Chitosan Coatings Modified with Nanostructured ZnO for the Preservation of Strawberries
Abstract
1. Introduction
2. Materials and Methods
2.1. Chitosan Synthesis
2.2. Infrared Spectroscopy Characterization
2.3. Synthesis of Nanostructured ZnO Using the Close-Spaced Sublimation Method
2.4. Preparation and Application of the Coating
2.5. Characterization of the Coating
2.6. Chitosan and Ch/ZnO-NPS Coating Functionality in the Preservation of Strawberries
2.6.1. Colorimetric Test
2.6.2. Texture Test
2.6.3. pH, Titratable Acidity, Soluble Solids, and Humidity Content Determination
2.6.4. Aerobic Mesophilic Bacteria, Fungi, and Yeast
2.7. Statistical Analysis
3. Results
3.1. X-ray Diffraction
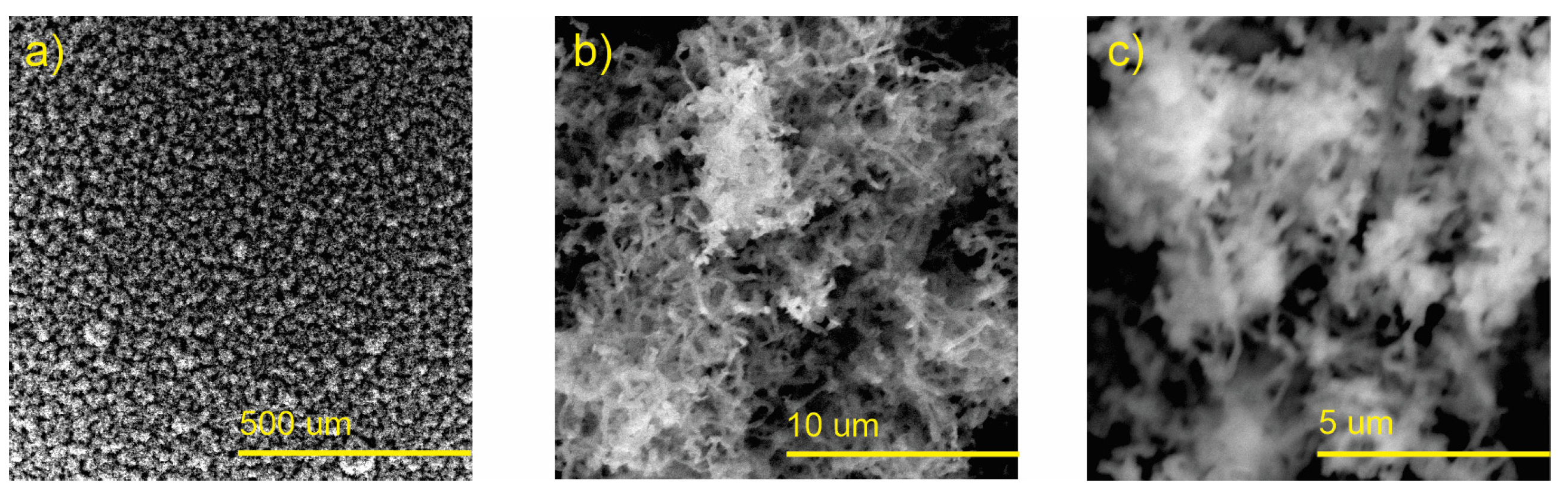
3.2. Scanning Electron Microscopy
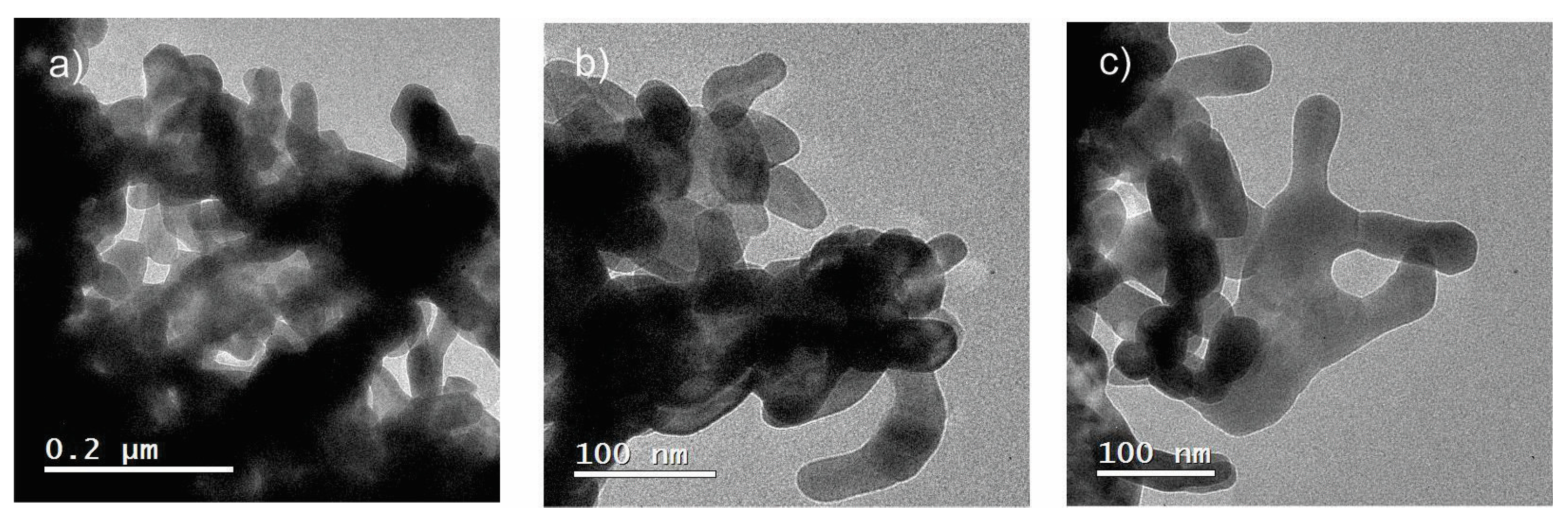
3.3. Transmission Electron Microscopy
3.4. Fourier Transform Infrared Spectroscopy Analysis
3.5. Energy Dispersive Spectroscopy Analysis
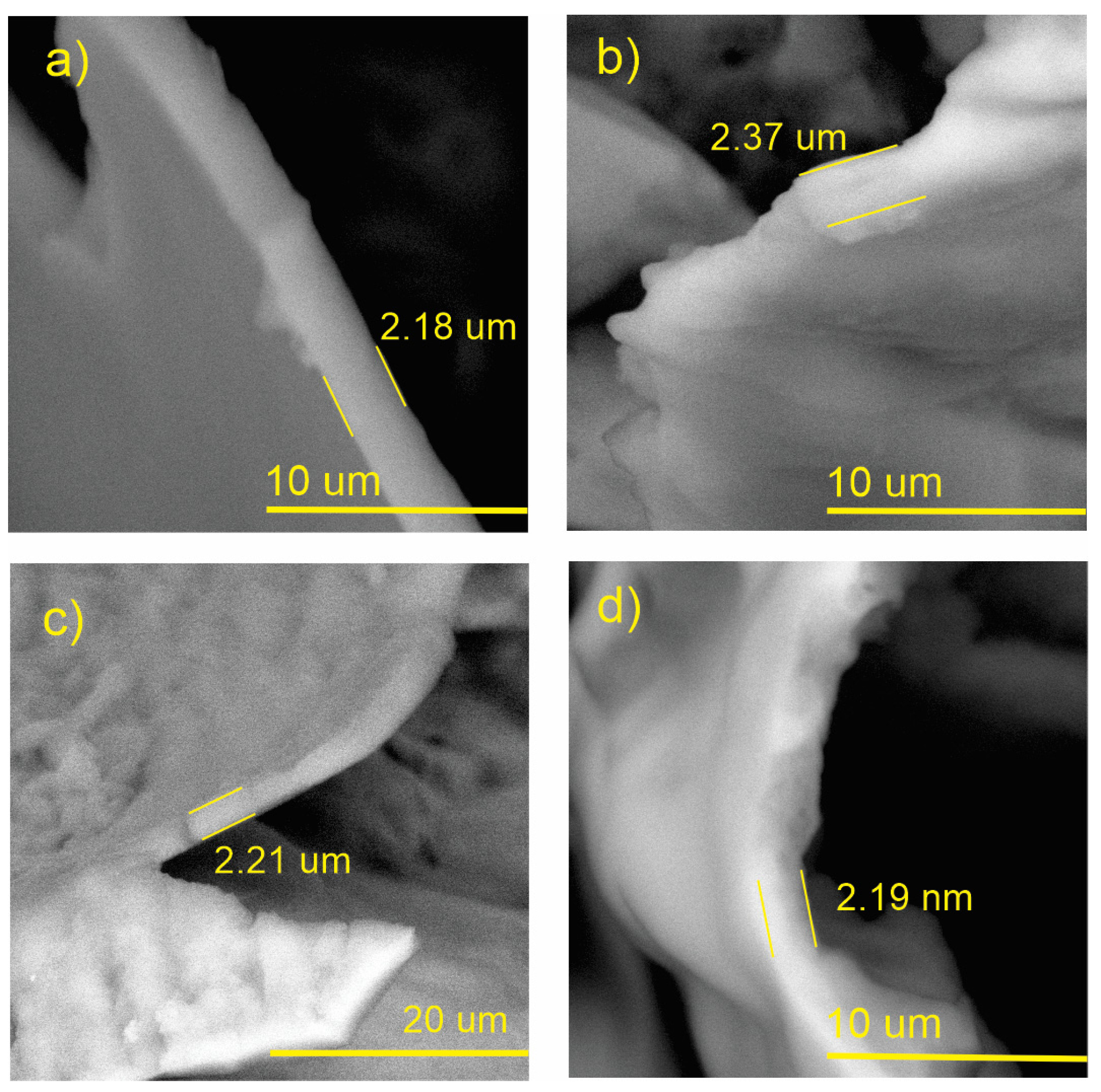
3.6. Coating Thickness
3.7. Functionality of the Ch/ZnO-NSs Coating in the Preservation of Strawberries
3.8. Microbiological Tests
3.9. Moisture Content
3.10. Texture Test Analysis
3.11. pH, Soluble Solids, and Titratable Acidity Analysis
3.12. Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: A review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Z.; Wang, R.; Liu, L.; Zhang, J.; Ma, F.; Khan, Z.H.; Zhao, D.; Liu, X. Characterization and antibacterial activity of edible films based on carboxymethyl cellulose, Dioscorea opposita mucilage, glycerol and ZnO nanoparticles. Food Chem. 2021, 349, 129208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Wang, Z.; Cai, R.; Yue, T.; Cui, L. Preparation and characterization of chitosan–nano-zno composite films for preservation of cherry tomatoes. Foods 2021, 10, 3135. [Google Scholar] [CrossRef] [PubMed]
