Manipulating Microbial Cell Morphology for the Sustainable Production of Biopolymers
Abstract
1. Introduction
2. Biopolymers
2.1. Polyhydroxyalkanoates
2.2. Polylactides
2.3. Polycaprolactones
2.4. Other Polymers
3. Sustainable Production of Biopolymers
3.1. Bioengineering of Microbial Cells
3.1.1. Cell Morphology
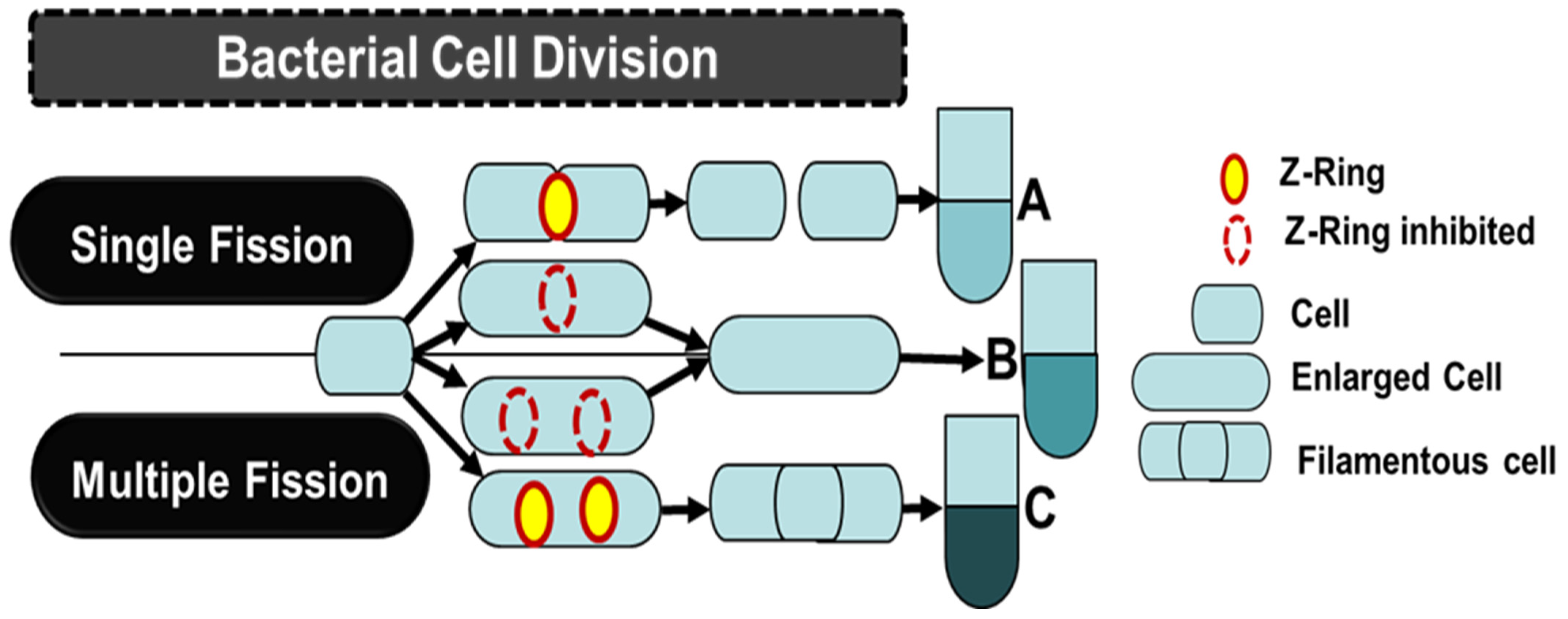
3.1.2. Cell Division
| Gene | Gene Product | Cell Function/Activity | Reference |
|---|---|---|---|
| dxs | 1-deoxyxylulose-5-phosphate synthase | Cell wall synthesis | [65] |
| glmU | N-acetylglucosamine-1-phosphate uridyltransferase | Cell wall synthesis | |
| murA | UDP- N -acetylglucosamine enolpyruvoyl transferase | Cell wall synthesis | |
| murC | UDP-N-acetylmuramate-alanine ligase | Cell wall synthesis | |
| murD | UDP-N-acetylmuramoyl-L-alanine:D-glutamate ligase | Cell wall synthesis | |
| murE | Ligase | Cell wall synthesis | |
| murJ | Putative lipid II flippase | Regulates peptidoglycan incorporation to the septum | [67] |
| ftsZ | Bacterial fission ring formation protein | Recruiting divisiome proteins and z-ring stabilization | [64,73,74] |
| ftsA | Cell division protein | Divisiome | [69] |
| ftsW | Peptidoglycan glycosyltransferase, lipid II flippase | Divisiome | [65] |
| ftsL, ftsN, ftsQ | Cell division proteins | Divisiome | [67] |
| sulA | Cell division inhibitor protein | Divisiome, induces FtsZ inhibition | [72,75,78] |
| slmA | Nucleoid-associated FtsZ binding protein | Divisiome | [60] |
| minC | Z-ring positioning protein | Divisiome: actin-related proteins, inhibits FtsZ polymerization | [57,76] |
| minD | Z-ring positioning protein | Divisiome: actin-related proteins, recruits MinC | [57,76] |
| envC | Murein hydrolase activator | Divisiome | [74] |
| zipA | Integral inner membrane protein | Divisiome | [62] |
| PBP1, PBP3 | Penicillin binding proteins | Divisiome | [68] |
| envC | Regulate amidase activity | Cell division | [68,77] |
| nlpD | Murein hydrolase activator, peptidoglycan degradation | Cell division | [74,77,78] |
| mreB | Dynamic cytoskeletal protein | Rod complex and cell division | [55,59,72] |
| RodZ | Transmembrane protein | Rod complex | [69] |
| RodA | Transglycosylase, lipid II flippase | Rod complex | [63] |
| PBP2 | Penicillin binding protein, murein DD-transpeptidase | Rod complex, cell elongation | [68] |
| gltA | Citrate synthase | Manipulate cell rigidity | [65] |
| idi | Isopentenyl diphosphate isomerase | Cell wall synthesis | [65] |
| mraY | Translocase 1, phosphor-N-acetylmuramoyl-pentapeptide transferase | Cell wall synthesis | [65] |
| pgi | Phosphoglucose isomerase | Cell wall synthesis | [65] |
| PBP5, PBP7 | DD-carboxypeptidases and DD-endopeptidases | Hydrolases | [61] |
| ampD, amiA, amiB | MurNAc-L-Ala amidases | Hydrolases | [61] |
| Organism | Gene Edited b | Characteristics Affected | Impact on Polyhydroxyalkanoate c Production | Reference |
|---|---|---|---|---|
| Escherichia coli JM109SGIK | sulA | Transformation of rod to filamentous cell with larger internal space | PHB accumulation showed 100% increase | [75] |
| E. coli JM109SGIK | sad, gabD, ispH folk, and sulA | Transformation of rod to filamentous cell with larger internal space | Copolymers of PHA [P(3HB-co-4HB)] were 10% higher (78% in cell dry weight, CDW). | [75] |
