Incorporation of Bayberry Tannin into a Locust Bean Gum/Carboxycellulose Nanocrystals/ZnO Coating: Properties and Its Application in Banana Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Equipment
2.2.1. Preparation of ZnO Clusters
2.2.2. Preparation of Composite Coatings
2.2.3. Characterizations
2.2.4. Performance Measurements
2.2.5. Fruits Preservation Effect Measurement
2.2.6. Statistics Analysis
3. Results and Discussion
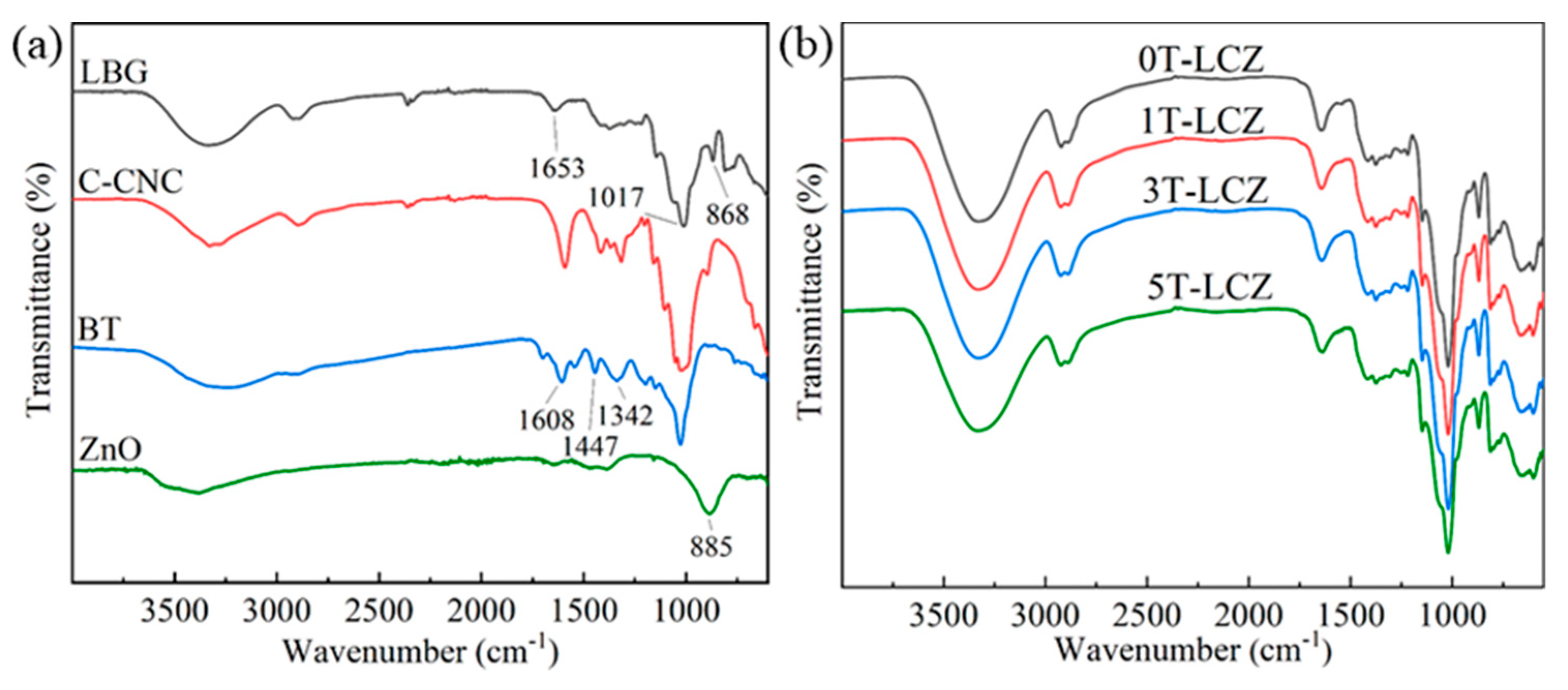
3.1. FTIR Analysis
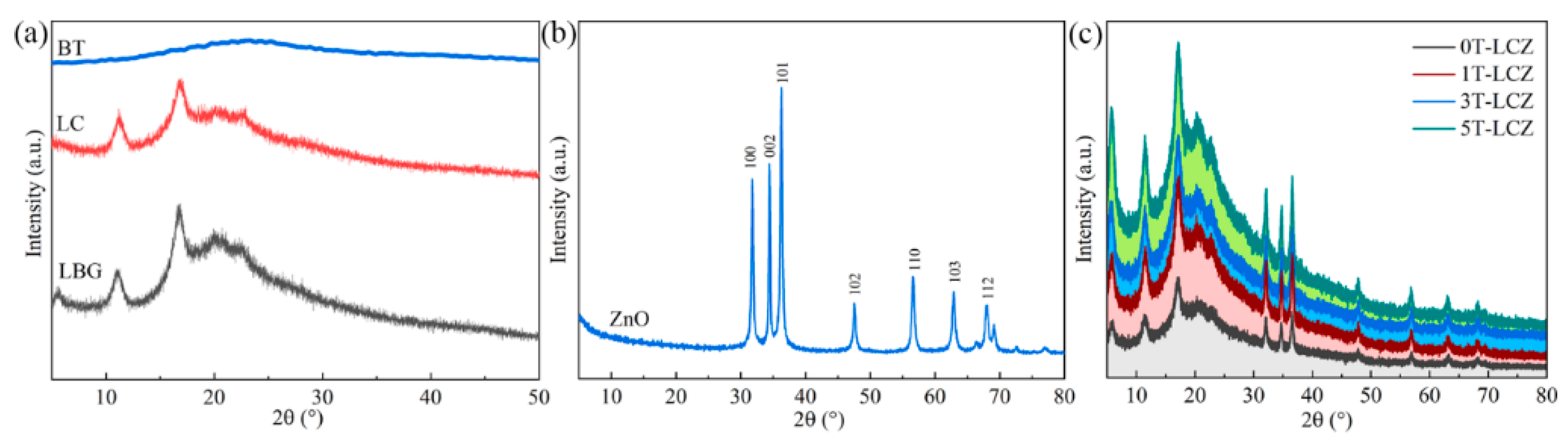
3.2. XRD Analysis
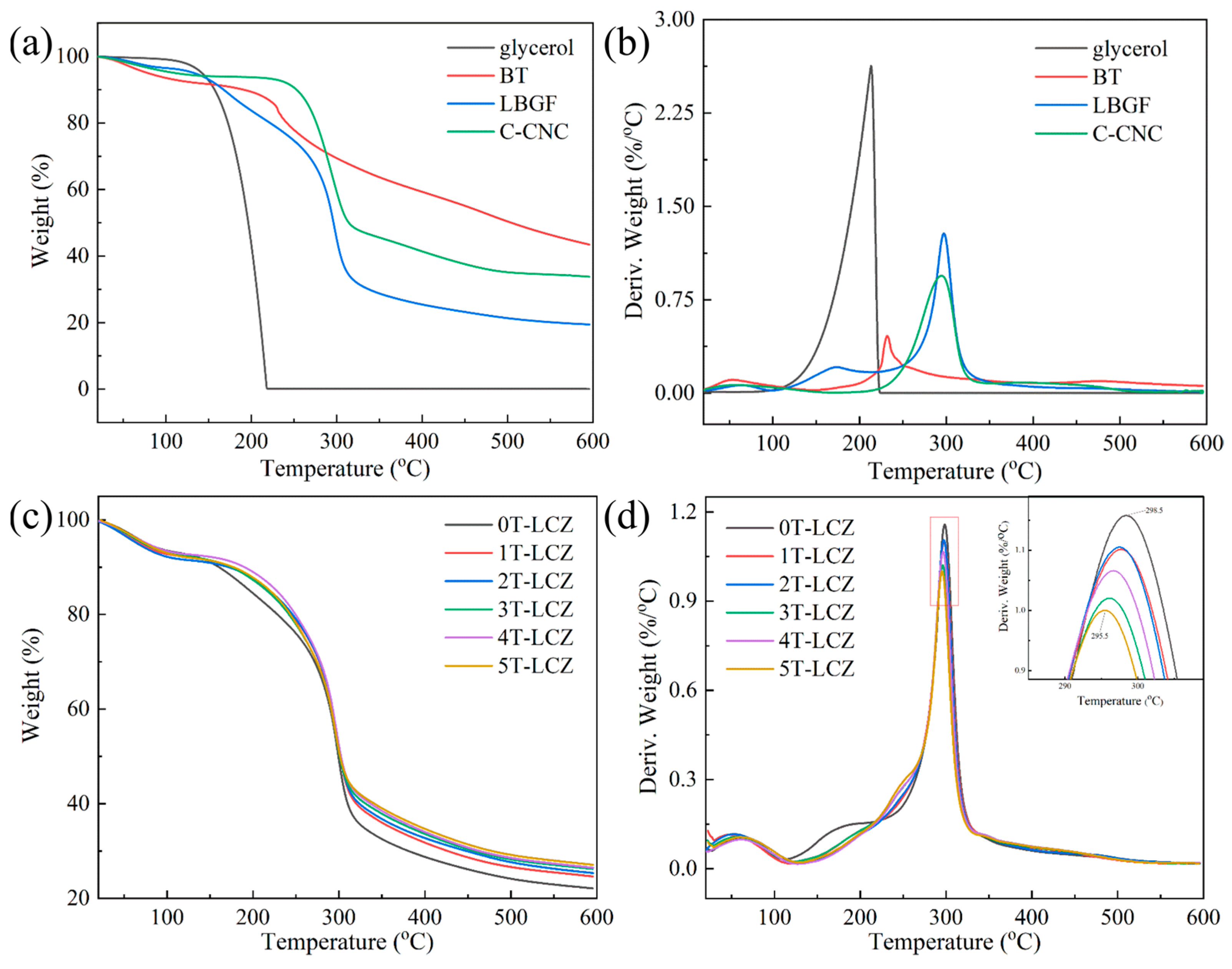
3.3. Thermal Stability
3.4. Physical Properties
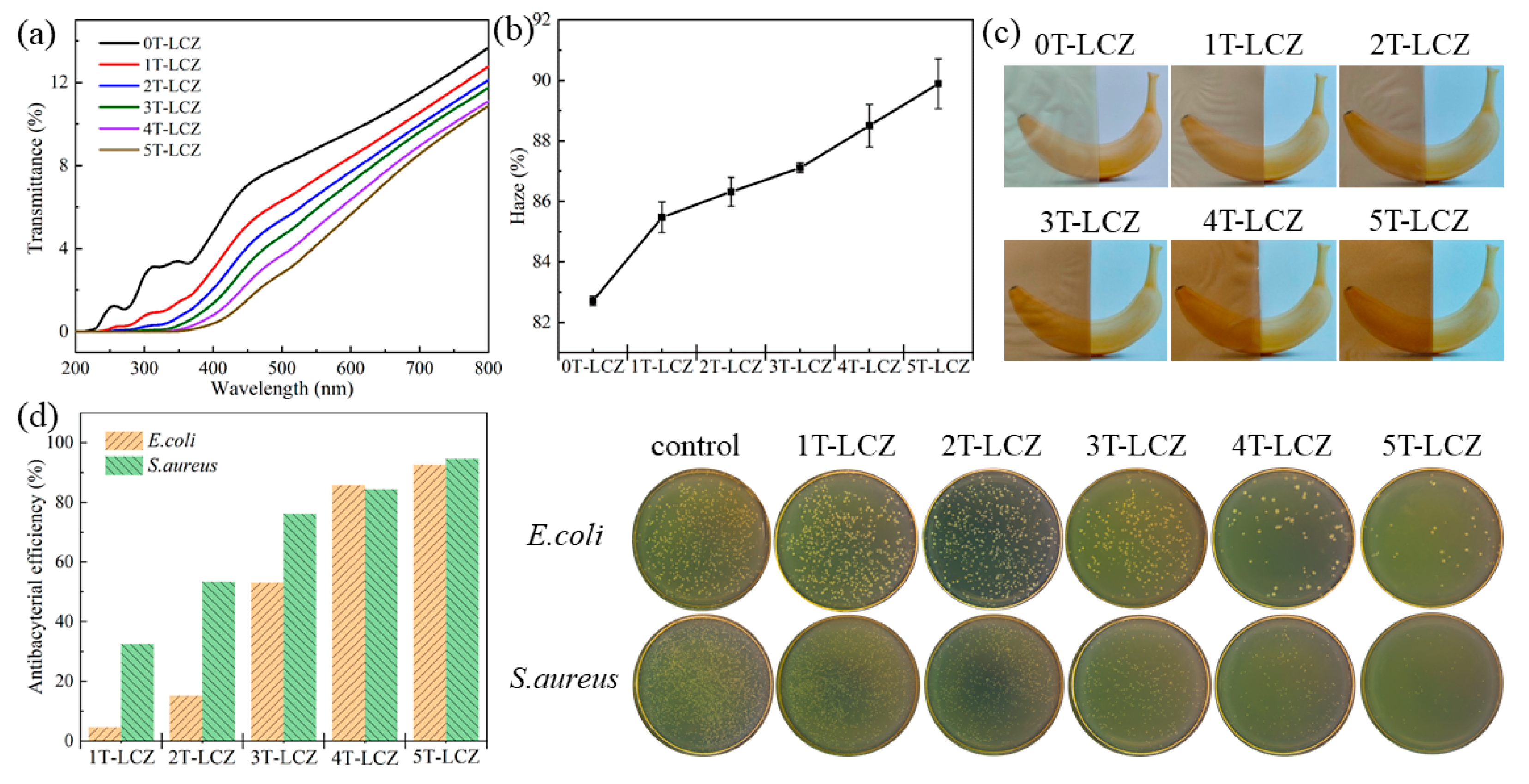
3.5. Optical Performance and Antibacterial Activity
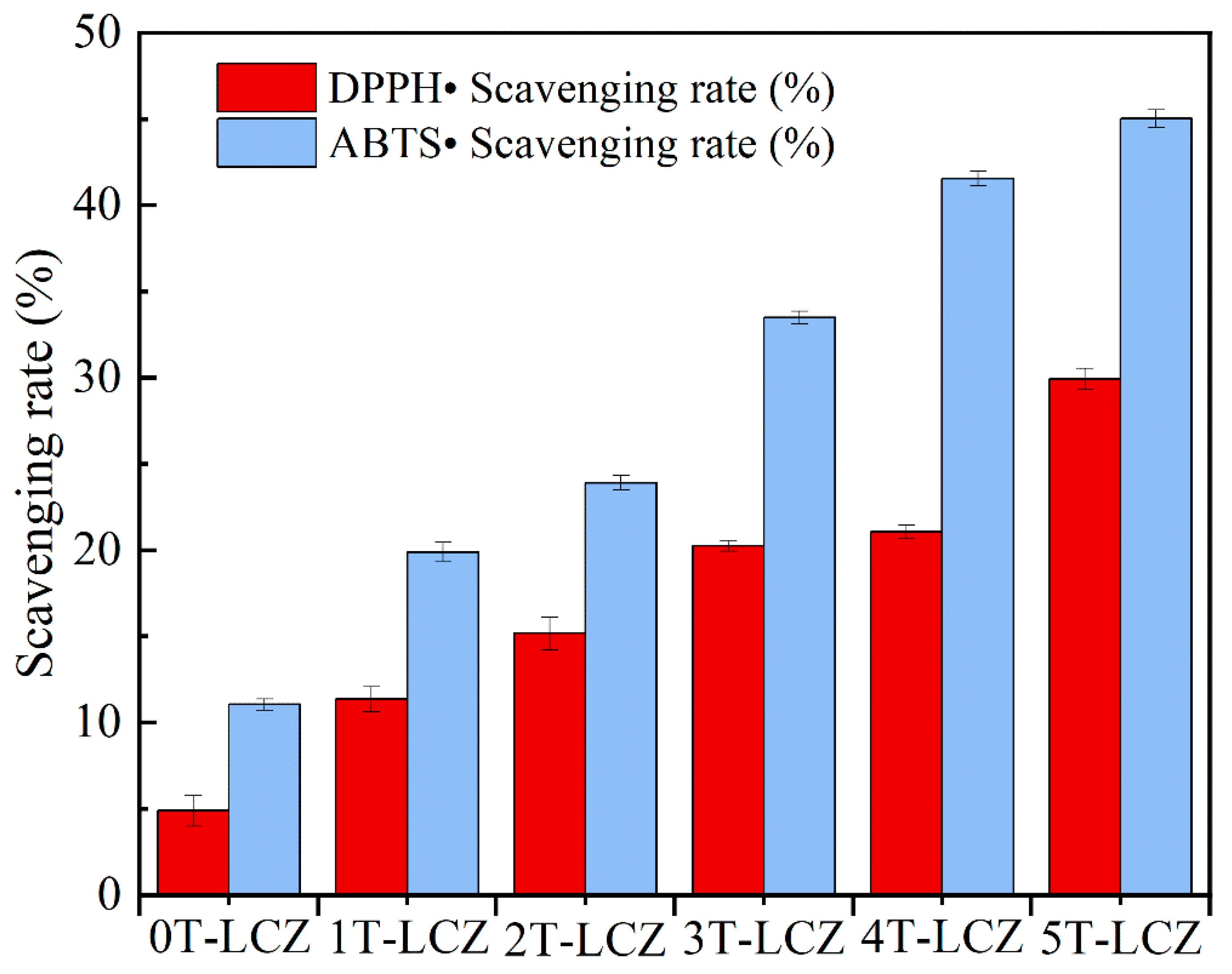
3.6. Antioxidation Property
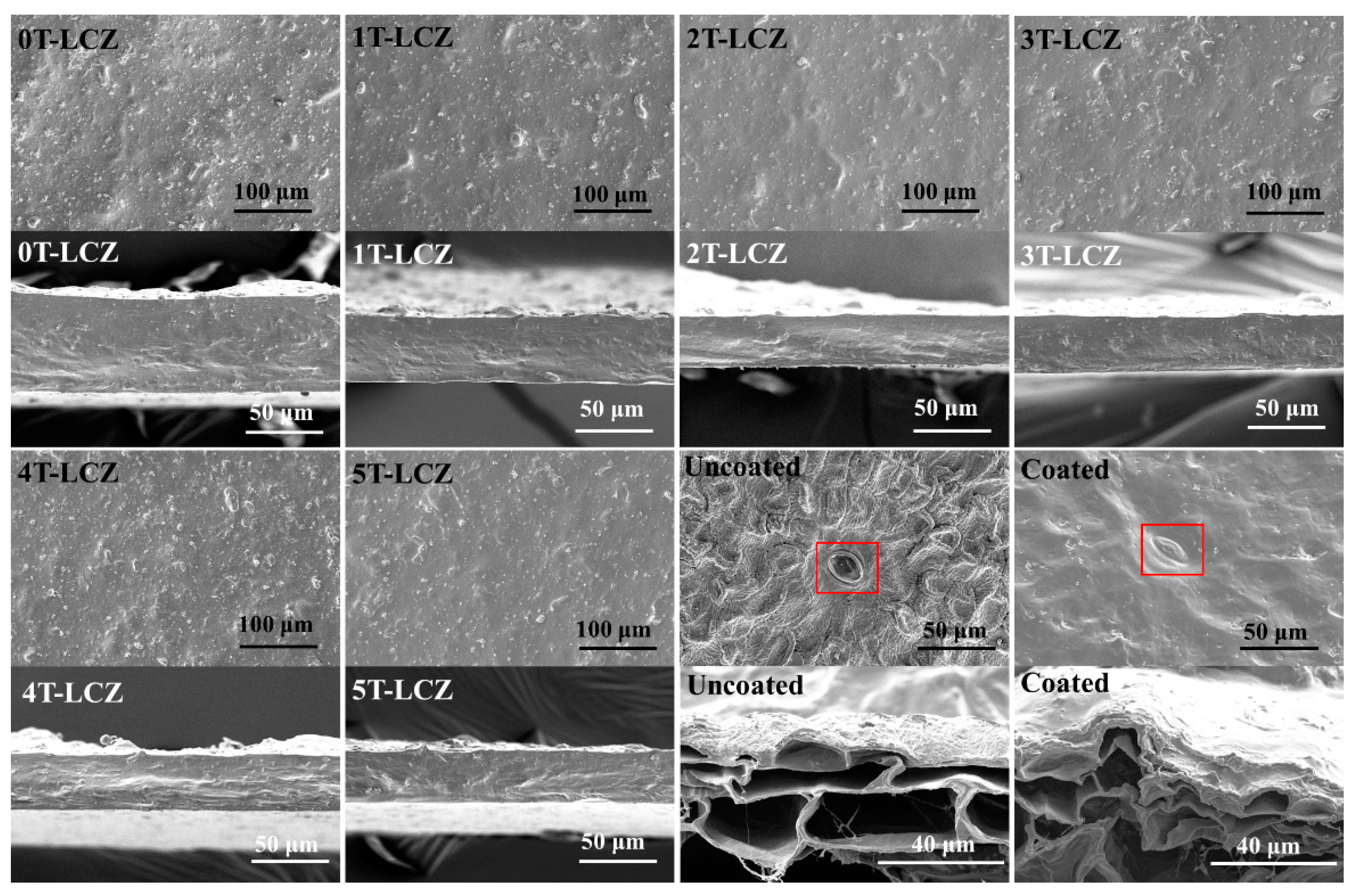
3.7. SEM
3.8. The Preservation Effect of Coatings on Bananas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Zhong, Y.; Li, L.; Jiang, K.; Gao, J.; Zhong, K.; Pan, M.; Yan, B. A multifunctional chitosan-derived conformal coating for the preservation of passion fruit. LWT 2022, 163, 113584. