One-Dimensional Croconate-Based Fe-CP as a High-Performance Anode Material for Lithium–Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Electrochemical Analyses
2.3. Synthesis of the Fe-CP
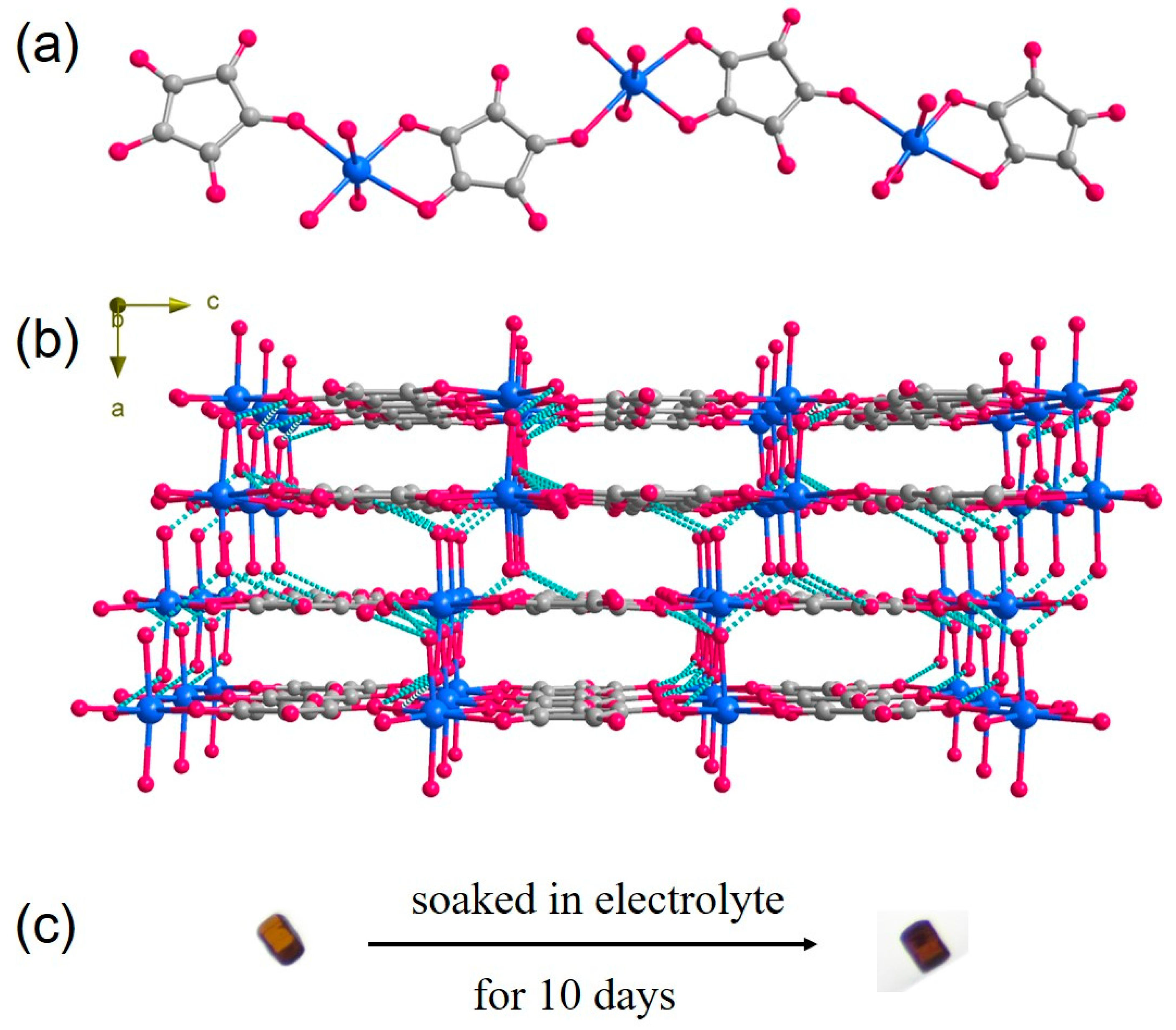
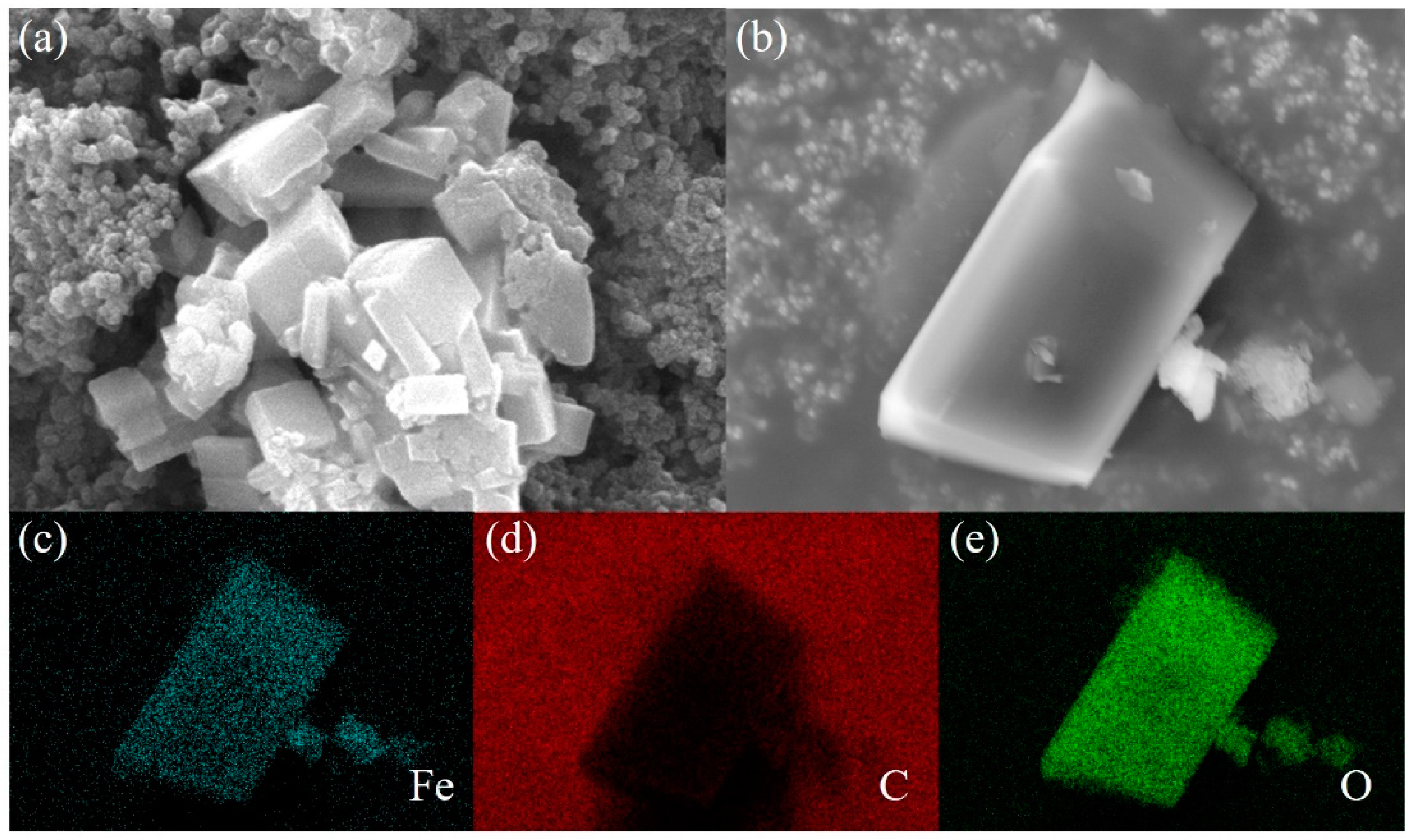
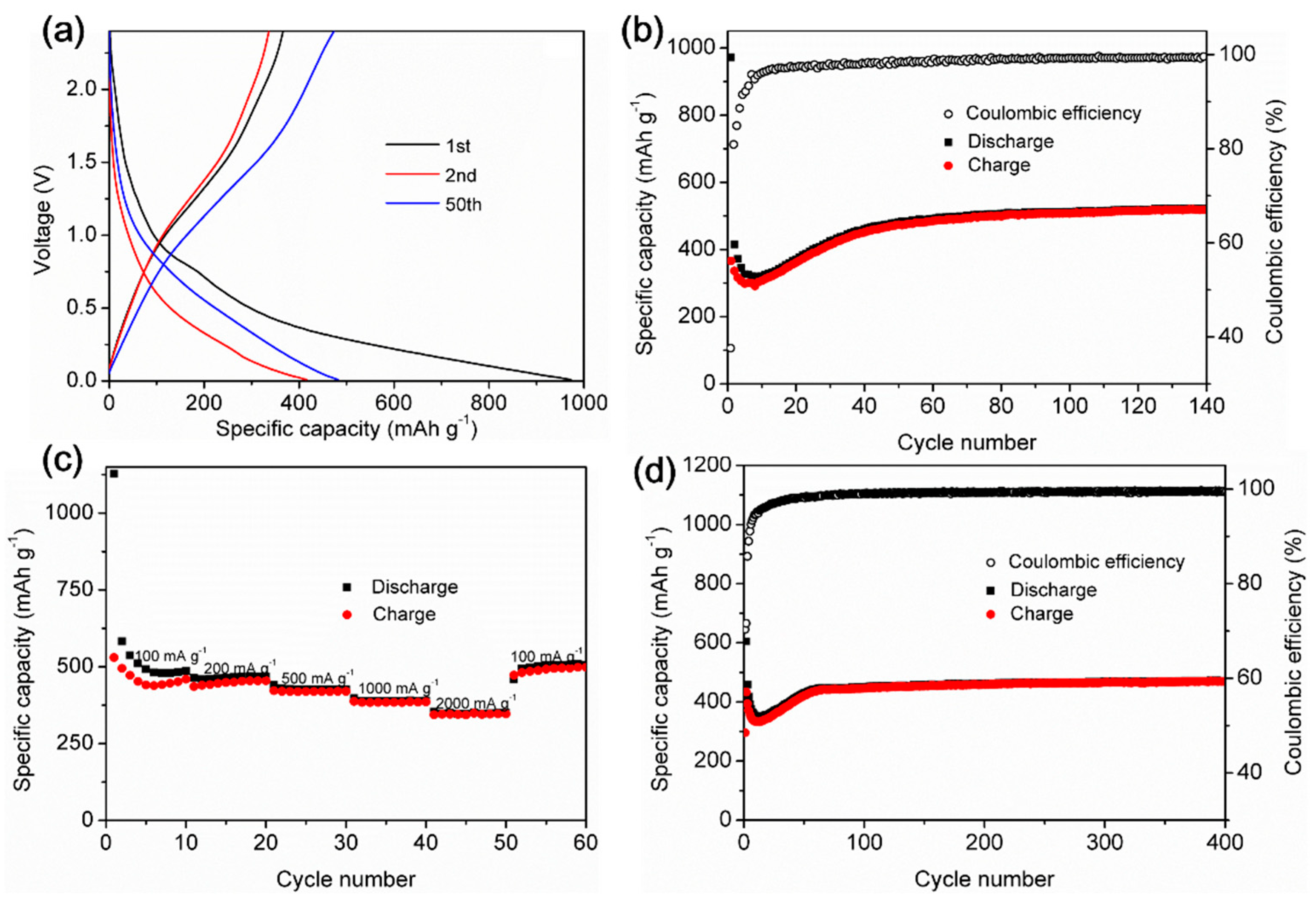
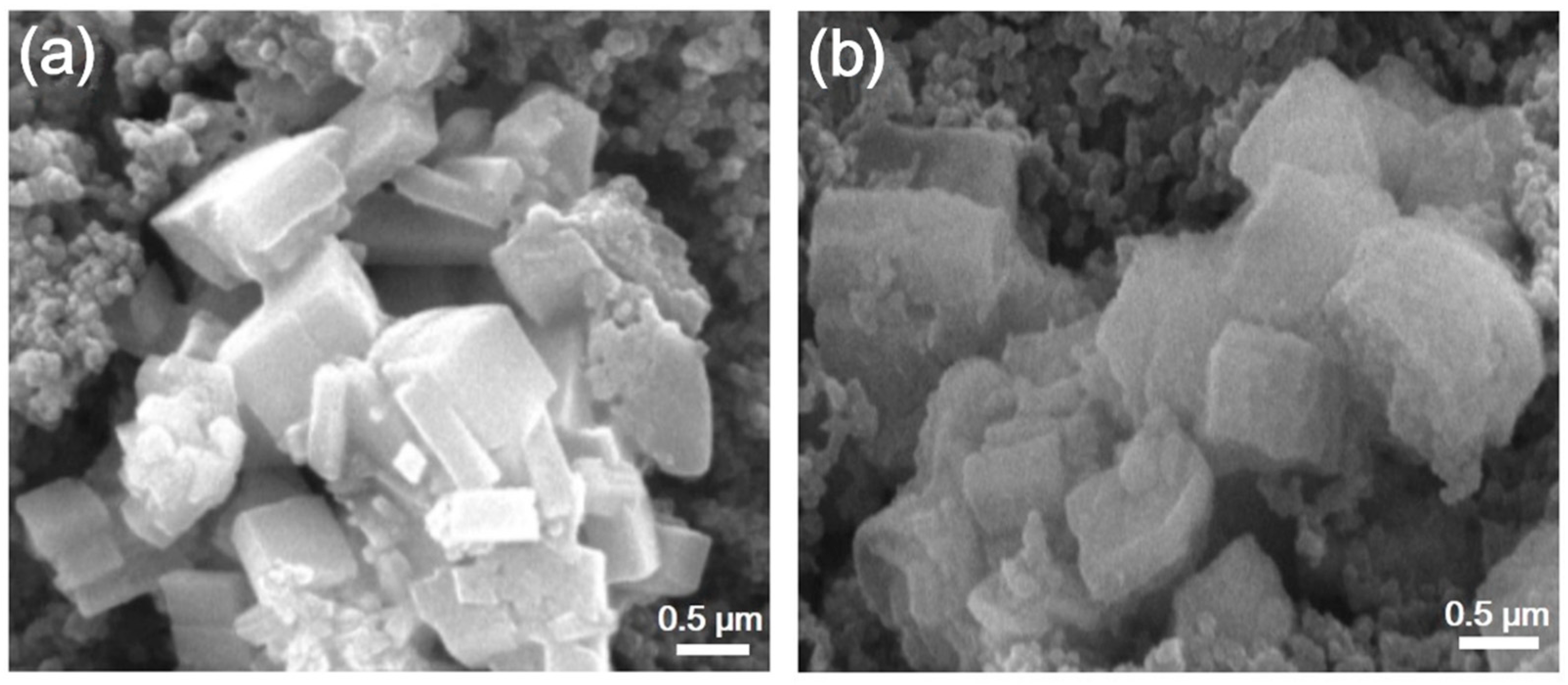
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Lv, Z.S.; Li, W.L.; Yang, L.; Loh, X.J.; Chen, X.D. Custom-Made Electrochemical Energy Storage Devices. ACS Energy Lett. 2019, 4, 606–614. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.M.; Li, K.; Lin, Y.P.; Chen, J.M.; Gao, L.J.; Nicolosi, V.; Xiao, X.; Lee, J.-M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354–1390. [Google Scholar] [CrossRef]
- Kong, L.J.; Liu, M.; Huang, H.; Xu, Y.H.; Bu, X.-H. Metal/Covalent-Organic Framework Based Cathodes for Metal-Ion Batteries. Adv. Energy Mater. 2022, 12, 2100172. [Google Scholar] [CrossRef]
- Li, J.L.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Liu, T.; Yang, X.-G.; Ge, S.H.; Stanley, N.V.; Rountree, E.S.; Leng, Y.J.; McCarthy, B.D. Fast charging of energy-dense lithium-ion batteries. Nature 2022, 611, 485–490. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.X.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Wang, K.; Zhang, G.F.; Li, S.N.; Xu, Y.N.; Zhang, X.D.; Zhang, X.; Zheng, S.H.; Sun, X.Z.; Ma, Y.W. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Liu, B.Y.; Jiang, K.; Zhu, K.; Liu, X.L.; Ye, K.; Yan, J.; Wang, J.L.; Cao, D.X. Cable-like polyimide@carbon nanotubes composite as a capable anode for lithium ion batteries. Chem. Eng. J. 2022, 446, 137208. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.Y.; Gao, G. Recent progress of advanced anode materials of lithium-ion batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kang, I.; Choi, J.; Jeong, H.; Yi, K.-W.; Hong, J.; Lee, M. Molecularly Tailored Lithium-Arene Complex Enables Chemical Prelithiation of High-Capacity Lithium-Ion Battery Anodes. Angew. Chem. Int. Ed. 2020, 59, 14473–14480. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bai, Y.L.; Wang, Y.; Xu, H.; Jin, H. Advances in transition-metal (Zn, Mn, Cu)-based MOFs and their derivatives for anode of lithium-ion batteries. Coordi. Chem. Rev. 2020, 410, 213221. [Google Scholar] [CrossRef]
