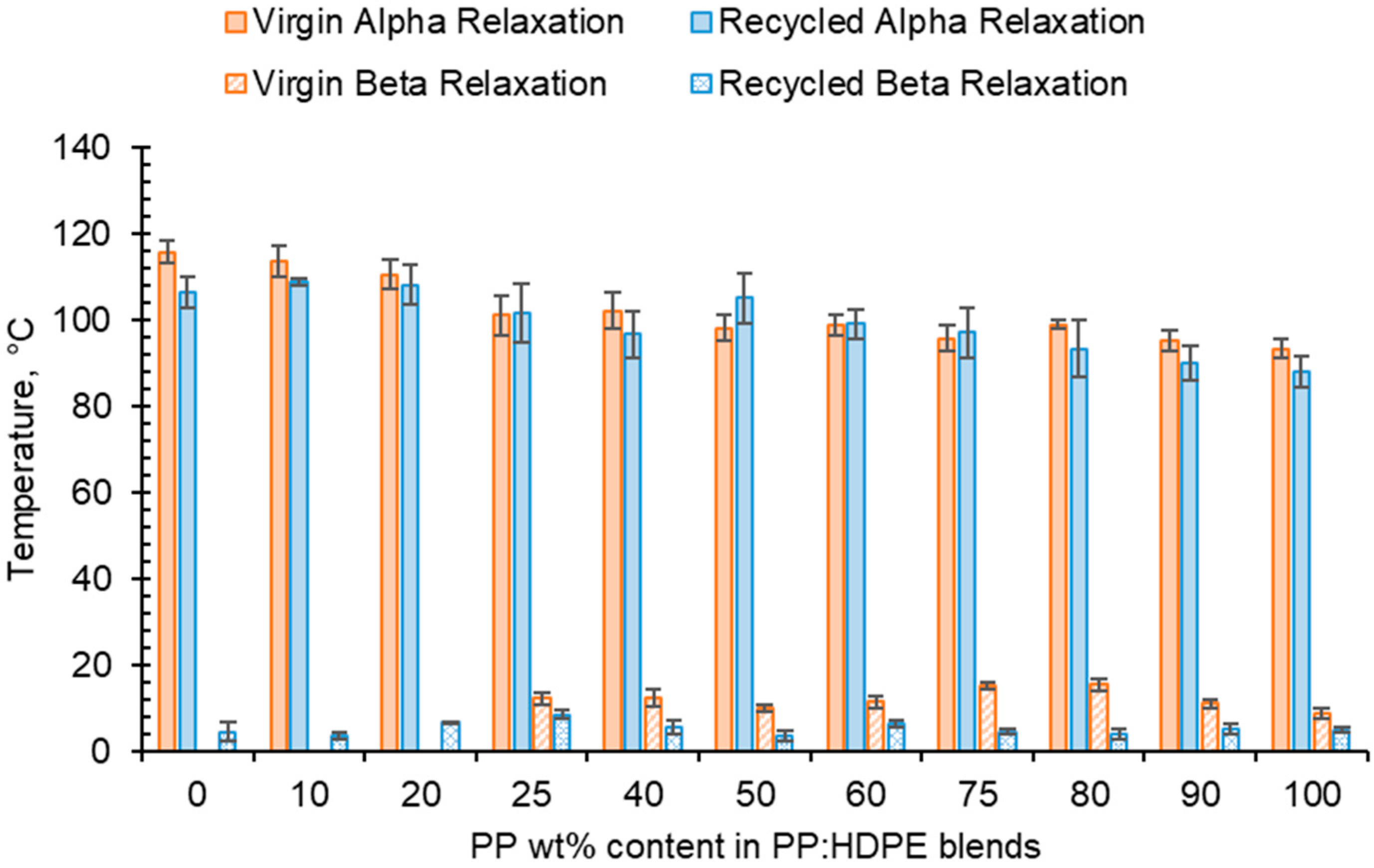
For both virgin and recycled PP, HDPE and their blends, as temperature increased, E′ decreased and E″ increased, shown in the tanδ vs. temperature graphs presented in Figure 5. This is due to material softening and the beginning of relaxation processes with increasing temperature [64]. HDPE and PP exhibit three relaxation processes: alpha (α), beta (β) and gamma (γ) [51,65]. Both the virgin and recycled PP:HDPE blends exhibited α and β relaxation processes in the tanδ vs. T graphs, as shown in Figure 5. γ relaxation was not observed as it typically occurs below −100 °C, which is outside the experimental temperature range. γ relaxation is associated with the motions of the side chain groups attached to the main chain in the amorphous region [65]. α relaxation is associated with the crystalline region, where the CH2 groups within the crystallites have vibrational and re-orientation motion. The chains are flexible and freely rotating [65,66,67]. The rHDPE and rPP alpha relaxation temperatures (Tα) were lower compared with the vHDPE and vPP, respectively, possibly caused by imperfect crystallite formation due to recycling (Figure 5 and Figure 6, see also Table S1) [55]. The higher Tα of HDPE compared with PP could be due to HDPE’s higher crystallinity and amount of crystalline domains compared with PP [68]. The Tα of the virgin blends decrease as the PP wt% increases, with a similar trend observed in the recycled blends. The Tα are intermediary between the Tα of PP and HDPE. As suggested by Karaagac et al. [32], the observed relaxation temperatures of the blends are likely following the rule mixtures, and caution must be taken before suggesting partial miscibility at the interface due to the observation of a single peak. As the Tα of PP and HDPE are close in value, it is possible that there is an overlap in the peaks causing the blend to have a broad Tα peak.
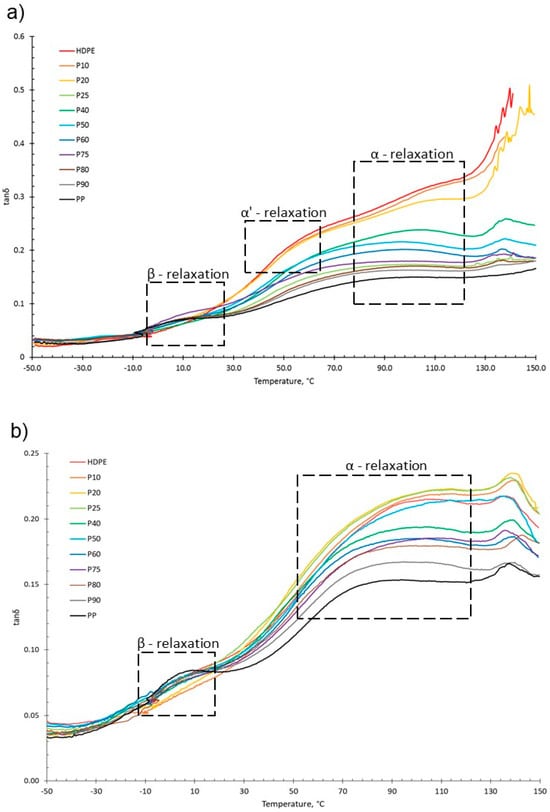
Figure 5.
tanδ graphs as a function of temperature obtained from DMA of (a) vPP:vHDPE blends and (b) rPP:rHDPE blends with the relaxation regions highlighted.
