Modern Approaches in Wounds Management
Abstract
:1. Introduction
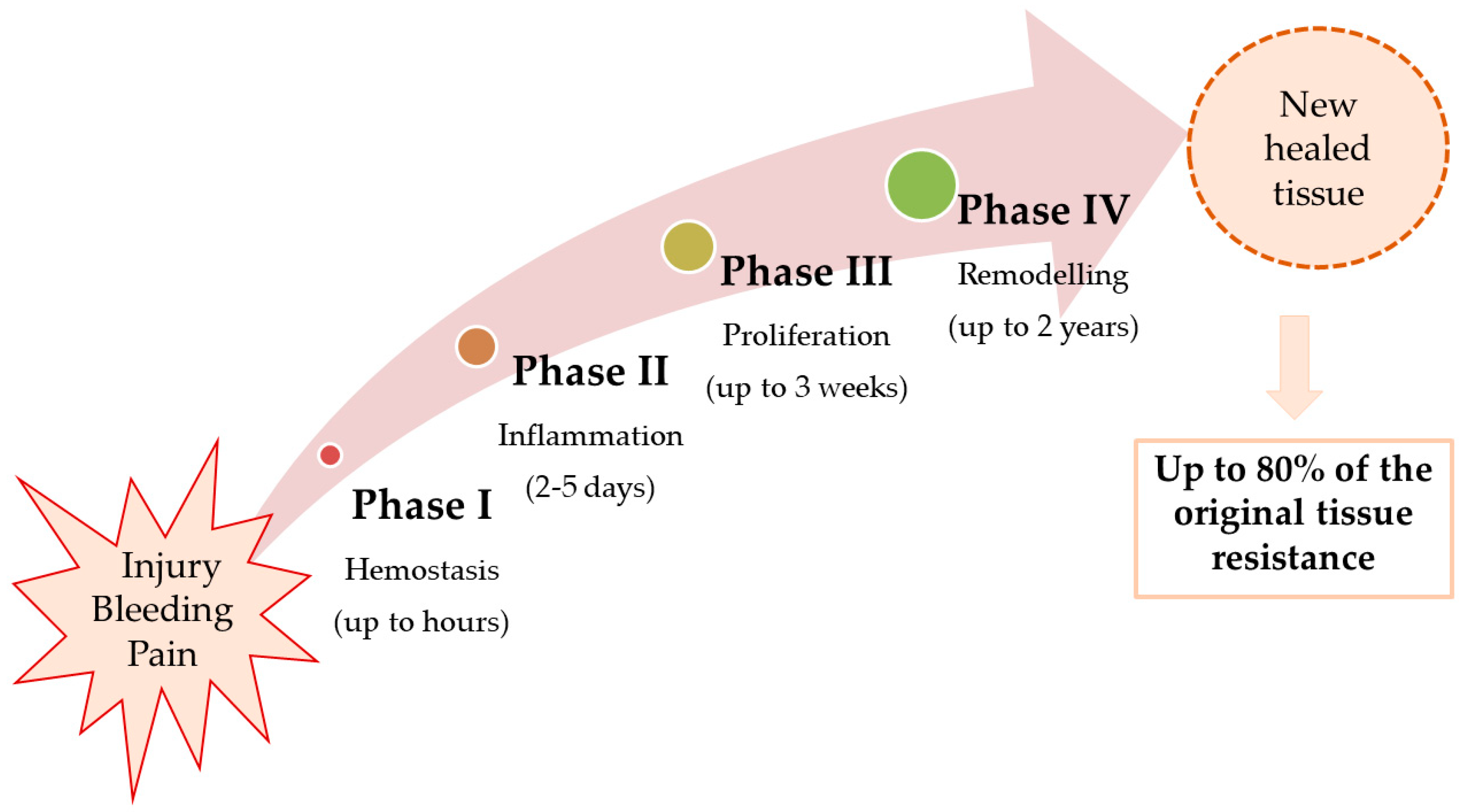
2. Pathophysiology of Wound Healing
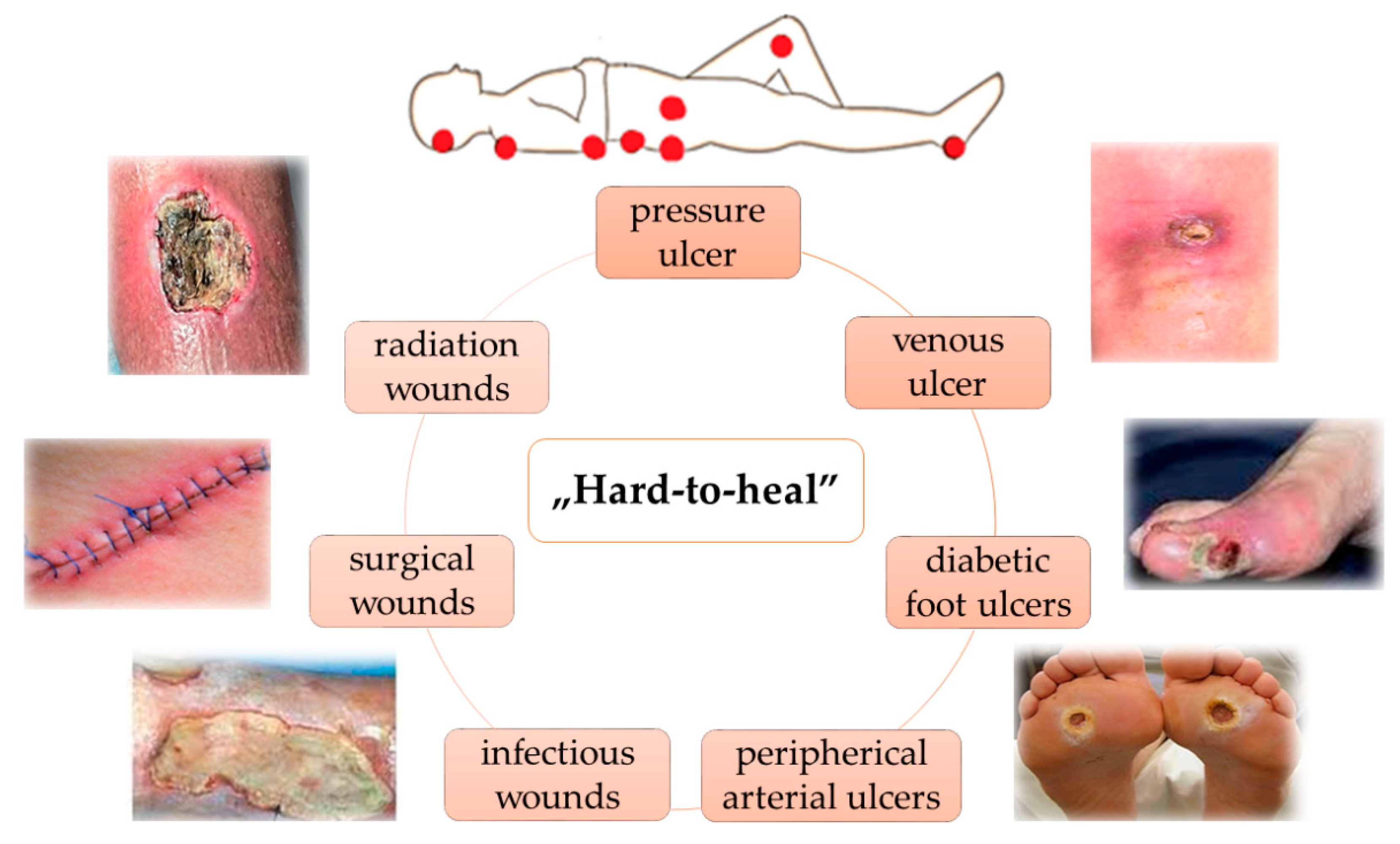
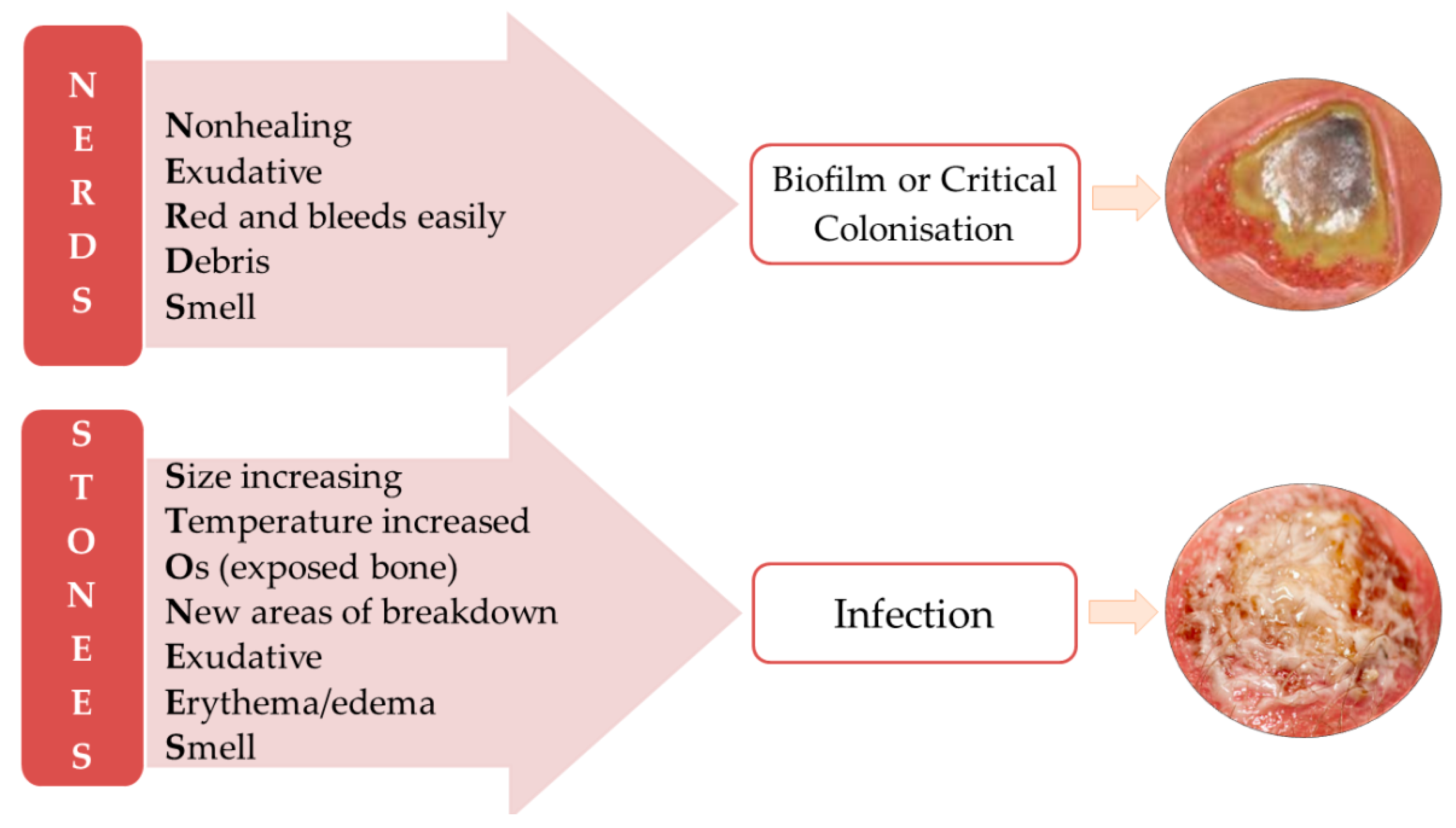
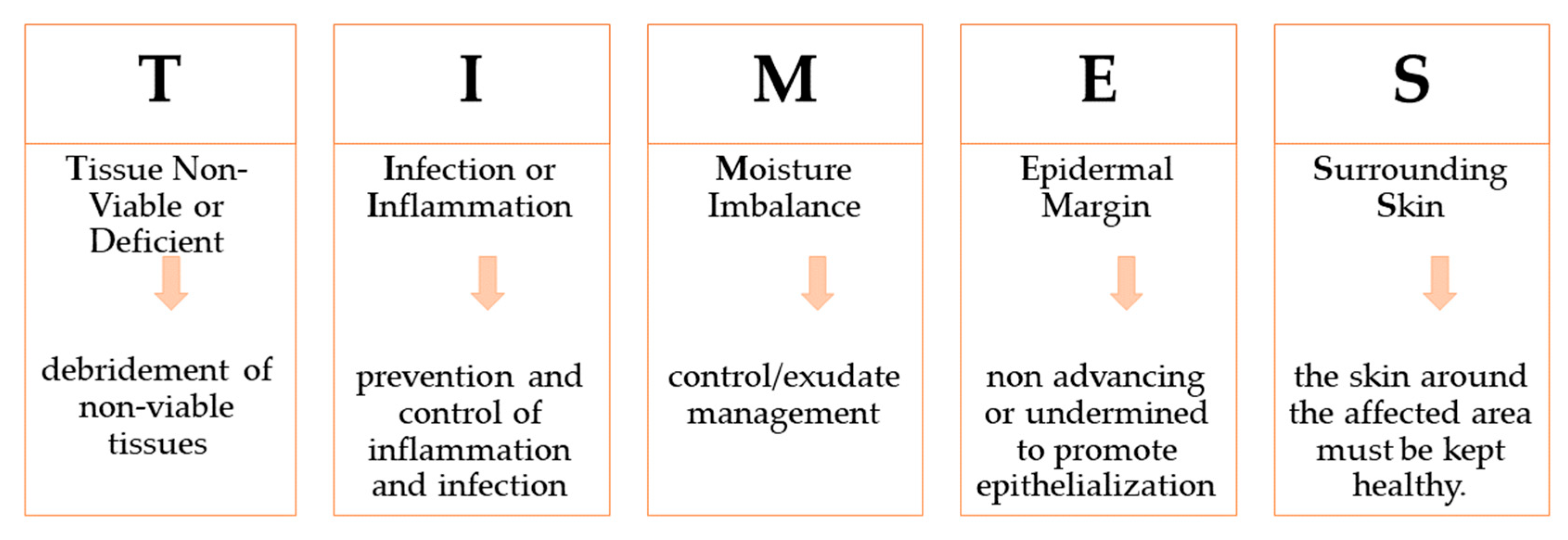
3. Wounds Care Management Protocol
4. Wound Care Treatment Approaches
4.1. Hydrogels as Modern Wound Dressings
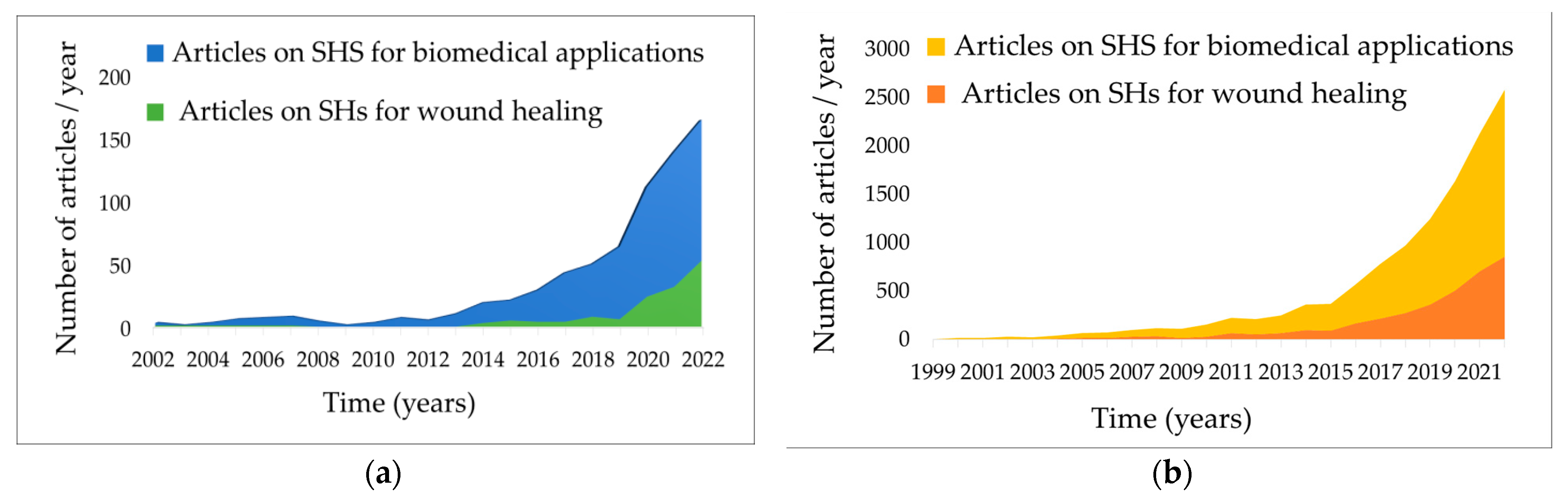
4.2. Smart Hydrogels as Innovative Wound Dressings
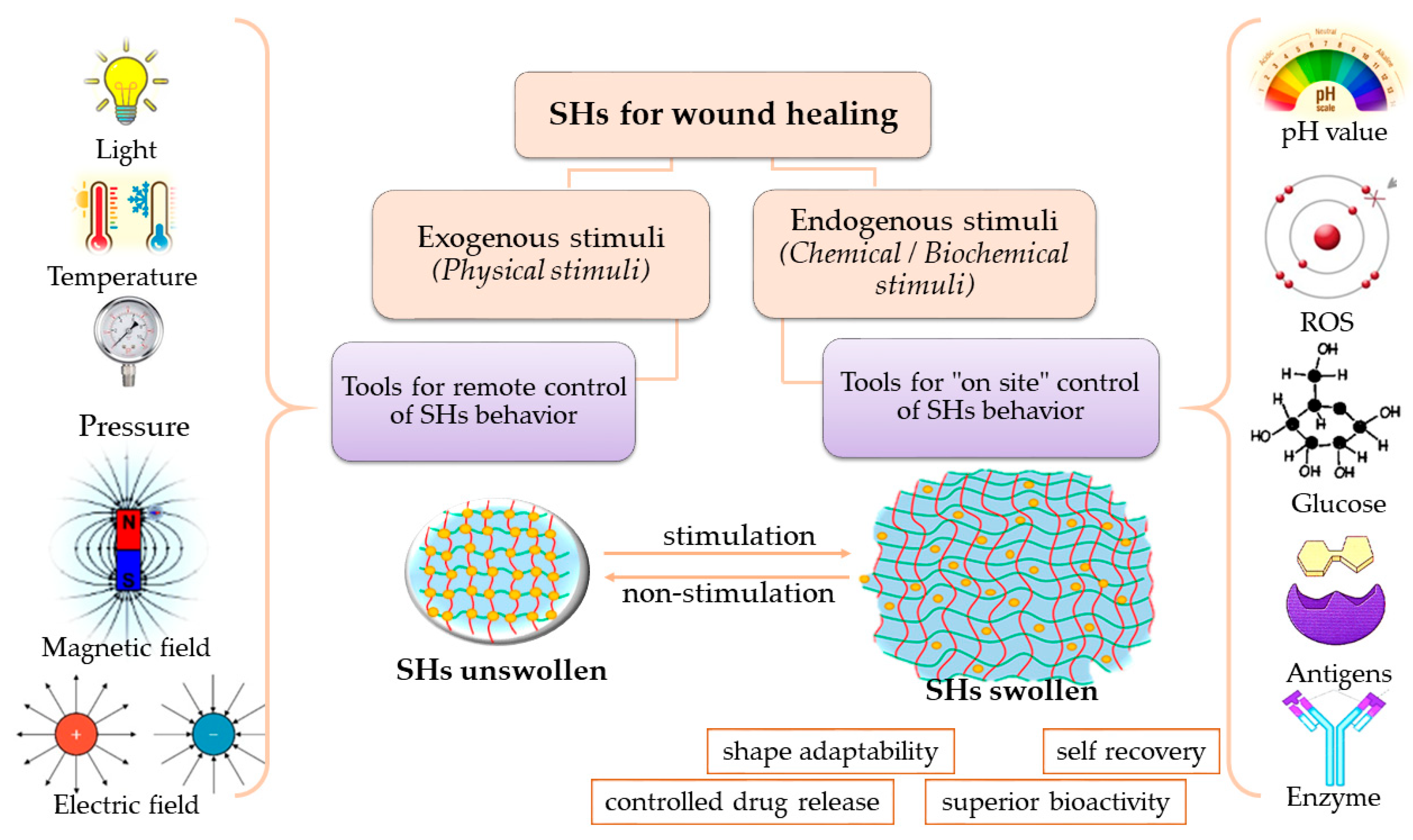
4.3. Stimuli-Responsive Hydrogels
4.3.1. Physical Stimuli-Responsive Hydrogels
Temperature-Responsive Hydrogels
Pressure-Responsive Hydrogels
Light-Responsive Hydrogels
Magnetic-Responsive Hydrogels
Electric-Responsive Hydrogels
4.3.2. Chemical Stimuli-Responsive Hydrogels
pH-Responsive Hydrogels
Reactive-Oxygen-Species-Responsive Hydrogels
