Supramolecular Responsive Chitosan Microcarriers for Cell Detachment Triggered by Adamantane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Chitosan Microspheres
2.3. Preparation of Cyclodextrin-Grafted Chitosan Microspheres (CSM-g-CD)
2.4. Preparation of Gelatin-Modified Responsive Microspheres (CSM-g-CD-Gel)
2.5. Physical Characterization of Chitosan Microspheres
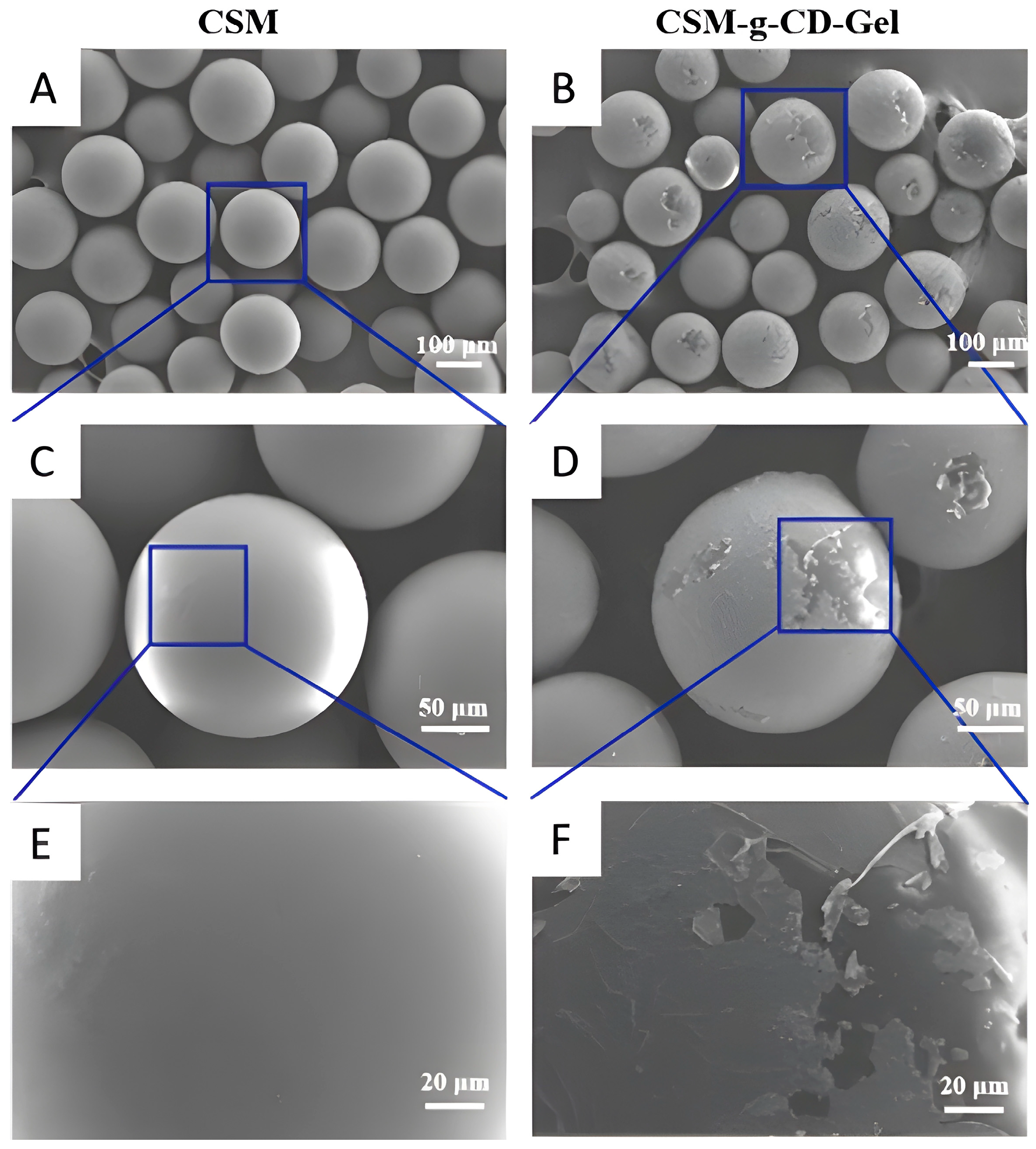
2.5.1. SEM
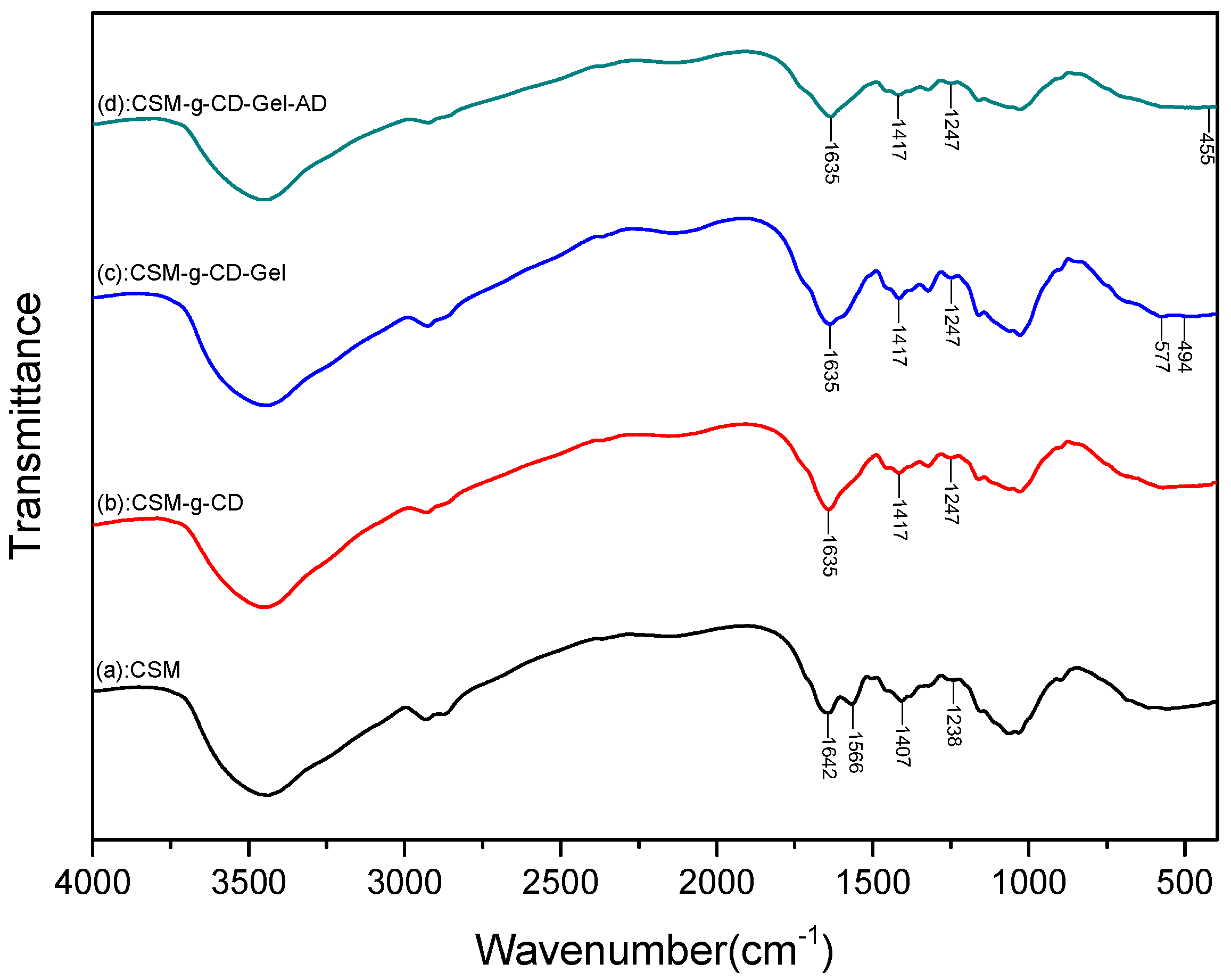
2.5.2. FTIR
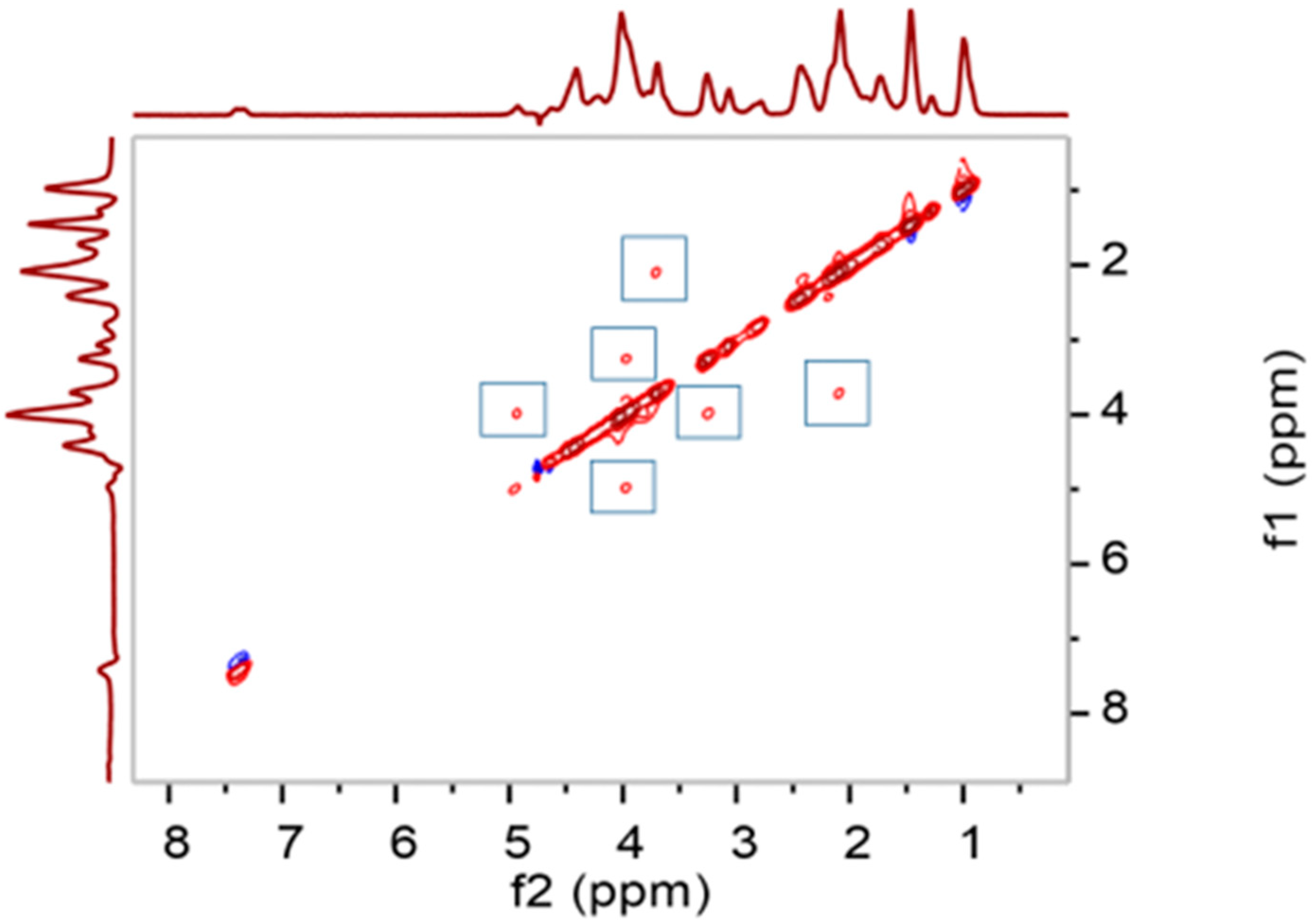
2.5.3. 2D NMR
2.5.4. Cytocompatibility Evaluation
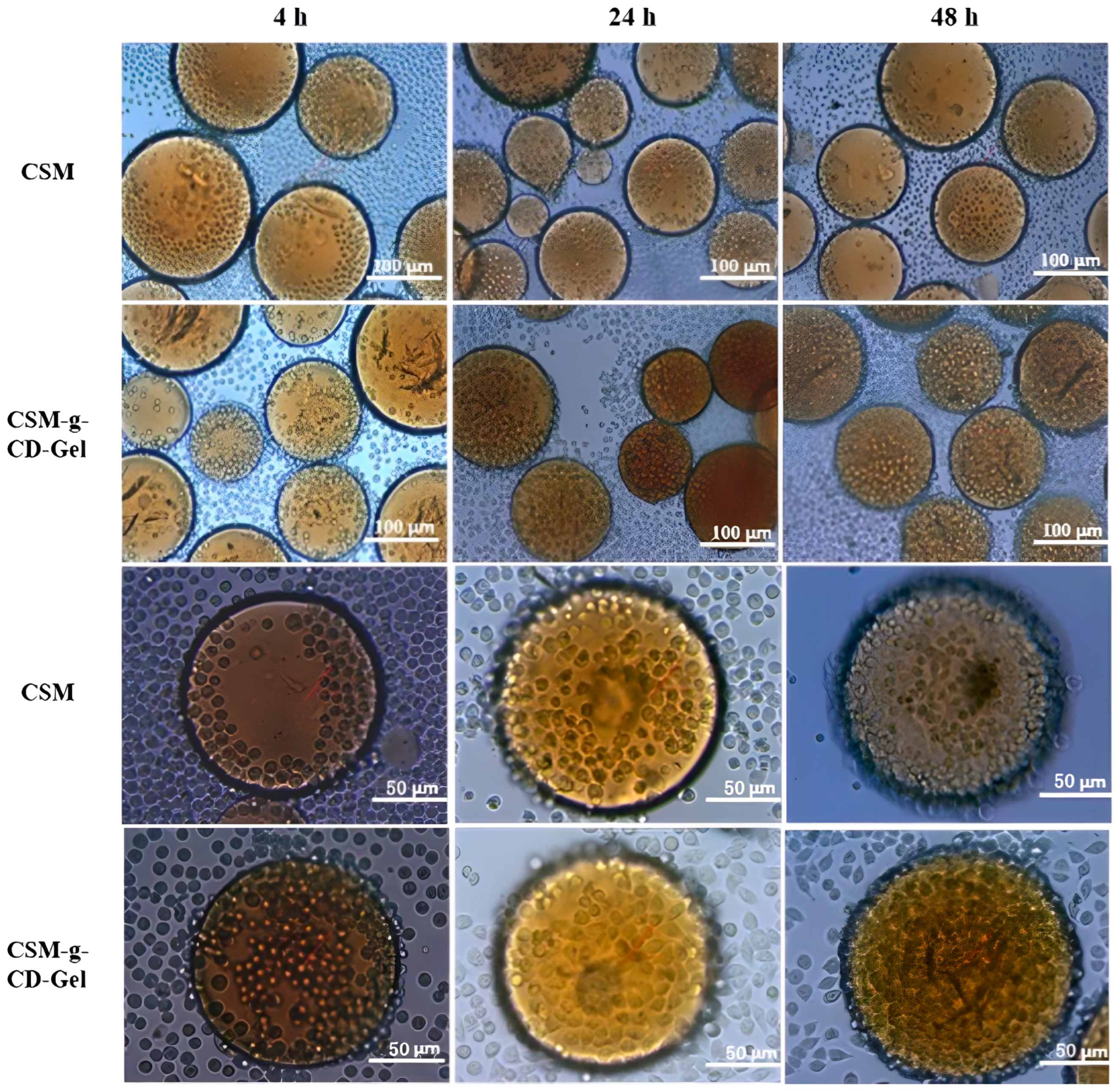
2.5.5. Cell Adhesion and Distribution on Microspheres
2.5.6. Responsive Detachment of Cells from CSM-g-CD-Gel Microspheres
2.6. Statistical Analysis
3. Results and Discussion
3.1. Morphological Analysis of the Microspheres
3.2. FTIR Analysis of the Microspheres
3.3. 2D NMR Analysis
3.4. Cytocompatibility
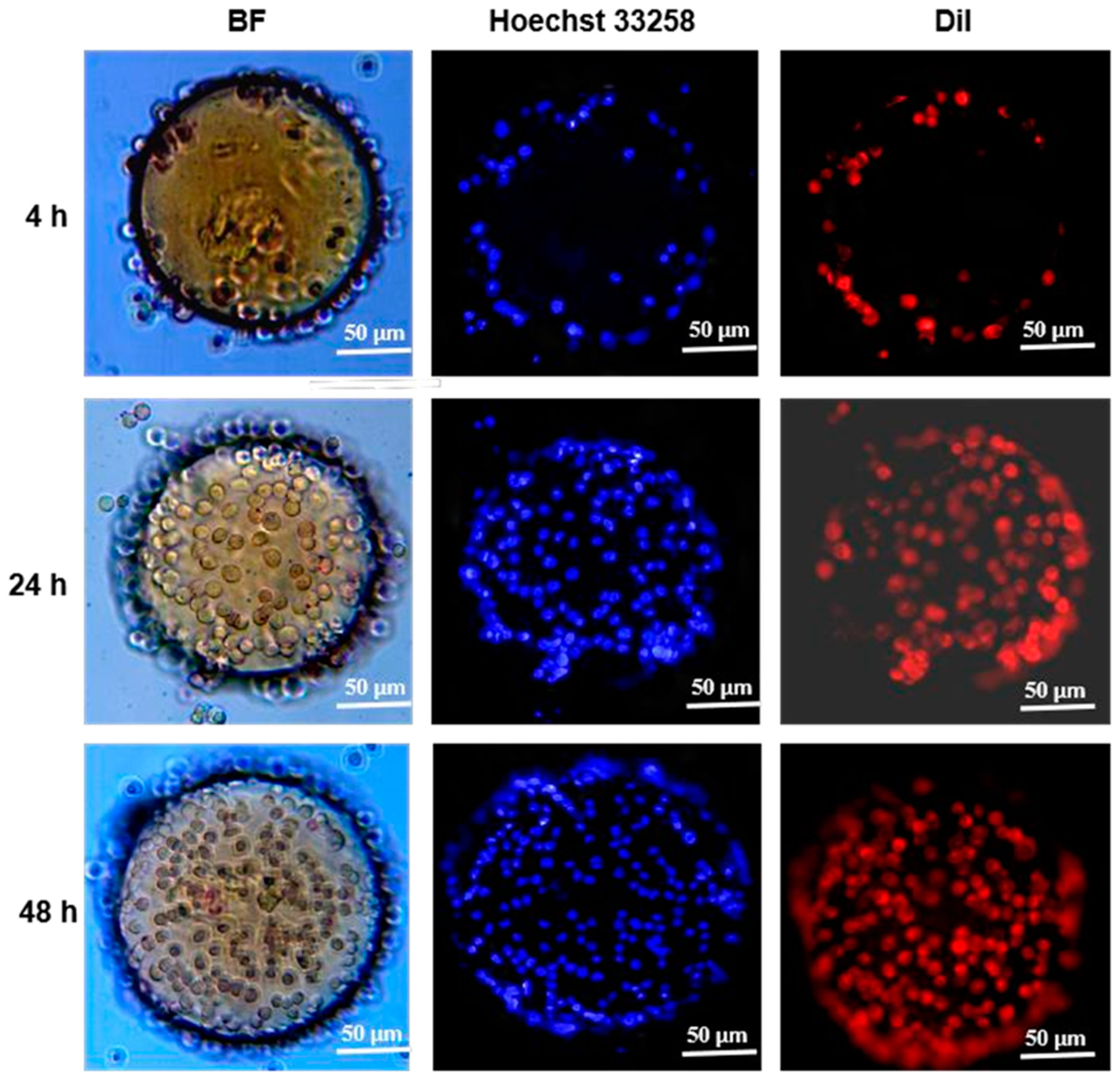
3.5. Cell Adhesion and Distribution on CSM-g-CD-Gel