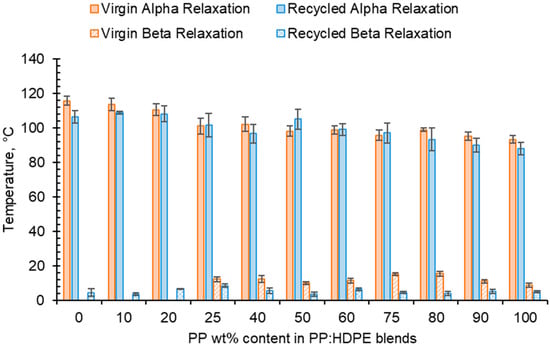
Figure 6.
Comparison of Tα and Tβ taken from tanδ traces for virgin and recycled PP:HDPE blends.
HDPE exhibits an additional relaxation process, α′, which is associated with the crystalline region and partially overlaps into the α region [51]. α′ is observed in vHDPE at approximately 40–50 °C (Figure 5a). As the vHDPE content in the virgin blends decreases, α′ decreases in prominence. α′ is not observed in the rHDPE, possibly due to the recycling process, which causes the formation of imperfect crystallites and the presence of contaminants, thus decreasing the peak prominence.
β relaxation is associated with the motion of the branches in the amorphous region and is connected to the Tg [63,66,69]. The PP β relaxation temperature (Tβ) is the Tg. There are many opposing viewpoints surrounding where the Tg of PE is: (a) in the β region just below 0 °C, (b) in the region of −81 °C and (c) in the γ region below −100 °C [65,70]. The magnitude of the Tβ is dependent on the amount of amorphous domains, as the relaxation occurs in the amorphous domain. The Tβ in HDPE may not always be observed due to the low proportion of amorphous domains compared with crystalline domains. Additionally, tie molecules between the crystalline and amorphous domains restrict the complete relaxation of amorphous chains [63,67]. The Tβ observed will be that of vPP as the vHDPE Tβ is not seen (Figure 5). The vPP Tβ is not visible in the vP10 and vP20 blends due to the small magnitude of the relaxation. The Tβ of PP becomes visible at 12.4 °C for vP25. The Tβ is present in the rHDPE due to the presence of PP impurities, which cannot be completely removed in the recycling process [53]. The Tβ of the recycled blends were lower than the virgin blends and had little variation. The recycling process results in a decrease in molecular weight. The presence of the low-molecular-weight chains causes an increase in free volume and reduced chain packing [71]. An increase in free volume lowers the Tβ as less thermal energy is required for chain mobility.
3.2.2. Tensile Measurements of Virgin and Recycled PP:HDPE Blends
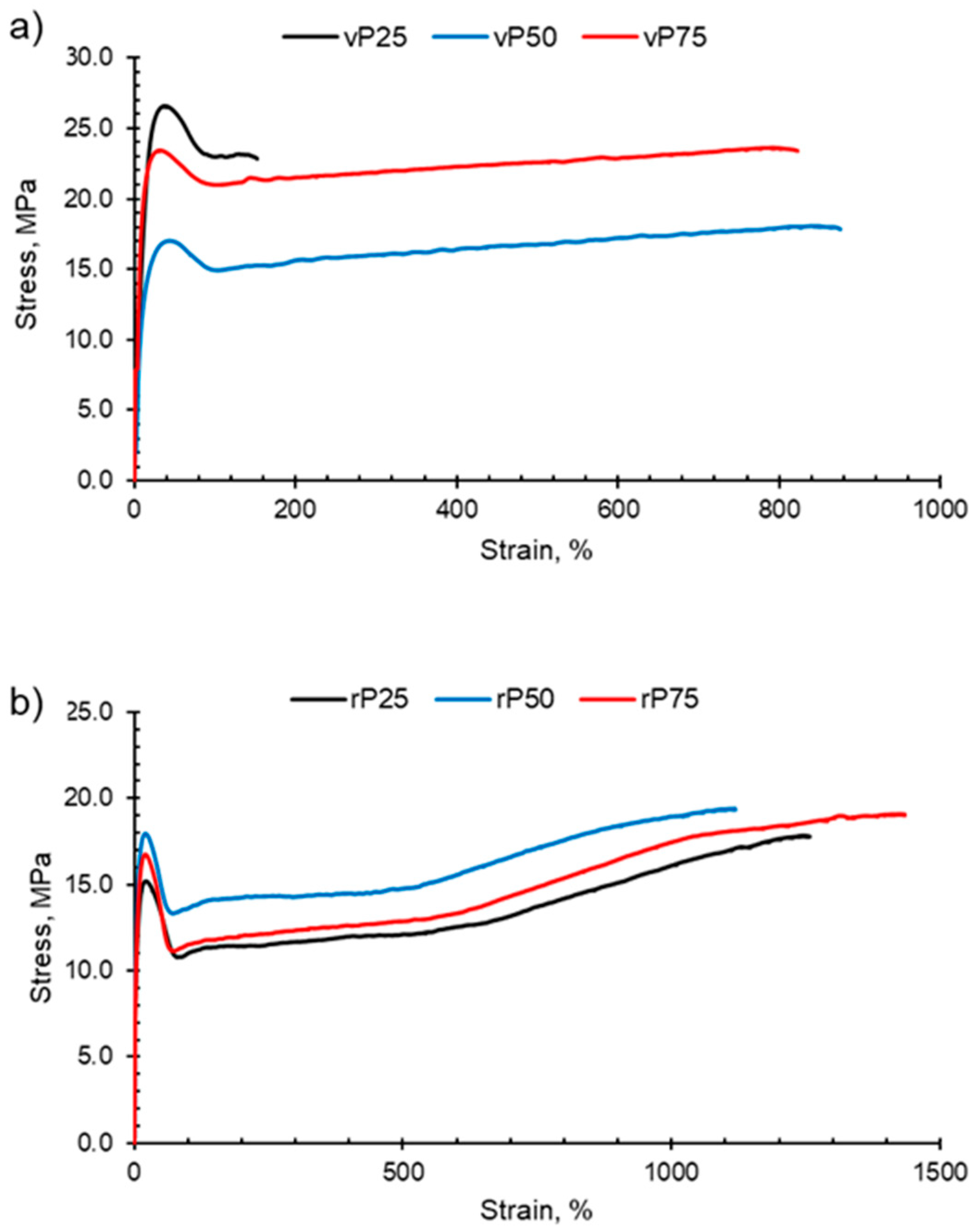
PP, HDPE and their blends undergo macroscopic deformation during a tensile test and typically exhibit strain hardening and a ductile fracture, as shown in Figure 7. Initially, the polymers undergo elastic deformation; however, as the force applied continues to increase, the polymer sample reaches the yield point and enters the region of plastic deformation. At the yield point, a small neck forms within the gauge section and the polymer chains align in the direction of elongation. Continuing beyond the yield point, the virgin and recycled PP, HDPE and their blends exhibit the strain-hardening phenomenon. Strain hardening occurs when there is resistance to deformation and the neck region propagates and extends, which is termed necking. The polymer chains continue to orientate and align in the direction of elongation, which results in an increase in the strength of the plastic. Necking continues until fracture.