Glucose-Responsive Hydrogels
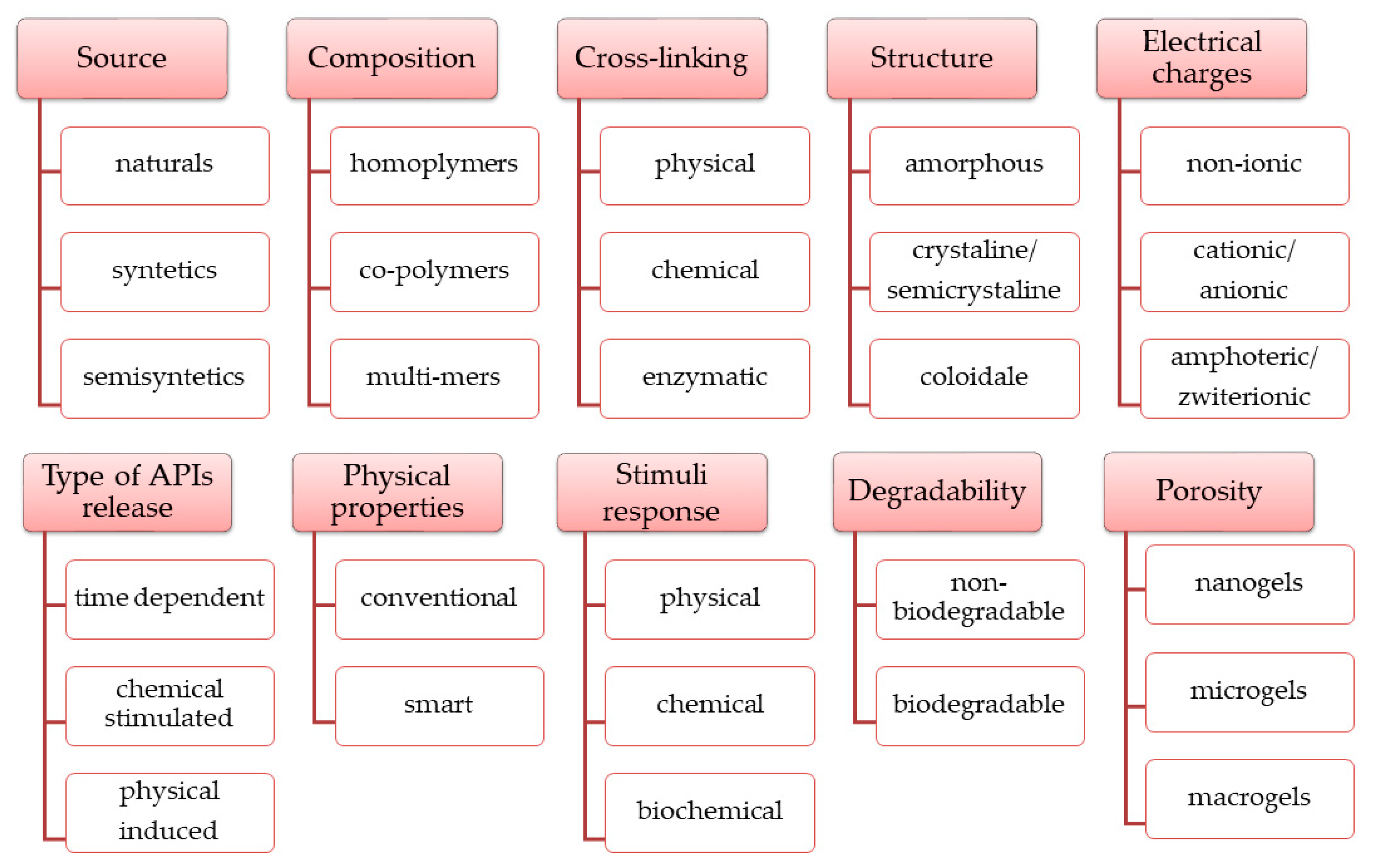
4.4. Polymers for SHs Formulation
4.4.1. Biopolymers
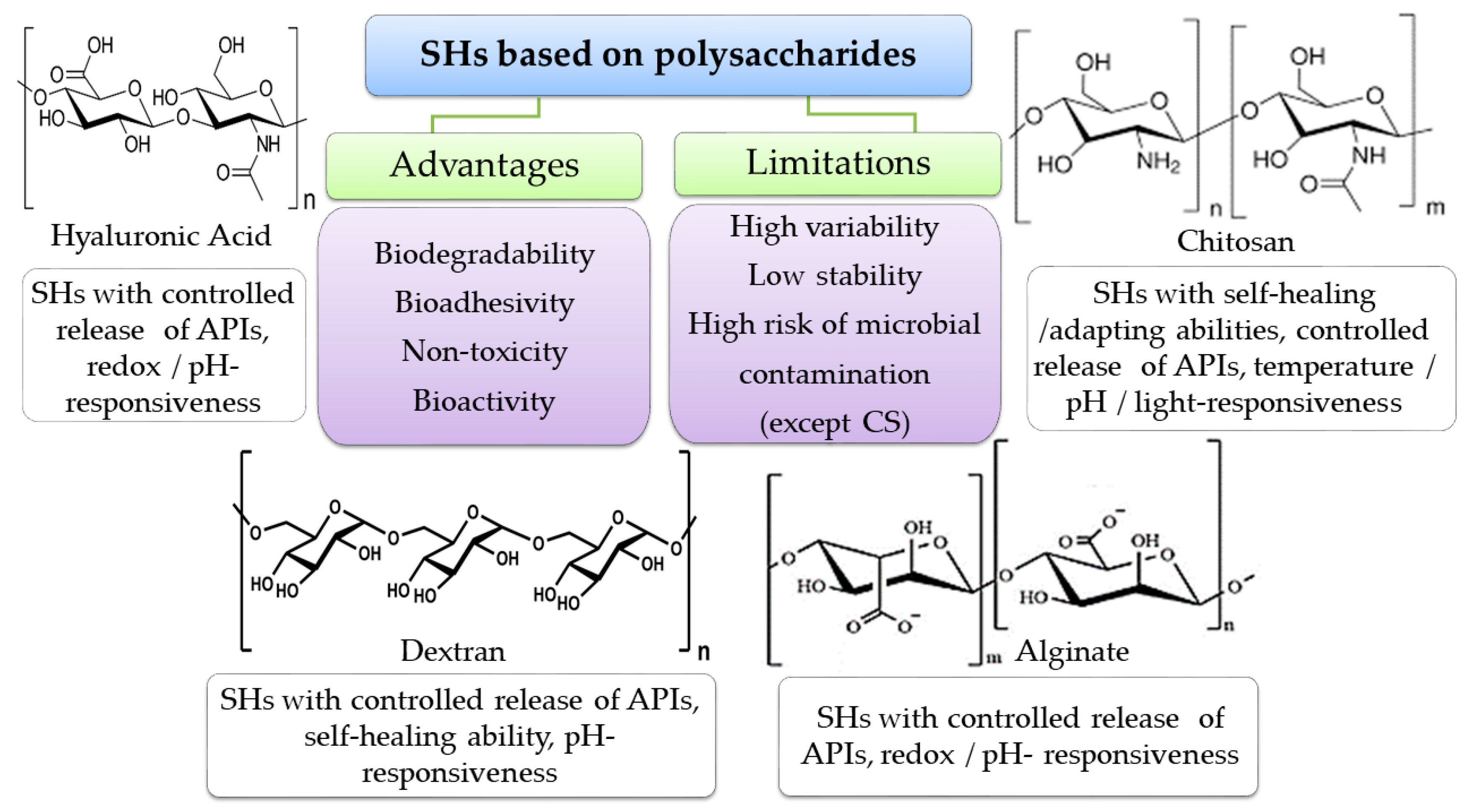
Polymers from Natural Sources
Biological-like Polymers
- Self-assembling peptides (SAPs)
- Deoxyribonucleic Acid
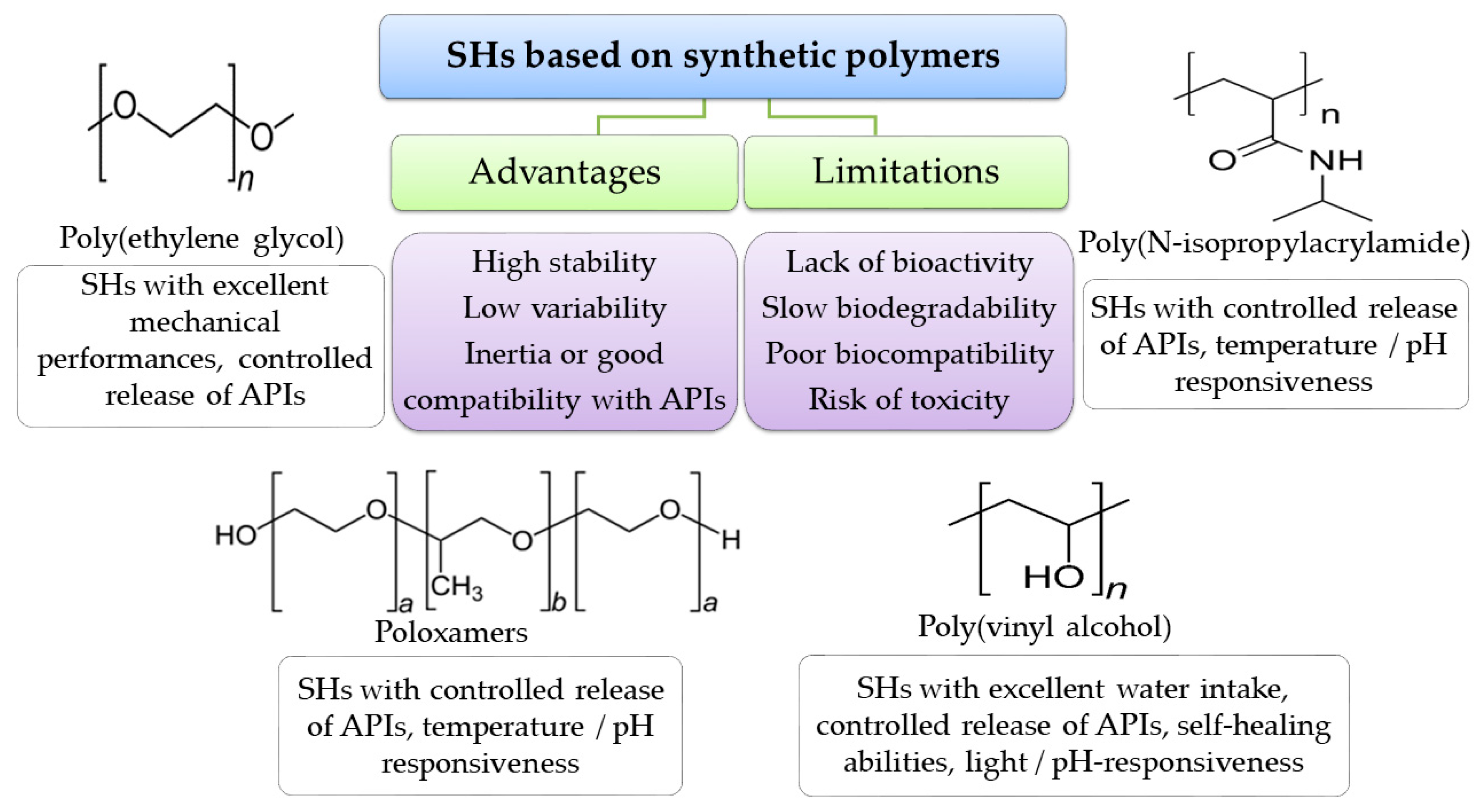
4.4.2. Synthetic Polymers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 1: Normal and Chronic Wounds: Biology, Causes, and Approaches to Care. Adv. Skin. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.; McIntosh, C.; Cundell, J. The Dissemination of Wound Management Guidelines: A National Survey. J. Wound Care 2011, 20, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Olsson, M.M.; Bajpai, R.; Järbrink, K.; Tang, W.E.; Car, J. Health-Related Quality of Life and Chronic Wound Characteristics among Patients