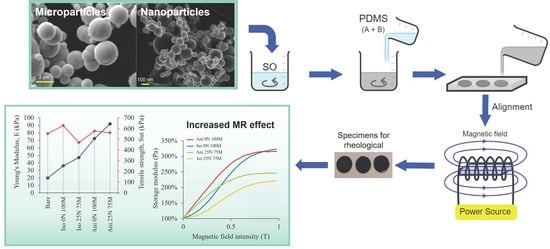
Magnetic and Viscoelastic Response of Magnetorheological Elastomers Based on a Combination of Iron Nano- and Microparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure for Manufacturing the MREs
2.3. Characterization Methods
3. Results
3.1. Chemical Analysis by FTIR Spectroscopy
3.2. Crystallographic Analysis by XRD
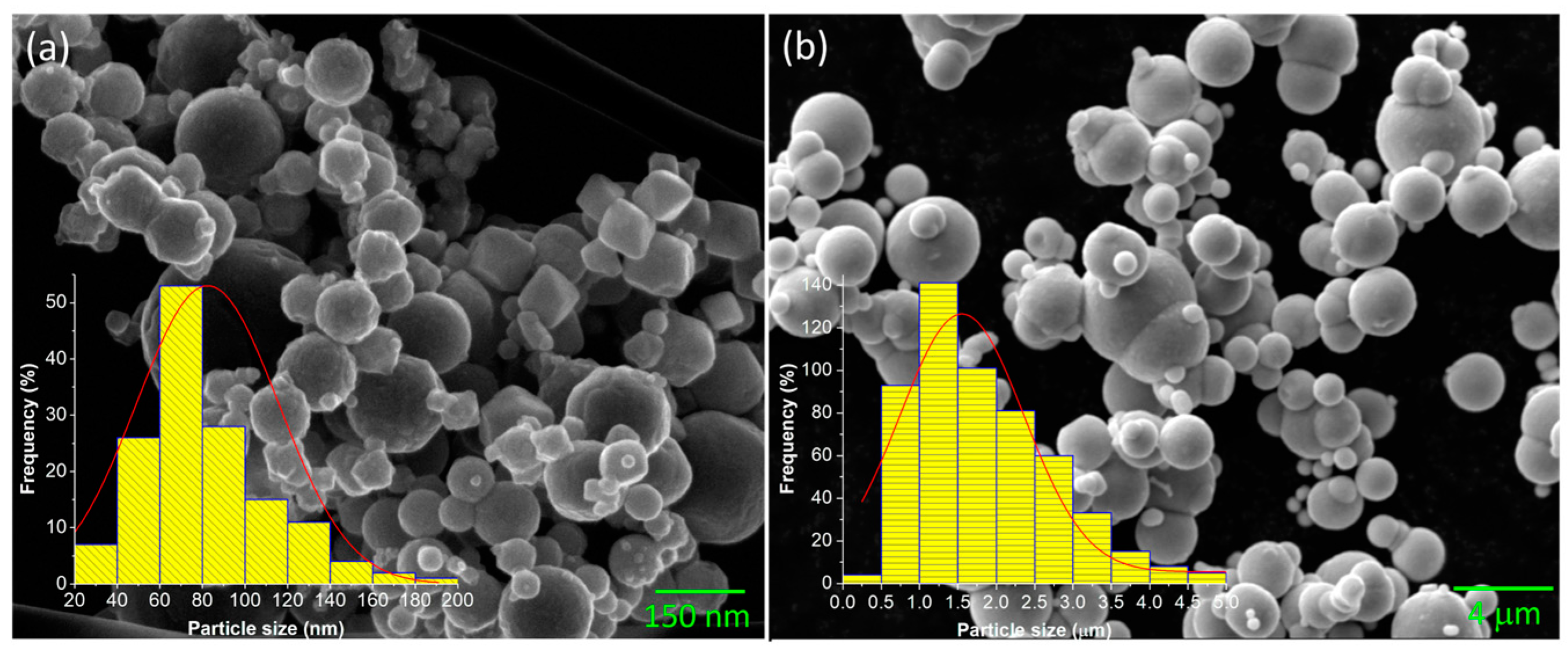
3.3. Morphological Analysis by SEM and Optical Microscopy
3.4. Thermal Stability by TGA
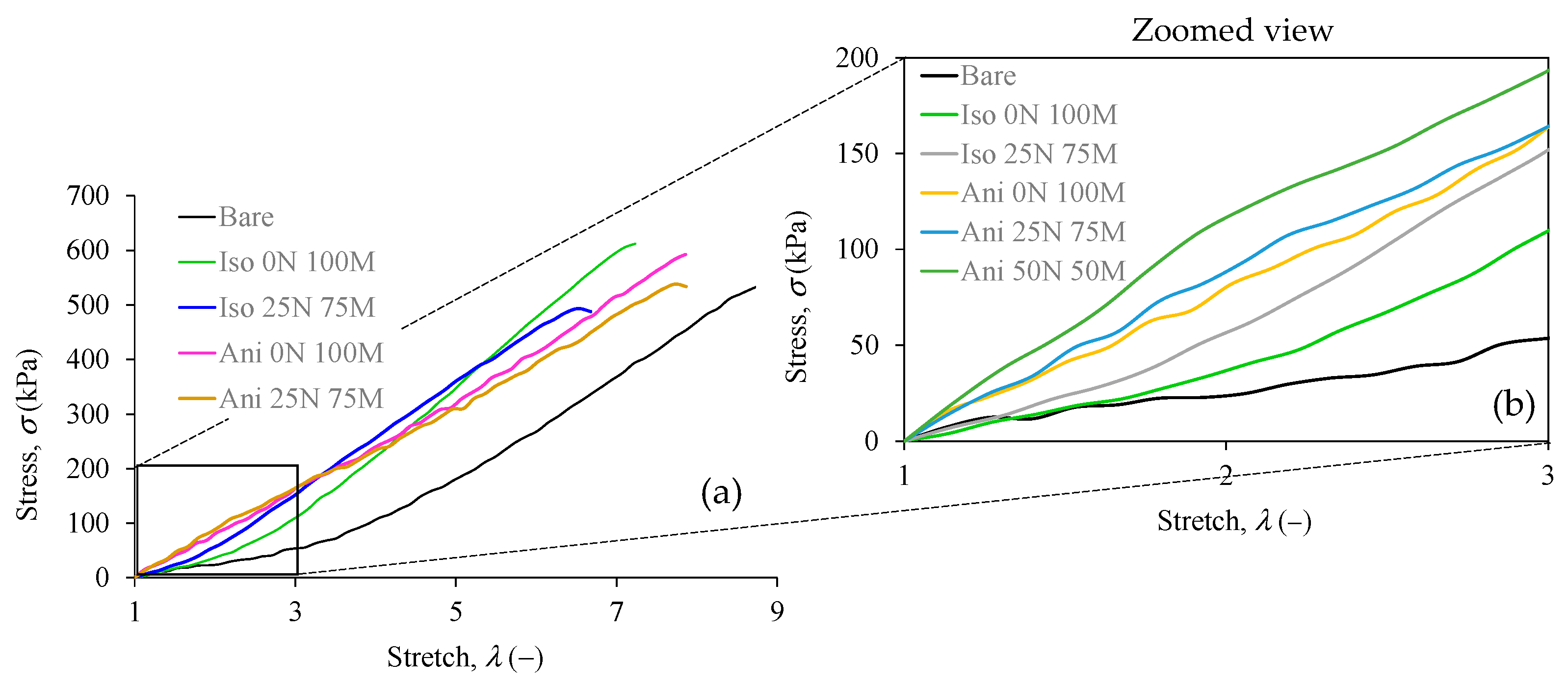
3.5. Uniaxial Extension Results
3.6. Rheological Test Results
3.7. Swelling Test Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurubasavaraju, T.M.; Kumar, H.; Arun, M. Evaluation of Optimal Parameters of MR Fluids for Damper Application Using Particle Swarm and Response Surface Optimisation. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 3683–3694. [Google Scholar] [CrossRef]
- Pramudya, I.; Sutrisno, J.; Fuchs, A.; Kavlicoglu, B.; Sahin, H.; Gordaninejad, F. Compressible Magnetorheological Fluids Based on Composite Polyurethane Microspheres. Macromol. Mater. Eng. 2013, 298, 888–895. [Google Scholar] [CrossRef]
- Cao, L.; Park, H.; Dodbiba, G.; Fujita, T. Synthesis of an Ionic Liquid-Based Magnetorheological Fluid Dispersing Fe84Nb3V4B9 Nanocrystalline Powders. Int. J. Mod. Phys. B 2010, 24, 1227–1234. [Google Scholar] [CrossRef]
- Tong, Y.; Li, X.; Zhao, P.; Dong, X.; Wu, Z.; Qi, M. Improved Magnetorheological Properties by Using Ionic Liquid as Carrier Liquid of Magnetorheological Fluids. Front. Mater. 2021, 8, 659998. [Google Scholar] [CrossRef]
