Preparation, Characterization, Dielectric Properties, and AC Conductivity of Chitosan Stabilized Metallic Oxides CoO and SrO: Experiments and Tight Binding Calculations
Abstract
:1. Introduction
2. Methodology
2.1. Materials and Instruments
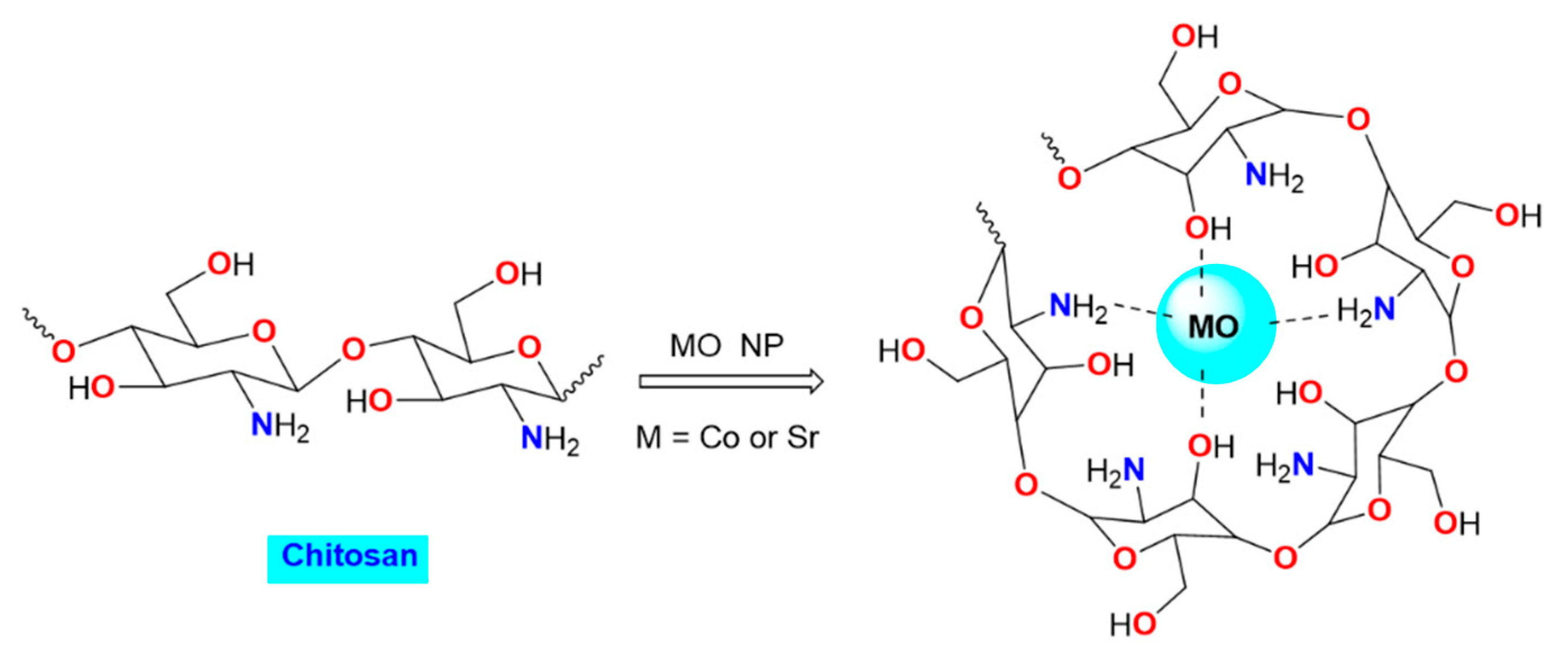
2.2. Preparation of CS-CoO and CS-SrO Nanocomposite Films
3. Results and Discussion
3.1. Characterization of the Nanocomposite Film Made of Chitosan and Metal Oxides
3.1.1. FTIR Characterization
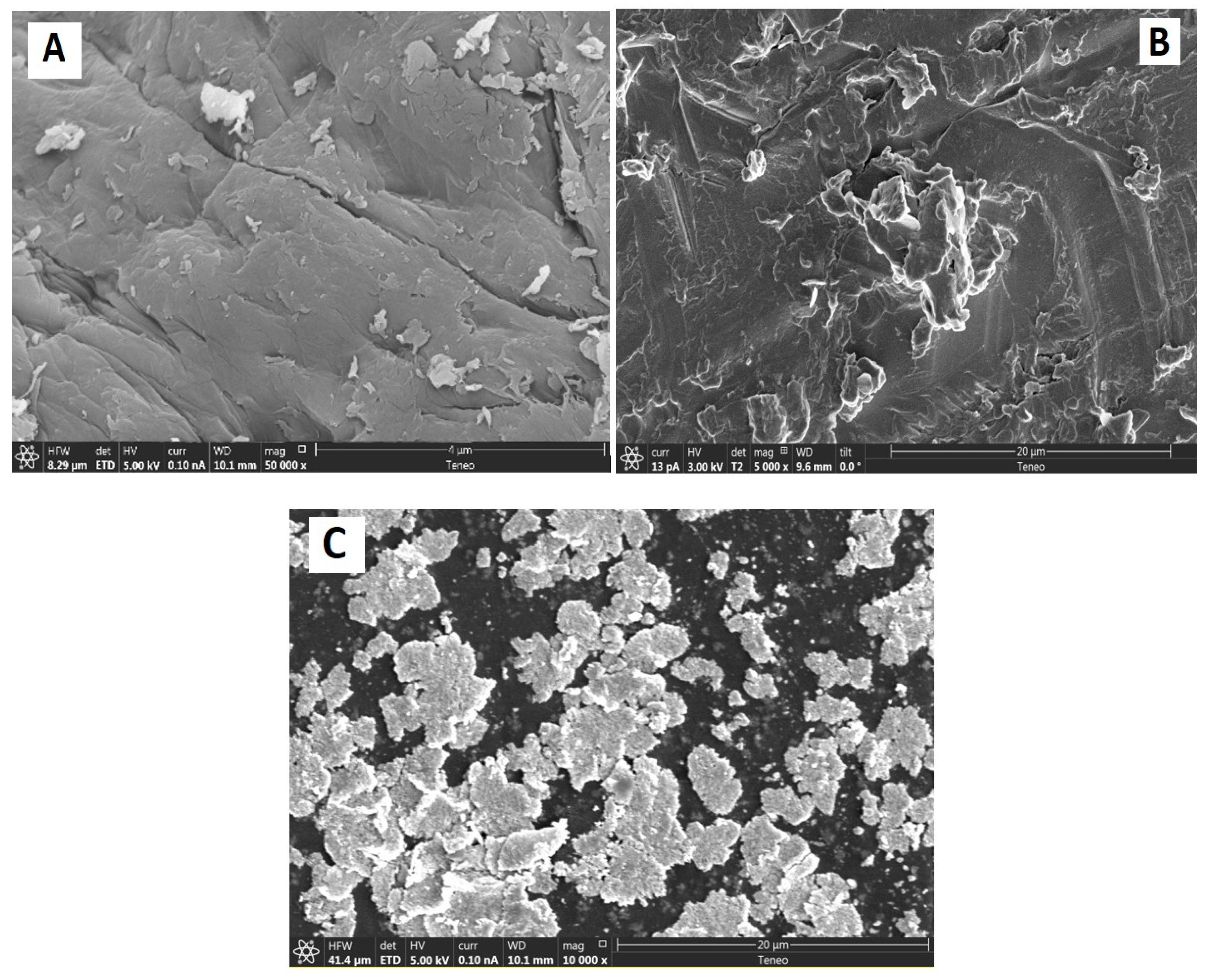
3.1.2. SEM and Morphological Characteristics
3.1.3. Estimating the Quantity of Cobalt and Strontium in the Polymer Matrix Using Energy-Dispersive X-ray Spectroscopy (EDS)
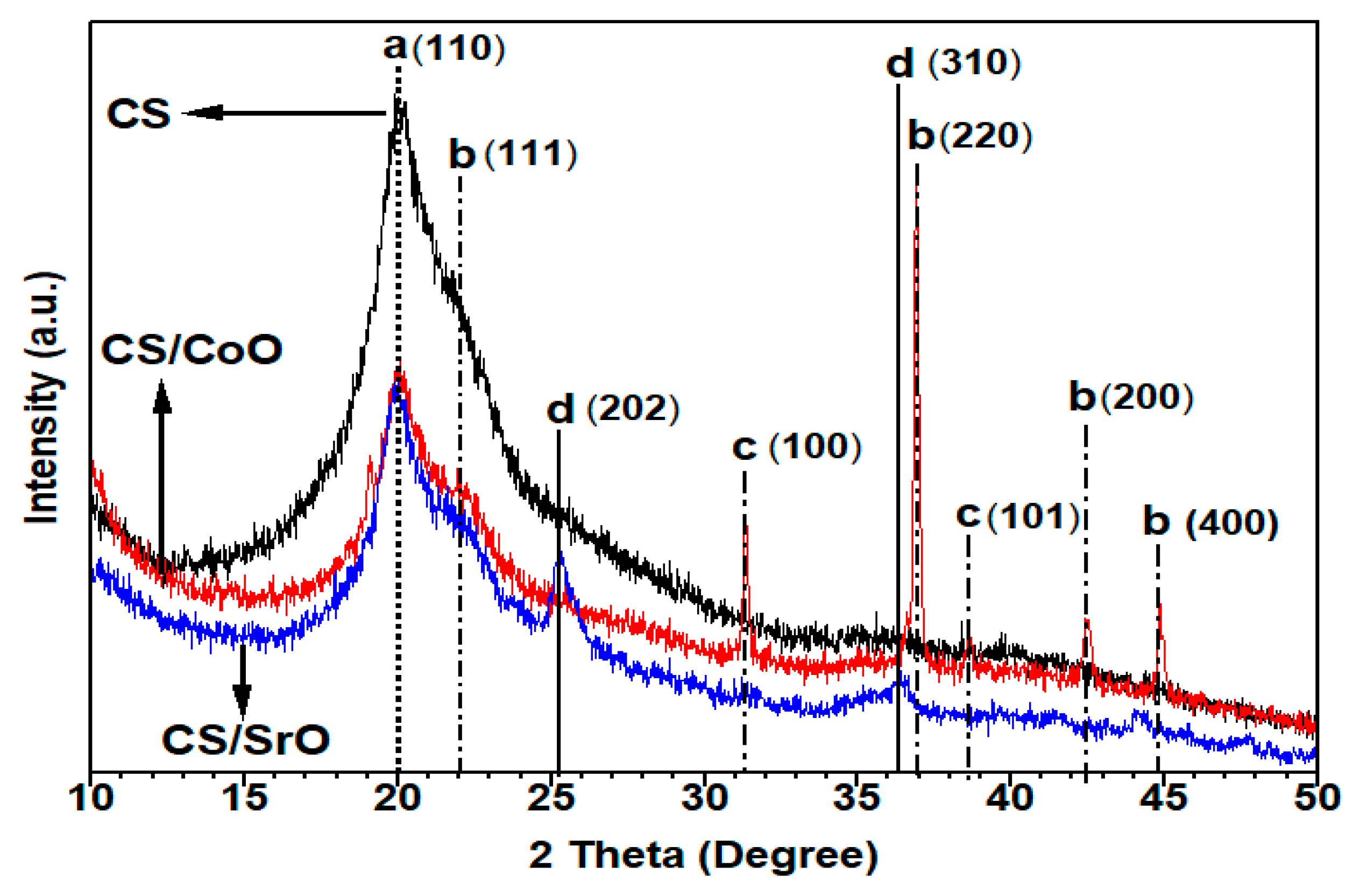
3.1.4. X-ray Diffraction
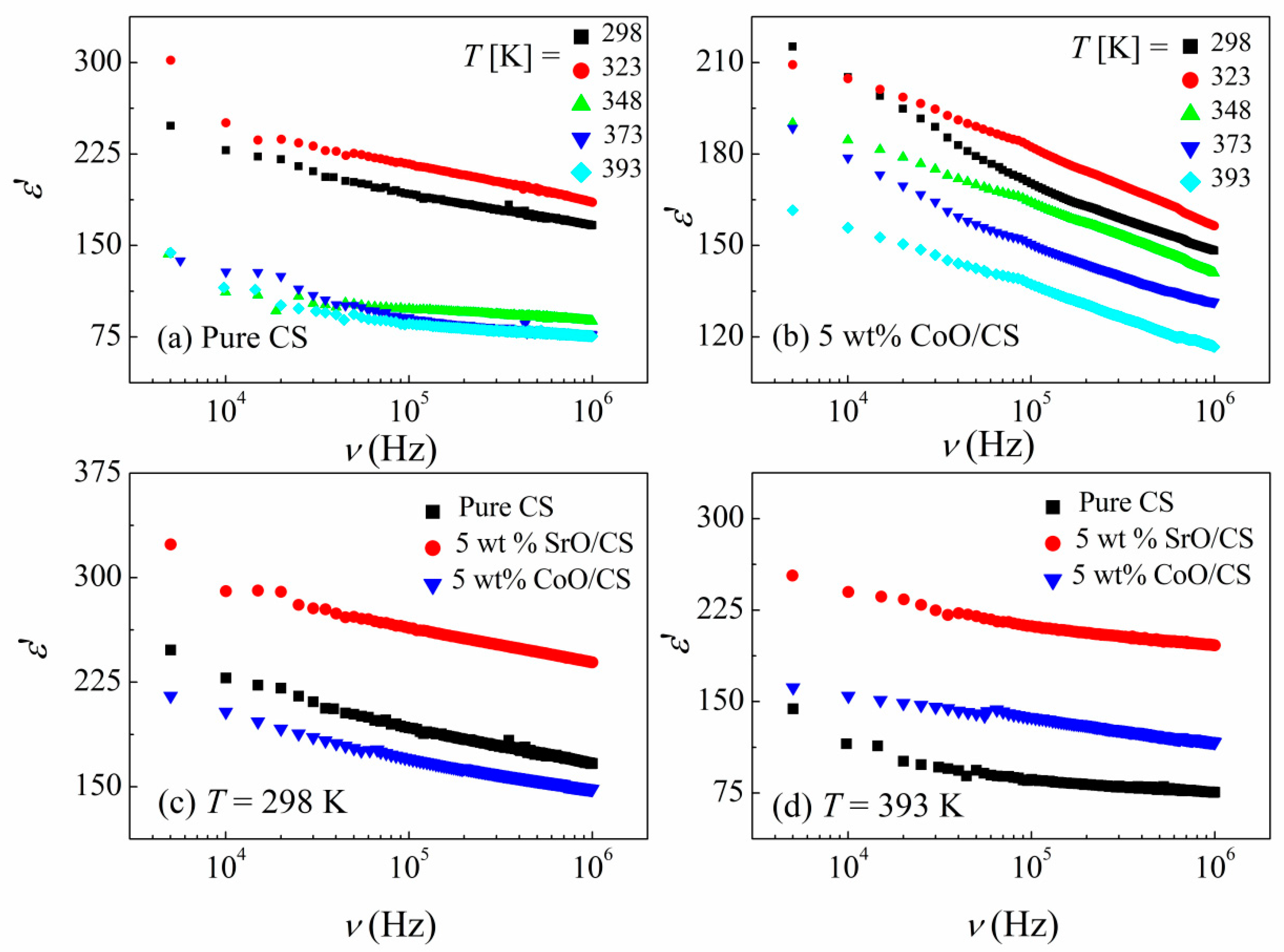
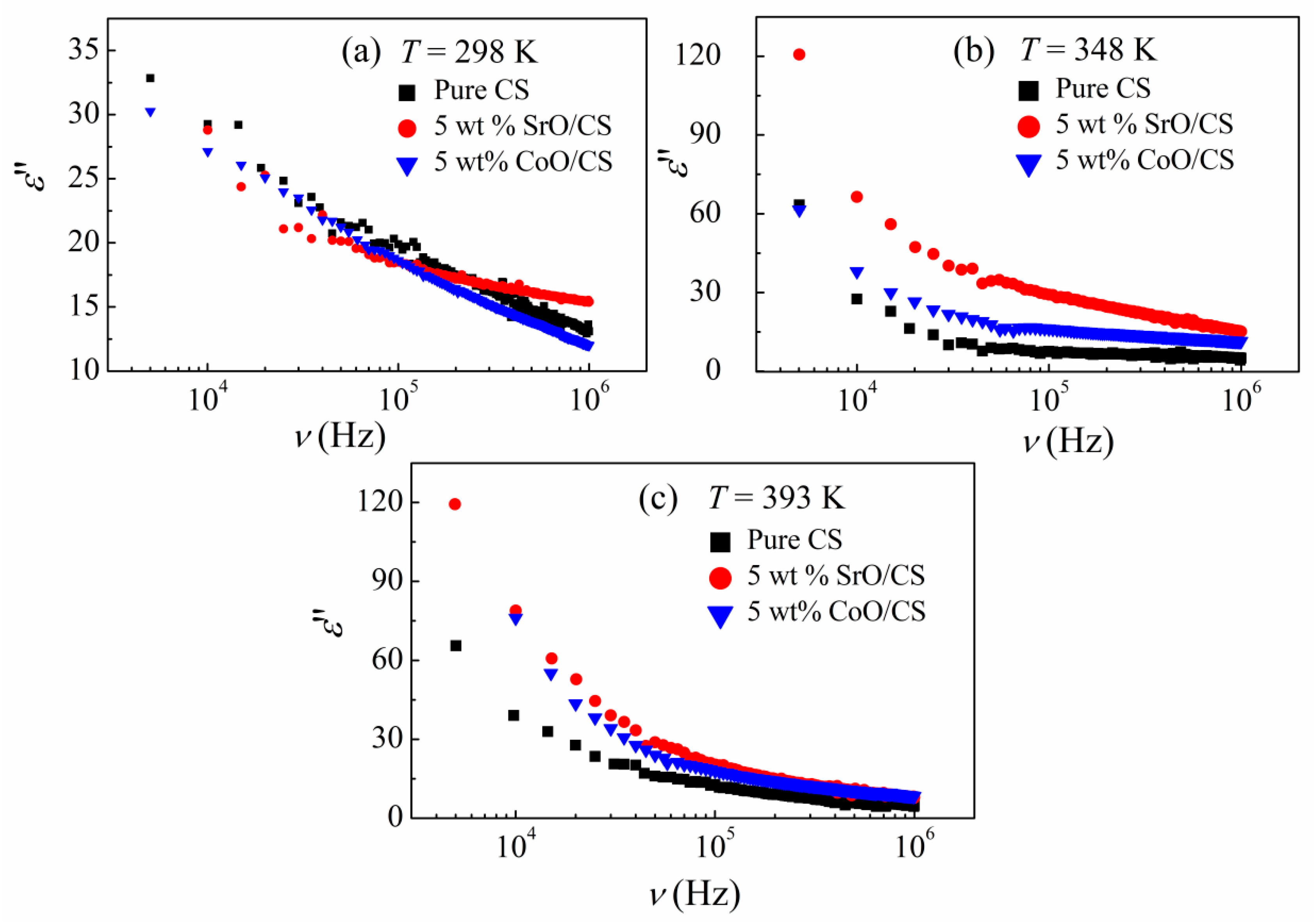
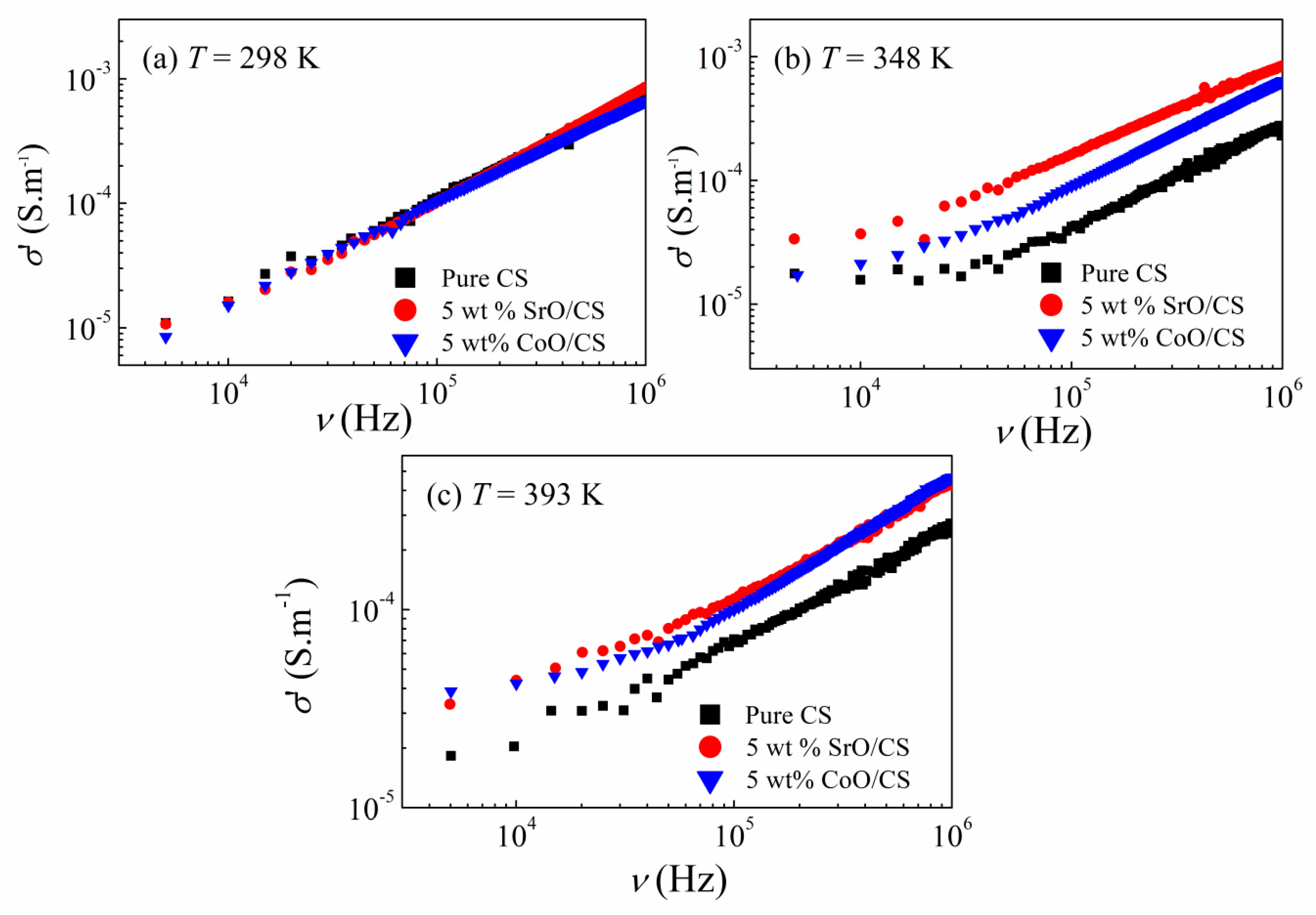
3.2. Dielectric Properties
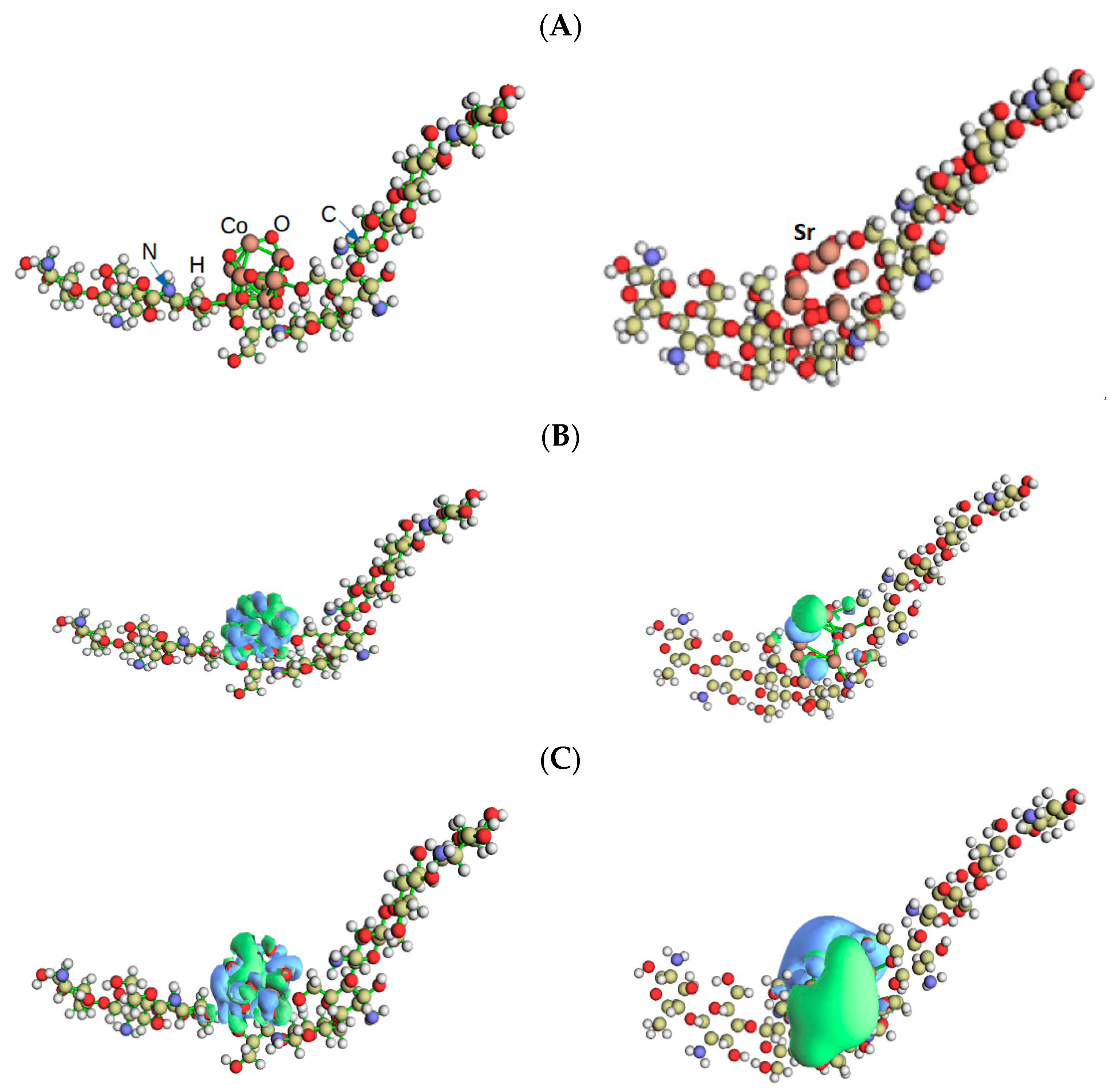
3.3. Tight-Binding Calculations of HOMO and LUMO and Other Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Adil, S.F.; Assal, M.E.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Liz-Marzán, L.M. Biogenic synthesis of metallic nanoparticles and prospects toward green chemistry. Dalton Trans. 2015, 44, 9709–9717. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Matsui, I. Nanoparticles for Electronic Device Applications: A Brief Review. J. Chem. Eng. Jpn. 2005, 38, 535–546. [Google Scholar] [CrossRef]
- Mohamed, A.T. Emerging Nanotechnology Applications in Electrical Engineering, 1st ed.; IGI Global Publisher of Timely Knowledge: Hershey, PA, USA, 2021; pp. 15–38. ISBN 9781799885368. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Wu, L.Q.; Gadre, A.P.; Yi, H.; Kastantin, M.J.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Voltage-dependent assembly of the polysaccharide chitosan onto an electrode surface. Langmuir 2002, 18, 8620–8625. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Khalil, K.D.; Bashal, A.H. Structural Properties and Catalytic Activity of Binary Poly (vinyl alcohol)/Al2O3 Nanocomposite Film for Synthesis of Thiazoles. Catalysts 2020, 10, 100. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Mollarasouli, F. Bismuth and Bismuth-Chitosan modified electrodes for determination of two synthetic food colorants by net analyte signal standard addition method. Cent. Eur. J. Chem. 2014, 12, 711–718. [Google Scholar] [CrossRef]
- Zhang, C.; Chi, Q.; Dong, J.; Cui, Y.; Wang, X.; Liu, L.; Lei, Q. Enhanced dielectric properties of poly (vinylidene fluoride) composites filled with nano iron oxide-deposited barium titanate hybrid particles. Sci. Rep. 2016, 6, 33508. [Google Scholar] [CrossRef]
- Bilkan, C.; Altındal, S.; Azizian-Kalandaragh, Y. Investigation of frequency and voltage dependence surface states and series resistance profiles using admittance measurements in Al/p-Si with Co3O4-PVA interlayer structures. Phys. B 2017, 515, 28–33. [Google Scholar] [CrossRef]
- Guo, P.; Yuan, S.; Guo, B.; Li, S.; Gao, Y. Chitosan-derived carbon supported CoO combined with CdS facilitates visible light catalytic hydrogen evolution. Catal. Sci. Technol. 2023, 13, 1128–1139. [Google Scholar] [CrossRef]
- Okla, M.; Kokilavani, S.; Mohebaldin, A.; Thomas, A.; Soufan, W.; Abdel-Maksoud, M.; Abdelgawad, H.; Raju, L.; Khan, S. Ag decorated CoO NPs supported on chitosan matrix for colorimetric detection of L-cysteine, antibacterial application and photocatalytic reduction of hexavalent chromium ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128318. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, C.; Liang, G.; Gu, A.; Wang, W. Polyester-imide solventless impregnating resin and its nano-silica modified varnishes with excellent corona resistance and thermal stability. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 372–379. [Google Scholar] [CrossRef]