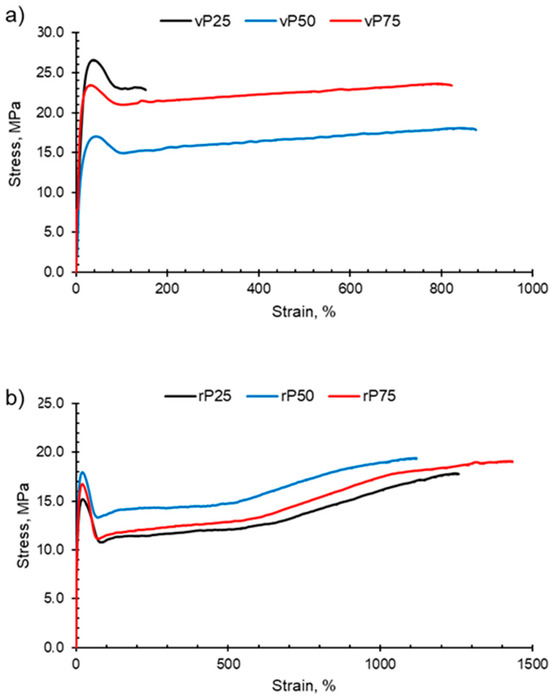
Figure 7.
Examples of typical stress–strain curves obtained for PP:HDPE blends. The blends P25, P50 and P75 have been shown for (a) vPP:vHDPE blends and (b) rPP:rHDPE blends.
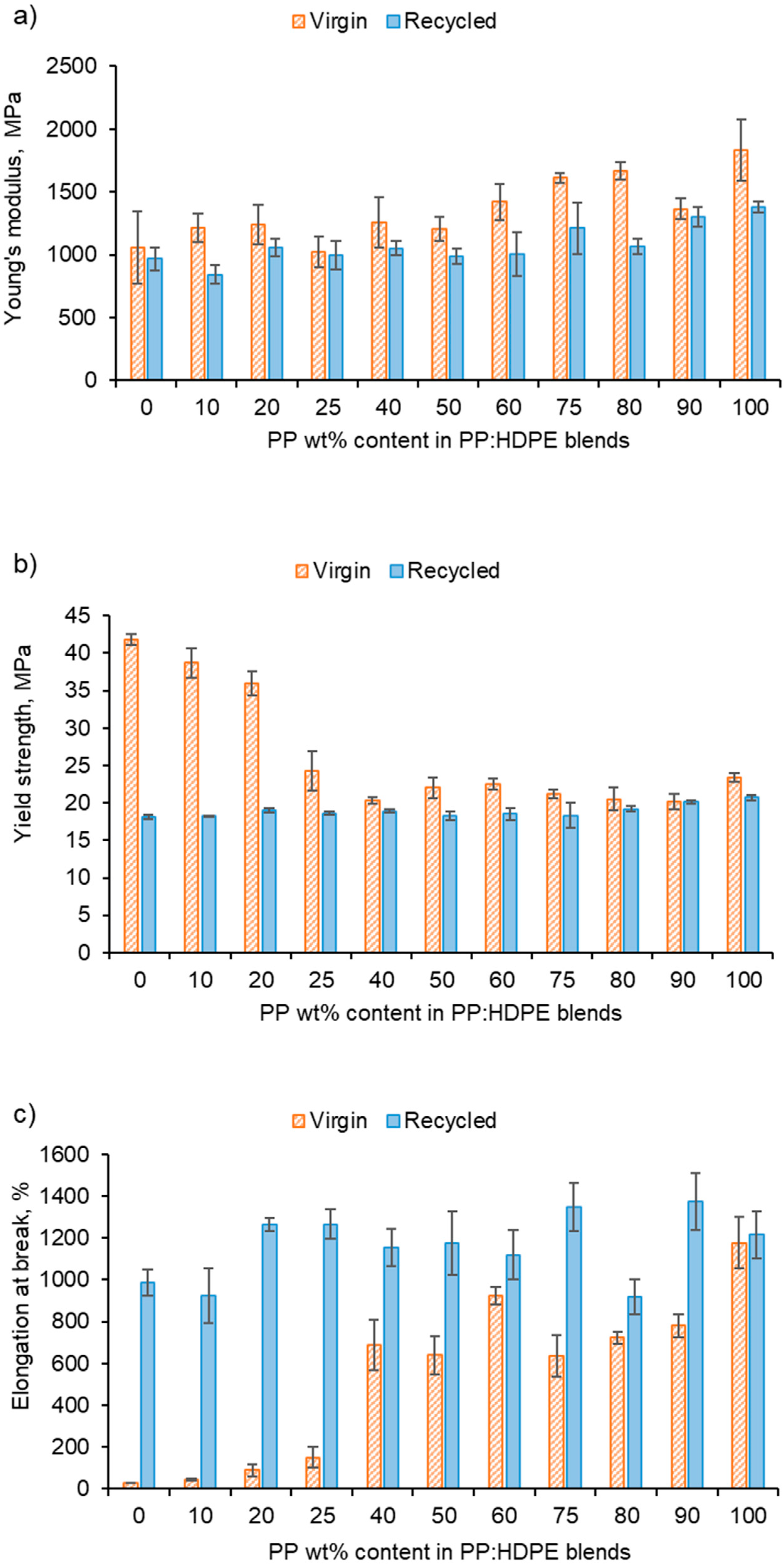
The recycled blends exhibited deteriorated tensile properties compared with the virgin blends in terms of Young’s modulus and yield strength (Figure 8). The Young’s modulus values of rPP and rHDPE were lower than those of the virgin polymers due to structural deterioration caused by the recycling process [60,72]. However, above rP75, the rPP:rHDPE blend’s yield strength values approach the virgin blend’s values. Studies have found that yield strength increases with crystallinity and lamellar thickness, with little or no effect of molecular weight [73]. The crystallinity of the recycled blends is lower compared with the virgin blends, and the recycling process results in the formation of imperfect crystals (which has been discussed in the thermal properties section), thus causing a reduction in the yield strength. Generally, there was little variation in the yield strength for the rPP:rHDPE blends.