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Juric, S.; Bures, M.S.; Vlahovicek-Kahlina, K.; Stracenski, K.S.; Fruk, G.; Jalsenjak, N.; Bandic, L.M. Chitosan-based layer-by-layer edible coatings application for the preservation of mandarin fruit bioactive compounds and organic acids. Food Chem. X 2023, 17, 100575. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, L.; Geng, C.; Lan, T.; Huang, X.; Xu, S.; Shen, Y.; Bian, H. Green and multifunctional chitosan-based conformal coating as a controlled release platform for fruit preservation. Int. J. Biol. Macromol. 2022, 219, 767–778. [Google Scholar] [CrossRef]
- Botreau, H.; Cohen, M.J. Gender inequality and food insecurity: A dozen years after the food price crisis, rural women still bear the brunt of poverty and hunger. Adv. Food Secur. Sustain. 2020, 5, 53–117. [Google Scholar]
- Hong, H.; Luo, Y.; Zhou, Z.; Bao, Y.; Lu, H.; Shen, H. Effects of different freezing treatments on the biogenic amine and quality changes of bighead carp (Aristichthys nobilis) heads during ice storage. Food Chem. 2013, 138, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Pace, B.; Cefola, M. Innovative Preservation Technology for the Fresh Fruit and Vegetables. Foods 2021, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.Y.; Zhang, J.; Niu, B.; Li, W. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kang, S.; Zhang, Q.; Chen, S.; Yang, Q.; Yan, B. Self-assembly fabrication of chitosan-tannic acid/MXene composite film with excellent antibacterial and antioxidant properties for fruit preservation. Food Chem. 2023, 410, 135405. [Google Scholar] [CrossRef]
- Karimi Sani, I.; Masoudpour-Behabadi, M.; Alizadeh Sani, M.; Motalebinejad, H.; Juma, A.S.M.; Asdagh, A.; Eghbaljoo, H.; Khodaei, S.M.; Rhim, J.W.; Mohammadi, F. Value-added utilization of fruit and vegetable processing by-products for the manufacture of biodegradable food packaging films. Food Chem. 2023, 405, 134964. [Google Scholar] [CrossRef]
- Li, T.; Chi, W.; Ning, Y.; Xu, S.; Wang, L. Locust bean gum/carboxycellulose nanocrystal coating incorporating ZnO clusters built by the accretion of micro spindles or sheets for strawberries preservation. Int. J. Biol. Macromol. 2023, 226, 267–278. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Priyadarshi, R.; Han, S. Cellulose nanofiber-based coating film integrated with nitrogen-functionalized carbon dots for active packaging applications of fresh fruit. Postharvest Biol. Technol. 2022, 186, 111845. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, M.A.; Nawaz, A.; Naz, S.; Shah, A.A.; Gohari, G.; Razavi, F.; Khaliq, G.; Razzaq, K. Carboxymethyl cellulose coating regulates cell wall polysaccharides disassembly and delays ripening of harvested banana fruit. Postharvest Biol. Technol. 2022, 191, 111978. [Google Scholar] [CrossRef]
- Petriccione, M.; Pasquariello, M.S.; Mastrobuoni, F.; Zampella, L.; Di Patre, D.; Scortichini, M. Influence of a chitosan coating on the quality and nutraceutical traits of loquat fruit during postharvest life. Sci. Hortic. 2015, 197, 287–296. [Google Scholar] [CrossRef]
- Ali, A.; Maqbool, M.; Alderson, P.G.; Zahid, N. Effect of gum arabic as an edible coating on antioxidant capacity of tomato (Solanum lycopersicum L.) fruit during storage. Postharvest Biol. Technol. 2013, 76, 119–124. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Bowyer, M.; Singh, S.P.; Scarlett, C.J.; Stathopoulos, C.E.; Vuong, Q.V. A starch edible surface coating delays banana fruit ripening. LWT 2019, 100, 341–347. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications—A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Eze, F.N.; Jayeoye, T.J.; Eze, R.C. Construction, characterization and application of locust bean gum/Phyllanthus reticulatus anthocyanin—Based plasmonic silver nanocomposite for sensitive detection of ferrous ions. Env. Res. 2023, 228, 115864. [Google Scholar] [CrossRef]