- De Vicente, J.; Klingenberg, D.J.; Hidalgo-Alvarez, R. Magnetorheological Fluids: A Review. Soft Matter 2011, 7, 3701–3710. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, H.J. Magnetic Carbonyl Iron Particle Dispersed in Viscoelastic Fluid and Its Magnetorheological Property. IEEE Trans. Magn. 2011, 47, 3173–3176. [Google Scholar] [CrossRef]
- Bossis, G.; Khuzir, P.; Lacis, S.; Volkova, O. Yield Behavior of Magnetorheological Suspensions. J. Magn. Magn. Mater. 2003, 258–259, 456–458. [Google Scholar] [CrossRef]
- Xu, Z.-D.; Chen, B.-B. Experimental and Numerical Study on Magnetorheological Fluids Based on Mixing Coated Magnetic Particles. J. Mater. Civil. Eng. 2016, 28, 04015198. [Google Scholar] [CrossRef]
- Choi, H.Y.; Choi, J.Y.; Park, H.H.; Kim, T.H.; Choi, H.J.; Lee, C.S. Effect of Reduced Graphene Oxide and MnFe2O4 Nanoparticles on Carbonyl Iron for Magnetorheological Fluids. J. Ind. Eng. Chem. 2021, 98, 140–147. [Google Scholar] [CrossRef]
- Rwei, S.P.; Lee, H.Y.; Yoo, S.D.; Wang, L.Y.; Lin, J.G. Magnetorheological Characteristics of Aqueous Suspensions That Contain Fe3O4 Nanoparticles. Colloid. Polym. Sci. 2005, 283, 1253–1258. [Google Scholar] [CrossRef]
- Park, B.J.; Song, K.H.; Choi, H.J. Magnetic Carbonyl Iron Nanoparticle Based Magnetorheological Suspension and Its Characteristics. Mater. Lett. 2009, 63, 1350–1352. [Google Scholar] [CrossRef]
- Yang, J.; Sun, S.S.; Du, H.; Li, W.H.; Alici, G.; Deng, H.X. A Novel Magnetorheological Elastomer Isolator with Negative Changing Stiffness for Vibration Reduction. Smart Mater. Struct. 2014, 23, 105023. [Google Scholar] [CrossRef]
