Effects of Modifying Agent and Conductive Hybrid Filler on Butyl Rubber Properties: Mechanical, Thermo-Mechanical, Dynamical and Re-Crosslinking Properties
Abstract
:1. Introduction
2. Experimental and Characterization
2.1. Materials
2.2. Compound Preparations
3. Characterization
3.1. Mechanical Properties
3.2. Morphologies
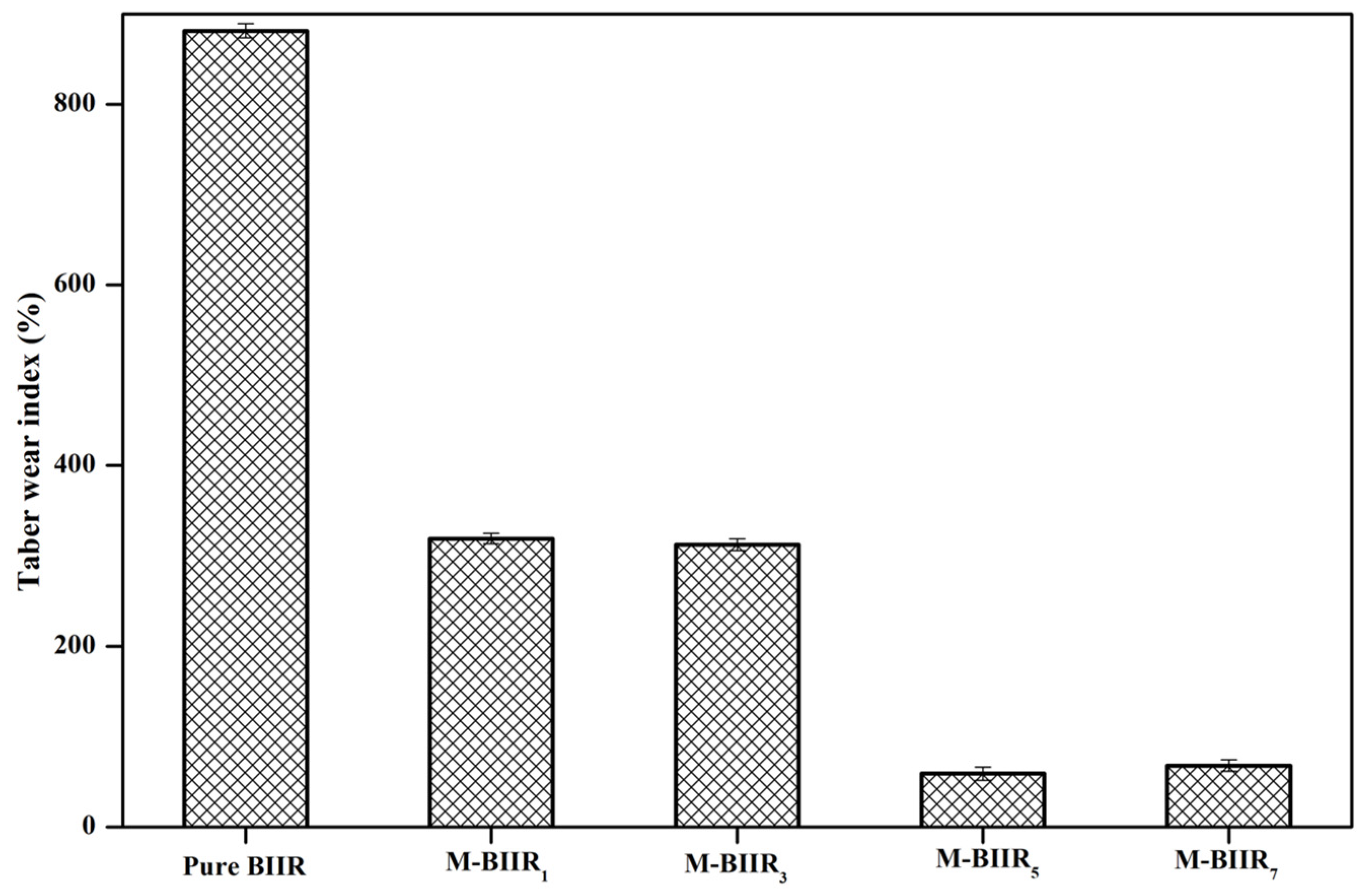
3.3. Abrasion Resistance
3.4. Thermo-Mechanical Properties
3.5. Dynamic Mechanical Properties
3.6. Thermal Stability
4. Results and Discussion
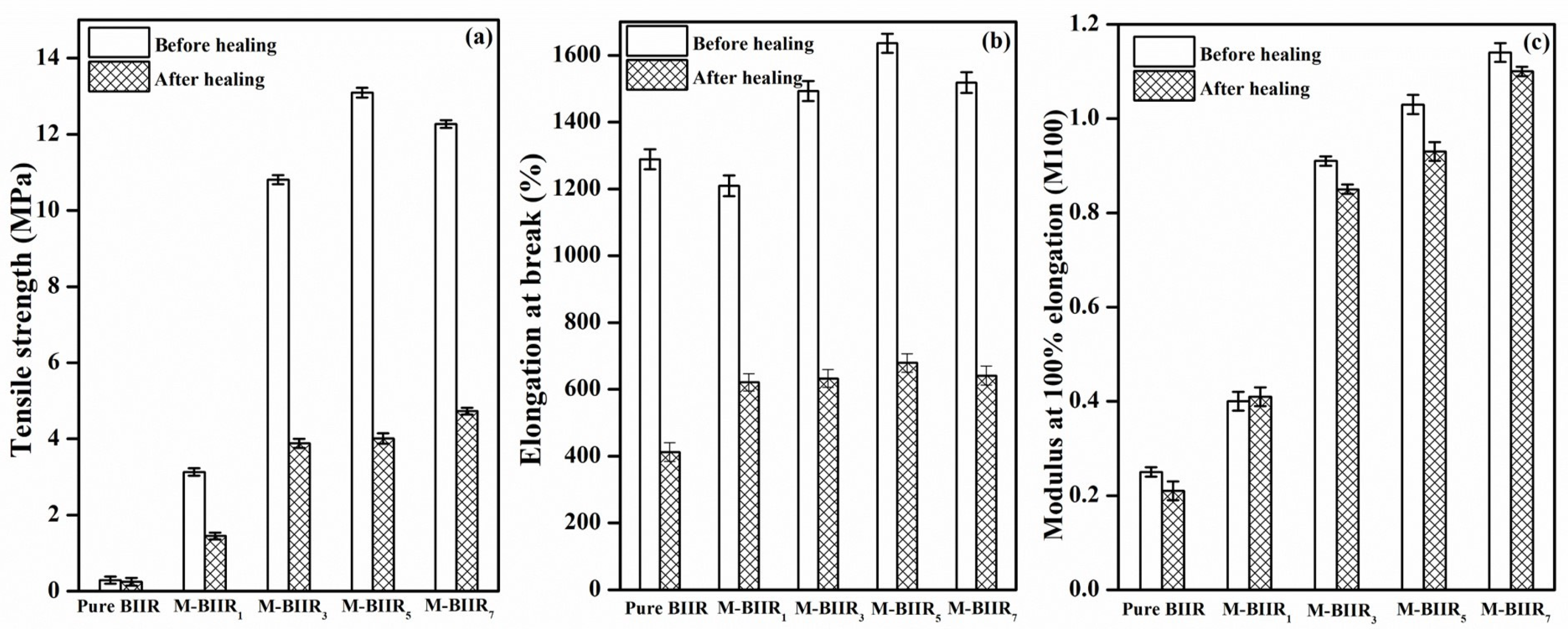
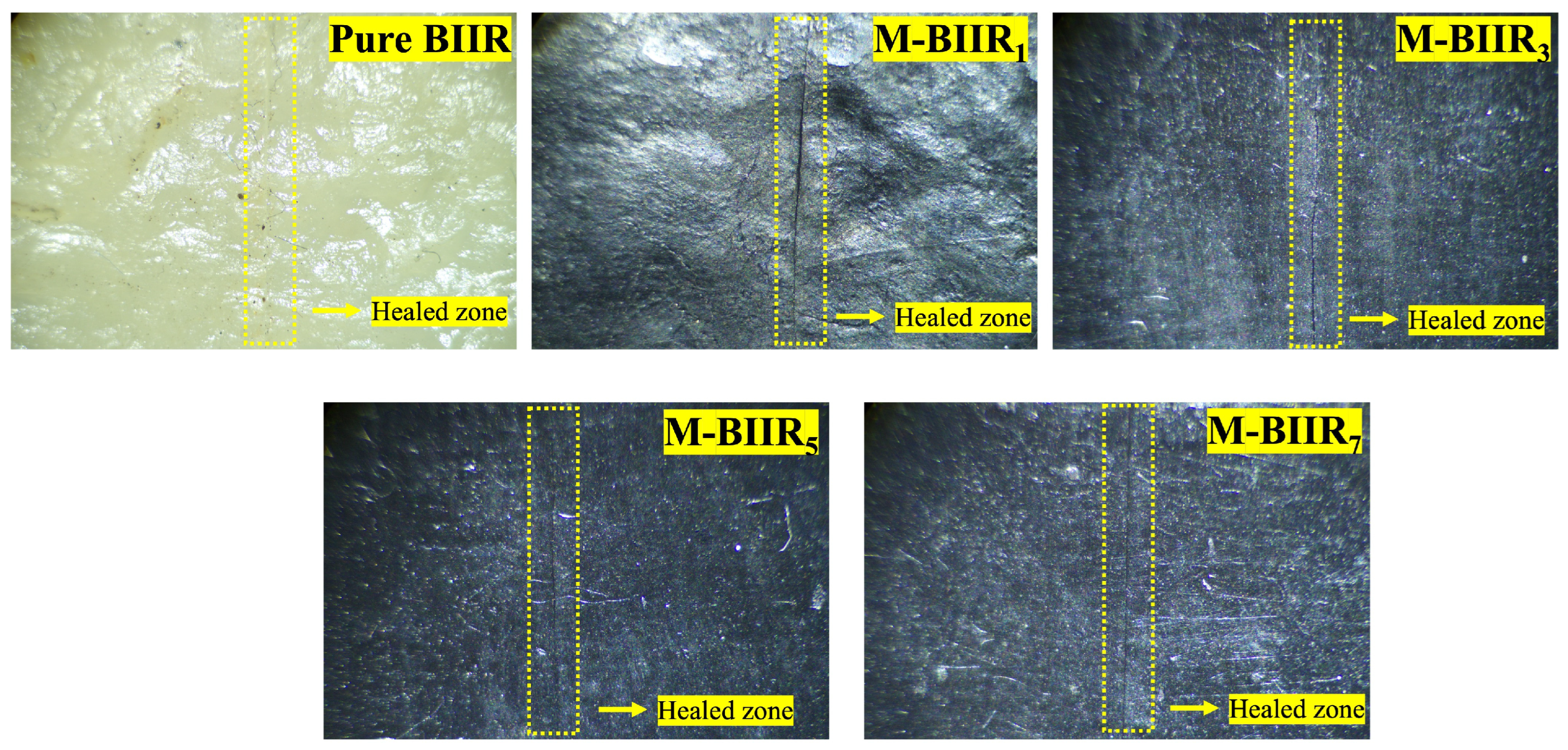
4.1. Tensile Properties and Morphologies
4.2. Abrasion Ability
4.3. Thermo-Mechanical Properties
4.4. Dynamic Mechanical Properties
4.5. Thermal Stability
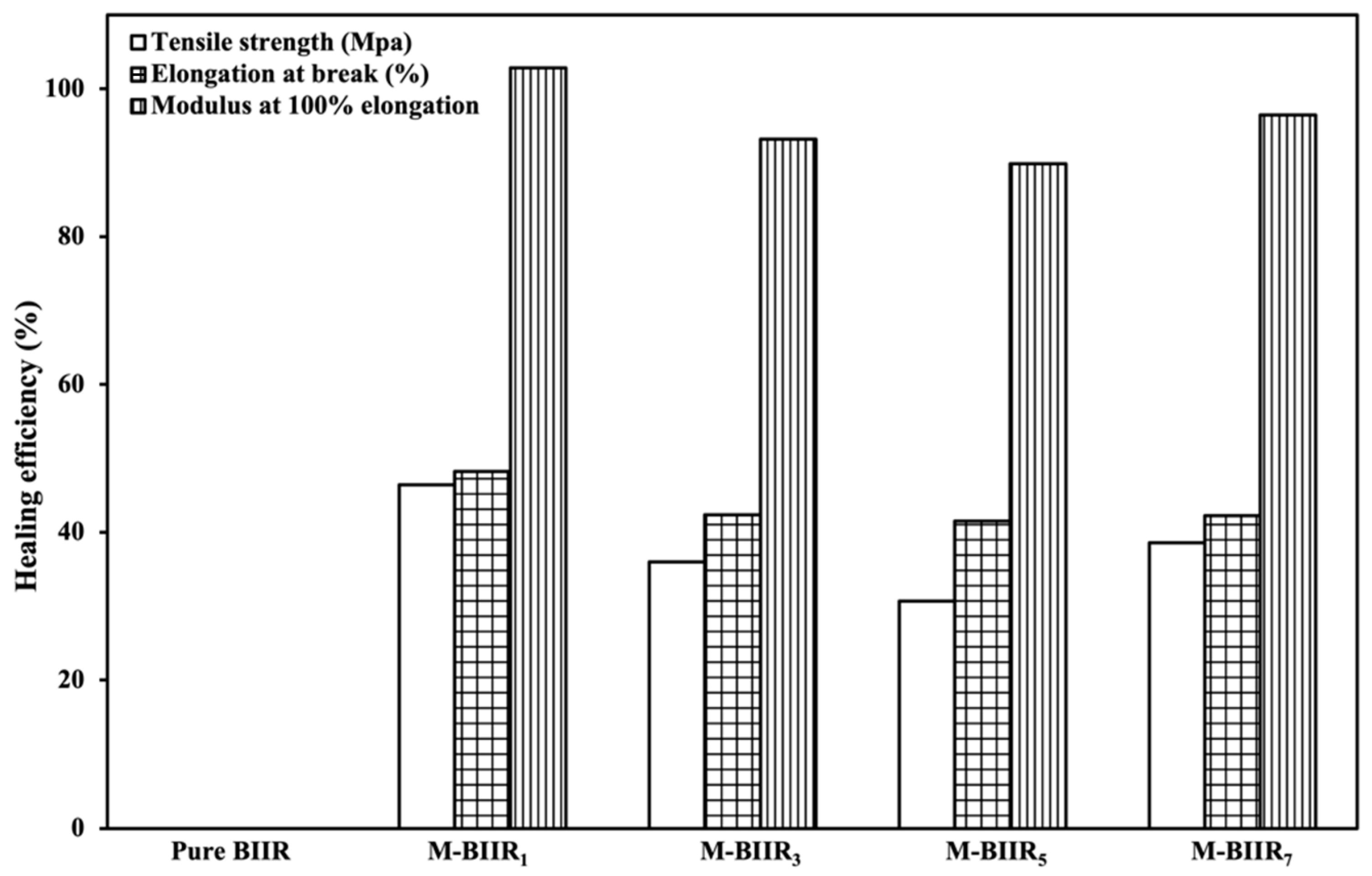
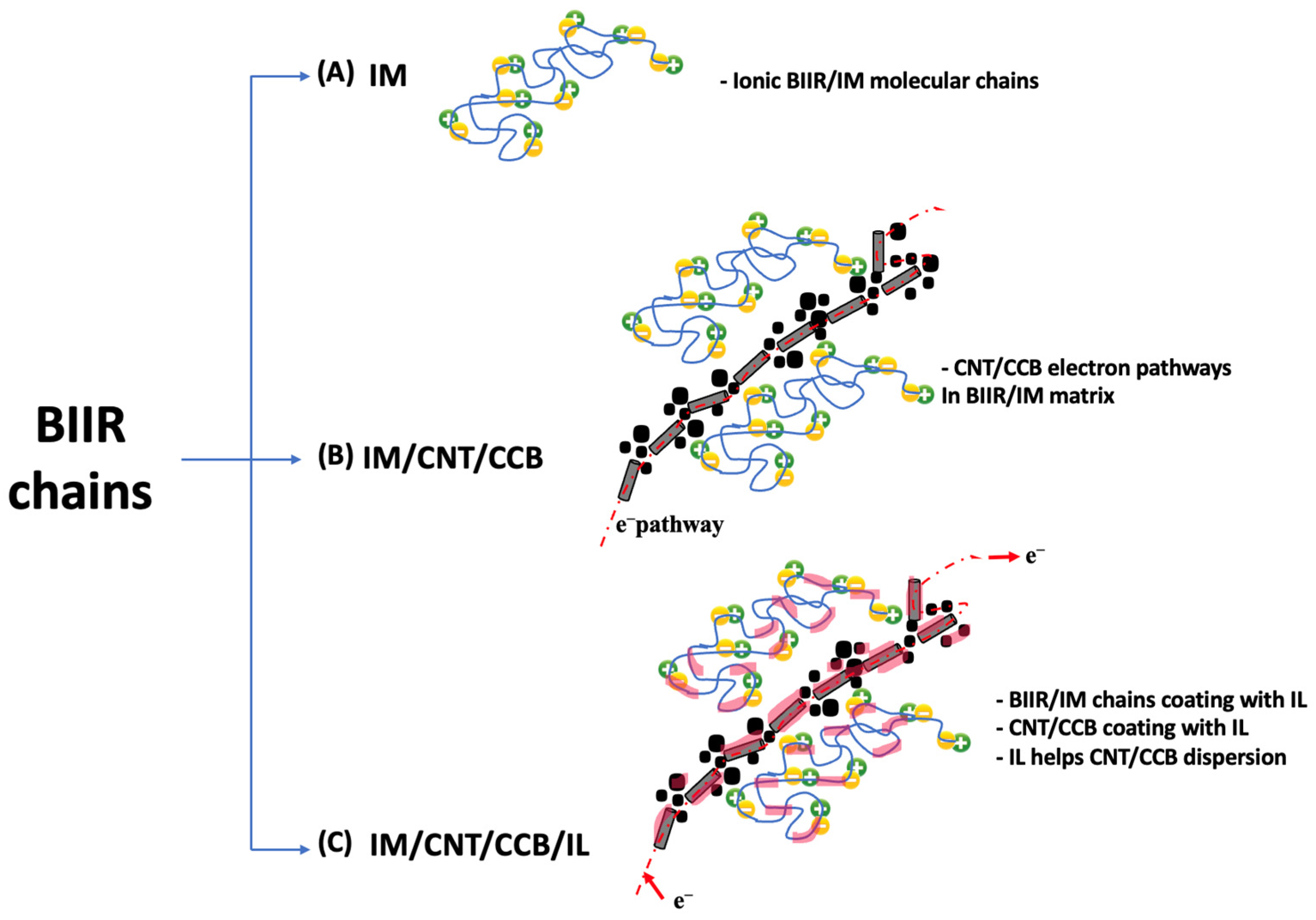
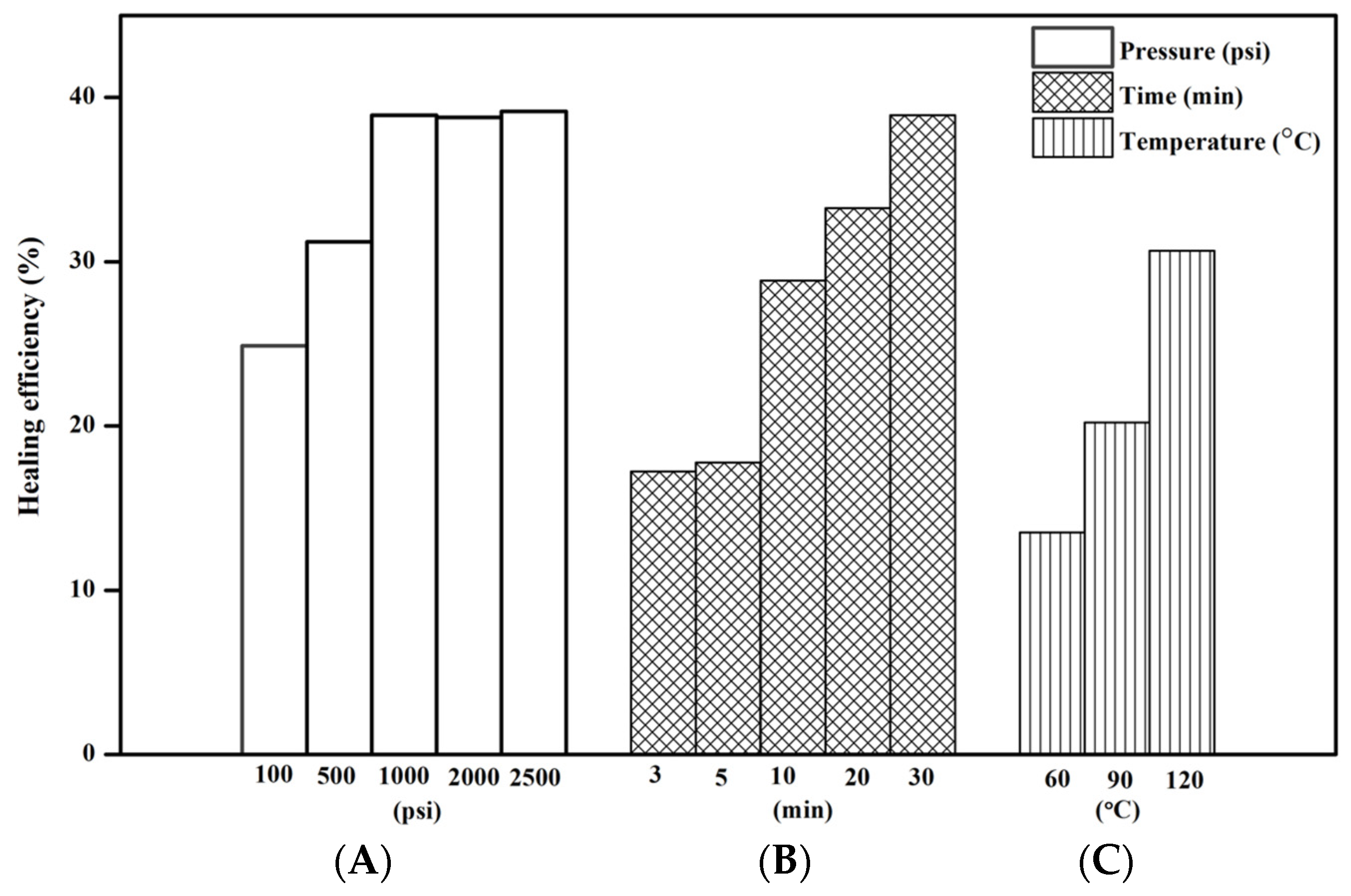