- Dong, C.L.; Dong, W.J.; Lin, X.Y.; Zhao, Y.T.; Li, R.Z.; Huang, F.Q. Recent progress and perspectives of defective oxide anode materials for advanced lithium ion battery. EnergyChem 2020, 2, 100045. [Google Scholar] [CrossRef]
- Xing, Z.Y.; Wang, S.; Yu, A.P.; Chen, Z.W. Aqueous intercalation-type electrode materials for grid-level energy storage: Beyond the limits of lithium and sodium. Nano Energy 2018, 50, 229–244. [Google Scholar] [CrossRef]
- Liu, J.L.; Xu, C.H.; Chen, Z.; Ni, S.B.; Shen, Z.X. Progress in aqueous rechargeable batteries. Green Energy Environ. 2018, 3, 20–41. [Google Scholar] [CrossRef]
- Cheng, Q.H.; Zhao, X.; Yang, G.Y.; Mao, L.; Liao, F.F.; Chen, L.Y.; He, P.G.; Pan, D.J.; Chen, S.W. Recent advances of metal phosphates-based electrodes for high-performance metal ion batteries. Energy Storage Mater. 2021, 41, 842. [Google Scholar] [CrossRef]
- Wu, B.; Xie, Y.; Meng, Y.Q.; Qian, C.; Chen, Y.Y.; Yuan, A.H.; Guo, X.M.; Yang, H.X.; Wan, S.J.; Lin, S.L. Construction of unique heterogeneous cobalt–manganese oxide porous microspheres for the assembly of long-cycle and high-rate lithium ion battery anodes. J. Mater. Chem. A 2019, 7, 6149–6160. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Zhu, C.X.; Yu, S.C.; Ge, D.H.; Zhou, H.S. Status and challenges facing representative anode materials for rechargeable lithium batteries. J. Energy Chem. 2022, 66, 260–294. [Google Scholar] [CrossRef]
- Vlad, A.; Chen, J.; Yao, Y. Organic Electrode Materials and Engineering for Electrochemical Energy Storage. Batter. Supercaps 2023, 6, e202300090. [Google Scholar] [CrossRef]
- Xu, D.Y.; Liang, M.X.; Qi, S.; Sun, W.W.; Lv, L.P.; Du, F.H.; Wang, B.F.; Chen, S.Q.; Wang, Y.; Yu, Y. The Progress and Prospect of Tunable Organic Molecules for Organic Lithium-Ion Batteries. ACS Nano 2021, 15, 47–80. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Liao, Y.C.; Hu, Q.; Zeng, Z.W.; Yi, L.; Wang, Y.D.; Lu, H.J.; Pan, M. Multi carbonyl polyimide as high capacity anode materials for lithium ion batteries. J. Power Sources 2020, 451, 227792. [Google Scholar] [CrossRef]
- Wang, H.; Yao, C.-J.; Nie, H.-J.; Wang, K.-Z.; Zhong, Y.-W.; Chen, P.W.; Mei, S.L.; Zhang, Q.C. Recent progress in carbonyl-based organic polymers as promising electrode materials for lithium-ion batteries (LIBs). J. Mater. Chem. A 2020, 8, 11906–11922. [Google Scholar] [CrossRef]