| E. coli JM109SG (ΔmreB/pTK-mreB/pBHR68) a | ftsZ, mreB, and sulA | Enlarged cell space due to reduced restriction on space. Larger volume to size ratio. | PHB d production was observed to increase from 5.72 g/L to (9.29 g/L, with a yield of 73.53% of CDW) e in a shake flask | [59] |
| E. coli | envC and nlpD | Switch from binary to multiple fission mode | PHB storing capacity enhanced from 51 to 70% | [74] |
| E. coli JM109 | ftsZ and mreB | Enlarged cell volume | Enhanced PHB accumulations (up to 80%) | [73] |
| E. coli JM109 | ftsW, dxs, glmU, idi, pgi, murA, murC, murD, murE, and mraY | Cell wall thickening | PHB accumulation of 93% in weakened cells and 25% in thickened cell walls | [65] |
| Pseudomonas mendocina NK-01 | ftsZ, mreB, sulA, minCD, and mreB | Modified bacterial shape and growth pattern | Increased mcl-PHA f yield by 45.62% and up to 60.87% | [78] |
| Halomonas bluephagenesis TD08 | minCD | Enlarged cells (1.4-fold longer than the parent) | PHB content enhanced from 69 to 82% | [57] |
| Halomonas campaniensis LS21 | ftsZ and mreB | Enlarged cell morphology | Increase in PHB yield accompanied by normal growth | [72] |
| H. bluephagenesis TDH4-minCD-ΔphaP1 | phaP1, phaP2, phaP3, and minCD | Bigger PHA granules and larger cell size | PHA granules up to 10 μm. PHA copolymer with 14% higher 4HB mol% | [16] |
3.1.3. Cytoskeletal Protein
3.2. Complementary Extraction Processes
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Liu, S.; Huang, J.; Cui, R.; Xu, Y.; Song, Z. Genetic engineering strategies for sustainable polyhydroxyalkanoate (PHA) production from carbon-rich wastes. Environ. Technol. Innov. 2023, 30, 103069. [Google Scholar] [CrossRef]
- Atanasova, N.; Stoitsova, S.; Paunova-Krasteva, T.; Kambourova, M. Plastic degradation by extremophilic bacteria. Int. J. Mol. Sci. 2021, 22, 5610. [Google Scholar] [CrossRef]
- Moses, N.E.; Erhianoh, C.; Anih, C.E. Modelling and simulation of waste plastic power plant: A theoretical framework. Am. J. Chem. Eng. 2018, 6, 94–98. [Google Scholar] [CrossRef]
- Mahmoud, Y.S.; Belhanche-Bensemra, N.; Safidine, Z. Impact of microcrystalline cellulose extracted from walnut and apricots shells on the biodegradability of poly(lactic acid). Front. Mater. 2022, 9, 1005387. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Plastic Waste Management | Plastics and the Environment Series. Available online: https://www.genevaenvironmentnetwork.org/resources/updates/plastic-waste-management/ (accessed on 27 December 2023).
- Lonca, G.; Lesage, P.; Majeau-Bettez, G.; Bernard, S.; Margni, M. Assessing scaling effects of circular economy strategies: A case study on plastic bottle closed-loop recycling in the USA PET market. Resour. Conserv. Recycl. 2020, 162, 105013. [Google Scholar] [CrossRef]