- Guan, X.; Dong, X.; Ou, J. Magnetostrictive Effect of Magnetorheological Elastomer. J. Magn. Magn. Mater. 2008, 320, 158–163. [Google Scholar] [CrossRef]
- Farshad, M.; Benine, A. Magnetoactive Elastomer Composites. Polym. Test. 2004, 23, 347–353. [Google Scholar] [CrossRef]
- Qiao, X.; Lu, X.; Gong, X.; Yang, T.; Sun, K.; Chen, X. Effect of Carbonyl Iron Concentration and Processing Conditions on the Structure and Properties of the Thermoplastic Magnetorheological Elastomer Composites Based on Poly(Styrene-b-Ethylene-Co-Butylene-b-Styrene) (SEBS). Polym. Test. 2015, 47, 51–58. [Google Scholar] [CrossRef]
- Bica, I. The Influence of the Magnetic Field on the Elastic Properties of Anisotropic Magnetorheological Elastomers. J. Ind. Eng. Chem. 2012, 18, 1666–1669. [Google Scholar] [CrossRef]
- Lee, C.J.; Kwon, S.H.; Choi, H.J.; Chung, K.H.; Jung, J.H. Enhanced Magnetorheological Performance of Carbonyl Iron/Natural Rubber Composite Elastomer with Gamma-Ferrite Additive. Colloid. Polym. Sci. 2018, 296, 1609–1613. [Google Scholar] [CrossRef]
- Bica, I.; Anitas, E.M.; Chirigiu, L. Magnetic Field Intensity Effect on Plane Capacitors Based on Hybrid Magnetorheological Elastomers with Graphene Nanoparticles. J. Ind. Eng. Chem. 2017, 56, 407–412. [Google Scholar] [CrossRef]
- Khayam, S.U.; Usman, M.; Umer, M.A.; Rafique, A. Development and Characterization of a Novel Hybrid Magnetorheological Elastomer Incorporating Micro and Nano Size Iron Fillers. Mater. Des. 2020, 192, 108748. [Google Scholar] [CrossRef]
- ASTM D-2393; Test Method for Viscosity of Epoxy Resins and Related Components. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D-2240; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2021.