- Zhang, S.N.; Ren, S.; Han, D.M.; Xiao, M.; Wang, S.J.; Sun, L.Y.; Meng, Y.Z. A Highly Immobilized Organic Anode Material for High Performance Rechargeable Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 36237–36246. [Google Scholar] [CrossRef]
- Deng, Q.J.; Luo, Z.B.; Wang, Y.M.; Liu, H.X.; Zhou, Y.Y.; Yang, R. N-contained organic carbonyl potassium salt with Mult-active sites as a High-capacity anode for Potassium-ion batteries. Mater. Lett. 2021, 301, 130247. [Google Scholar] [CrossRef]
- Zhang, C.J.; Chen, S.; Zhou, G.Y.; Hou, Q.; Wang, Y.H.; Shi, G. A Polythiophene Material Featuring a Conjugated Carbonyl Side Group as an Anode for Lithium-Ion Batteries. ChemistrySelect 2022, 7, e202201699. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.W.; Shi, W.; Cheng, P. Synthesis strategies and potential applications of metal-organic frameworks for electrode materials for rechargeable lithium ion batteries. Coordi. Chem. Rev. 2019, 388, 293–309. [Google Scholar] [CrossRef]
- Tong, Y.H.; Xu, H.Y.; Li, T.; Kong, Z.; Li, J.W.; Fan, Q.H.; Xu, H.; Jin, H.; Wang, K.L. A novel flower-like hierarchical aluminum-based MOF anode for high-performance lithium-ion batteries. CrystEngComm 2022, 24, 6944–6952. [Google Scholar] [CrossRef]
- Liu, K.Y.; Li, C.; Yan, L.J.; Fan, M.Q.; Wu, Y.C.; Meng, X.H.; Ma, T.L. MOFs and their derivatives as Sn-based anode materials for lithium/sodium ion batteries. J. Mater. Chem. A 2021, 9, 27234–27251. [Google Scholar] [CrossRef]
- Liu, J.W.; Xie, D.X.; Shi, W.; Cheng, P. Coordination compounds in lithium storage and lithium-ion transport. Chem. Soc. Rev. 2020, 49, 1624–1642. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Zheng, M.X.; Wu, S.Y.; Zhang, L. Design strategies for coordination polymers as electrodes and electrolytes in rechargeable lithium batteries. Coordi. Chem. Rev. 2023, 483, 215084. [Google Scholar] [CrossRef]
- Zhang, Q.; Cui, X.; Hao, S.M.; Zhang, Q.; Guo, Z.P.; Li, H.Q.; Lin, Z.Q.; Yang, Y.K. Chain engineering of carbonyl polymers for sustainable lithium-ion batteries. Mater. Today 2021, 50, 170–198. [Google Scholar] [CrossRef]
- Guo, L.Z.; Sun, J.F.; Sun, X.; Zhang, J.Y.; Hou, L.R.; Yuan, C.Z. Construction of 1D conductive Ni-MOF nanorods with fast Li+ kinetic diffusion and stable high-rate capacities as an anode for lithium ion batteries. Nanoscale Adv. 2019, 1, 4688–4691. [Google Scholar] [CrossRef]