3.6. Responsive Detachment of Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Xiao, L.; Jung Poudel, A.; Li, J.; Zhou, P.; Gauthier, M.; Liu, H.; Wu, Z.; Yang, G. Porous chitosan microspheres as microcarriers for 3D cell culture. Carbohydr. Polym. 2018, 202, 611–620. [Google Scholar] [CrossRef]
- Huang, L.; Abdalla, A.M.E.; Xiao, L.; Yang, G. Biopolymer-based microcarriers for three-dimensional cell culture and engineered tissue formation. Int. J. Mol. Sci. 2020, 21, 1895. [Google Scholar]
- Chen, X.; Chen, J.; Tong, X.; Mei, J.; Chen, Y.; Mou, X. Recent advances in the use of microcarriers for cell cultures and their ex vivo and in vivo applications. Biotechnol. Lett. 2020, 42, 1–10. [Google Scholar]
- Wei, F.; Zhang, X.; Cui, P.; Gou, X.; Wang, S. Cell-based 3D bionic screening by mimicking the drug-receptor interaction environment in vivo. J. Mater. Chem. B. Mater. Biol. Med. 2021, 3, 9. [Google Scholar] [CrossRef]
- Niederau, C.; Fronhoffs, K.; Klonowski, H.; Schulz, H.U. Active pancreatic digestive enzymes show striking differences in their potential to damage isolated rat pancreatic acinar cells. J. Lab. Clin. Med. 1995, 125, 265–275. [Google Scholar]