- Kim, M.O.; Park, J.K.; Han, T.H.; Seo, J.; Lee, H. Influence of polyethylene terephthalate powder on hydration of portland cement. Polymers 2012, 13, 2551. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Encarnação, T.; Tavares, R.; Todo Bom, T.; Mateus, A. Bioplastics: Innovation for green transition. Polymers 2023, 15, 517. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Edit. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, Y.; Wu, Q.; Wang, Y.; Chen, G.Q. Synthetic biology and genome-editing tools for improving PHA metabolic engineering. Trends Biotechnol. 2020, 38, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, J.-C.; Chen, G.-Q. Controlling microbial PHB synthesis via CRISPRi. Appl. Microbiol. Biot. 2017, 101, 5861–5867. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Jiang, X.R. Next generation industry biotechnology based on extremophiles. Curr. Opin. Biotechnol. 2018, 50, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; Wang, H.; Hajnal, I.; Wu, Q.; Guo, Y.; Chen, G.Q. Increasing oxygen availability for improving poly(3-hydroxybutyrate) production by Halomonas. Metab. Eng. 2018, 45, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Landick, R.; Raman, S. A regulatory NADH/NAD+ redox biosensor for bacteria. ACS Synth. Biol. 2019, 8, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Ning, Z.Y.; Lan, Y.X.; Chen, J.C.; Chen, G.Q. Manipulation of polyhydroxyalkanoate granular sizes in Halomonas bluephagenesis. Metab. Eng. 2019, 54, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent developments. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as source of polyhydroxyalkanoates (PHAs) —A review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, P.; Patel, S.K.S.; Kalia, V.C. Production of polyhydroxyalkanoate co-polymer by Bacillus thuringiensis. Indian J. Microbiol. 2013, 53, 77–83. [Google Scholar] [CrossRef]
- Singh, M.; Patel, S.K.S.; Kalia, V.C. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb. Cell Fact. 2009, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Gopi, K.; Muthuvelan, B. A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bioplastics. Algal Res. 2013, 2, 278–285. [Google Scholar] [CrossRef]
- Singh, G.; Kumari, A.; Mittal, A.; Goel, V.; Yadav, A.; Aggarwal, N.K. Cost effective production of poly-β-hydroxybutyrate by Bacillus subtilis NG05 using sugar industry waste water. J. Polym. Environ. 2013, 21, 441–449. [Google Scholar] [CrossRef]
- Singh, A.K.; Mallick, N. Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol. Lett. 2017, 364, fnx189. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, P.; Ray, S.; Kalia, V.C. Challenges and opportunities for customizing polyhydroxyalkanoates. Indian J. Microbiol. 2015, 55, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Peelman, N.; Ragaert, R.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Fd. Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Biomedical applications of polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Ray, S.; Patel, S.K.; Singh, M.; Singh, G.P. The dawn of novel biotechnological applications of polyhydroxyalkanoates. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 1–11. [Google Scholar] [CrossRef]
- Venkatachalam, H.; Palaniswamy, R. Bioplastic World: A Review. J. Adv. Sci. Res. 2020, 11, 43–53. [Google Scholar]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides-methods of synthesis and characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.A.; Waymouth, R.M. Ion pairing effects in the zwitterionic ring opening polymerization of δ-valerolactone. Polym. Chem. 2015, 6, 5212–5218. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.C.; Södergård, A. From lactic acid to poly(lactic acid) (PLA): Characterization and analysis of PLA and Its precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef]