- Yue, X.-Y.; Yao, Y.-X.; Zhang, J.; Yang, S.Y.; Li, Z.H.; Yan, C.; Zhang, Q. Unblocked Electron Channels Enable Efficient Contact Prelithiation for Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2110337. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.B.; Chen, C.C.; Ma, T.; Chen, J. Nanostructured organic electrode materials grown on graphene with covalent-bond interaction for high-rate and ultra-long-life lithium-ion batteries. Nano Res. 2017, 10, 4245–4255. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Li, L.; Niu, Z.Q.; Chen, J. Design Strategies toward Enhancing the Performance of Organic Electrode Materials in Metal-Ion Batteries. Chem 2018, 4, 2786–2813. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, F.Y.; Shi, W.; Chen, J.; Cheng, P. Transition-Metal-Triggered High-Efficiency Lithium Ion Storage via Coordination Interactions with Redox-Active Croconate in One-Dimensional Metal-Organic Anode Materials. ACS Appl. Mater. Interfaces 2018, 10, 6398–6406. [Google Scholar] [CrossRef]
- West, R.; Niu, H.Y. New aromatic anions. VI. Complexes of croconate ion with some divalent and trivalent metals. J. Am. Chem. Soc. 1963, 85, 2586–2588. [Google Scholar] [CrossRef]
- Cornia, A.; Fabretti, A.C.; Giusti, A.; Ferraro, F.; Gatteschi, D. Molecular structure and magnetic properties of copper(II), manganese(II) and iron(II) croconate tri-hydrate. Inorg. Chim. Acta 1993, 212, 87–94. [Google Scholar] [CrossRef]
- Deguenon, D.; Bernardinelli, G.; Tuchagues, J.P.; Castan, P. Molecular crystal structure and magnetic properties of (croconato)- and (oxalato)manganese(II) complexes. Inorg. Chem. 1990, 29, 3031–3037. [Google Scholar] [CrossRef]
- Sanati-Tirgan, P.; Eshghi, H.; Mohammadinezhad, A. Designing a new method for growing metal-organic framework (MOF) on MOF: Synthesis, characterization and catalytic applications. Nanoscale 2023, 15, 4917–4931. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Yang, H.; Liang, F.; Xue, D.F. Microwave-irradiation-assisted combustion toward modified graphite as lithium ion battery anode. ACS Appl. Mater. Interfaces 2018, 10, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.W.; Jiang, Z.J.; Wang, P.X.; Jensen, L.R.; Zhang, Y.F.; Yue, Y.Z. Optimized assembling of MOF/SnO2/Graphene leads to superior anode for lithium ion batteries. Nano Energy 2020, 74, 104868. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, Y. Multi-channel graphite for high-rate lithium ion battery. J. Electrochem. Soc. 2018, 165, A1104. [Google Scholar] [CrossRef]