- Li, T.; Liu, R.; Zhang, C.; Meng, F.; Wang, L. Developing a green film from locust bean gum/carboxycellulose nanocrystal for fruit preservation. Future Foods 2021, 4, 100072. [Google Scholar] [CrossRef]
- Huang, X.; Ji, Y.; Guo, L.; Xu, Q.; Jin, L.; Fu, Y.; Wang, Y. Incorporating tannin onto regenerated cellulose film towards sustainable active packaging. Ind. Crops Prod. 2022, 180, 114710. [Google Scholar] [CrossRef]
- Bajaj, K.; Adhikary, T.; Gill, P.P.S.; Kumar, A. Edible coatings enriched with plant-based extracts preserve postharvest quality of fruits: A review. Prog. Org. Coat. 2023, 182, 107669. [Google Scholar]
- Hou, X.; Xue, Z.; Liu, J.; Yan, M.; Xia, Y.; Ma, Z. Characterization and property investigation of novel eco-friendly agar/carrageenan/TiO2 nanocomposite films. J. Appl. Polym. Sci. 2018, 136, 47113. [Google Scholar] [CrossRef]
- Kassab, Z.; Aziz, F.; Hannache, H.; Ben Youcef, H.; El Achaby, M. Improved mechanical properties of k-carrageenan-based nanocomposite films reinforced with cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 123, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.T.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Souza, B.W.S.; Vicente, A.A. Synergistic effects between κ-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll. 2012, 29, 280–289. [Google Scholar] [CrossRef]