- Vert, M. Degradable and bioresorbable polymers in surgery and in pharmacology: Beliefs and facts. J. Mater. Sci. Mater. Med. 2009, 20, 437–446. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Isroi, I.; Cifriadi, A.; Panji, T.; Wibowo, N.A.; Syamsu, K. Bioplastic production from cellulose of oil palm empty fruit bunch. IOP Conf. Ser. Earth Environ. Sci. 2017, 65, 012011. [Google Scholar] [CrossRef]
- El-malek, F.A.; Khairy, H.; Farag, A.; Omar, S. The sustainability of microbial bioplastics, production and applications. Int. J. Biol. Macromol. 2020, 157, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Luengo, J.M.; García, B.; Sandoval, A.; Naharro, G.; Olivera, E.R. Bioplastics from microorganisms. Curr. Opin. Microbiol. 2003, 6, 251–260. [Google Scholar] [CrossRef]
- Karpušenkaite, A.; Varžinskas, V. Bioplastics: Development, possibilities and difficulties. Environ. Res. Eng. Manag. 2014, 68, 69–78. [Google Scholar] [CrossRef]
- Lackner, M. Bioplastics. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–41. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental impact of bioplastic use: A review. Heliyon. 2021, 7, e07918. [Google Scholar] [CrossRef]
- Nandakumar, A.; Chuah, J.A.; Sudesh, K. Bioplastics: A boon or bane? Renew. Sustain. Energy Rev. 2021, 147, 111237. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Jerez, A.; Partal, P.; Martínez, I.; Gallegos, C.; Guerrero, A. Protein-based bioplastics: Effect of thermo-mechanical processing. Rheol. Acta. 2007, 46, 711–720. [Google Scholar] [CrossRef]
- Costa, A.A.; Gameiro, F.; Potêncio, A.; Silva, D.P.; Carreira, P.; Martinez, J.C.; Pascoal-Faria, P.; Mateus, A.; Mitchell, G.R. Evaluating the injection moulding of plastic parts using in situ Time-Resolved Small-Angle X-ray Scattering Techniques. Polymers 2022, 14, 4745. [Google Scholar] [CrossRef]
- Eisoldt, L.; Smith, A.; Scheibel, T. Decoding the secrets of spider silk. Materialstoday 2011, 14, 80–86. [Google Scholar] [CrossRef]
- Qiao, X.; Qian, Z.; Li, J.; Sun, H.; Han, Y.; Xia, X.; Zhou, J.; Wang, C.; Wang, Y.; Wang, C. Synthetic engineering of spider silk fiber as implantable optical waveguides for low-loss light guiding. ACS Appl. Mater. Interfaces 2017, 9, 14665–14676. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Rising, A. Doing what spiders cannot-a road map to supreme artificial silk fibers. ACS Nano 2021, 15, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Chen, X.-Y.; Wu, F.-Q.; Chen, J.-C. Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polymer Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Ademakinwa, A.N.; Ayinla, Z.A.; Agboola, F.K. Strain improvement and statistical optimization as a combined strategy for improving fructosyltransferase production by Aureobasidium pullulans NAC8. J. Genet. Eng. Biotechnol. 2017, 15, 345–358. [Google Scholar] [CrossRef]
- Cheng, K.K.; Zhao, X.B.; Zeng, J.; Wu, R.C.; Xu, Y.Z.; Liu, D.H.; Zhang, J.A. Downstream processing of biotechnological produced succinic acid. Appl. Microbiol. Biotechnol. 2012, 95, 841–850. [Google Scholar] [CrossRef]