- Son, D.K.; Kim, J.; Raj, M.R.; Lee, G. Elucidating the structural redox behaviors of nanostructured expanded graphite anodes toward fast-charging and high-performance lithium-ion batteries. Carbon 2021, 175, 187–201. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, Y.E.; Kim, H. Improved fast charging capability of graphite anodes via amorphous Al2O3 coating for high power lithium ion batteries. J. Power Sources 2019, 422, 18–24. [Google Scholar] [CrossRef]
- Wang, J.X.; Xia, Y.; Liu, Y.; Li, W.; Zhao, D.Y. Mass production of large-pore phosphorus-doped mesoporous carbon for fast-rechargeable lithium-ion batteries. Energy Storage Mater. 2019, 22, 147–153. [Google Scholar] [CrossRef]
- Mei, J.; Yi, T.F.; Li, X.Y.; Zhu, Y.R.; Xie, Y.; Zhang, C.F. Robust strategy for crafting Li5Cr7Ti6O25@CeO2 composites as high-performance anode material for lithium-ion battery. ACS Appl. Mater. Interfaces 2017, 9, 23662–23671. [Google Scholar] [CrossRef]
- Liu, G.Y.; Jin, B.; Zhang, R.X.; Bao, K.Y.; Xie, H.Q.; Guo, J.L.; Wei, M.; Jiang, Q. Synthesis of Ti2Nb10O29/C composite as an anode material for lithium-ion batteries. Int. J. Hydrogen Energy 2016, 41, 14807–14812. [Google Scholar] [CrossRef]
- Wang, L.F.; Wei, G.; Dong, X.Y.; Zhao, Y.L.; Xing, Z.; Hong, H.P.; Ju, Z.C. Hollow α-Fe2O3 Nanotubes Embedded in Graphene Aerogel as High-Performance Anode Material for Lithium-Ion Batteries. ChemistrySelect 2019, 4, 11370–11377. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, R.X.; Chen, Y.L.; Jiang, H.; Zeng, Y.B.; Chen, X.; Zhang, Y.; Huang, N.M.; Guo, H. MOF-derived transition metal oxide encapsulated in carbon layer as stable lithium ion battery anodes. J. Alloys Compd. 2019, 797, 83–91. [Google Scholar] [CrossRef]
- Liu, F.; Liu, S.Y.; Meng, J.S.; Xia, F.J.; Xiao, Z.T.; Liu, Z.A.; Li, Q.; Wu, J.S.; Mai, L.Q. Stabilizing conversion reaction electrodes by MOF derived N-doped carbon shell for highly reversible lithium storage. Nano Energy 2020, 73, 104758. [Google Scholar] [CrossRef]
- Liu, F.; Liao, S.Y.; Lin, H.; Yin, Y.W.; Liu, Y.D.; Meng, H.; Min, Y. A Facile Strategy for Synthesizing Organic Tannic Metal Salts as Advanced Energy Storage Anodes. ChemElectroChem 2021, 8, 2686–2692. [Google Scholar] [CrossRef]