- Irman, N.; Abd Latif, N.H.; Brosse, N.; Gambier, F.; Syamani, F.A.; Hussin, M.H. Preparation and characterization of formaldehyde-free wood adhesive from mangrove bark tannin. Int. J. Adhes. Adhes. 2022, 114, 103094. [Google Scholar] [CrossRef]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Akkaya, A.; Şahin, B.; Aydın, R.; Çetin, H.; Ayyıldız, E. Solution-processed nanostructured ZnO/CuO composite films and improvement its physical properties by lustrous transition metal silver doping. J. Mater. Sci. Mater. Electron. 2020, 31, 14400–14410. [Google Scholar] [CrossRef]
- Taşdemir, A.; Aydin, R.; Akkaya, A.; Akman, N.; Altınay, Y.; Çetin, H.; Şahin, B.; Uzun, A.; Ayyıldız, E. A green approach for the preparation of nanostructured zinc oxide: Characterization and promising antibacterial behaviour. Ceram. Int. 2021, 47, 19362–19373. [Google Scholar]
- Zubair, N.; Akhtar, K. Morphology controlled synthesis of ZnO nanoparticles for in-vitro evaluation of antibacterial activity. Trans. Nonferrous Met. Soc. China 2020, 30, 1605–1614. [Google Scholar] [CrossRef]