- Ecuyer, J.L.; Audet, N.; Shink, D.; Triboulet, R.; Benz, K.W.; Fiederle, M.; Lincot, D.; Tomashik, V.N.; Tomashik, Z.F. Crystal Growth and Surfaces. In CdTe and Related Compounds; Physics, Defects, Hetero- and Nano-Structures, Crystal Growth, Surfaces and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; pp. 1–144. [Google Scholar]
- Espitia, P.J.P.; Batista, R.A.; Otoni, C.G.; Soares, N.F.F. Antimicrobial Food Packaging Incorporated with Triclosan: Potential Uses and Restrictions. In Antimicrobial Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 417–423. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Guo, J.; Gu, A.Z.; Li, D.; Chen, J. Toxicity assessment of nano-ZnO exposure on the human intestinal microbiome, metabolic functions, and resistome using an in vitro colon simulator. Environ. Sci. Technol. 2021, 55, 6884–6896. [Google Scholar] [CrossRef]
- Rajan, M.; Anthuvan, A.J.; Muniyandi, K.; Kalagatur, N.K.; Shanmugam, S.; Sathyanarayanan, S.; Chinnuswamy, V.; Thangaraj, P.; Narain, N. Comparative Study of Biological (Phoenix loureiroi Fruit) and Chemical Synthesis of Chitosan-Encapsulated Zinc Oxide Nanoparticles and their Biological Properties. Arab. J. Sci. Eng. 2020, 45, 15–28. [Google Scholar] [CrossRef]
- Strand, S.P.; Issa, M.M.; Christensen, B.E.; Vårum, K.M.; Artursson, P. Tailoring of chitosans for gene delivery: Novel self-branched glycosylated chitosan oligomers with improved functional properties. Biomacromolecules 2008, 9, 3268–3276. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Jiang, W. Effects of chitosan coating on shelf life of cold-stored litchi fruit at ambient temperature. LWT 2005, 38, 757–761. [Google Scholar] [CrossRef]