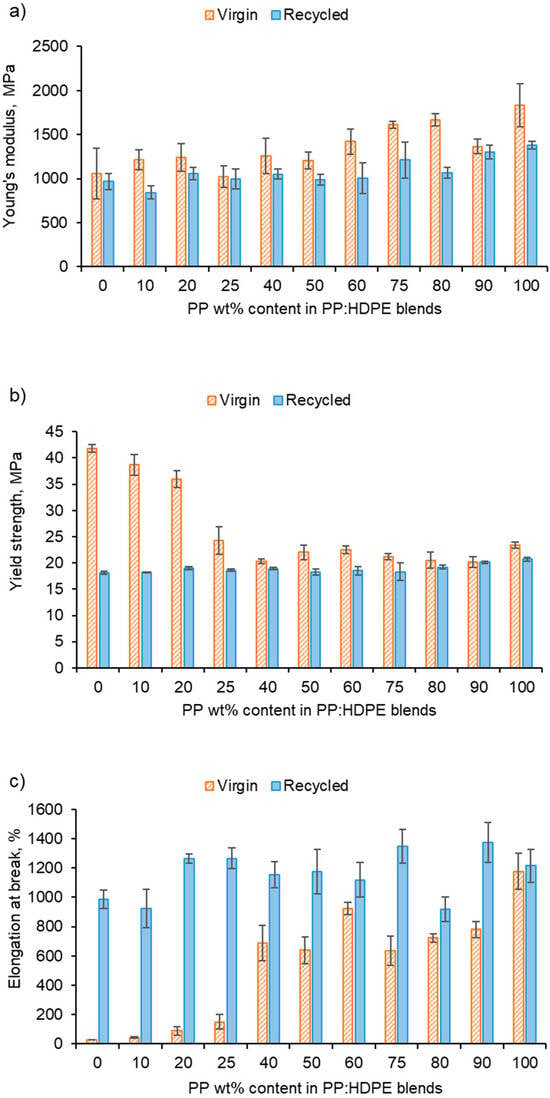
Figure 8.
Tensile properties of vPP:vHDPE and rPP:rHDPE blends: (a) Young’s modulus, (b) yield strength and (c) elongation at break.
The vHDPE demonstrated unexpected behaviour in yield strength (42 MPa) and elongation at break (29%) (Figure 8). vHDPE did not show typical necking behaviour and a brittle fracture was observed. No change in crystallinity was found by DSC when comparing the crystallinity of the HDPE before and after extrusion and injection moulding. During the injection moulding process, the polymer melt is exposed to a strong shear and elongational flow in which the chains are stretched and become highly orientated [74]. Flow-induced crystallisation increases HDPE’s crystallisation rate and forms a highly orientated shish-kebab structure, which improves the strength of HDPE [75]. Lei et al. [76] found no necking behaviour when vHDPE was blended with 4% ultra-high molecular weight PE prepared by twin screw extruder and dynamic injection moulding. An increase in the tensile strength in the flow direction was observed from 23 to 76 MPa, which was caused by the formation of a web-like shish-kebab morphology and chain orientation. Therefore, the high chain orientation of the vHDPE could result in an interlocking of the shish-kebab to form a rigid structure, which affected the yield strength and elongation at break up to the vP25 blend (Figure 8) [76,77,78]. The rHDPE did not exhibit the same unexpected behaviour as vHDPE in its yield strength and elongation at break. This is most likely due to the presence of lower-molecular-weight chains caused by the recycling processing, which have a reduced packing ability and degree of orientation. Additionally, the presence of micro-voids can result in a decrease in compatibility between polymeric components [79].
The comparison between the elongation at break for the virgin and recycled blends presents interesting results (Figure 8c). It was expected that recycled blends would have a lower elongation at break compared with the virgin blends due to the structural deterioration during recycling causing a reduction in molecular weight [80]. For example, Fang et al. [47] found that with the addition of rPP up to 15 wt% in a PP:PE blend, the elongation at break decreased, and with over 30 wt%, the elongation at break reached a minimum. However, the longer elongation at break observed for the recycled blend could be due to the presence of lower-molecular-weight polymer chains caused by the recycling process [81]. It is possible that the low-molecular-weight polymer chains locate at the interface between PP and HDPE phases and lower the interfacial tension [81]. Additionally, the lower-molecular-weight chains increase the capability of molecules sliding over each other, resulting in an increase in deformability [80]. The vHDPE up to vP25 presented extremely low elongation at break and samples exhibited brittle fractures. The data sheet provided by Ineos suggests an elongation at break value of 800% for vHDPE at 2 in min−1. As discussed, this behaviour could be due to the formation of a very rigid crystalline structure for vHDPE (and up to vP25), which would explain the brittle fracture observed. Due to this behaviour, a comparison between the virgin and recycled blends is more complex.
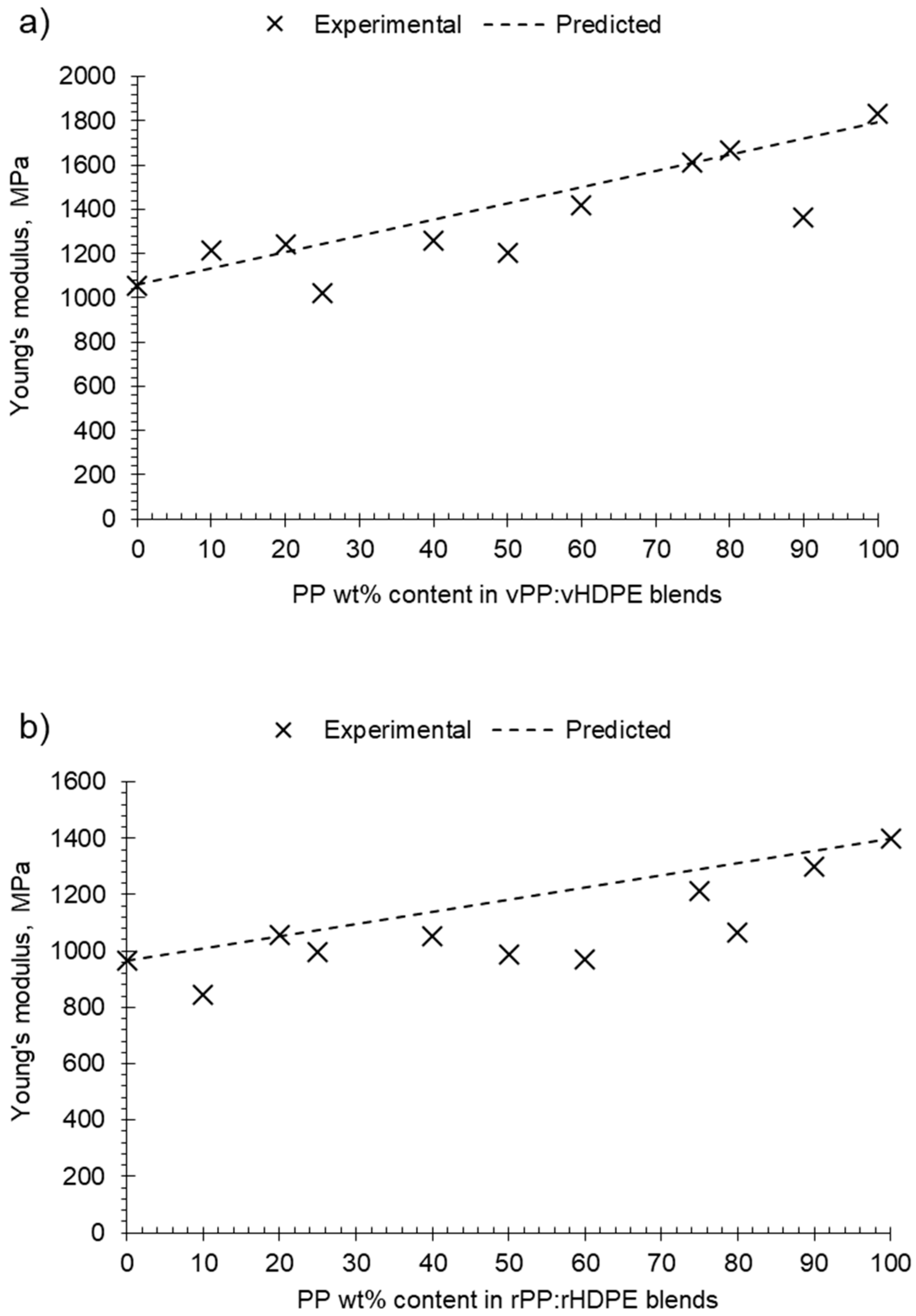
The virgin and recycled PP:HDPE blends gave intermediary Young’s moduli values between PP and HDPE. Comparing the predicted rules of mixtures to the experimental Young’s modulus shows a negative deviation for most recycled blends (Figure 9b). A negative deviation suggests poor compatibility and weak adhesion between the phases [6]. It is important to note that the rule of mixtures does not take into account interactions between components. The Young’s modulus values of the virgin blends showed positive and negative deviations from the rule of mixtures with composition (Figure 9a): positive deviation for blends vP10 and vP20, negative deviation between vP25 and vP60, a minor positive deviation at vP75 and vP80, and a negative deviation at vP90. Lovinger and Williams [82] observed a maximum deviation at P80 and suggested that PE can play the role of stiffener to the PP matrix; in sufficient quantities, it enhances the intercrystalline links. The alternating changes of positive and negative deviations suggest a complex interplay of morphological factors and crystallinity as the composition varied.