with Chronic Wounds Treated in Primary Care: A Cross-Sectional Study in Singapore. Int. Wound J. 2022, 19, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Niu, J.; Cheng, B. Prevalence of Chronic Skin Wounds and Their Risk Factors in an Inpatient Hospital Setting in Northern China. Adv. Skin Wound Care 2020, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and Incidence of Chronic Wounds and Related Complications: A Protocol for a Systematic Review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic Wounds: Treatment Consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Jupiter, D.C.; Thorud, J.C.; Buckley, C.J.; Shibuya, N. The Impact of Foot Ulceration and Amputation on Mortality in Diabetic Patients. I: From Ulceration to Death, a Systematic Review. Int. Wound J. 2016, 13, 892–903. [Google Scholar] [CrossRef]
- Cheun, T.J.; Jayakumar, L.; Sideman, M.J.; Ferrer, L.; Mitromaras, C.; Miserlis, D.; Davies, M.G. Short-Term Contemporary Outcomes for Staged versus Primary Lower Limb Amputation in Diabetic Foot Disease. J. Vasc. Surg. 2020, 72, 658–666.e2. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- BuganzaTepole, A.; Kuhl, E. Systems-Based Approaches toward Wound Healing. Pediatr. Res. 2013, 73, 553–563. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic Wounds: Current Status, Available Strategies and Emerging Therapeutic Solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The Humanistic and Economic Burden of Chronic Wounds: A Systematic Review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Nagle, S.M.; Stevens, K.A.; Wilbraham, S.C. Wound Assessment. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482198/ (accessed on 11 August 2023).