4.6. Factors to Healing Efficiency of Composites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathalie, K.G.; Kim, K.O.; Zhou, J.; Stefan, H.; Friedrich, G.S.; Christopher, B. Current trends in the field of Self-Healing Material. Macromol. Chem. Phys. 2012, 213, 131–143. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiao, X.; Pan, K.; Pang, H. Development and application of self-healing materials in smart batteries and supercapacitors. Chem. Eng. J. 2020, 380, 122565. [Google Scholar] [CrossRef]
- Li, C.H.; Zuo, J.L. Self-Healing Polymers Based on Coordination Bonds. Adv. Mater. 2019, 32, 1903762. [Google Scholar] [CrossRef] [PubMed]
- Amit, D.; Aladdin, S.; Frank, B.; Marcus, S.; Debdipta, B.; Sven, W.; Klaus, W.S.; Brigitte, V.; Gert, H. Ionic Modification Turns Commercial Rubber into a Self-healing Material. Adv. Mater. 2015, 7, 20623–20630. [Google Scholar] [CrossRef]
- Sobia, I.; Muhammad, S.; Ayesha, K.; Sedra, T.M.; Jaweria, A.; Iram, B. A review featuring fabrication, properties and applications of carbon nanotubes (CNTs) reinforced polymer and epoxy nanocomposites. Chin. J. Polym. Sci. 2017, 7, 20623–20630. [Google Scholar] [CrossRef]
- Claudia, K.; Norbert, V.; Sven, P.; Achim, S.; Yeampon, N. Preparation and properties of carbon-nanotube composites with natural rubber and epoxidized natural rubber. Polimery 2014, 59, 811–818. [Google Scholar] [CrossRef]
- Yasser, Z. Assumption of interphase properties in classical Christensen–Lo model for Young’s modulus of polymer nanocomposites reinforced with spherical nanoparticles. RSC Adv. 2015, 5, 95532–95538. [Google Scholar] [CrossRef]
- Suradet, M.; Charoen, N.; Azizon, K. Electrical and Mechanical Properties of Conductive Carbon Black Filled Epoxidized Natural Rubber. Adv. Mater. Res. 2013, 844, 255–258. [Google Scholar] [CrossRef]
- Kunakorn, C.; Ekwipoo, K.; Jobish, J.; Karnda, S.; Yeampon, N. Combination of Self-Healing Butyl Rubber and Natural Rubber Composites for Improving the Stability. Polymers 2021, 13, 443. [Google Scholar] [CrossRef]