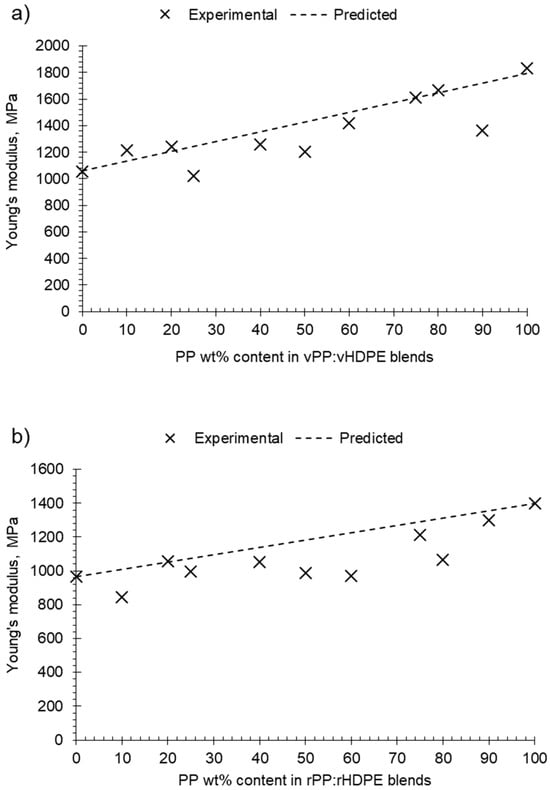
Figure 9.
Plot of experimental and predicted Young’s modulus against PP wt% content in PP:HDPE blends: (a) vPP:vHDPE blends and (b) rPP:rHDPE blends.
4. Conclusions
This study investigated the thermal and mechanical properties of virgin and recycled PP:HDPE blends. Thermal studies carried out by DSC confirmed that both the virgin and recycled PP:HDPE blends were immiscible. The recycling process was found to lower the Tm values of the rPP:rHDPE blends due to structural deterioration and the formation of imperfect crystallites. Interestingly, there was little difference in the Tc values when comparing the virgin and recycled blends. The PP and HDPE crystallinities were dependent upon the blend composition. As the ratio of PP increased, the crystallinity of PP increased and that of HDPE decreased in the PP:HDPE blends. Generally, the rPP:rHDPE blends had a lower overall crystallinity compared with the vPP:vHDPE blends, suggesting the formation of imperfect crystallites and a heterogeneous crystalline morphology. However, the crystallisation of the individual polymers was more complex. vPP crystallinity was enhanced (compared with rPP) at higher PP content; conversely, rHDPE crystallinity was enhanced (compared with vHDPE) at higher PP content. rPP and rHDPE could contain contaminants due to the difficulty of separating PP and HDPE during the recycling process, thus affecting the crystallinity behaviour.
DMA analysis found little variation in the E′ of the virgin and recycled blends with composition. However, a decreasing trend was observed for the virgin blends E″ as the PP wt% increased, while the recycled blends’ E″ was relatively constant. The interplay between the structure and dissipation mechanisms can be complex. Chain scission caused by the recycling process can introduce plasticising shorter chains. However, impurities can act as fillers in the recycled materials that impose a mechanical restraint. Recycled blends were found to have lower Tα and Tβ due to structural deterioration caused by the recycling process. Recycled blends gave a reduced Young’s modulus and yield strength in comparison with virgin blends due to deterioration during the recycling process. Generally, the recycled blends gave a higher elongation at break compared with the virgin blends, possibly due to the plasticity effect of the low-molecular-weight chain fragments. However, a comparison between the virgin and recycled blends’ elongation at break was not straightforward in all cases due to the highly orientated vHDPE induced by the injection moulding.
This work explored the variability in the thermomechanical behaviour of vPP:vHDPE and rPP:rHDPE blends without the addition of other components. Understanding the performance variability in recycled blends is key to increasing the quantity of recycled material re-entering the consumer market to contribute towards a circular plastic economy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym15214200/s1, Table S1: Summary of the crystallinities obtained from the first and second heating ramps for vPP:vHDPE blends; Table S2: Summary of the crystallinities obtained from the first and second heating ramps for rPP:rHDPE blends; Table S3: Summary of Tα and Tβ taken from tanδ traces for virgin and recycled PP:HDPE blends; Figure S1: Melting temperatures of vPP, rPP, vHDPE and rHDPE of vPP:vHDPE and rPP:rHDPE blends obtained from DSC; Figure S2: Enthalpy of fusion of vPP, rPP, vHDPE and rHDPE of vPP:vHDPE and rPP:rHDPE blends obtained from DSC; Figure S3: Crystallisation temperatures of vPP, rPP, vHDPE and rHDPE of vPP:vHDPE and rPP:rHDPE blends obtained from DSC; Figure S4: Enthalpy of crystallisation of vPP:vHDPE and rPP:rHDPE blends obtained from DSC; Figure S5: Percentage crystallinity of vPP, rPP, vHDPE and rHDPE, and total crystallinity of vPP:vHDPE and rPP:rHDPE blends obtained from DSC.