- Derakhti, S.; Safiabadi-Tali, S.H.; Amoabediny, G.; Sheikhpour, M. Attachment and detachment strategies in microcarrier-based cell culture technology: A comprehensive review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109782. [Google Scholar]
- Dabiri, S.M.H.; Samiei, E.; Shojaei, S.; Karperien, L.; Khun Jush, B.; Walsh, T.; Jahanshahi, M.; Hassanpour, S.; Hamdi, D.; Seyfoori, A.; et al. Multifunctional Thermoresponsive Microcarriers for High-Throughput Cell Culture and Enzyme-Free Cell Harvesting. Small 2021, 17, 2103192. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Zhao, Z.; Sun, L.; Zou, M.; Ren, J.; Zhao, Y. Responsive graphene oxide hydrogel microcarriers for controllable cell capture and release. Sci. China Mater. 2018, 61, 1314–1324. [Google Scholar] [CrossRef]
- Li, X.; Ji, X.; Chen, K.; Ullah, M.W.; Li, B.; Cao, J.; Xiao, L.; Xiao, J.; Yang, G. Immobilized thrombin on X-ray radiopaque polyvinyl alcohol/chitosan embolic microspheres for precise localization and topical blood coagulation. Bioact. Mater. 2021, 6, 2105–2119. [Google Scholar]
- Agarwal, T.; Chiesa, I.; Costantini, M.; Lopamarda, A.; Tirelli, M.C.; Borra, O.P.; Varshapally, S.V.S.; Kumar, Y.A.V.; Koteswara Reddy, G.; De Maria, C.; et al. Chitosan and its derivatives in 3D/4D (bio) printing for tissue engineering and drug delivery applications. Int. J. Biol. Macromol. 2023, 246, 125669. [Google Scholar]