- Sharma, S.; Barman, K.; Siddiqui, M.W. Chitosan: Properties and roles in postharvest quality preservation of horticultural crops. In Eco-Friendly Technology for Postharvest Produce Quality; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 269–296. [Google Scholar] [CrossRef]
- Folta, K.M.; Davis, T.M. Strawberry genes and genomics. Crit. Rev. Plant Sci. 2006, 25, 399–415. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Indumathi, M.P.; Saral Sarojini, K.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef]
- Thanh Huong, Q.T.; Hoai Nam, N.T.; Duy, B.T.; An, H.; Hai, N.D.; Kim Ngan, H.T.; Ngan, L.T.; Nhi, T.L.H.; Linh, D.T.Y.; Khanh, T.N.; et al. Structurally natural chitosan films decorated with Andrographis paniculata extract and selenium nanoparticles: Properties and strawberry preservation. Food Biosci. 2023, 53, 102647. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Karkar, B.; Patır, İ.; Eyüboğlu, S.; Şahin, S. Development of an edible active chitosan film loaded with Nigella sativa L. extract to extend the shelf life of grapes. Biocatal. Agric. Biotechnol. 2023, 50, 102708. [Google Scholar] [CrossRef]
- Kang, S.H.; Cha, H.J.; Jung, S.W.; Lee, S.J. Application of chitosan-ZnO nanoparticle edible coating to wild-simulated Korean ginseng root. Food Sci. Biotechnol. 2022, 31, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Lavinia, M.; Hibaturrahman, S.N.; Harinata, H.; Wardana, A.A. Antimicrobial activity and application of nanocomposite coating from chitosan and ZnO nanoparticle to inhibit microbial growth on fresh-cut papaya. Food Res. 2020, 4, 307–311. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Chelliah, R.; Oh, D.H. Development of antimicrobial edible coating based on modified chitosan for the improvement of strawberries shelf life. Food Sci. Biotechnol. 2019, 28, 1257–1264. [Google Scholar] [CrossRef]