- Okuzumi, S.; Murakami, Y.; Nagao, M.; Sekiguchi, Y.; Reddy, C.C.; Murata, Y. DC Breakdown Strength and Conduction Current of MgO/LDPE Composite Influenced by Filler Size. In Proceedings of the Conference on Electrical Insulation and Dielectric Phenomena, Quebec, QC, Canada, 26–29 October 2008; pp. 722–725. [Google Scholar]
- Zazoum, B.; Frechette, M.; David, E. LDPE/TiO2 nanocomposites: Effect of poss on structure and dielectric properties. IEEE Trans. Dielectr. Electr. Insul. 2016, 3, 2505–2507. [Google Scholar] [CrossRef]
- Katayama, J.; Ohki, Y.; Fuse, N.; Kozako, M.; Tanaka, T. Effects of nanofiller materials on the dielectric properties of epoxy nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 157–165. [Google Scholar] [CrossRef]
- Jeong, J.W.; Hwang, H.S.; Choi, D.; Ma, B.C.; Jung, J.; Chang, M. Hybrid Polymer/Metal Oxide Thin Films for High Performance, Flexible Transistors. Micromachines 2020, 11, 264. [Google Scholar] [CrossRef]
- Ben Amor, I.; Hemmami, H.; Laouini, S.E.; Mahboub, M.S.; Barhoum, A. Sol-Gel Synthesis of ZnO Nanoparticles Using Different Chitosan Sources: Effects on Antibacterial Activity and Photocatalytic Degradation of AZO Dye. Catalysts 2022, 12, 1611. [Google Scholar] [CrossRef]
- Mondal, K. Recent Advances in the Synthesis of Metal Oxide Nanofibers and Their Environmental Remediation Applications. Inventions 2017, 2, 9. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Khalil, K.D.; Bashal, A.H. Chitosan Capped Copper Oxide Nanocomposite: Efficient, Recyclable, Heterogeneous Base Catalyst for Synthesis of Nitroolefins. Catalysts 2022, 12, 964. [Google Scholar] [CrossRef]
- Drasovean, R.; Condurache-Bota, S.; Tigau, N. Structural and electrical characterization of cobalt oxide semiconductors. J. Sci. Arts 2010, 2, 379–384. [Google Scholar]
- Bhargava, R.; Khan, S.; Ahmad, N.; Ansari, M.M.N. Investigation of structural, optical and electrical properties of Co3O4 nanoparticles. AIP Conf. Proc. 2018, 1953, 030034. [Google Scholar] [CrossRef]