- Piyawadee, L.; Kunakorn, C.; Ekwipoo, K.; Yeampon, N. Increase in Properties and Self-Healing Ability of Conductive Butyl Rubber/Epoxidized Natural Rubber Composites by Using Bis (triethoxysilylpropyl) tetrasulfide Coupling Agent. Polimery 2023, 15, 547. [Google Scholar] [CrossRef]
- Jaime, O.; Ignacio, M.V.; Ranjita, K.B.; Francesco, P.; Mario, E.F.; Rodrigo, A.H. Self-Healing Polymer Nanocomposite Materials by Joule Effect. Polymers 2021, 13, 649. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Liu, R.; Liang, Z.; Yang, J.; Zhang, R.; Zhou, Z.; Nie, Y. Design of self-healing rubber by introducing ionic interaction to construct a network composed of ionic and covalent cross-linking. Ind. Eng. Chem. Res. 2019, 58, 14848–14858. [Google Scholar] [CrossRef]
- Yeampon, N.; Skurat, P.; Suwalak, W.; Charoen, N. Hybrid carbon nanotubes and conductive carbon black in natural rubber composites to enhance electrical conductivity by reducing gaps separating carbon nanotube encapsulates. Eur. Polym. J. 2017, 90, 467–484. [Google Scholar] [CrossRef]
- Jie, C.; Xue, C.D.; Wen, B.Z.; Jing, H.Y.; Nan, Z.; Ting, H.; Yong, W. Synergistic effect of carbon nanotubes and carbon black on electrical conductivity of PA6/ABS blend. Compos. Sci. Technol. 2013, 81, 1–8. [Google Scholar] [CrossRef]
- Shubham, C.A.; Gopal, L.D.; Bharat, P.; Amit, D.; Sakrit, H.; Gedam, R.S.; Rajkumar, K.; Chayan, D. Enhancing the material performance of chloroprene rubber (CR) by strategic incorporation of zirconia. Mater. Adv. 2022, 3, 2434–2446. [Google Scholar] [CrossRef]
- Suradet, M.; Azizon, K.; Norbert, V.; Claudia, K.; Charoen, N. Effects of imidazolium ionic liquid on cure characteristics, electrical conductivity and other related properties of epoxidized natural rubber vulcanizates. Eur. Polym. J. 2017, 87, 344–359. [Google Scholar] [CrossRef]