Author Contributions
Conceptualization, V.K., D.R., C.S.H. and M.K.; methodology, V.K., D.R. and J.M.; software, H.J.; validation, H.J. and D.R.; formal analysis, H.J.; investigation, H.J.; resources, V.K., D.R. and C.S.H.; data curation, H.J.; writing—original draft preparation, H.J.; writing—review and editing, V.K., D.R, H.J., J.M., C.S.H. and M.K.; visualization, H.J.; supervision, V.K. and D.R.; project administration, V.K., D.R. and M.K.; funding acquisition, V.K. and H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EPSRC SOFI CDT (Grant ref No. EP/L015536/1) and partially by Impact Solutions.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data available upon request.
Acknowledgments
We thank Impact Solutions for their partial financial support of the project as well as providing the materials used in the experiments. We acknowledge the financial support of the EPSRC and the SOFI CDT (Grant ref No. EP/L015536/1). For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.
Conflicts of Interest
Author M.K. was employed by the company Impact Solutions. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.; Saffar, F.; Koutsos, V.; Ray, D. Polyolefins and Polyethylene Terephthalate Package Wastes: Recycling and Use in Composites. Energies 2021, 14, 7306. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Kumar, S.; Panda, A.K.; Singh, R.K. A review on tertiary recycling of high-density polyethylene to fuel. Resour. Conserv. Recycl. 2011, 55, 893–910. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Pulikkalparambil, H.; Sanjay, M.R.; Siengchin, S. Polypropylene/high-density polyethylene based blends and nanocomposites with improved toughness. Mater. Res. Express 2019, 6, 075334. [Google Scholar] [CrossRef]
- Jose, S.; Aprem, A.S.; Francis, B.; Chandy, M.C.; Werner, P.; Alstaedt, V.; Thomas, S. Phase morphology, crystallisation behaviour and mechanical properties of isotactic polypropylene/high density polyethylene blends. Eur. Polym. J. 2004, 40, 2105–2115. [Google Scholar] [CrossRef]
- Graziano, A.; Jaffer, S.; Sain, M. Review on modification strategies of polyethylene/polypropylene immiscible thermoplastic polymer blends for enhancing their mechanical behavior. J. Elastomers Plast. 2019, 51, 291–336. [Google Scholar] [CrossRef]
- Strapasson, R.; Amico, S.C.; Pereira, M.F.R.; Sydenstricker, T.H.D. Tensile and impact behavior of polypropylene/low density polyethylene blends. Polym. Test. 2005, 24, 468–473. [Google Scholar] [CrossRef]
- Madi, N.K. Thermal and mechanical properties of injection molded recycled high density polyethylene blends with virgin isotactic polypropylene. Mater. Des. 2013, 46, 435–441. [Google Scholar] [CrossRef]
- Feldman, D. Polyblend Nanocomposites. J. Macromol. Sci. Part A 2015, 52, 648–658. [Google Scholar] [CrossRef]
- Salih, S.E.; Hamood, A.F.; Alsabih, A.H. Comparison of the Characteristics of LDPE:PP and HDPE:PP Polymer Blends. Mod. Appl. Sci. 2013, 7, 33–42. [Google Scholar] [CrossRef]
- Ajji, A.; Utracki, L.A. Interphase and compatibilization of polymer blends. Polym. Eng. Sci. 1996, 36, 1574–1585. [Google Scholar] [CrossRef]
- John, B.; Varughese, K.T.; Oommen, Z.; Pötschke, P.; Thomas, S. Dynamic mechanical behavior of high-density polyethylene/ethylene vinyl acetate copolymer blends: The effects of the blend ratio, reactive compatibilization, and dynamic vulcanization. J. Appl. Polym. Sci. 2003, 87, 2083–2099. [Google Scholar] [CrossRef]
- Scott, C.E.; Macosko, C.W. Morphology development during the initial stages of polymer-polymer blending. Polymer 1995, 36, 461–470. [Google Scholar] [CrossRef]
- Souza, A.M.C.; Demarquette, N.R. Influence of composition on the linear viscoelastic behavior and morphology of PP/HDPE blends. Polymer 2002, 43, 1313–1321. [Google Scholar] [CrossRef]
- Souza, A.M.C.; Demarquette, N.R. Influence of coalescence and interfacial tension on the morphology of PP/HDPE compatibilized blends. Polymer 2002, 43, 3959–3967. [Google Scholar] [CrossRef]
- Favis, B.D. The effect of processing parameters on the morphology of an immiscible binary blend. J. Appl. Polym. Sci. 1990, 39, 285–300. [Google Scholar] [CrossRef]
- Jordan, A.M.; Kim, K.; Soetrisno, D.; Hannah, J.; Bates, F.S.; Jaffer, S.A.; Lhost, O.; Macosko, C.W. Role of Crystallization on Polyolefin Interfaces: An Improved Outlook for Polyolefin Blends. Macromolecules 2018, 51, 2506–2516. [Google Scholar] [CrossRef]
- Albano, C.; González, J.; Ichazo, M.; Rosales, C.; Urbina de Navarro, C.; Parra, C. Mechanical and morphological behavior of polyolefin blends in the presence of CaCO3. Compos. Struct. 2000, 48, 49–58. [Google Scholar] [CrossRef]
- Kallel, T.; Massardier-Nageotte, V.; Jaziri, M.; Gérard, J.-F.; Elleuch, B. Compatibilization of PE/PS and PE/PP blends. I. Effect of processing conditions and formulation. J. Appl. Polym. Sci. 2003, 90, 2475–2484. [Google Scholar] [CrossRef]
- Cao, W.; Wang, K.; Zhang, Q.; Du, R.; Fu, Q. The hierarchy structure and orientation of high density polyethylene obtained via dynamic packing injection molding. Polymer 2006, 47, 6857–6867. [Google Scholar] [CrossRef]
- Huang, D.E.; Kotula, A.P.; Snyder, C.R.; Migler, K.B. Crystallization Kinetics in an Immiscible Polyolefin Blend. Macromolecules 2022, 55, 10921–10932. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shanks, R.A.; Long, Y. Mechanical properties and morphology of polyethylene–polypropylene blends with controlled thermal history. J. Appl. Polym. Sci. 2000, 76, 1151–1164. [Google Scholar] [CrossRef]