- Lampert, C.M.; Granqvist, C.G. (Eds.) Large Area Chromogenics: Material and Devices for Transmittance Control; SPIE IS 4; Optical Engineering Press: Bellingham, WA, USA, 1990; p. 2. [Google Scholar]
- Alimuddin, A.; Rafeeq, M. Synthesis and Characterization of Strontium Oxide Nano Particle by Sol-Gel Method. Orient. J. Chem. 2021, 37, 177–180. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Mehl, R.F.; Medalist, A. The role of nanosized particles. A frontier in modern materials science, from nanoelectronics to environmental problems. Metall. Mater. Trans. A 1998, 29, 713–725. [Google Scholar] [CrossRef]
- Atkins, P.W.; Overton, T.L.; Rourke, J.P.; Weller, M.T.; Armstrong, F.A. The structures of simple solids. In Shiver & Atkins’ Inorganic Chemistry, 4th ed.; McGraw Hill: New York, NY, USA, 2008; p. 84. [Google Scholar]
- Tiwary, R.; Narayan, S.; Pandey, O. Preparation of strontium oxide from celestite: A review. Mater. Sci. 2007, 3, 201–211. [Google Scholar]
- Athar, T. Synthesis and Characterization of Strontium Oxide Nanoparticles via Wet Process. Mater. Focus 2013, 2, 450–453. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Alkayal, N.S.; Bashal, A.H.; Alharbi, K.H.; Alharbi, W. Chitosan-Strontium Oxide Nanocomposite: Preparation, Characterization, and Catalytic Potency in Thiadiazoles Synthesis. Polymers 2022, 14, 2827. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Singh, A.K.; Jyoti, K. Review article on 1,3,4-thiadiazole derivatives and its pharmacological activities. Int. J. Chem. Technol. Res. 2011, 3, 1380–1393. [Google Scholar]
- Vernardou, D.; Kazas, A.; Apostolopoulou, M.; Katsarakis, N.; Koudoumas, E. Cationic Effect on the Electrochemical Characteristics of the Hydrothermally Grown Manganese Dioxide. J. Electron. Mater. 2017, 46, 2232–2240. [Google Scholar] [CrossRef]
- Mallahi, M.; Shokuhfar, A.; Vaezi, M.R.; Esmaeilirad, A.; Mazinani, V. Synthesis and characterization of Bismuth oxide nanoparticles via sol-gel method. Am. J. Eng. Res. 2014, 3, 162–165. [Google Scholar]