- Woo, K.Y.; Sibbald, R.G. A Cross-Sectional Validation Study of Using NERDS and STONEES to Assess Bacterial Burden. Ostomy. Wound Manag. 2009, 55, 40–48. [Google Scholar]
- Calling on NERDS for Critically Colonized Wounds: Nursing. 2023. Available online: https://journals.lww.com/nursing/Citation/2007/05000/Calling_on_NERDS_for_critically_colonized_wounds.17.aspx (accessed on 2 April 2023).
- Wound Bed Preparation: Employing the TIME Acronym. Available online: https://www.independentnurse.co.uk/content/clinical/wound-bed-preparation-employing-the-time-acronym/ (accessed on 2 April 2023).
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Varma, A.; Warghane, A.; Dhiman, N.K.; Paserkar, N.; Upadhye, V.; Modi, A.; Saini, R. The Role of Nanocomposites against Biofilm Infections in Humans. Front. Cell. Infect. Microbiol. 2023, 13, 1104615. [Google Scholar] [CrossRef] [PubMed]
- Doughty, D. Dressings and More: Guidelines for Topical Wound Management. Nurs. Clin. N. Am. 2005, 40, 217–231. [Google Scholar] [CrossRef]
- Junker, J.P.E.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef]
- Wound-Management-Guidelines. Available online: https://www.bcpft.nhs.uk/documents/policies/w/1444-wound-management-guidelines/file (accessed on 17 January 2023).
- Ward, J.; Holden, J.; Grob, M.; Soldin, M. Management of Wounds in the Community: Five Principles. Br. J. Community Nurs. 2019, 24, S20–S23. [Google Scholar] [CrossRef]
- Dowsett, C.; Swanson, T.; Karlsmark, T. A Focus on the Triangle of Wound Assessment-Addressing the Gap Challenge and Identifying Suspected Biofilm in Clinical Practice. Clin. Pract. 2019, 10, 34–39. [Google Scholar]
- Gil, S.B. Implementing the Triangle of Wound Assessment Framework to Transform the Care Pathway for Diabetic Foot Ulcers. J Wound Care 2020, 29, 363–369. [Google Scholar] [CrossRef]
- Dowsett, C.; Bain, K.; Bain, M. Closing the Gap between the Evidence and Clinical Practice-a Consensus Report on Exudate Management. Wounds 2020, 11, 64–68. [Google Scholar]
- Wound Assessment Tools: An Introduction to PUSH, NPUAP and Wagner. Available online: https://www.woundsource.com/blog/wound-assessment-tools-basic-introduction-push-npuap-and-wagner (accessed on 2 April 2023).
- Moore, Z.E.; Webster, J. Dressings and Topical Agents for Preventing Pressure Ulcers. Cochrane Database Syst. Rev. 2018, 12, CD009362. [Google Scholar] [CrossRef]
- Advanced Wound Care Market–Table of Content, List of Tables and List of Figures. Available online: https://www.factmr.com/report/advance-wound-care-market/toc (accessed on 2 April 2023).
- Derwin, R.; Patton, D.; Avsar, P.; Strapp, H.; Moore, Z. The Impact of Topical Agents and Dressing on PH and Temperature on Wound Healing: A Systematic, Narrative Review. Int. Wound J. 2022, 19, 1397–1408. [Google Scholar] [CrossRef]
- Barbu, A.; Neamtu, B.; Zăhan, M.; Iancu, G.M.; Bacila, C.; Mireșan, V. Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds. J. Pers. Med. 2021, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Ruiz, P.B.; Lima, A.F.C. Average Direct Costs of Outpatient, Hospital, and Home Care Provided to Patients with Chronic Wounds. Rev. Esc. Enferm. 2022, 56, e20220295. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent Advances in Smart Hydrogels for Biomedical Applications: From Self-Assembly to Functional Approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater. Today Bio. 2022, 13, 100186. [Google Scholar] [CrossRef]
- Thakur, S.; Thakur, V.K.; Arotiba, O.A. History, Classification, Properties and Application of Hydrogels: An Overview. In Hydrogels; Thakur, V.K., Thakur, M.K., Eds.; Gels Horizons: From Science to Smart Materials; Springer Singapore: Singapore, 2018; pp. 29–50. ISBN 978-981-10-6076-2. [Google Scholar]
- Hu, C.; Yang, L.; Wang, Y. Recent Advances in Smart-Responsive Hydrogels for Tissue Repairing. MedComm.–Biomater. Appl. 2022, 1, e23. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-Responsive Hydrogels: Fabrication and Biomedical Applications. VIEW 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.-B.; Mateescu, A.L.; Stan, D. Wound Healing Applications of Creams and “Smart” Hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and Future Scope of Hydrogels in Wound Healing: Synthesis, Materials and Evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, Classification, Properties and Potential Applications—A Brief Review. J Polym Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Baby, D.K. Rheology of Hydrogels. In Rheology of Polymer Blends and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 193–204. ISBN 978-0-12-816957-5. [Google Scholar]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-Healing Hyaluronic Acid Hydrogels Based on Dynamic Schiff Base Linkages as Biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar] [CrossRef]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff Bases: A Short Review of Their Antimicrobial Activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Gavalyan, V.B. Synthesis and Characterization of New Chitosan-Based Schiff Base Compounds. Carbohydr. Polym. 2016, 145, 37–47. [Google Scholar] [CrossRef]
- Yin, N.; Santos, T.M.A.; Auer, G.K.; Crooks, J.A.; Oliver, P.M.; Weibel, D.B. Bacterial Cellulose as a Substrate for Microbial Cell Culture. Appl. Environ. Microbiol. 2014, 80, 1926–1932. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical Crosslinking of Biopolymeric Scaffolds: Current Knowledge and Future Directions of Crosslinked Engineered Bone Scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of Physical and Chemical Crosslinked Hydrogels for Bone Tissue Engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical Functionalization of Polysaccharides—Towards Biocompatible Hydrogels for Biomedical Applications. Chemistry 2018, 24, 1231–1240. [Google Scholar] [CrossRef]
- Zarzycki, R.; Modrzejewska, Z.; Nawrotek, K. Drug Release from Hydrogel Matrices. Ecol. Chem. Eng. 2010, 17, 117–136. [Google Scholar]
- Shahinpoor, M. Conceptual Design, Kinematics and Dynamics of Swimming Robotic Structures Using Ionic Polymeric Gel Muscles. Smart Mater. Struct. 1992, 1, 91–94. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- AliakbarAhovan, Z.; Esmaeili, Z.; Eftekhari, B.S.; Khosravimelal, S.; Alehosseini, M.; Orive, G.; Dolatshahi-Pirouz, A.; Pal Singh Chauhan, N.; Janmey, P.A.; Hashemi, A.; et al. Antibacterial Smart Hydrogels: New Hope for Infectious Wound Management. Mater. Today Bio. 2022, 17, 100499. [Google Scholar] [CrossRef]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Chopra, H.; Kumar, S.; Singh, I. Bioadhesive Hydrogels and Their Applications. In Bioadhesives in Drug Delivery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 147–170. ISBN 978-1-119-64024-0. [Google Scholar]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A Review of the Properties and Applications of Bioadhesive Hydrogels. Polym. Chem. 2021, 12, 3721–3739. [Google Scholar] [CrossRef]
- Sundriyal, P.; Pandey, M.; Bhattacharya, S. Plasma-Assisted Surface Alteration of Industrial Polymers for Improved Adhesive Bonding. Int. J. Adhes. Adhes. 2020, 101, 102626. [Google Scholar] [CrossRef]