- Jungbauer, A. Continuous downstream processing of biopharmaceuticals. Trends Biotechnol. 2013, 31, 479–492. [Google Scholar] [CrossRef]
- Kreyenschulte, D.; Krull, R.; Margaritis, A. Recent advances in microbial biopolymer production and purification. Crit. Rev. Biotechnol. 2014, 34, 1–15. [Google Scholar] [CrossRef]
- Jiang, X.R.; Chen, G.Q. Morphology engineering of bacteria for bio-production. Biotechnol. Adv. 2016, 34, 435–440. [Google Scholar] [CrossRef] [PubMed]
- van Teeffelen, S.; Gitai, Z. Rotate into shape: MreB and bacterial morphogenesis. EMBO J. 2011, 30, 4856–4857. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wu, Q.; Chen, J.C.; Chen, G.Q. Engineering Halomonas TD01 for the lowcost production of polyhydroxyalkanoates. Metab. Eng. 2014, 26, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Bisson-Filho, A.W.; Discola, K.F.; Castellen, P.; Blasios, V.; Martins, A.; Sforca, M.L.; Garcia, W.; Zeri, A.C.; Erickson, H.P.; Dessen, A.; et al. FtsZ filament capping by MciZ, a developmental regulator of bacterial division. Proc. Natl. Acad. Sci. USA 2015, 112, E2130–E2138. [Google Scholar] [CrossRef]
- Jiang, X.R.; Wang, H.; Shen, R.; Chen, G.Q. Engineering the bacterial shapes for enhanced inclusion bodies accumulation. Metab. Eng. 2015, 29, 227–237. [Google Scholar] [CrossRef]
- Bernhardt, T.G.; de Boer, P.A.J. SlmA, a Nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol. Cell. 2005, 18, 555–564. [Google Scholar] [CrossRef]
- Van Heijenoort, J. Peptidoglycan hydrolases of Escherichia coli. Microbiol. Mol. Biol. Rev. 2011, 75, 636–663. [Google Scholar] [CrossRef]
- Shiomi, D.; Niki, H. A mutation in the promoter region of zipA, a component of the divisome, suppresses the shape defect of RodZ-deficient cells. MicrobiologyOpen 2013, 2, 798–810. [Google Scholar] [CrossRef]
- Shiomi, D.; Toyoda, A.; Aizu, T.; Ejima, F.; Fujiyama, A.; Shini, T.; Kohara, Y.; Niki, H. Mutations in cell elongation genes mreB, mrdA and mrdB suppress the shape defect of RodZ-deficient cells. Mol. Microbiol. 2013, 87, 1029–1044. [Google Scholar] [CrossRef]
- Loose, M.; Mitchison, T.J. The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat. Cell Biol. 2014, 16, 38–46. [Google Scholar] [CrossRef]
- Zhang, X.C.; Guo, Y.; Liu, X.; Chen, X.G.; Wu, Q.; Chen, G.Q. Engineering cell wall synthesis mechanism for enhanced PHB accumulation in E. coli. Metab. Eng. 2018, 45, 32–42. [Google Scholar] [CrossRef]
- Turner, R.D.; Hurd, A.F.; Cadby, A.; Hobbs, J.K.; Foster, S.J. Cell wall elongation mode in Gram-negative bacteria is determined by peptidoglycan architecture. Nat. Commun. 2013, 4, 1496. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.M.; Pereira, A.R.; Reichmann, N.T.; Saraiva, B.M.; Fernandes, P.B.; Veiga, H.; Tavares, A.C.; Santos, M.; Ferreira, M.T.; Macario, V.; et al. Peptidoglycan synthesis drives an FtsZ-treadmilling-independent step of cytokinesis. Nature 2018, 554, 528–532. [Google Scholar] [CrossRef] [PubMed]
- van Teeffelen, S.; Renner, L.D. Recent advances in understanding how rod-like bacteria stably maintain their cell shapes. F1000Research 2018, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ling, C.; Chen, Y.; Jiang, X.; Chen, G.-Q. Microbial engineering for easy downstream processing. Biotechnol. Adv. 2019, 37, 107365. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Parveen, S.; SaiSree, L.; Reddy, M. Regulated proteolysis of a crosslink-specific peptidoglycan hydrolase contributes to bacterial morphogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 10956–10961. [Google Scholar] [CrossRef]