- Vallejo-Domínguez, D.; Rubio-Rosas, E.; Aguila-Almanza, E.; Hernández-Cocoletzi, H.; Ramos-Cassellis, M.E.; Luna-Guevara, M.L.; Rambabu, K.; Manickam, S.; Munawaroh, H.S.H.; Show, P.L. Ultrasound in the deproteinization process for chitin and chitosan production. Ultrason. Sonochem. 2021, 72, 105417. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Guerrero-Beltrán, J.Á. Postharvest quality of peeled prickly pear fruit treated with acetic acid and chitosan. Postharvest Biol. Technol. 2014, 92, 139–145. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Zhang, J.; Gao, Y.; Hui, G. Physicochemical properties enhancement of Chinese kiwi fruit (Actinidia chinensis Planch) via chitosan coating enriched with salicylic acid treatment. J. Food Meas. Charact. 2017, 11, 184–191. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wu, W.; Yang, J.; Yang, Q. Preparation and characterization of chitosan/Nano-ZnO composite film with antimicrobial activity. Bioprocess Biosyst. Eng. 2021, 44, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Camacho Feria, D.M.; Caviedes Rubio, D.I.; Delgado, D.R. Tratamientos para la remoción de antibacteriales y agentes antimicrobiales presentes en aguas residuales. Logos Cienc. Y Tecnol. 2017, 9, 43–62. [Google Scholar] [CrossRef]
- Boura-Theodoridou, O.; Giannakas, A.; Katapodis, P.; Stamatis, H.; Ladavos, A.; Barkoula, N.M. Performance of ZnO/chitosan nanocomposite films for antimicrobial packaging applications as a function of NaOH treatment and glycerol/PVOH blending. Food Packag. Shelf Life 2020, 23, 100456. [Google Scholar] [CrossRef]
- Mote, V.D.; Purushotham, Y.; Dole, B.N. Williamson-Hall Analysis in Estimation of Lattice Strain in Nanometer-Sized ZnO Particles. 2012. Available online: http://www.jtaphys.com/content/2251-7235/6/1/6 (accessed on 7 January 2023).