- Zheng, K.; Tong, Y.; Zhang, S.; He, R.; Xiao, L.; Iqbal, Z.; Zhang, Y.; Gao, J.; Zhang, L.; Jiang, L.; et al. Flexible Bicolorimetric Polyacrylamide/Chitosan Hydrogels for Smart Real-Time Monitoring and Promotion of Wound Healing. Adv. Funct. Mater. 2021, 31, 2102599. [Google Scholar] [CrossRef]
- Jahanmir, G.; Chau, Y. Chapter 9–Mathematical Models of Drug Release from Degradable Hydrogels. In Biomedical Applications of Nanoparticles; Grumezescu, A.M., Ed.; William Andrew Publishing: New York, NY, USA, 2019; pp. 233–269. ISBN 978-0-12-816506-5. [Google Scholar]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Fan, W.; Yin, J.; Yi, C.; Xia, Y.; Nie, Z.; Sui, K. Nature-Inspired Sequential Shape Transformation of Energy-Patterned Hydrogel Sheets. ACS Appl. Mater. Interfaces 2020, 12, 4878–4886. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Fu, Y.; Wei, Y.; Zhao, L.; Tao, L. Self-Adapting Hydrogel to Improve the Therapeutic Effect in Wound-Healing. ACS Appl. Mater. Interfaces 2018, 10, 26046–26055. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, R.; Li, Q.; Dai, F.; Lan, G.; Shang, S.; Lu, F. A Self-Adapting Hydrogel Based on Chitosan/Oxidized KonjacGlucomannan/AgNPs for Repairing Irregular Wounds. Biomater. Sci. 2020, 8, 1910–1922. [Google Scholar] [CrossRef]
- Le, T.M.D.; Duong, H.T.T.; Thambi, T.; Giang Phan, V.H.; Jeong, J.H.; Lee, D.S. Bioinspired PH- and Temperature-Responsive Injectable Adhesive Hydrogels with Polyplexes Promotes Skin Wound Healing. Biomacromolecules 2018, 19, 3536–3548. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A Dual Synergistic of Curcumin and Gelatin on Thermal-Responsive Hydrogel Based on Chitosan-P123 in Wound Healing Application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, B.; Sun, L.; Jiang, L.; Li, Q.; Jin, P. Smart Thermosensitive Poloxamer Hydrogels Loaded with Nr-CWs for the Treatment of Diabetic Wounds. PLoS ONE 2022, 17, e0279727. [Google Scholar] [CrossRef]
- Paneysar, J.S.; Barton, S.; Ambre, P.; Coutinho, E. Novel Temperature Responsive Films Impregnated with Silver Nano Particles (Ag-NPs) as Potential Dressings for Wounds. J. Pharm. Sci. 2022, 111, 810–817. [Google Scholar] [CrossRef]
- Zhou, L.; Pi, W.; Cheng, S.; Gu, Z.; Zhang, K.; Min, T.; Zhang, W.; Du, H.; Zhang, P.; Wen, Y. Multifunctional DNA Hydrogels with Hydrocolloid-Cotton Structure for Regeneration of Diabetic Infectious Wounds. Adv. Funct. Mater. 2021, 31, 2106167. [Google Scholar] [CrossRef]
- Si, Y.; Wang, L.; Wang, X.; Tang, N.; Yu, J.; Ding, B. Ultrahigh-Water-Content, Superelastic, and Shape-Memory Nanofiber-Assembled Hydrogels Exhibiting Pressure-Responsive Conductivity. Adv. Mater. 2017, 29, 1700339. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fei, X.; Xu, L.; Wang, Y.; Tian, J.; Li, Y. Pressure-Sensitive Antibacterial Hydrogel Dressing for Wound Monitoring in Bed Ridden Patients. J. Colloid Interface Sci. 2022, 627, 942–955. [Google Scholar] [CrossRef]
- de Alencar Fonseca Santos, J.; Campelo, M.B.D.; de Oliveira, R.A.; Nicolau, R.A.; Rezende, V.E.A.; Arisawa, E.Â.L. Effects of Low-Power Light Therapy on the Tissue Repair Process of Chronic Wounds in Diabetic Feet. Photomed. Laser Surg. 2018, 36, 298–304. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Lubart, R. A Possible Mechanism for Visible Light-Induced Wound Healing. Lasers Surg. Med. 2008, 40, 509–514. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Chapter Twelve–Multifunctional Materials Based on Smart Hydrogels for Biomedical and 4D Applications. In Advanced Lightweight Multifunctional Materials; Costa, P., Costa, C.M., Lanceros-Mendez, S., Eds.; Woodhead Publishing in Materials; Woodhead Publishing: Thorston, UK, 2021; pp. 407–467. ISBN 978-0-12-818501-8. [Google Scholar]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A Near-Infrared Light-Responsive Multifunctional Nanocomposite Hydrogel for Efficient and Synergistic Antibacterial Wound Therapy and Healing Promotion. J. Mater. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The Production and Application of Hydrogels for Wound Management: A Review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Arenbergerova, M.; Arenberger, P.; Bednar, M.; Kubat, P.; Mosinger, J. Light-Activated Nanofibre Textiles Exert Antibacterial Effects in the Setting of Chronic Wound Healing. Exp. Dermatol. 2012, 21, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart Hydrogel-Based DVDMS/BFGF Nanohybrids for Antibacterial Phototherapy with Multiple Damaging Sites and Accelerated Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, J.; Zhen, C.; Wang, Y.; Wei, Y.; Ren, W.; Shang, P. Magnetic Fields as a Potential Therapy for Diabetic Wounds Based on Animal Experiments and Clinical Trials. Cell Prolif. 2021, 54, e12982. [Google Scholar] [CrossRef] [PubMed]
- Ekici, Y.; Aydogan, C.; Balcik, C.; Haberal, N.; Kirnap, M.; Moray, G.; Haberal, M. Effect of Static Magnetic Field on Experimental Dermal Wound Strength. Indian J. Plast. Surg. 2012, 45, 215–219. [Google Scholar] [CrossRef]
- Nezami, S.; Sadeghi, M.; Mohajerani, H. A Novel PH-Sensitive and Magnetic Starch-Based Nanocomposite Hydrogel as a Controlled Drug Delivery System for Wound Healing. Polym. Degrad. Stab. 2020, 179, 109255. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small 2022, 18, 2104368. [Google Scholar] [CrossRef]
- Xu, J.; Tsai, Y.-L.; Hsu, S. Design Strategies of Conductive Hydrogel for Biomedical Applications. Molecules 2020, 25, 5296. [Google Scholar] [CrossRef]
- Verdes, M.; Mace, K.; Margetts, L.; Cartmell, S. Status and Challenges of Electrical Stimulation Use in Chronic Wound Healing. Curr. Opin. Biotechnol. 2022, 75, 102710. [Google Scholar] [CrossRef]
- Tai, G.; Tai, M.; Zhao, M. Electrically Stimulated Cell Migration and Its Contribution to Wound Healing. Burns Trauma. 2018, 6, 20. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, R.; Guo, B. Stimuli-Responsive Conductive Hydrogels: Design, Properties, and Applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration during Wound Healing. Small Weinh. Bergstr. Ger. 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Zhang, S.; Ge, G.; Qin, Y.; Li, W.; Dong, J.; Mei, J.; Ma, R.; Zhang, X.; Bai, J.; Zhu, C.; et al. Recent Advances in Responsive Hydrogels for Diabetic Wound Healing. Mater. Today Bio 2023, 18, 100508. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Xu, T.; Zhang, X. Multifunctional Hydrogel as Wound Dressing for Intelligent Wound Monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory PH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Shi, W.; Kong, Y.; Su, Y.; Kuss, M.A.; Jiang, X.; Li, X.; Xie, J.; Duan, B. Tannic Acid-Inspired, Self-Healing, and Dual Stimuli Responsive Dynamic Hydrogel with Potent Antibacterial and Anti-Oxidative Properties. J. Mater. Chem. B 2021, 9, 7182–7195. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.; Xu, L.; Lu, H.; Chen, Y.; Wu, C.; Hu, P. A Self-Healing Hydrogel Based on Crosslinked Hyaluronic Acid and Chitosan to Facilitate Diabetic Wound Healing. Int. J. Biol. Macromol. 2022, 220, 326–336. [Google Scholar] [CrossRef]
- Li, L.; Lei, D.; Zhang, J.; Xu, L.; Li, J.; Jin, L.; Pan, L. Dual-Responsive Alginate Hydrogel Constructed by Sulfhdryl Dendrimer as an Intelligent System for Drug Delivery. Molecules 2022, 27, 281. [Google Scholar] [CrossRef]