- Ansari, M.T.; Murteza, S.; Ahsan, M.N.; Hasnain, M.S.; Nayak, A.K. Chitosan as a responsive biopolymer in drug delivery. In Chitosan in Drug Delivery; Academic Press: Cambridge, MA, USA, 2022; pp. 389–410. [Google Scholar]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torre, R.; Rodrigue, R.; Fernandez-Lafuent, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Monsan, P. Optimization of glutaraldehyde activation of a support for enzyme immobilization. J. Mol. Catal Chem. 1978, 3, 371–384. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, Z.; Field, R.; Moloney, M.G.; Rimmer, S.; Ye, H. Thermo-responsive microcarriers based on poly(N-isopropylacrylamide). Eur. Polym. J. 2015, 67, 346–364. [Google Scholar]
- Tamura, A.; Kobayashi, J.; Yamato, M.; Okano, T. Thermally responsive microcarriers with optimal poly(N-isopropylacrylamide) grafted density for facilitating cell adhesion/detachment in suspension culture. Acta Biomater. 2012, 8, 3904–3939. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Althumayri, K.; Fouda, A.; Hamad, N.A. Synthesis and Characterization of Functionalized Chitosan Nanoparticles with Pyrimidine Derivative for Enhancing Ion Sorption and Application for Removal of Contaminants. Materials. 2022, 15, 4676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Guo, J.; Huang, C.; Hu, Y. Polysaccharide supramolecular hydrogel microparticles based on carboxymethyl β-cyclodextrin/chitosan complex and EDTA-chitosan for controlled release of protein drugs. Polym. Bull. 2022, 79, 6087–6097. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-based hybrid scaffolds: Promising wound dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef] [PubMed]
- Niquini, F.M.; Moura, A.L.S.; Machado, P.H.; Oliveira, K.M.; Correa, R.S. Synthesis, infrared and molecular structure of adamantane-1-ammonium picrate monohydrate: A derivative of the antiviral symmetrel. Crystallogr. Rep. 2020, 65, 879–884. [Google Scholar] [CrossRef]