- Richard, J.P.; Ivan, P. The thermo-oxidation of chlorinated and brominated isobutylene-co-isoprene polymers: Activation energies and reactions from room temperature to 100 °C. Polym. Degrad. Stab. 2015, 121, 311–320. [Google Scholar] [CrossRef]
- Norbert, V. Thermoplastic Elastomers || Characterization of Thermoplastic Elastomers by Means of Temperature Scanning Stress Relaxation Measurements. Thermoplast. Elastom. 2012, 1, 347–370. [Google Scholar] [CrossRef]
- Zi, K.L.; Shun, L.S.; Yi, W. Fundamentals of Thermal Expansion and Thermal Contraction. Materials 2017, 10, 410. [Google Scholar] [CrossRef]



| Samples | IM | CNT | CB | IL |
|---|---|---|---|---|
| (phr) | (phr) | (phr) | (phr) | |
| Pure BIIR | 0.0 | 0.0 | 0.0 | 0.0 |
| M-BIIR1 | 1.0 | 1.0 | 1.5 | 1.5 |
| M-BIIR3 | 3.0 | 3.0 | 4.5 | 4.5 |
| M-BIIR5 | 5.0 | 5.0 | 7.5 | 7.5 |
| M-BIIR7 | 7.0 | 7.0 | 10.5 | 10.5 |
| Samples | Peak Areas |
|---|---|
| (MPa.K) | |
| Pure BIIR | 1.75 |