- Zheng, C.; Cao, C.; Ali, Z.; Hou, J. Enhanced electrochemical performance of ball milled CoO for supercapacitor applications. J. Mater. Chem. A 2014, 2, 16467–16473. [Google Scholar] [CrossRef]
- Wang, S.; Chen, M.; Xie, Y.; Fan, Y.; Wang, D.; Jiang, J.-J.; Grützmacher, H.; Su, C.-Y. Nanoparticle Cookies Derived from Metal-Organic Frameworks: Controlled Synthesis and Application in Anode Materials for Lithium-Ion Batteries. Small 2016, 12, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Naor, E.O.; Koberg, M.; Gedanken, A. Nonaqueous synthesis of SrO nanopowder and SrO/SiO2 composite and their application for biodiesel production via microwave irradiation. Renew. Energy 2017, 101, 493–499. [Google Scholar] [CrossRef]
- Narmani, A.; Jafari, S.M. Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydr. Polym. 2021, 272, 118464. [Google Scholar] [CrossRef]
- Fu, S.; Yang, R.; Zhang, L.; Liu, W.; Du, G.; Cao, Y.; Xu, Z.; Cui, H.; Kang, Y.; Peng, X. Biomimetic CoO@AuPt nanozyme responsive to multiple tumor microenvironmental clues for augmenting chemodynamic therapy. Biomaterials 2020, 257, 120279. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.D.; Ibrahim, E.I.; Al-Sagheer, F.A. A novel, efficient, and recyclable biocatalyst for Michael addition reactions and its iron(iii) complex as promoter for alkyl oxidation reactions. Catal. Sci. Technol. 2016, 6, 1410–1416. [Google Scholar] [CrossRef]
- Aljuhani, A.; Riyadh, S.M.; Khalil, K.D. Chitosan/CuO nanocomposite films mediated regioselective synthesis of 1,3,4-trisubstituted pyrazoles under microwave irradiation. J. Saud. Chem. Soc. 2021, 25, 101276. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3]triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef]
- Allaedini, G.; Muhammad, A. Study of influential factors in synthesis and characterization of cobalt oxide nanoparticles. J. Nanostruct. Chem. 2013, 3, 77. [Google Scholar] [CrossRef]