- Xiao, F.Y.; Liu, P.; Li, J.; Zhang, Y.W.; Liu, Y.J.; Xu, M.W. A Small Molecule Organic Compound Applied as an Advanced Anode Material for Lithium-Ion Batteries. Chem. Commun. 2022, 58, 697–700. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Hou, S.; Eidson, N.; Fan, X.L.; Liang, Y.J.; Deng, T.; Jiang, J.J.; Wang, C.S. Azo Compounds Derived from Electrochemical Reduction of Nitro Compounds for High Performance Li-Ion Batteries. Adv. Mater. 2018, 30, 1706498. [Google Scholar] [CrossRef]
- Lakraychi, A.E.; Dolhem, F.; Djedaïni-Pilard, F.; Becuwe, M. Substituent Effect on Redox Potential of Terephthalate-Based Electrode Materials for Lithium Batteries. Electrochem. Commun. 2018, 93, 71–75. [Google Scholar] [CrossRef]
- Lei, Z.D.; Chen, X.D.; Sun, W.W.; Zhang, Y.; Wang, Y. Exfoliated Triazine-Based Covalent Organic Nanosheets with Multielectron Redox for High-Performance Lithium Organic Batteries. Adv. Energy Mater. 2019, 9, 1801010. [Google Scholar] [CrossRef]
- Wu, M.M.; Zhao, Y.; Zhang, H.T.; Zhu, J.; Ma, Y.F.; Li, C.X.; Zhang, Y.M.; Chen, Y.S. A 2D Covalent Organic Framework with Ultra-Large Interlayer Distance as High-Rate Anode Material for Lithium-Ion Batteries. Nano Res. 2021, 15, 9779–9784. [Google Scholar] [CrossRef]
- Buyukcakir, O.; Ryu, J.; Joo, S.H.; Kang, J.; Yuksel, R.; Lee, J.; Jiang, Y.; Choi, S.; Lee, S.H.; Kwak, S.K.; et al. Lithium Accommodation in a Redox-Active Covalent Triazine Framework for High Areal Capacity and Fast-Charging Lithium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 2003761. [Google Scholar] [CrossRef]
- Fei, H.L.; Liu, X.; Li, Z.W.; Feng, W.J. Metal dicarboxylates: New anode materials for lithium-ion batteries with good cycling performance. Dalton Trans. 2015, 44, 9909–9914. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.D.; Xia, Q.H.; Xue, X.; Liu, Q.; Liu, H.J. Synthesis of cobalt-based layered coordination polymer nanosheets and their application in lithium-ion batteries as anode materials. RSC Adv. 2016, 6, 4442–4447. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, H.D.; Huang, J.M.; Zhang, L. MOF-derived α-MnSe/C composites as anode materials for Li-ion batteries. Ceram. Int. 2019, 45, 23765–23771. [Google Scholar] [CrossRef]