| M-BIIR1 | 13.51 |
| M-BIIR3 | 27.47 |
| M-BIIR5 | 31.85 |
| M-BIIR7 | 32.03 |
| Sample | Tg (°C) | Tanmax | Tan0 | Tan60 |
|---|---|---|---|---|
| Pure BIIR | −17.20 | 1.44 | 1.15 | 0.16 |
| M-BIIR1 | −23.96 | 1.12 | 0.79 | 0.15 |
| M-BIIR3 | −24.13 | 1.11 | 0.65 | 0.15 |
| M-BIIR5 | −28.16 | 0.99 | 0.53 | 0.19 |
| M-BIIR7 | −26.10 | 0.93 | 0.52 | 0.20 |
| Samples | Decomposition (%) | TDR (°C) | ||
|---|---|---|---|---|
| Step 1 (N2) | End Step 1 (O2) | * T0 | * TE | |
| Pure BIIR | 93.9 | 4.1 | 362.1 | 429.2 |
| M-BIIR1 | 91.5 | 7.0 | 363.4 | 432.6 |
| M-BIIR3 | 89.8 | 8.1 | 359.9 | 437.9 |
| M-BIIR5 | 86.5 | 11.1 | 358.8 | 437.8 |
| M-BIIR7 | 81.4 | 17.9 | 358.9 | 440.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luangchuang, P.; Sornanankul, T.; Nakaramontri, Y. Effects of Modifying Agent and Conductive Hybrid Filler on Butyl Rubber Properties: Mechanical, Thermo-Mechanical, Dynamical and Re-Crosslinking Properties. Polymers 2023, 15, 4023. https://doi.org/10.3390/polym15194023
Luangchuang P, Sornanankul T, Nakaramontri Y. Effects of Modifying Agent and Conductive Hybrid Filler on Butyl Rubber Properties: Mechanical, Thermo-Mechanical, Dynamical and Re-Crosslinking Properties. Polymers. 2023; 15(19):4023. https://doi.org/10.3390/polym15194023
Chicago/Turabian StyleLuangchuang, Piyawedee, Tanawat Sornanankul, and Yeampon Nakaramontri. 2023. "Effects of Modifying Agent and Conductive Hybrid Filler on Butyl Rubber Properties: Mechanical, Thermo-Mechanical, Dynamical and Re-Crosslinking Properties" Polymers 15, no. 19: 4023. https://doi.org/10.3390/polym15194023
APA StyleLuangchuang, P., Sornanankul, T., & Nakaramontri, Y. (2023). Effects of Modifying Agent and Conductive Hybrid Filler on Butyl Rubber Properties: Mechanical, Thermo-Mechanical, Dynamical and Re-Crosslinking Properties. Polymers, 15(19), 4023. https://doi.org/10.3390/polym15194023