- Cai, H.; Wei, Q.; Xiao, H.; Liu, H.; Wang, J. Preparation and microwave absorption properties of petal CoO/CNFs composites. J. Mater. Sci. Mater. Electron. 2020, 31, 7606–7615. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kimura, S.; Wada, M.; Kuga, S. Crystal analysis and high-resolution imaging of microfibrillar α-chitin from Phaeocystis. J. Struct. Biol. 2010, 171, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of Chitin Hydrogel Under Mild Conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Deori, K.; Deka, S. Morphology oriented surfactant dependent CoO and reaction time dependent Co3O4 nanocrystals from single synthesis method and their optical and magnetic properties. CrystEngComm 2013, 15, 8465. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Dash, S.K.; Kar Mahapatra, S.; Tripathy, S.; Ghosh, T.; Das, B.; Pramanik, P.; Roy, S. Chitosan-modified cobalt oxide nanoparticles stimulate TNF-α-mediated apoptosis in human leukemic cells. JBIC J. Biol. Inorg. Chem. 2014, 19, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, H.; Li, Y.; Zhao, M. Porous Co3O4 column as a high-performance Lithium anode material. J. Porous Mater. 2021, 28, 889–894. [Google Scholar] [CrossRef]
- Suresh, R.; Indira Priyadharshini, T.; Thirumal Valavan, K.; Justin Paul, M. Role of annealing temperature on the properties of SrO nanoparticles by precipitation method. NanoNEXT 2020, 1, 18–23. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. (Eds.) Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003; pp. 35–57. ISBN 978-3-642-56120-7. [Google Scholar]
- El Sayed, A.M.; Mohamad, A.D.M. Synthesis, structural, thermal, optical and dielectric properties of chitosan biopolymer; influence of PVP and α-Fe2O3 Nanorods. J. Polym. Res. 2018, 25, 175. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Krohns, S.; Lunkenheimer, J.; Lu, P.; Brize, V.; Autret-Lambert, C.; Gervais, M.; Gervais, F.; Bouree, F.; Porcher, F.; Loidl, A. Correlations of structural, magnetic, and dielectric properties of undoped and doped CaCu3Ti4O12. Eur. Phys. J. B 2009, 72, 173–182. [Google Scholar] [CrossRef]