- Lin, X.M.; Niu, J.L.; Lin, J.; Wei, L.M.; Hu, L.; Zhang, G.; Cai, Y.P. Lithium-ion-battery anode materials with improved capacity from a metal-organic framework. Inorg. Chem. 2016, 55, 8244–8247. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Pramanik, A.; Manju, U.; Mahanty, S. Cu3(1,3,5-benzenetricarboxylate)2 metal-organic framework: A promising anode material for lithium-ion battery. Microporous Mesoporous Mater. 2016, 226, 353–359. [Google Scholar] [CrossRef]
- Mutahir, S.; Wang, C.X.; Song, J.J.; Wang, L.; Lei, W.; Jiao, X.Y.; Khan, M.A.; Zhou, B.J.; Zhong, Q.; Hao, Q.L. Pristine Co(BDC)TED0.5 a pillared-layer biligand cobalt based metal organic framework asimproved anode material for lithium-ion batteries. Appl. Mater. Today 2020, 21, 100813. [Google Scholar] [CrossRef]
- Hu, L.; Lin, X.M.; Mo, J.T.; Lin, J.; Gan, H.L.; Yang, X.L.; Cai, Y.P. Lead-based Metal-Organic framework with stable lithium anodic performance. Inorg. Chem. 2017, 56, 4289–4295. [Google Scholar] [CrossRef]
- Liu, J.W.; Zheng, M.X.; Dang, S.F.; Zhang, L.; Wu, S.Y. Design of 3D Metal-Organic Material with Multiple Redox-Active Sites for High-Performance Lithium-Ion Batteries. Energy Fuels 2023. [Google Scholar] [CrossRef]
- Chen, J.X.; Mu, X.X.; Du, M.J.; Lou, Y.B. Porous rod-shaped Co3O4 derived from Co-MOF-74 as high-performance anode materials for lithium ion batteries. Inorg. Chem. Commun. 2017, 84, 241–245. [Google Scholar]
- Li, G.H.; Yang, H.; Li, F.C.; Du, J.; Shi, W.; Cheng, P. Facile formation of a nanostructured NiP2@C material for advanced lithium-ion battery anode using adsorption property of metal-organic framework. J. Mater. Chem. A 2016, 4, 9593–9599. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.K.; Zhang, Y.S.; Hou, R.H.; Zhang, X.L.; Xue, C.; Wang, S.B.; Zhu, B.C.; Li, N.; Shao, G.S. Photogenerated electron transfer process in heterojunctions: In situ irradiation XPS. Small Methods 2020, 4, 2000214. [Google Scholar] [CrossRef]
- Bi, H.B.; Wang, C.X.; Lin, Q.Z.; Jiang, X.D.; Jiang, C.L.; Bao, L. Combustion behavior, kinetics, gas emission characteristics and artificial neural network modeling of coal gangue and biomass via TG-FTIR. Energy 2020, 213, 118790. [Google Scholar] [CrossRef]
- Yan, W.; Fan, K.; Zheng, L.-M.; Jin, Z. Cluster-Bridging-Coordinated Bimetallic Metal-Organic Framework as High-Performance Anode Material for Lithium-Ion Storage. Small Struct. 2021, 2, 2100122. [Google Scholar] [CrossRef]

| Type of Anode Material | Typical Examples | Current Density (mA g−1) | Capacity (mA h g−1) | Cycle Number | Ref. |