- Priyadarshi, R.; Negi, Y.S. Effect of Varying Filler Concentration on Zinc Oxide Nanoparticle Embedded Chitosan Films as Potential Food Packaging Material. J. Polym. Environ. 2017, 25, 1087–1098. [Google Scholar] [CrossRef]
- Zahiri Oghani, F.; Tahvildari, K.; Nozari, M. Novel Antibacterial Food Packaging Based on Chitosan Loaded ZnO Nano Particles Prepared by Green Synthesis from Nettle Leaf Extract. J. Inorg. Organomet. Polym. Mater. 2021, 31, 43–54. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, D.; Ren, D.; Zeng, K.; Wu, X. Green synthesis of zinc oxide nanoparticles using Citrus sinensis peel extract and application to strawberry preservation: A comparison study. LWT 2020, 126, 109297. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Narasimha Rao, K.; Nanda, K.K. Effect of oxygen partial pressure on the growth of zinc micro and nanostructures. J. Cryst. Growth 2009, 311, 4329–4333. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A.; Hallaj, R. Influence of nano-ZnO on microbial growth, bioactive content and postharvest quality of strawberries during storage. Innov. Food Sci. Emerg. Technol. 2016, 35, 168–176. [Google Scholar] [CrossRef]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V. Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020; 616p. [Google Scholar]
- Varma, R.; Vasudevan, S. Extraction, characterization, and antimicrobial activity of chitosan from horse mussel modiolus modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of chitosan / zinc oxide nanoparticles stabilized by chitosan via microwave heating. Bull. Chem. React. Eng. Catal. 2019, 14, 450–458. [Google Scholar] [CrossRef]
- Chagas, P.M.B.; Caetano, A.A.; Tireli, A.A.; Cesar, P.H.S.; Corrêa, A.D.; Guimarães, I.D.R. Use of an Environmental Pollutant from Hexavalent Chromium Removal as a Green Catalyst in The Fenton Process. Sci. Rep. 2019, 9, 12819. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef]
- Saral Sarojini, K.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar] [CrossRef]
- Restrepo, J.I.; Aristizábal, I.D. Conservación de fresa (Fragaria x ananassa Duch cv. Camarosa) mediante la aplicación de recubrimientos comestibles de gel mucilaginoso de penca sábila (Aloe barbadensis Miller) y cera de carnaúba. Vitae 2010, 17, 252–263. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Effects of various antimicrobial polyvinyl alcohol/tea polyphenol composite films on the shelf life of packaged strawberries. LWT 2019, 113, 108297. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, S.M.; Lee, J.S.; Lee, Y.S. Development of antimicrobial polyolefin films containing lauroyl arginate and their use in the packaging of strawberries. J. Food Meas. Charact. 2017, 11, 1706–1716. [Google Scholar] [CrossRef]
- Lakshmeesha, T.R.; Murali, M.; Ansari, M.A.; Udayashankar, A.C.; Alzohairy, M.A.; Almatroudi, A.; Alomary, M.N.; Asiri, S.M.M.; Ashwini, B.; Kalagatur, N.K.; et al. Biofabrication of zinc oxide nanoparticles from Melia azedarach and its potential in controlling soybean seed-borne phytopathogenic fungi. Saudi J. Biol. Sci. 2020, 27, 1923–1930. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involve in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Sucharitha, K.V.; Beulah, A.M.; Ravikiran, K. Effect of chitosan coating on storage stability of tomatoes (Lycopersicon esculentum Mill). Int. Food Res. J. 2018, 25, 93–99. [Google Scholar]