- Şirin, K.; Doğan, F.; Çanlı, M.; Yavuz, M. Mechanical properties of polypropylene (PP) + high-density polyethylene (HDPE) binary blends: Non-isothermal degradation kinetics of PP + HDPE (80/20) Blends. Polym. Adv. Technol. 2013, 24, 715–722. [Google Scholar] [CrossRef]
- Xie, M.; Chen, J.; Li, H. Morphology and mechanical properties of injection-molded ultrahigh molecular weight polyethylene/polypropylene blends and comparison with compression molding. J. Appl. Polym. Sci. 2009, 111, 890–898. [Google Scholar] [CrossRef]
- Aumnate, C.; Rudolph, N.; Sarmadi, M. Recycling of Polypropylene/Polyethylene Blends: Effect of Chain Structure on the Crystallization Behaviors. Polymers 2019, 11, 1456. [Google Scholar] [CrossRef]
- Lin, J.-H.; Pan, Y.-J.; Liu, C.-F.; Huang, C.-L.; Hsieh, C.-T.; Chen, C.-K.; Lin, Z.-I.; Lou, C.-W. Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends. Materials 2015, 8, 8850–8859. [Google Scholar] [CrossRef]
- Chiu, F.-C.; Yen, H.-Z.; Lee, C.-E. Characterization of PP/HDPE blend-based nanocomposites using different maleated polyolefins as compatibilizers. Polym. Test. 2010, 29, 397–406. [Google Scholar] [CrossRef]
- Kazemi, Y.; Ramezani Kakroodi, A.; Rodrigue, D. Compatibilization efficiency in post-consumer recycled polyethylene/polypropylene blends: Effect of contamination. Polym. Eng. Sci. 2015, 55, 2368–2376. [Google Scholar] [CrossRef]
- Sutar, H.; Sahoo, P.C.; Sahu, P.S.; Sahoo, S.; Murmu, R.; Swain, S.; Mishra, S.C. Mechanical, Thermal and Crystallization Properties of Polypropylene (PP) Reinforced Composites with High Density Polyethylene (HDPE) as Matrix. Mater. Sci. Appl. 2018, 9, 502–515. [Google Scholar] [CrossRef]
- Hammache, Y.; Serier, A.; Chaoui, S. The effect of thermoplastic starch on the properties of polypropylene/high density polyethylene blend reinforced by nano-clay. Mater. Res. Express 2020, 7, 025308. [Google Scholar] [CrossRef]
- Karaagac, E.; Koch, T.; Archodoulaki, V.-M. The effect of PP contamination in recycled high-density polyethylene (rPE-HD) from post-consumer bottle waste and their compatibilization with olefin block copolymer (OBC). Waste Manag. 2021, 119, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Aurrekoetxea, J.; Sarrionandia, M.A.; Urrutibeascoa, I.; Maspoch, M.L. Effects of recycling on the microstructure and the mechanical properties of isotactic polypropylene. J. Mater. Sci. 2001, 36, 2607–2613. [Google Scholar] [CrossRef]
- Yin, S.; Tuladhar, R.; Shi, F.; Shanks, R.A.; Combe, M.; Collister, T. Mechanical reprocessing of polyolefin waste: A review. Polym. Eng. Sci. 2015, 55, 2899–2909. [Google Scholar] [CrossRef]
- da Costa, H.M.; Ramos, V.D.; de Oliveira, M.G. Degradation of polypropylene (PP) during multiple extrusions: Thermal analysis, mechanical properties and analysis of variance. Polym. Test. 2007, 26, 676–684. [Google Scholar] [CrossRef]
- Ha, K.H.; Kim, M.S. Application to refrigerator plastics by mechanical recycling from polypropylene in waste-appliances. Mater. Des. 2012, 34, 252–257. [Google Scholar] [CrossRef]
- Oliveira, T.A.; Oliveira, R.R.; Barbosa, R.; Azevedo, J.B.; Alves, T.S. Effect of reprocessing cycles on the degradation of PP/PBAT-thermoplastic starch blends. Carbohydr. Polym. 2017, 168, 52–60. [Google Scholar] [CrossRef]
- Bunjes, A.; Arndt, J.-H.; Geertz, G.; Barton, B. Characterization and chemometric modelling of mechanically recycled polypropylene for automotive manufacturing. Polymer 2022, 249, 124823. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Lin, Q.-B.; Su, Q.-Z.; Zhong, H.-N.; Nerin, C. Identification of recycled polyethylene and virgin polyethylene based on untargeted migrants. Food Packag. Shelf Life 2021, 30, 100762. [Google Scholar] [CrossRef]
- Jmal, H.; Bahlouli, N.; Wagner-Kocher, C.; Leray, D.; Ruch, F.; Munsch, J.-N.; Nardin, M. Influence of the grade on the variability of the mechanical properties of polypropylene waste. Waste Manag. 2018, 75, 160–173. [Google Scholar] [CrossRef]
- Jin, H.; Gonzalez-Gutierrez, J.; Oblak, P.; Zupančič, B.; Emri, I. The effect of extensive mechanical recycling on the properties of low density polyethylene. Polym. Degrad. Stab. 2012, 97, 2262–2272. [Google Scholar] [CrossRef]
- Oblak, P.; Gonzalez-Gutierrez, J.; Zupančič, B.; Aulova, A.; Emri, I. Processability and mechanical properties of extensively recycled high density polyethylene. Polym. Degrad. Stab. 2015, 114, 133–145. [Google Scholar] [CrossRef]
- Saikrishnan, S.; Jubinville, D.; Tzoganakis, C.; Mekonnen, T.H. Thermo-mechanical degradation of polypropylene (PP) and low-density polyethylene (LDPE) blends exposed to simulated recycling. Polym. Degrad. Stab. 2020, 182, 109390. [Google Scholar] [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Vorst, K.L.; Curtzwiler, G.W. The effect of post-consumer recycled polyethylene (PCRPE) on the properties of polyethylene blends of different densities. Polym. Degrad. Stab. 2021, 190, 109627. [Google Scholar] [CrossRef]
- Noor Hasanah, T.I.T.; Wijeyesekera, D.C.; Lim, A.J.M.S.; Ismail, B. Recycled PP/HDPE Blends: A Thermal Degradation and Mechanical Properties Study. Appl. Mech. Mater. 2014, 465–466, 932–936. [Google Scholar] [CrossRef]
- Atiqah, A.A.S.M.; Salmah, H.; Firuz, Z.; Uy Lan, D.N. Properties of Recycled High Density Polyethylene/Recycled Polypropylene Blends: Effect of Maleic Anhydride Polypropylene. Key Eng. Mater. 2014, 594–595, 837–841. [Google Scholar] [CrossRef]
- Fang, C.; Nie, L.; Liu, S.; Yu, R.; An, N.; Li, S. Characterization of polypropylene–polyethylene blends made of waste materials with compatibilizer and nano-filler. Compos. Part B Eng. 2013, 55, 498–505. [Google Scholar] [CrossRef]
- ISO 527-2; Tensile Testing for Plastics. International Organization for Standardization: Geneva, Switzerland, 2012.