- Araujo, F.P.; Trigueiro, P.; Honorio, L.M.C.; Oliveira, D.M.; Almeida, L.C.; Garcia, R.P.; Lobo, A.O.; Cantanhede, W.; Silva-Filho, E.C.; Osajima, J.A. Eco-friendly synthesis and photocatalytic application of flowers-like ZnO structures using Arabic and Karaya Gums. Int. J. Biol. Macromol. 2020, 165, 2813–2822. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Yu, Y.; Zhao, K.; Hou, X.; Zhang, Y. Multifunctional cotton fabric with directional water transport, UV protection and antibacterial properties based on tannin and laser treatment. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131131. [Google Scholar] [CrossRef]

| Samples | TS (MPa) | EB (%) | OP (cm3 mm m−2 Day−1 atm−1) | WVP (×10−11 g m−1 s−1 Pa−1) |
|---|---|---|---|---|
| 0T-LCZ | 31.80 ± 1.36 b | 24.80 ± 0.51 ab | 0.72 ± 0.13 ab | 1.90 ± 0.11 b |
| 1T-LCZ | 25.18 ± 2.41 a | 25.80 ± 1.33 bc | 1.27 ± 0.18 c | 2.50 ± 0.02 c |
| 2T-LCZ | 29.09 ± 0.95 b | 26.76 ± 1.02 c | 0.81 ± 0.05 ab | 2.01 ± 0.07 b |
| 3T-LCZ | 44.57 ± 2.58 d | 24.84 ± 1.13 ab | 0.66 ± 0.04 a | 1.67 ± 0.05 a |
| 4T-LCZ | 40.09 ± 2.89 c | 23.84 ± 0.39 a | 0.83 ± 0.01 ab | 1.70 ± 0.05 a |
| 5T-LCZ | 39.17 ± 2.02 c | 24.84 ± 0.79 ab | 0.92 ± 0.03 b | 1.71 ± 0.02 a |
| Bananas | Titratable Acid (%) | Vc Contents (mg/100 g) |
|---|---|---|
| Fresh | 0.57 ± 0.01 c | 7.37 ± 0.49 b |