- Emamifar, A.; Bavaisi, S. Nanocomposite coating based on sodium alginate and nano-ZnO for extending the storage life of fresh strawberries (Fragaria × ananassa Duch.). J. Food Meas. Charact. 2020, 14, 1012–1024. [Google Scholar] [CrossRef]
- Emamifar, A.; Mohammadizadeh, M. Preparation and Application of LDPE/ZnO Nanocomposites for Extending Shelf Life of Fresh Strawberries. Food Technol. Biotechnol. 2015, 53, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Pastor, C.; Sanchez-Gonzalez, L.; Marcilla, A.; Chiralt, A.; Cháfer, M.; Gonzalez-Martinez, C. Quality and safety of table grapes coated with hydroxypropylmethylcellulose edible coatings containing propolis extract. Postharvest Biol. Technol. 2011, 60, 64–70. [Google Scholar] [CrossRef]
- Sanaa, S.; Entsar, N.; Entsar, S. Effect of Edible Coating on Extending the Shelf Life and Quality of Fresh Cut Taro. Am. J. Food Technol. 2017, 12, 124–131. [Google Scholar] [CrossRef][Green Version]
- Hernández Suárez, M.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Chemical composition of tomato (Lycopersicon esculentum) from Tenerife, the Canary Islands. Food Chem. 2008, 106, 1046–1056. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.M.B.; Sargent, S.A. Physicochemical changes during strawberry development in the field compared with those that occur in harvested fruit during storage. J. Sci. Food Agric. 2006, 86, 180–190. [Google Scholar] [CrossRef]
- Varasteh, F.; Arzani, K.; Barzegar, M.; Zamani, Z. Pomegranate (Punica granatum L.) Fruit Storability Improvement Using Pre-storage Chitosan Coating Technique. J. Agric. Sci. Tech. 2017, 389–400, 54746420. [Google Scholar]
- Tanada-Palmu, P.S.; Grosso, C.R.F. Effect of edible wheat gluten-based films and coatings on refrigerated strawberry (Fragaria ananassa) quality. Postharvest Biol. Technol. 2005, 36, 199–208. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, L.; Hu, Y.; Zhu, Z.; Zhuang, C.; Zhao, Y.; Zhong, Y. The preservation performance of chitosan coating with different molecular weight on strawberry using electrostatic spraying technique. Int. J. Biol. Macromol. 2020, 151, 278–285. [Google Scholar] [CrossRef]
- Huber, D.J. Strawberry Fruit Softening: The Potential Roles of Polyuronides and Hemicelluloses. J. Food Sci. 1984, 49, 1310–1315. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Zamindar, N.; Paidari, S.; Ibrahim, S.A.; Mohammadi Nafchi, A. The synergistic effects of aloe vera gel and modified atmosphere packaging on the quality of strawberry fruit. J. Food Process. Preserv. 2021, 45, e16003. [Google Scholar] [CrossRef]
- Alvarado-Ambriz, S.; Lobato-Calleros, C.; Hernández-Rodríguez, L.; Vernon-Carter, E.J. Wet processing coffee waste as an alternative to produce extracts with antifungal activity: In vitro and in vivo valorization. Rev. Mex. De Ing. Quim. 2020, 19, 135–149. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Ocio, M.J.; Gavara, R. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria x ananassa). Postharvest Biol. Technol. 2006, 39, 247–253. [Google Scholar] [CrossRef]