- Palacios-Pineda, L.M.; Perales-Martínez, I.A.; Moreno-Guerra, M.R.; Elías-Zúñiga, A. An Optimum Specimen Geometry for Equibiaxial Experimental Tests of Reinforced Magnetorheological Elastomers with Iron Micro- and Nanoparticles. Nanomaterials 2017, 7, 254. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Toru, I.; Okuyama, K. Correlation between Particle Size/Domain Structure and Magnetic Properties of Highly Crystalline Fe3O4 Nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef] [PubMed]
- Perales-Martínez, I.A.; Rodríguez-González, V.; Obregon-Alfaro, S.; Lee, S.W. Facile Synthesis of Decahedral Particles of Anatase TiO2 with Exposed {001} Facets. J. Nanosci. Nanotechnol. 2015, 15, 7351–7356. [Google Scholar] [CrossRef] [PubMed]
- Pickering, K.L.; Raa Khimi, S.; Ilanko, S. The Effect of Silane Coupling Agent on Iron Sand for Use in Magnetorheological Elastomers Part 1: Surface Chemical Modification and Characterization. Compos. Part. A Appl. Sci. Manuf. 2015, 68, 377–386. [Google Scholar] [CrossRef]
- Palacios-Pineda, L.M.; Perales-Martinez, I.A.; Lozano-Sanchez, L.M.; Martínez-Romero, O.; Puente-Córdova, J.; Segura-Cárdenas, E.; Elías-Zúñiga, A. Experimental Investigation of the Magnetorheological Behavior of PDMS Elastomer Reinforced with Iron Micro/Nanoparticles. Polymers 2017, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Treloar, L.R.G. The Elasticity and Related Properties of Rubbers. Rep. Progress Phys. 1973, 36, 755–826. [Google Scholar] [CrossRef]
- ISO37-2011; Rubber, vulcanized or thermoplastic—Determination of tensile stress-strain properties. ISO: Geneva, Switzerland, 2011.
- Hanoosh, W.S.; Abdelrazaq, E.M. Polydimethyl Siloxane Toughened Epoxy Resins: Tensile Strength and Dynamic Mechanical Analysis. Malays. Polym. J. 2009, 4, 52–61. [Google Scholar]
- Perales-Martínez, I.A.; Palacios-Pineda, L.M.; Lozano-Sánchez, L.M.; Martínez-Romero, O.; Puente-Cordova, J.G.; Elías-Zúñiga, A. Enhancement of a Magnetorheological PDMS Elastomer with Carbonyl Iron Particles. Polym. Test. 2017, 57, 78–86. [Google Scholar] [CrossRef]
- Siniecki, B.; Mieroslawski, W.; Szczerkowska, Z. Polymer Data Handbokk. Pediatr. Pol. 1975, 50, 235–237. [Google Scholar]