- Sugane, K.; Maruoka, Y.; Shibata, M. Self-healing epoxy networks based on cyclodextrin–adamantane host–guest interactions. J. Polym. Res. 2021, 28, 423. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, W.; Huang, L.; Liu, J.; Yang, G. Nanocomposite pastes of gelatin and cyclodextrin-grafted chitosan nanoparticles as potential postoperative tumor therapy. Adv. Compos. Hybrid. Mater. 2023, 6, 15. [Google Scholar] [CrossRef]
- Liang, J.; Guo, Z.; Timmerman, A.; Grijpma, D.; Poot, A. Enhanced mechanical and cell adhesive properties of photo-crosslinked PEG hydrogels by incorporation of gelatin in the networks. Biomed. Mater. 2019, 14, 024102. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q. Targeting integrin pathways: Mechanisms and advances in therapy. Sig. Transduct. Target. Ther. 2023, 8, 1. [Google Scholar]
- Boraschi-Diaz, I.; Wang, J.; Mort, J.; Komarova, S. Collagen Type I as a Ligand for Receptor-Mediated Signaling. Front. Phys. 2017, 5, 12. [Google Scholar]
- Azreq, M.A.E.; Naci, D.; Aoujit, F. Collagen/β1 integrin signaling up-regulates the ABCC1/MRP-1 transporter in an ERK/MAPK-dependent manner. Mol. Biol. Cell. 2012, 23, 3437–3684. [Google Scholar] [CrossRef]