| Uncoated | 0.30 ± 0.01 a | 4.77 ± 0.19 a |
| 3T-LCZ | 0.44 ± 0.01 b | 5.31 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, W.; Li, T.; Wei, N.; Pan, Z.; Wang, L. Incorporation of Bayberry Tannin into a Locust Bean Gum/Carboxycellulose Nanocrystals/ZnO Coating: Properties and Its Application in Banana Preservation. Polymers 2023, 15, 3364. https://doi.org/10.3390/polym15163364
Chi W, Li T, Wei N, Pan Z, Wang L. Incorporation of Bayberry Tannin into a Locust Bean Gum/Carboxycellulose Nanocrystals/ZnO Coating: Properties and Its Application in Banana Preservation. Polymers. 2023; 15(16):3364. https://doi.org/10.3390/polym15163364
Chicago/Turabian StyleChi, Wenrui, Tingting Li, Na Wei, Zijing Pan, and Lijuan Wang. 2023. "Incorporation of Bayberry Tannin into a Locust Bean Gum/Carboxycellulose Nanocrystals/ZnO Coating: Properties and Its Application in Banana Preservation" Polymers 15, no. 16: 3364. https://doi.org/10.3390/polym15163364
APA StyleChi, W., Li, T., Wei, N., Pan, Z., & Wang, L. (2023). Incorporation of Bayberry Tannin into a Locust Bean Gum/Carboxycellulose Nanocrystals/ZnO Coating: Properties and Its Application in Banana Preservation. Polymers, 15(16), 3364. https://doi.org/10.3390/polym15163364