- Launer, P.J.; Arkles, B. Infrared Analysis of Organosilicon Compounds: Spectra-Structure Correlations. Silicone Compd. Regist. Rev. 1987, 100, 100–103. [Google Scholar]
- De Souza Neto, F.N.; Araújo, O.A.; Guilherme, L.R.; Garg, V.K.; Oliveira, A.C.; De Souza, P.E.N.; Franco Júnior, A. Particles That Slide over the Water Surface: Synthesis and Characterization of Iron Oxides Particles Coated with PDMS, with Hydrophobic and Magnetic Properties. Mater. Chem. Phys. 2015, 162, 100–105. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Vatani, A.; Mohammadi, T. Synergistic Interactions between POSS and Fumed Silica and Their Effect on the Properties of Crosslinked PDMS Nanocomposite Membranes. RSC Adv. 2015, 5, 82460–82470. [Google Scholar] [CrossRef]
- Manglam, M.K.; Mallick, J.; Kumari, S.; Pandey, R.; Kar, M. Crystal Structure and Magnetic Properties Study on Barium Hexaferrite (BHF) and Cobalt Zinc Ferrite (CZF) in Composites. Solid. State Sci. 2021, 113, 106529. [Google Scholar] [CrossRef]
- Puente-Córdova, J.G.; Reyes-Melo, M.E.; Palacios-Pineda, L.M.; Martínez-Perales, I.A.; Martínez-Romero, O.; Elías-Zúñiga, A. Fabrication and Characterization of Isotropic and Anisotropic Magnetorheological Elastomers, Based on Silicone Rubber and Carbonyl Iron Microparticles. Polymers 2018, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gong, X.; Fan, Y.; Xia, H. Anisotropic Polyurethane Magnetorheological Elastomer Prepared through in Situ Polycondensation under a Magnetic Field. Smart Mater. Struct. 2010, 19, 105007. [Google Scholar] [CrossRef]
- Plachy, T.; Rohrer, P.; Holcapkova, P. Gelatine-Coated Carbonyl Iron Particles and Their Utilization in Magnetorheological Suspensions. Materials 2021, 14, 2503. [Google Scholar] [CrossRef]
- Chahal, R.S.; Kao, W.-P. Donald Patterson Thermodynamics of Polydimethylsiloxane Solutions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1973, 69, 1834–1848. [Google Scholar] [CrossRef]
- Schuld, N.; Wolf, B. The Wiley Database of Polymer Properties; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]









| Sample | T10% (°C) | Tp1 (°C) | Tp2 (°C) | Tp3 (°C) | Rp1 (%/°C) | Rp2 (%/°C) | Rp3 (%/°C) | Residue (%) |