|---|---|---|---|---|---|
| Conventional inorganic materials | Multichannel graphite | 0.1C | 365 | 3000 | [46] |
| Expanded graphite | 100 | 338 | 500 | [47] | |
| Al2O3@graphite | 100 | 335 | 100 | [48] | |
| P-doped mesoporous C | 0.5C | 500 | 200 | [49] | |
| Li5Cr7Ti6O25@CeO2 | 5C | 100.5 | 100 | [50] | |
| Ti2Nb10O29/C | 10C | 194 | 100 | [51] | |
| Fe2O3 nanotubes on 3DG aerogel | 100 | 1406 | 65 | [52] | |
| Co3O4@C | 250 | 721 | 500 | [53] | |
| ZnO@C | 250 | 526 | 500 | [53] | |
| Zn2SiO4 nanowires | 100 | 455.8 | 100 | [54] | |
| Zn2SiO4@NC nanowire | 100 | 685.2 | 100 | [54] | |
| Organic anode materials | LiTA | 100 | 133.5 | 100 | [55] |
| CMH | 200 | 404.6 | 250 | [56] | |
| NBALS | 0.5C | 153 | 100 | [57] | |
| Li2-DBT | 0.1C | 122 | 50 | [58] | |
| Li2-DMoT | 0.1C | 95 | 50 | [58] | |
| Li2-DAT | 0.1C | 98 | 50 | [58] | |
| E-CIN-1/CNT | 100 | 1005 | 250 | [59] | |
| E-SNW-1/CNT | 100 | 920 | 250 | [59] | |
| PA-TA | 1000 | 543 | 400 | [60] | |
| rCTF | 300 | 1190 | 1000 | [61] | |
| CP-based anode materials | Mn(2,5-FDC)·3H2O | 300 | 503 | 326 | [62] |
| Mn(3,5-PDC)·2H2O | 100 | 554 | 240 | [62] | |
| Co-LCP | 50 | 545 | 50 | [63] | |
| Mn-BTC | 100 | 845 | 100 | [64] | |
| [Cd(HTCPPA)·2H2O]n | 100 | 302 | 100 | [65] | |
| Cu3(BTC)2 | 383 | 474 | 50 | [66] | |
| Co-BDC | 100 | 1090 | 1000 | [67] | |
| [Pb(4,4′-ocppy)2]·7H2O | 100 | 489 | 500 | [68] | |
| Mn-3D | 100 | 692 | 160 | [69] | |
| Co-MOF | 100 | 400 | 80 | [70] | |
| Fe-CP | 100 | 521 | 140 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, X.; Gui, Y. One-Dimensional Croconate-Based Fe-CP as a High-Performance Anode Material for Lithium–Ion Batteries. Polymers 2023, 15, 3728. https://doi.org/10.3390/polym15183728
Zhang L, Zhang X, Gui Y. One-Dimensional Croconate-Based Fe-CP as a High-Performance Anode Material for Lithium–Ion Batteries. Polymers. 2023; 15(18):3728. https://doi.org/10.3390/polym15183728
Chicago/Turabian StyleZhang, Lin, Xiaofei Zhang, and Yingcai Gui. 2023. "One-Dimensional Croconate-Based Fe-CP as a High-Performance Anode Material for Lithium–Ion Batteries" Polymers 15, no. 18: 3728. https://doi.org/10.3390/polym15183728
APA StyleZhang, L., Zhang, X., & Gui, Y. (2023). One-Dimensional Croconate-Based Fe-CP as a High-Performance Anode Material for Lithium–Ion Batteries. Polymers, 15(18), 3728. https://doi.org/10.3390/polym15183728