- Wu, J.; Nan, C.-W.; Lin, Y.; Deng, Y. Giant dielectric permittivity observed in Li and Ti doped NiO. Phys. Rev. Lett. 2002, 89, 217601. [Google Scholar] [CrossRef]
- Alshammari, F.H. Physical characterization and dielectric properties of chitosan incorporated by zinc oxide and graphene oxide nanoparticles prepared via laser ablation route. J. Mater. Res. Technol. 2022, 20, 740–747. [Google Scholar] [CrossRef]
- Neagu, A.M.; Curecheriu, L.P.; Cazacu, A.; Mitoseriu, L. Impedance analysis and tunability of BaTiO3–chitosan composites: Towards active dielectrics for flexible electronics. Compos. Part B 2014, 66, 109–116. [Google Scholar] [CrossRef]
- Bhatt, S.; Bhat, D.K.; Santosh, M.S. Electrochemical properties of chitosan–Co3O4 nanocomposite films. J. Electroanal. Chem. 2011, 657, 135–143. [Google Scholar] [CrossRef]
- Almond, D.P.; Hunter, C.C.; West, A.R. The extraction of ionic conductivities and hopping rates from a.c. conductivity data. J. Mater. Sci. 1984, 19, 3236. [Google Scholar] [CrossRef]
- Jonscher, A.K. The ‘universal’ dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Elliot, S.R. A.c. conduction in amorphous chalcogenide and pnictide semiconductors. Adv. Phys. 1987, 36, 135–217. [Google Scholar] [CrossRef]
- Pike, G.E. AC Conductivity of Scandium Oxide and a New Hopping Model for Conductivity. Phys. Rev. B 1972, 6, 1572–1579. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Viscosity–molecular weight and viscosity–temperature relationship for polystyrene and polyisobutylene. J. Am. Chem. Soc. 1948, 70, 2384–2395. [Google Scholar] [CrossRef]
- Lewis, T.J. Charge transport, charge injection and breakdown in polymeric insulators. J. Phys. D Appl. Phys. 1990, 23, 1469. [Google Scholar] [CrossRef]