- Egan, A.J.; Vollmer, W. The physiology of bacterial cell division. Ann. N. Y. Acad. Sci. 2013, 1277, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.R.; Yao, Z.H.; Chen, G.Q. Controlling cell volume for efficient PHB production by Halomonas. Metab. Eng. 2017, 44, 30–37. [Google Scholar] [CrossRef]
- Elhadi, D.; Lv, L.; Jiang, X.R.; Wu, H.; Chen, G.Q. CRISPRi engineering E. coli for morphology diversification. Metab. Eng. 2016, 38, 358e369. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Chen, G.Q. Engineering the growth pattern and cell morphology for enhanced PHB production by Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 9907–9916. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Jiang, X.; Chen, G.Q. Engineering Escherichia coli for enhanced production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in larger cellular space. Metab. Eng. 2014, 25, 183–193. [Google Scholar] [CrossRef]
- Ghosal, D.; Trambaiolo, D.; Amos, L.A.; Lowe, J. MinCD cell division proteins form alternating copolymeric cytomotive filaments. Nat. Commun. 2014, 5, 5341. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Dinh, T.; Bernhardt, T.G. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J. Bacteriol. 2009, 191, 5094–5107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Gong, T.; Liu, X.; Fan, X.; Huang, R.; Ma, T.; Wang, S.; Gao, W.; Yang, C. Morphology engineering for enhanced production of medium-chain-length polyhydroxyalkanoates in Pseudomonas mendocina NK-01. Appl. Microbiol. Biotechnol. 2019, 103, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Strahl, H.; Burmann, F.; Hamoen, L.W. The actin homologue MreB organizes the bacterial cell membrane. Nat. Commun. 2014, 5, 3442. [Google Scholar] [CrossRef] [PubMed]
- Morgenstein, R.M.; Bratton, B.P.; Nguyen, J.P.; Ouzounov, N.; Shaevitz, J.W.; Gitai, Z. RodZ links MreB to cell wall synthesis to mediate MreB rotation and robust morphogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 12510–12515. [Google Scholar] [CrossRef] [PubMed]
- Colavin, A.; Shi, H.; Huang, K.C. RodZ modulates geometric localization of the bacterial actin MreB to regulate cell shape. Nat. Commun. 2018, 9, 1280. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Cress, B.F.; Jones, J.A. Rapid generation of CRISPR/dCas9-regulated, orthogonally repressible hybrid T7-lac promoters for modular, tuneable control of metabolic pathway fluxes in Escherichia coli. Nucleic Acids Res. 2016, 44, 4472–4485. [Google Scholar] [CrossRef]
- Peternel, S. Bacterial cell disruption: A crucial step in protein production. New Biotechnol. 2013, 30, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Feng, X.; Xian, M.; Wang, Q.; Zhao, G. Inducible cell lysis systems in microbial production of bio-based chemicals. Appl. Microbiol. Biotechnol. 2013, 97, 7121–7129. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Lee, S.Y. Efficient and economical recovery of poly(3-hy-droxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol. Bioeng. 1999, 62, 546–553. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Z.; Fang, Q.; Zheng, J.; Hu, M.; Jiao, X. An auto-inducible Escherichia coli lysis system controlled by magnesium. J. Microbiol. Methods 2009, 79, 199–204. [Google Scholar] [CrossRef]