| Ctrl | FRCh-1 | FRBN-1 | |
|---|---|---|---|
| Day 1 |  |  |  |
| Day 8 |  |  |  |
| Ctrl | FRCh-1 | FRBN-1 | |
|---|---|---|---|
| Day 1 |  |  |  |
| Day 8 |  |  |  |
| Strawberry | ts (Days) | T (°C) | AMB (CFU/g) | Molds and Yeasts (CFU/g) |
|---|---|---|---|---|
| Ctrl | 1 | 5 | 6.0 × 102 ± 112 aC | 1.5 × 102 ± 10 aB |
| 25 | 2.0 × 102 ± 12 bA | 3.0 × 102 ± 2.6 aA | ||
| 8 | 5 | INC | INC | |
| 25 | 4.0 × 103 ± 22 bB | 1.04 × 105 ± 1.6 aB | ||
| FRCh-1 | 1 | 5 | 50 ± 1.2 aA | 0 ± 0.00 aA |
| 25 | 2.0 × 102 ± 7.2 bB | 0 ± 0.00 aA | ||
| 8 | 5 | 1.3 × 106 ± 15,612 aB | 3.9 × 106 ± 13,212 bB | |
| 25 | 4.3 × 103 ± 182 bC | 2.9 × 104 ± 1122 cC | ||
| FRCh-2 | 1 | 5 | 3.0 × 102 aB | 0 ± 0.00 aA |
| 25 | 0 bC | 0 ± 0.00 aA | ||
| 8 | 5 | 1.3 × 106 aA | 3.9 × 106 ± 10,100 bB | |
| 25 | 2.4 × 103 bA | 3.4 × 103 ± 123 cC | ||
| * FRBN-1 | 1 | 5 | 0 ± 0.00 aA | 0 ± 0.00 aA |
| 25 | 0 ± 0.00 aA | 0 ± 0.00 aA | ||
| 8 | 5 | 7.9 × 105 ± 600 bC | 1.2 × 106 ± 1200 bB | |
| 25 | 0 ± 0.00 aA | 0 ± 0.00 Aa | ||
| FRBN-2 | 1 | 5 | 0 ± 0.00 aA | 0 ± 0.00 aA |
| 25 | 0 ± 0.00 aA | 0 ± 0.00 aA | ||
| 8 | 5 | 6.3 × 105 ± 0.00 bC | 1.2 × 106 ± 1300 bB | |
| 25 | 0 ± 0.00 aA | 0 ± 0.00 aA |
| ts (Days) | T (°C) | Moisture (%) | ||||
|---|---|---|---|---|---|---|
| Ctrl | * FRCh-1 | FRCh-2 | FRBN-1 | FRBN-2 | ||
| 1 | 5 | 91.9 ± 2.0 aA | 92.6 ± 2.2 aB | 92.2 ± 0.9 aAB | 93.2 ± 1.1 Aa | 91.6 ± 1.3 aAB |
| 25 | 89.1 ± 0.6 aA | 89.0 ± 1.3 aA | 82.0 ± 9.9 aB* | 89.0 ± 2.2 aA | 92.7 ± 4.0 aAC | |
| 8 | 5 | 89.3 ± 1.1 aA | 92.3 ± 1.4 aA | 91.4 ± 1.0 aA | 92.9 ± 0.8 Aa | 91.0 ± 1.4 aA |
| 25 | 45.3 ± 3.7 bA* | 69.8 ± 1.5 bB | 67.4 ± 2.7 bB | 83.0 ± 1.6 Bc | 84.5 ± 3.3 bC | |
| Strawberry | ts (Days) | T (°C) | pH | TSS (°Bx) | TA(%) |
|---|---|---|---|---|---|
| Ctrl | 1 | 5 | 3.59 ± 0.18 aA | 8.5 ± 0.2 aA | 0.76 ± 0.04 aA |
| 25 | 3.17 ± 0.03 bB | 8.1 ± 0.1 aA | 0.80 ± 0.02 aA | ||
| 8 | 5 | 3.31 ± 0.01 aC | 8.0 ± 0.1 aA | 0.76 ± 0.04 aA | |
| 25 | 3.68 ± 0.03 bA | 7.7 ± 0.2 bA | 0.60 ± 0.01 cB | ||
| * FRCh-1 | 1 | 5 | 3.47 ± 0.02 aA | 8.1 ± 0.1 aA | 0.77 ± 0.05 aA |
| 25 | 3.38 ± 0.08 aC | 8.2 ± 0.2 aA | 0.79 ± 0.02 aA | ||
| 8 | 5 | 3.32 ± 0.01 aC | 8.0 ± 0.1 aA | 0.61 ± 0.01 aA | |
| 25 | 3.76 ± 0.01 bB | 6.9 ± 0.2 bB | 0.69 ± 0.03 bA | ||
| FRCh-2 | 1 | 5 | 3.53 ± 0.06 aA | 8.5 ± 0.5 aA | 0.77 ± 0.03 aA |
| 25 | 3.31 ± 0.01 bC | 7.8 ± 0.3 bA | 0.80 ± 0.01 aA | ||
| 8 | 5 | 3.67 ± 0.06 cA | 8.5 ± 0.3 aA | 0.60 ± 0.01 bB | |
| 25 | 3.80 ± 0.18 cB | 6.7 ± 1.0 bB | 0.68 ± 0.02 cA | ||
| * FRBN-1 | 1 | 5 | 3.48 ± 0.01 aA | 9.1 ± 0.2 cA | 0.80 ± 0.04 aCA |
| 25 | 3.37 ± 0.01 aC | 9.5 ± 0.3 aCA | 0.82 ± 0.02 aCA | ||
| 8 | 5 | 3.61 ± 0.01 bA | 8.6 ± 0.2 bA | 0.70 ± 0.01 bA | |
| 25 | 3.85 ± 0.02 aB | 8.5 ± 0.2 Ba | 0.77 ± 0.02 abA | ||
| FRBN-2 | 1 | 5 | 3.22 ± 0.02 aB | 8.9 ± 0.1 aA | 0.80 ± 0.01 aA |
| 25 | 3.28 ± 0.02 aB | 10.3 ± 0.5 bCA | 0.80 ± 0.01 aA | ||
| 8 | 5 | 3.54 ± 0.05 bA | 8.3 ± 0.3 aA | 0.69 ± 0.01 bA | |