- Watt, E.; Abdelwahab, M.A.; Snowdon, M.R.; Mohanty, A.K.; Khalil, H.; Misra, M. Hybrid biocomposites from polypropylene, sustainable biocarbon and graphene nanoplatelets. Sci. Rep. 2020, 10, 10714. [Google Scholar] [CrossRef]
- Shrivastava, A. 3-Plastic Properties and Testing. In Introduction to Plastics Engineering; Shrivastava, A., Ed.; William Andrew Publishing: Oxford, UK, 2018; pp. 49–110. [Google Scholar]
- Wetton, R.E. Thermal analysis. In Polymer Characterisation; Hunt, B.J., James, M.I., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1993; pp. 178–221. [Google Scholar]
- Nielsen, L.E. Predicting the Properties of Mixtures: Mixture Rules in Science and Engineering; Marcel Dekker: New York, NY, USA, 1978. [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Momanyi, J.; Herzog, M.; Muchiri, P. Analysis of Thermomechanical Properties of Selected Class of Recycled Thermoplastic Materials Based on Their Applications. Recycling 2019, 4, 33. [Google Scholar] [CrossRef]
- Gu, J.; Xu, H.; Wu, C. Thermal and Crystallization Properties of HDPE and HDPE/PP Blends Modified with DCP. Adv. Polym. Technol. 2014, 33, 21384. [Google Scholar] [CrossRef]
- Mileva, D.; Tranchida, D.; Gahleitner, M. Designing polymer crystallinity: An industrial perspective. Polym. Cryst. 2018, 1, e10009. [Google Scholar] [CrossRef]
- Samanta, P.; Srivastava, R.; Nandan, B.; Chen, H.-L. Crystallization behavior of crystalline/crystalline polymer blends under confinement in electrospun nanofibers of polystyrene/poly(ethylene oxide)/poly(ε-caprolactone) ternary mixtures. Soft Matter 2017, 13, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Stangenberg, F.; Ågren, S.; Karlsson, S. Quality Assessments of Recycled Plastics by Spectroscopy and Chromatography. Chromatographia 2004, 59, 101–106. [Google Scholar] [CrossRef]
- Ruj, B.; Pandey, V.; Jash, P.; Srivastava, V. Sorting of plastic waste for effective recycling. Int. J. Appl. Sci. Eng. Res. 2015, 4, 564–571. [Google Scholar]
- Pinheiro, L.A.; Chinelatto, M.A.; Canevarolo, S.V. The role of chain scission and chain branching in high density polyethylene during thermo-mechanical degradation. Polym. Degrad. Stab. 2004, 86, 445–453. [Google Scholar] [CrossRef]
- Shirkavand, M.J.; Azizi, H.; Ghasemi, I.; Karabi, M. Effect of Molecular Structure Parameters on Crystallinity and Environmental Stress Cracking Resistance of High-Density Polyethylene/TiO2 Nanocomposites. Adv. Polym. Technol. 2018, 37, 770–777. [Google Scholar] [CrossRef]
- Vilaplana, F.; Karlsson, S. Quality Concepts for the Improved Use of Recycled Polymeric Materials: A Review. Macromol. Mater. Eng. 2008, 293, 274–297. [Google Scholar] [CrossRef]
- Sewda, K.; Maiti, S.N. Dynamic mechanical properties of high density polyethylene and teak wood flour composites. Polym. Bull. 2013, 70, 2657–2674. [Google Scholar] [CrossRef]
- Hidalgo-Salazar, M.A.; Correa-Aguirre, J.P.; García-Navarro, S.; Roca-Blay, L. Injection Molding of Coir Coconut Fiber Reinforced Polyolefin Blends: Mechanical, Viscoelastic, Thermal Behavior and Three-Dimensional Microscopy Study. Polymers 2020, 12, 1507. [Google Scholar] [CrossRef]
- McCrum, N.G.; Read, B.E.; Williams, G. Anelastic and Dielectric Effects in Polymeric Solids; John Wiley: London, UK; New York, NY, USA, 1967. [Google Scholar]
- Sethi, M.; Gupta, N.K.; Srivastava, A.K. Dynamic mechanical analysis of polyethylene and ethylene vinylacetate copolymer blends irradiated by electron beam. J. Appl. Polym. Sci. 2002, 86, 2429–2434. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Micic, M.; Milicevic, D. Structural Changes and Dielectric Relaxation Behavior of Uniaxially Oriented High Density Polyethylene. J. Eng. Fibers Fabr. 2013, 8, 131–143. [Google Scholar] [CrossRef]
- Popli, R.; Glotin, M.; Mandelkern, L.; Benson, R.S. Dynamic mechanical studies of α and β relaxations of polyethylenes. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 407–448. [Google Scholar] [CrossRef]
- Khanna, Y.P.; Turi, E.A.; Taylor, T.J.; Vickroy, V.V.; Abbott, R.F. Dynamic mechanical relaxations in polyethylene. Macromolecules 1985, 18, 1302–1309. [Google Scholar] [CrossRef]
- Fakirov, S.; Krasteva, B. On the Glass Transition Temperature of Polyethylene as Revealed by Microhardness Measurements. J. Macromol. Sci. Part B 2000, 39, 297–301. [Google Scholar] [CrossRef]
- Morris, B.A. 4-Commonly Used Resins and Substrates in Flexible Packaging. In The Science and Technology of Flexible Packaging; Morris, B.A., Ed.; William Andrew Publishing: Oxford, UK, 2017; pp. 69–119. [Google Scholar]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Chivers, R.A.; Moore, D.R. The effect of molecular weight and crystallinity on the mechanical properties of injection moulded poly(aryl-ether-ether-ketone) resin. Polymer 1994, 35, 110–116. [Google Scholar] [CrossRef]
- Yang, H.-R.; Lei, J.; Li, L.; Fu, Q.; Li, Z.-M. Formation of Interlinked Shish-Kebabs in Injection-Molded Polyethylene under the Coexistence of Lightly Cross-Linked Chain Network and Oscillation Shear Flow. Macromolecules 2012, 45, 6600–6610. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; Huang, Y.; Cong, Y.; Ma, Z.; Shao, C.; An, H.; Yan, T.; Li, L. Inducing Crystallization of Polymer through Stretched Network. Macromolecules 2009, 42, 1428–1432. [Google Scholar] [CrossRef]
- Lei, J.; Zhang, Z.; Jiang, C.; Shen, K. Bi-axial self-reinforcement of high-density polyethylene induced by high-molecular weight polyethylene through dynamic packing injection molding. Polym. Int. 2006, 55, 1021–1026. [Google Scholar] [CrossRef]
- Deng, C.; Lei, J.; Gao, X.; Chen, Z.; Shen, K. Study on the Improvement of Crystallization in HDPE Induced by High–Molecular-Weight Polyethylene Through Dynamic Packing Injection Molding. Polym. Plast. Technol. Eng. 2008, 47, 716–721. [Google Scholar] [CrossRef]
- Bayer, R.K.; Baltá Calleja, F.J.; López Cabarcos, E.; Zachiviann, H.G.; Paulsen, A.; Brüning, F.; Meins, W. Properties of elongational flow injection moulded polyethylene. J. Mater. Sci. 1989, 24, 2643–2652. [Google Scholar] [CrossRef]
- Murugan, D.; Varughese, S.; Swaminathan, T. Recycled Polyolefin-Based Plastic Wastes for Sound Absorption. Polym. Plast. Technol. Eng. 2006, 45, 885–888. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Botta, L.; Mistretta, M.C.; Di Fiore, A.; Titone, V. Recycling of a Biodegradable Polymer Blend. Polymers 2020, 12, 2297. [Google Scholar] [CrossRef] [PubMed]
- Dorigato, A. Recycling of polymer blends. Adv. Ind. Eng. Polym. Res. 2021, 4, 53–69. [Google Scholar] [CrossRef]
- Lovinger, A.J.; Williams, M.L. Tensile properties and morphology of blends of polyethylene and polypropylene. J. Appl. Polym. Sci. 1980, 25, 1703–1713. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).