- Hajnal, I.; Chen, X.; Chen, G.Q. A novel cell autolysis system for cost-competitive downstream processing. Appl. Microbiol. Biotechnol. 2016, 100, 9103–9110. [Google Scholar] [CrossRef]
- Martínez, V.; García, P.; García, J.L.; Prieto, M.A. Controlled autolysis facilitates the polyhydroxyalkanoate recovery in Pseudomonas putida KT2440. Microb. Biotechnol. 2011, 4, 533–547. [Google Scholar] [CrossRef]
- Borrero-De Acuna, J.M.; Hidalgo-Dumont, C.; Pacheco, N.; Cabrera, A.; Poblete-Castro, I. A novel programmable lysozyme-based lysis system in Pseudomonas putida for biopolymer production. Sci. Rep. 2017, 7, 4373. [Google Scholar] [CrossRef]
- Poblete-Castro, I.; Aravena-Carrasco, C.; Orellana-Saez, M.; Pacheco, N.; Cabrera, A.; Borrero-de Acuña, J.M. Engineering the osmotic state of Pseudomonas putida KT2440 for efficient cell disruption and downstream processing of poly(3-Hydroxyalkanoates). Front Bioeng Biotechnol. 2020, 8, 161. [Google Scholar] [CrossRef]
- Morita, M.; Tanji, Y.; Mizoguchi, K.; Soejima, A.; Orito, Y.; Unno, H. Antibacterial activity of Bacillus amyloliquefaciens phage endolysin without holing conjugation. J. Biosci. Bioeng. 2001, 91, 469–473. [Google Scholar] [CrossRef]
- Hori, K.; Kaneko, M.; Tanji, Y.; Xing, X.H.; Unno, H. Construction of self disruptive Bacillus megaterium in response to substrate exhaustion for polyhydroxybutyrate production. Appl. Microbiol. Biotechnol. 2002, 59, 211–216. [Google Scholar] [CrossRef]
- Salzberg, L.I.; Helmann, J.D. An antibiotic inducible cell wall associated protein that protects Bacillus subtilis from autolysis. J. Bacteriol. 2007, 189, 4671–4680. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Continuous flocculation-sedimentation for harvesting Nannochloropsis salina biomass. J. Biotechnol. 2016, 222, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Hao, X.M.; Lin, Y.H.; Bai, F.W. Redox potential driven aeration during very-high-gravity ethanol fermentation by using flocculating yeast. Sci. Rep. 2016, 6, 25763. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, M.M.; Xue, C.; Bai, F.W.; Zhao, X.Q. Development of stress tolerant Saccharomyces cerevisiae strains by metabolic engineering: New aspects from cell flocculation and zinc supplementation. J. Biosci. Bioeng. 2017, 123, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Qiao, G.Q.; Shuai, B.W.; Song, K.N.; Yao, W.X.; Jiang, X.R.; Chen, G.Q. Engineering self-flocculating Halomonas campaniensis for wastewaterless open and continuous fermentation. Biotechnol. Bioeng. 2018, 116, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Established and advanced approaches for recovery of microbial polyhydroxyalkanoate (PHA) biopolyesters from surrounding microbial biomass. EuroBiotech. J. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Rebocho, A.T.; Pereira, J.R.; Neves, L.A.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Freitas, F.; Reis, M.A.M. Preparation and characterization of films based on a natural P(3HB)/mcl-PHA blend obtained through the co-culture of Cupriavidus necator and Pseudomonas citronellolis in apple pulp waste. Bioengineering 2020, 7, 34. [Google Scholar] [CrossRef]