| 25 | 3.98 ± 0.02 aC | 9.2 ± 0.5 bC | 0.75 ± 0.04 abA |
| Treatment | ts (Days) | T (°C) | L | a* | b* | Browning Index |
|---|---|---|---|---|---|---|
| Ctrl | 1 | 5 | 18.21 ± 0.87 aA | 20.48 ± 2.43 aA | 6.01 ± 1.08 aA | 108.9 ± 11.1 aA |
| 25 | 22.42 ± 3.42 bC | 22.28 ± 0.95 aC | 6.57 ± 0.47 bA | 97.7 ± 8.70 bB | ||
| 8 | 5 | 22.18 ± 3.70 bC | 17.22 ± 1.10 aD | 7.08 ± 1.17 aA | 89.6 ± 8.30 aB | |
| 25 | 20.05 ± 8.35 bB | 08.01 ± 3.74 bE | 3.95 ± 0.41 bB | 56.0 ± 27.2 bC | ||
| FRCh-1 | 1 | 5 | 18.57 ± 4.00 aA | 20.26 ± 5.10 aA | 5.87 ± 2.69 aC | 104.0 ± 13.5 aA |
| 25 | 18.83 ± 1.43 aA | 17.71 ± 2.58 bD | 6.40 ± 2.83 bA | 101.2 ± 22.2 aA | ||
| 8 | 5 | 22.17 ± 1.63 bB | 21.78 ± 3.42 aB | 11.88 ± 10.29 aD | 148.5 ± 99.1 aD | |
| 25 | 18.42 ± 1.97 aA | 13.36 ± 2.14 bE | 4.34 ± 0.91 bC | 73.6 ± 5.8 bEC | ||
| FRCh-2 | 1 | 5 | 19.59 ± 0.61 aAB | 22.41 ± 0.88 aC | 6.96 ± 1.12 aA | 114.0 ± 7.5 aA |
| 25 | 21.65 ± 1.31 bBC | 19.55 ± 2.20 aD | 6.25 ± 1.29 aA | 91.1 ± 8.8 bB | ||
| 8 | 5 | 20.32 ± 3.13 aB | 49.03 ± 1.8 aF | 11.97 ± 10.35 aD | 152.1 ± 88.2 aD | |
| 25 | 15.66 ± 1.29 bD | 37.78 ± 0.86 bG | 2.50 ± 0.67 bB | 50.6 ± 3.2 Bc | ||
| FRBN-1 | 1 | 5 | 20.36 ± 3.10 aAB | 22.06 ± 1.40 aC | 6.84 ± 1.18 aA | 108.4 ± 6.2 aA |
| 25 | 19.30 ± 2.38 aAB | 17.81 ± 3.90 bD | 5.17 ± 1.54 aAC | 88.6 ± 12.5 bB | ||
| 8 | 5 | 21.71 ± 5.35 aBC | 61.88 ± 1.9 aH | 5.68 ± 2.41 aAC | 93.9 ± 5.4 aB | |
| 25 | 17.50 ± 2.50 bD | 11.04 ± 3.5 bI | 3.31 ± 0.88 Bb | 61.5 ± 9.2 bC | ||
| FRBN-2 | 1 | 5 | 20.12 ± 1.32 aB | 24.32 ± 1.17 aC | 7.15 ± 0.40 Aa | 117.4 ± 4.6 aA |
| 25 | 20.89 ± 1.65 bB | 18.21 ± 0.77 bD | 5.74 ± 1.15 bAC | 87.6 ± 4.0 bB | ||
| 8 | 5 | 21.88 ± 2.07 aBC | 21.88 ± 1.91 aC | 7.31 ± 1.44 aA | 103.1 ± 5.1 aA | |
| 25 | 19.00 ± 2.03 Bab | 12.07 ± 1.27 bE | 8.82 ± 0.34 bA | 124.6 ± 103.2 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, D.J.; Pérez-Sánchez, G.F.; Hernández-Cocoletzi, H.; Sánchez-Arzubide, M.G.; Luna-Guevara, M.L.; Rubio-Rosas, E.; Krishnamoorthy, R.; Morán-Raya, C. Chitosan Coatings Modified with Nanostructured ZnO for the Preservation of Strawberries. Polymers 2023, 15, 3772. https://doi.org/10.3390/polym15183772
García-García DJ, Pérez-Sánchez GF, Hernández-Cocoletzi H, Sánchez-Arzubide MG, Luna-Guevara ML, Rubio-Rosas E, Krishnamoorthy R, Morán-Raya C. Chitosan Coatings Modified with Nanostructured ZnO for the Preservation of Strawberries. Polymers. 2023; 15(18):3772. https://doi.org/10.3390/polym15183772
Chicago/Turabian StyleGarcía-García, Dulce J., G. F. Pérez-Sánchez, H. Hernández-Cocoletzi, M. G. Sánchez-Arzubide, M. L. Luna-Guevara, E. Rubio-Rosas, Rambabu Krishnamoorthy, and C. Morán-Raya. 2023. "Chitosan Coatings Modified with Nanostructured ZnO for the Preservation of Strawberries" Polymers 15, no. 18: 3772. https://doi.org/10.3390/polym15183772
APA StyleGarcía-García, D. J., Pérez-Sánchez, G. F., Hernández-Cocoletzi, H., Sánchez-Arzubide, M. G., Luna-Guevara, M. L., Rubio-Rosas, E., Krishnamoorthy, R., & Morán-Raya, C. (2023). Chitosan Coatings Modified with Nanostructured ZnO for the Preservation of Strawberries. Polymers, 15(18), 3772. https://doi.org/10.3390/polym15183772