- Teyssedre, G.; Laurent, C. Charge transport modeling in insulating polymers: From molecular to macroscopic scale. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 857. [Google Scholar] [CrossRef]
- Mujeeb Rahman, P.; Abdul Mujeeb, V.M.; Muraleedharan, K.; Thomas, S.K. Chitosan/nano ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Arab. J. Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Abou Elfadl, A.; El Sayed, A.M.; Ibrahim, I.M. Improving the optical, dielectric properties and antimicrobial activity of Chitosan–PEO by GO/MWCNTs: Nanocomposites for energy storage and food packaging applications. Polymer 2023, 267, 125650. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfadl, A.A.; Bashal, A.H.; Habeeb, T.H.; Khalafalla, M.A.H.; Alkayal, N.S.; Khalil, K.D. Preparation, Characterization, Dielectric Properties, and AC Conductivity of Chitosan Stabilized Metallic Oxides CoO and SrO: Experiments and Tight Binding Calculations. Polymers 2023, 15, 4132. https://doi.org/10.3390/polym15204132
Elfadl AA, Bashal AH, Habeeb TH, Khalafalla MAH, Alkayal NS, Khalil KD. Preparation, Characterization, Dielectric Properties, and AC Conductivity of Chitosan Stabilized Metallic Oxides CoO and SrO: Experiments and Tight Binding Calculations. Polymers. 2023; 15(20):4132. https://doi.org/10.3390/polym15204132
Chicago/Turabian StyleElfadl, Azza Abou, Ali H. Bashal, Talaat H. Habeeb, Mohammed A. H. Khalafalla, Nazeeha S. Alkayal, and Khaled D. Khalil. 2023. "Preparation, Characterization, Dielectric Properties, and AC Conductivity of Chitosan Stabilized Metallic Oxides CoO and SrO: Experiments and Tight Binding Calculations" Polymers 15, no. 20: 4132. https://doi.org/10.3390/polym15204132
APA StyleElfadl, A. A., Bashal, A. H., Habeeb, T. H., Khalafalla, M. A. H., Alkayal, N. S., & Khalil, K. D. (2023). Preparation, Characterization, Dielectric Properties, and AC Conductivity of Chitosan Stabilized Metallic Oxides CoO and SrO: Experiments and Tight Binding Calculations. Polymers, 15(20), 4132. https://doi.org/10.3390/polym15204132