- Ojha, N.; Das, N. Process optimization and characterization of polyhydroxyalkanoate copolymers produced by marine Pichia kudriavzevii VIT-NN02 using banana peels and chicken feather hydrolysate. Biocat. Agri. Biotechnol. 2020, 27, 101616. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Chiellini, E.; Braunegg, G. Extraction of short-chain- length poly-(I-hydroxyalkanoates) (scl-PHA) by the “anti-solvent” acetone under elevated temperature and pressure. Biotechnol. Lett. 2013, 35, 1023–1028. [Google Scholar] [CrossRef]
- Cerrone, F.; Radivojevic, J.; Nikodinovic-Runic, J.; Walsh, M.; Kenny, S.T.; Babu, R.; O’Connor, K.E. Novel sodium alkyl-1, 3-disulfates, anionic biosurfactants produced from microbial polyesters. Colloid Surface B 2019, 182, 110333. [Google Scholar] [CrossRef]
- Tang, S.; Baker, G.A.; Zhao, H. Ether- and alcohol-functionalized task-specific ionic liquids: Attractive properties and applications. Chem. Soc. Rev. 2012, 41, 4030–4066. [Google Scholar] [CrossRef]
- Fujita, K.; Kobayashi, D.; Nakamura, N.; Ohno, H. Direct dissolution of wet and saliferous marine microalgae by polar ionic liquids without heating. Enzyme Microb. Tech. 2013, 52, 199–202. [Google Scholar] [CrossRef]
- Kobayashi, D.; Fujita, K.; Nakamura, N.; Ohno, H. A simple recovery process for biodegradable plastics accumulated in cyanobacteria treated with ionic liquids. Appl. Microbiol. Biotechnol. 2015, 99, 1647–1653. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Lee, J.-K. Exploiting polyhydroxyalkanoates for biomedical applications. Polymers 2023, 15, 1937. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Gamero, J.E.; Favaro, L.; Pizzocchero, V.; Lomolino, G.; Basaglia, M.; Casella, S. Nuclease expression in efficient polyhydroxyalkanoates-producing bacteria could yield cost reduction during downstream processing. Bioresour. Technol. 2018, 261, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Kalia, V.C. Polyhydroxyalkanoate production and degradation patterns in Bacillus species. Indian J. Microbiol. 2017, 57, 387–392. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalia, V.C.; Patel, S.K.S.; Karthikeyan, K.K.; Jeya, M.; Kim, I.-W.; Lee, J.-K. Manipulating Microbial Cell Morphology for the Sustainable Production of Biopolymers. Polymers 2024, 16, 410. https://doi.org/10.3390/polym16030410
Kalia VC, Patel SKS, Karthikeyan KK, Jeya M, Kim I-W, Lee J-K. Manipulating Microbial Cell Morphology for the Sustainable Production of Biopolymers. Polymers. 2024; 16(3):410. https://doi.org/10.3390/polym16030410
Chicago/Turabian StyleKalia, Vipin C., Sanjay K. S. Patel, Kugalur K. Karthikeyan, Marimuthu Jeya, In-Won Kim, and Jung-Kul Lee. 2024. "Manipulating Microbial Cell Morphology for the Sustainable Production of Biopolymers" Polymers 16, no. 3: 410. https://doi.org/10.3390/polym16030410
APA StyleKalia, V. C., Patel, S. K. S., Karthikeyan, K. K., Jeya, M., Kim, I.-W., & Lee, J.-K. (2024). Manipulating Microbial Cell Morphology for the Sustainable Production of Biopolymers. Polymers, 16(3), 410. https://doi.org/10.3390/polym16030410