|---|---|---|---|---|---|---|---|---|
| Bare | 365 | 414 | 478 | 510 | 0.531 | 0.186 | 0.125 | 36.8 |
| Iso 0 N 100 M | 401 | 427 | 589 | --- | 0.136 | 0.12 | --- | 61.3 |
| Ani 0 N 100 M | 399 | 432 | 602 | --- | 0.14 | 0.076 | --- | 64.7 |
| Iso 25 N 75 M | 389 | 424 | 516 | --- | 0.122 | 0.098 | --- | 70.6 |
| Ani 25 N 75 M | 396 | 439 | 512 | --- | 0.141 | 0.128 | --- | 67.2 |
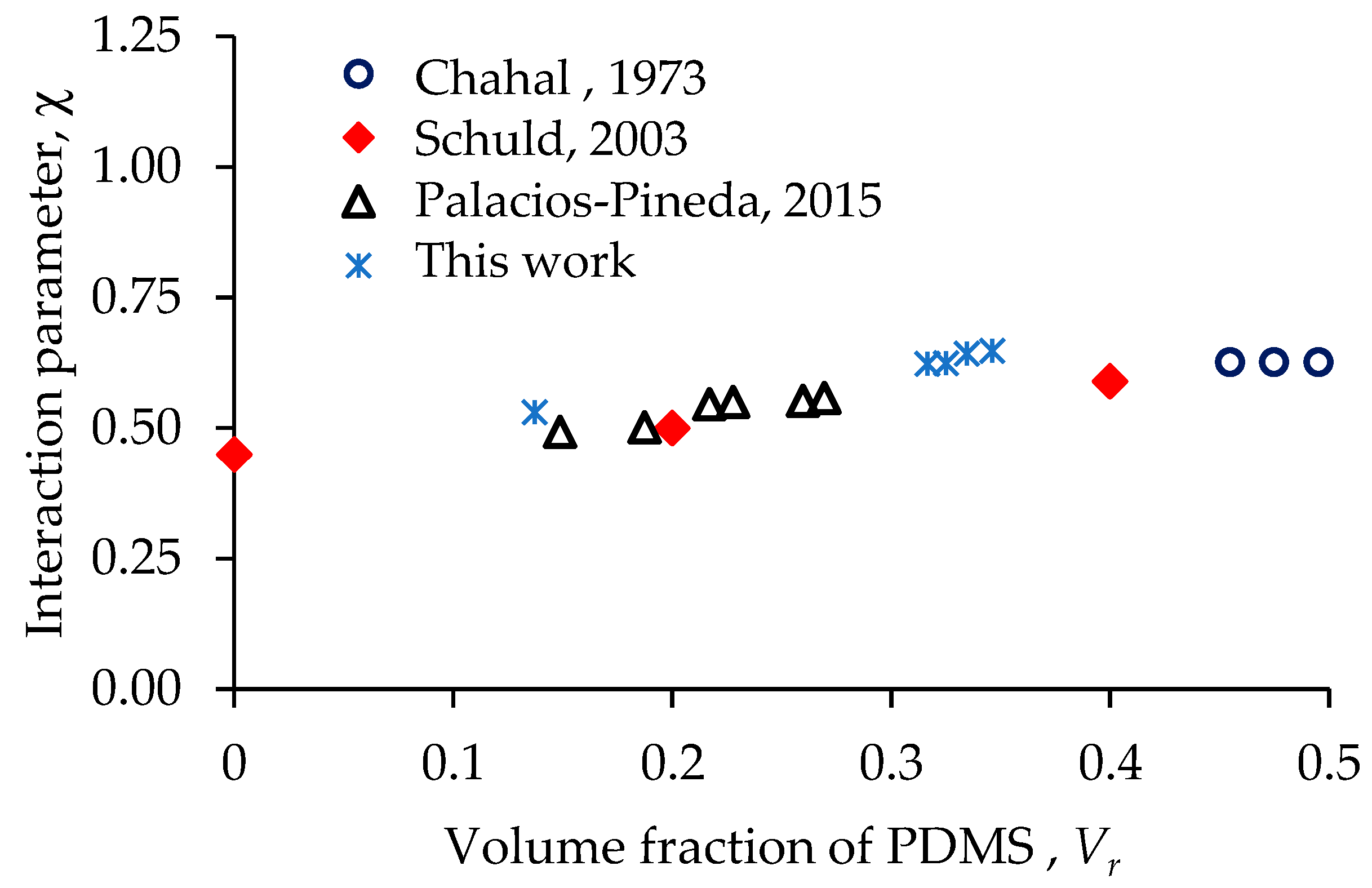
| Material | [X] [mol/m3] | Vr [-] | [-] |
|---|---|---|---|
| Bare | 8.1 | 0.137 | 0.531 |
| Ani 0 N 100 M | 29.7 | 0.317 | 0.623 |
| Ani 25 N 75 M | 37.7 | 0.325 | 0.624 |
| Iso 0 N 100 M | 14.8 | 0.335 | 0.643 |
| Iso 25 N 75 M | 19.3 | 0.346 | 0.648 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perales-Martínez, I.A.; Palacios-Pineda, L.M.; Elías-Zúñiga, A.; Olvera-Trejo, D.; Del Ángel-Sánchez, K.; Cruz-Cruz, I.; Ramírez-Herrera, C.A.; Martínez-Romero, O. Magnetic and Viscoelastic Response of Magnetorheological Elastomers Based on a Combination of Iron Nano- and Microparticles. Polymers 2023, 15, 3703. https://doi.org/10.3390/polym15183703
Perales-Martínez IA, Palacios-Pineda LM, Elías-Zúñiga A, Olvera-Trejo D, Del Ángel-Sánchez K, Cruz-Cruz I, Ramírez-Herrera CA, Martínez-Romero O. Magnetic and Viscoelastic Response of Magnetorheological Elastomers Based on a Combination of Iron Nano- and Microparticles. Polymers. 2023; 15(18):3703. https://doi.org/10.3390/polym15183703
Chicago/Turabian StylePerales-Martínez, Imperio Anel, Luis Manuel Palacios-Pineda, Alex Elías-Zúñiga, Daniel Olvera-Trejo, Karina Del Ángel-Sánchez, Isidro Cruz-Cruz, Claudia Angélica Ramírez-Herrera, and Oscar Martínez-Romero. 2023. "Magnetic and Viscoelastic Response of Magnetorheological Elastomers Based on a Combination of Iron Nano- and Microparticles" Polymers 15, no. 18: 3703. https://doi.org/10.3390/polym15183703
APA StylePerales-Martínez, I. A., Palacios-Pineda, L. M., Elías-Zúñiga, A., Olvera-Trejo, D., Del Ángel-Sánchez, K., Cruz-Cruz, I., Ramírez-Herrera, C. A., & Martínez-Romero, O. (2023). Magnetic and Viscoelastic Response of Magnetorheological Elastomers Based on a Combination of Iron Nano- and Microparticles. Polymers, 15(18), 3703. https://doi.org/10.3390/polym15183703