- Zaman, M.H. Understanding the Molecular Basis for Differential Binding of Integrins to Collagen and Gelatin. Biophys. J. 2007, 92, 17–19. [Google Scholar]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Jiang, Y.; Chen, X.; Zhang, W.; Luo, Q.; Chen, S.; Wang, S.; Weng, F.; Xiao, L. Supramolecular Responsive Chitosan Microcarriers for Cell Detachment Triggered by Adamantane. Polymers 2023, 15, 4024. https://doi.org/10.3390/polym15194024
Huang L, Jiang Y, Chen X, Zhang W, Luo Q, Chen S, Wang S, Weng F, Xiao L. Supramolecular Responsive Chitosan Microcarriers for Cell Detachment Triggered by Adamantane. Polymers. 2023; 15(19):4024. https://doi.org/10.3390/polym15194024
Chicago/Turabian StyleHuang, Lixia, Yifei Jiang, Xinying Chen, Wenqi Zhang, Qiuchen Luo, Siyan Chen, Shuhan Wang, Fangqing Weng, and Lin Xiao. 2023. "Supramolecular Responsive Chitosan Microcarriers for Cell Detachment Triggered by Adamantane" Polymers 15, no. 19: 4024. https://doi.org/10.3390/polym15194024
APA StyleHuang, L., Jiang, Y., Chen, X., Zhang, W., Luo, Q., Chen, S., Wang, S., Weng, F., & Xiao, L. (2023). Supramolecular Responsive Chitosan Microcarriers for Cell Detachment Triggered by Adamantane. Polymers, 15(19), 4024. https://doi.org/10.3390/polym15194024