- Zhang, J.; Hurren, C.; Lu, Z.; Wang, D. PH-Sensitive Alginate Hydrogel for Synergistic Anti-Infection. Int. J. Biol. Macromol. 2022, 222, 1723–1733. [Google Scholar] [CrossRef]
- Qiao, B.; Pang, Q.; Yuan, P.; Luo, Y.; Ma, L. Smart Wound Dressing for Infection Monitoring and NIR-Triggered Antibacterial Treatment. Biomater. Sci. 2020, 8, 1649–1657. [Google Scholar] [CrossRef]
- Guo, P.; Liang, J.; Li, Y.; Lu, X.; Fu, H.; Jing, H.; Guan, S.; Han, D.; Niu, L. High-Strength and PH-Responsive Self-Healing Polyvinyl Alcohol/Poly 6-Acrylamidohexanoic Acid Hydrogel Based on Dual Physically Cross-Linked Network. Colloids Surf. A Physicochem. Eng. Asp. 2019, 571, 64–71. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Mingot, J.; Torras, J.; Alemán, C.; Armelin, E. Recent Advances in Poly(N-isopropylacrylamide) Hydrogels and Derivatives as Promising Materials for Biomedical and Engineering Emerging Applications. Adv. Eng. Mater. 2023, 25, 2201303. [Google Scholar] [CrossRef]
- Han, X.; Yang, R.; Wan, X.; Dou, J.; Yuan, J.; Chi, B.; Shen, J. Antioxidant and Multi-Sensitive PNIPAAm/Keratin Double Network Gels for Self-Stripping Wound Dressing Application. J. Mater. Chem. B 2021, 9, 6212–6225. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; You, S.; Mao, R.; Cai, E.; Pan, W.; Zhang, C.; Hu, R.; Shen, J. Mild Hyperthermia-Assisted ROS Scavenging Hydrogels Achieve Diabetic Wound Healing. ACS Macro Lett. 2022, 11, 861–867. [Google Scholar] [CrossRef]
- Gao, F.; Xiong, Z. Reactive Oxygen Species Responsive Polymers for Drug Delivery Systems. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, R.; Chen, B.; Liu, X.; Jia, Q.; Wang, X.; Yang, Z.; Ning, P.; Wang, Z.; Yang, Y. Injectable Reactive Oxygen Species-Responsive Hydrogel Dressing with Sustained Nitric Oxide Release for Bacterial Ablation and Wound Healing. Adv. Funct. Mater. 2022, 32, 2202857. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-Responsive Injectable Hydrogels Encapsulating Drug-Loaded Micelles for on-Demand Antimicrobial Activity and Accelerated Wound Healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, J.; Cao, L.; Jiao, Q.; Zhou, J.; Yang, L.; Zhang, H.; Wei, Y. Antifouling Antioxidant Zwitterionic Dextran Hydrogels as Wound Dressing Materials with Excellent Healing Activities. ACS Appl. Mater. Interfaces. 2021, 13, 7060–7069. [Google Scholar] [CrossRef]
- Bas, Y.; Sanyal, R.; Sanyal, A. Hyaluronic-Acid Based Redox-Responsive Hydrogels Using the Diels-Alder Reaction for on-Demand Release of Biomacromolecules. J. Macromol. Sci. Part A 2023, 60, 246–254. [Google Scholar] [CrossRef]
- Webber, M.J.; Anderson, D.G. Smart Approaches to Glucose-Responsive Drug Delivery. J. Drug Target 2015, 23, 651–655. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Huang, J.; Wu, J. Novel Glucose-Responsive Antioxidant Hybrid Hydrogel for Enhanced Diabetic Wound Repair. ACS Appl. Mater. Interfaces 2022, 14, 7680–7689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Duan, Z.; Zhao, J.; Fu, R.; Zhu, C.; Fan, D. Glucose and MMP-9 Dual-Responsive Hydrogel with Temperature Sensitive Self-Adaptive Shape and Controlled Drug Release Accelerates Diabetic Wound Healing. Bioact. Mater. 2022, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, W.; Xu, P.; Fu, X.; Yu, X.; Chen, L.; Leng, F.; Yu, C.; Yang, Z. Glucose-Responsive Multifunctional Metal–Organic Drug-Loaded Hydrogel for Diabetic Wound Healing. Acta Biomater. 2022, 140, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Gardikiotis, I.; Cojocaru, F.-D.; Mihai, C.-T.; Balan, V.; Dodi, G. Borrowing the Features of Biopolymers for Emerging Wound Healing Dressings: A Review. Int. J. Mol. Sci. 2022, 23, 8778. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Yang, Y.; Qiao, L.; Hu, J.; Zhang, J.; Guo, B. Antibacterial Adhesive Self-Healing Hydrogels to Promote Diabetic Wound Healing. Acta Biomater. 2022, 146, 119–130. [Google Scholar] [CrossRef]
- Vahedi, M.; Barzin, J.; Shokrolahi, F.; Shokrollahi, P. Self-Healing, Injectable Gelatin Hydrogels Cross-Linked by Dynamic Schiff Base Linkages Support Cell Adhesion and Sustained Release of Antibacterial Drugs. Macromol. Mater. Eng. 2018, 303, 1800200. [Google Scholar] [CrossRef]
- Gutierrez-Reyes, J.E.; Caldera-Villalobos, M.; Claudio-Rizo, J.A.; Cabrera-Munguía, D.A.; Becerra-Rodriguez, J.J.; Soriano-Corral, F.; Herrera-Guerrero, A. Smart Collagen/Xanthan Gum-Based Hydrogels with Antibacterial Effect, Drug Release Capacity and Excellent Performance in Vitro Bioactivity for Wound Healing Application. Biomed. Mater. 2023, 18, 035011. [Google Scholar] [CrossRef]
- Hu, C.; Liu, W.; Long, L.; Wang, Z.; Yuan, Y.; Zhang, W.; He, S.; Wang, J.; Yang, L.; Lu, L.; et al. Microenvironment-Responsive Multifunctional Hydrogels with Spatiotemporal Sequential Release of Tailored Recombinant Human Collagen Type III for the Rapid Repair of Infected Chronic Diabetic Wounds. J. Mater. Chem. B 2021, 9, 9684–9699. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, C.; Peng, X.; Zhang, K.; Yu, X. A Self-Healing and Injectable Oxidized Quaternized Guar Gum/Carboxymethyl Chitosan Hydrogel with Efficient Hemostatic and Antibacterial Properties for Wound Dressing. Colloids Surf. B Biointerfaces 2022, 209, 112207. [Google Scholar] [CrossRef]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin Signaling in Wound Repair. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 248–257. [Google Scholar] [CrossRef]
- Kawabata, S.; Kanda, N.; Hirasawa, Y.; Noda, K.; Matsuura, Y.; Suzuki, S.; Kawai, K. The Utility of Silk-Elastin Hydrogel as a New Material for Wound Healing. Plast. Reconstr. Surg.–Glob. Open 2018, 6, e1778. [Google Scholar] [CrossRef] [PubMed]
- Stojic, M.; Ródenas-Rochina, J.; López-Donaire, M.L.; González De Torre, I.; González Pérez, M.; Rodríguez-Cabello, J.C.; Vojtová, L.; Jorcano, J.L.; Velasco, D. Elastin-Plasma Hybrid Hydrogels for Skin Tissue Engineering. Polymers 2021, 13, 2114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Desai, M.S.; Wang, T.; Lee, S.-W. Elastin-Based Thermoresponsive Shape-Memory Hydrogels. Biomacromolecules 2020, 21, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shah, S.A.-M.; Basharat, K.; Qamar, S.A.; Raza, A.; Mohamed, A.; Bilal, M.; Iqbal, H.M.N. Silk-Based Nano-Hydrogels for Futuristic Biomedical Applications. J. Drug Deliv. Sci. Technol. 2022, 72, 103385. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules 2022, 12, 1852. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional Silk Fibroin Hydrogels: Preparation, Properties and Applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef]
- Römer, L.; Scheibel, T. The Elaborate Structure of Spider Silk: Structure and Function of a Natural High Performance Fiber. Prion 2008, 2, 154–161. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-Based Hydrogels: Present Status and Application Prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Lin, F.; Zheng, J.; Guo, W.; Zhu, Z.; Wang, Z.; Dong, B.; Lin, C.; Huang, B.; Lu, B. Smart Cellulose-Derived Magnetic Hydrogel with Rapid Swelling and Deswelling Properties for Remotely Controlled Drug Release. Cellulose 2019, 26, 6861–6877. [Google Scholar] [CrossRef]
- SCOGS (Select Committee on GRAS Substances). Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS&sort=Sortsubstance&order=ASC&startrow=1&type=basic&search=ALGINATE (accessed on 3 April 2023).
- Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm?event=BasicSearch.page (accessed on 3 April 2023).
- Soeiro, V.C.; Melo, K.R.T.; Alves, M.G.C.F.; Medeiros, M.J.C.; Grilo, M.L.P.M.; Almeida-Lima, J.; Pontes, D.L.; Costa, L.S.; Rocha, H.A.O. Dextran: Influence of Molecular Weight in Antioxidant Properties and Immunomodulatory Potential. Int. J. Mol. Sci. 2016, 17, 1340. [Google Scholar] [CrossRef]
- Luanda, A.; Badalamoole, V. Past, Present and Future of Biomedical Applications of Dextran-Based Hydrogels: A Review. Int. J. Biol. Macromol. 2023, 228, 794–807. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Shen, Y.-I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran Hydrogel Scaffolds Enhance Angiogenic Responses and Promote Complete Skin Regeneration during Burn Wound Healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, M.; Liu, Q.; Yan, S.; Feng, L.; Lan, Y.; Shan, G.; Xue, W.; Guo, R. Enhanced Healing Activity of Burn Wound Infection by a Dextran-HA Hydrogel Enriched with Sanguinarine. Biomater. Sci. 2018, 6, 2472–2486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, L.; Wei, C.; Guo, R. A Bioactive Dextran-Based Hydrogel Promote the Healing of Infected Wounds via Antibacterial and Immunomodulatory. Carbohydr. Polym. 2022, 291, 119558. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Kung, H.N.; Tsai, Y.S.; Tseng, T.N.; Hsu, K.D.; Cheng, K.C. Novel Dextran Modified Bacterial Cellulose Hydrogel Accelerating Cutaneous Wound Healing. Cellulose 2017, 24, 4927–4937. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and Conductive Injectable Hydrogels Based on Quaternized Chitosan-Graft-Polyaniline/Oxidized Dextran for Tissue Engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, G.; Lei, M.; Lei, J.; Li, D.; Zheng, H. A PH-Sensitive Oxidized-Dextran Based Double Drug-Loaded Hydrogel with High Antibacterial Properties. Int. J. Biol. Macromol. 2021, 182, 385–393. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Wang, S.; Dong, C.; Zhang, X.; Zhang, R.; Yang, L. Dextran and Peptide-Based PH-Sensitive Hydrogel Boosts Healing Process in Multidrug-Resistant Bacteria-Infected Wounds. Carbohydr. Polym. 2022, 278, 118994. [Google Scholar] [CrossRef]
- Guo, C.; Wu, Y.; Li, W.; Wang, Y.; Kong, Q. Development of a Microenvironment-Responsive Hydrogel Promoting Chronically Infected Diabetic Wound Healing through Sequential Hemostatic, Antibacterial, and Angiogenic Activities. ACS Appl. Mater. Interfaces 2022, 14, 30480–30492. [Google Scholar] [CrossRef]
- Crini, G. Historical Review on Chitin and Chitosan Biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A Review of Molecular Structure, Bioactivities and Interactions with the Human Body and Micro-Organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wei, Y.; Tao, L. Chitosan-Based Self-Healing Hydrogel for Bioapplications. Chin. Chem. Lett. 2017, 28, 2053–2057. [Google Scholar] [CrossRef]
- Iacob, A.-T.; Drăgan, M.; Ghețu, N.; Pieptu, D.; Vasile, C.; Buron, F.; Routier, S.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives. Polymers 2018, 10, 607. [Google Scholar] [CrossRef]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan Membrane as a Wound-Healing Dressing: Characterization and Clinical Application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a Chitosan-Based Wound Dressing with Improved Hemostatic and Antimicrobial Properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Piątkowski, M.; Deineka, V.; Janus, Ł.; Korniienko, V.; Husak, E.; Holubnycha, V.; Liubchak, I.; Zhurba, V.; Sierakowska, A.; et al. Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties—Synthesis and Characterization. Molecules 2019, 24, 2629. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound Healing and Antimicrobial Effect of Active Secondary Metabolites in Chitosan-Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Guo, X.; Sun, T.; Zhong, R.; Ma, L.; You, C.; Tian, M.; Li, H.; Wang, C. Effects of Chitosan Oligosaccharides on Human Blood Components. Front. Pharmacol. 2018, 9, 1412. [Google Scholar] [CrossRef]
- AbdEl-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan Preparations for Wounds and Burns: Antimicrobial and Wound-Healing Effects. Expert Rev. Anti Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Yu, H.; Long, H.; Zhang, H.; Hao, C.; Shi, L.; Du, Y.; Jiao, S.; Guo, A.; Ma, L.; et al. Low Deacetylation Degree Chitosan Oligosaccharide Protects against IL-1β Induced Inflammation and Enhances Autophagy Activity in Human Chondrocytes. J. Biomater. Sci. Polym. Ed. 2022, 33, 517–531. [Google Scholar] [CrossRef]
- Satitsri, S.; Muanprasat, C. Chitin and Chitosan Derivatives as Biomaterial Resources for Biological and Biomedical Applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, B.; Li, W.; Han, Z.; Chen, S.; Wang, H. Bacterial Cellulose Reinforced Chitosan-Based Hydrogel with Highly Efficient Self-Healing and Enhanced Antibacterial Activity for Wound Healing. Int. J. Biol. Macromol. 2022, 217, 77–87. [Google Scholar] [CrossRef]
- Li, H.; Wei, X.; Yi, X.; Tang, S.; He, J.; Huang, Y.; Cheng, F. Antibacterial, Hemostasis, Adhesive, Self-Healing Polysaccharides-Based Composite Hydrogel Wound Dressing for the Prevention and Treatment of Postoperative Adhesion. Mater. Sci. Eng. C 2021, 123, 111978. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas Aeruginosa -Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef] [PubMed]
- Tatarusanu, S.-M.; Sava, A.; Profire, B.-S.; Pinteala, T.; Jitareanu, A.; Iacob, A.-T.; Lupascu, F.; Simionescu, N.; Rosca, I.; Profire, L. New Smart Bioactive and Biomimetic Chitosan-Based Hydrogels for Wounds Care Management. Pharmaceutics 2023, 15, 975. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Henríquez, L.; Castro-Alpízar, J.; Lopretti-Correa, M.; Vega-Baudrit, J. Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing. Int. J. Mol. Sci. 2021, 22, 1408. [Google Scholar] [CrossRef]
- Rusu, A.G.; Chiriac, A.P.; Nita, L.E.; Rosca, I.; Pinteala, M.; Mititelu-Tartau, L. Chitosan Derivatives in Macromolecular Co-Assembly Nanogels with Potential for Biomedical Applications. Biomacromolecules 2020, 21, 4231–4243. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Roquero, D.M.; Katz, E. “Smart” Alginate Hydrogels in Biosensing, Bioactuation and Biocomputing: State-of-the-Art and Perspectives. Sens. Actuators Rep. 2022, 4, 100095. [Google Scholar] [CrossRef]
- Ueno, M.; Oda, T. Biological Activities of Alginate. Adv. Food Nutr. Res. 2014, 72, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs. 2020, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-G.; Kim, S.-C.; Kim, T.-H.; Je, J.-Y.; Lee, B.; Lee, S.G.; Kim, Y.-M.; Kang, H.W.; Qian, Z.-J.; Kim, N.; et al. Ishophloroglucin A-Based Multifunctional Oxidized Alginate/Gelatin Hydrogel for Accelerating Wound Healing. Int. J. Biol. Macromol. 2023, 245, 125484. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Nagao, M.; Elias, A.; Narain, R.; Chung, H.-J. A PH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings. Polymers 2017, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-Y.; Weng, C.-C.; Lai, J.-Y.; Lin, S.-Y.; Tsai, H.-C. Design of an Interpenetrating Polymeric Network Hydrogel Made of Calcium-Alginate from a Thermos-Sensitive Pluronic Template as a Thermal-Ionic Reversible Wound Dressing. Polymers 2020, 12, 2138. [Google Scholar] [CrossRef]
- Yao, Z.; Qian, Y.; Jin, Y.; Wang, S.; Li, J.; Yuan, W.-E.; Fan, C. Biomimetic Multilayer Polycaprolactone/Sodium Alginate Hydrogel Scaffolds Loaded with Melatonin Facilitate Tendon Regeneration. Carbohydr. Polym. 2022, 277, 118865. [Google Scholar] [CrossRef]
- Xu, K.; Deng, S.; Zhu, Y.; Yang, W.; Chen, W.; Huang, L.; Zhang, C.; Li, M.; Ao, L.; Jiang, Y.; et al. Platelet Rich Plasma Loaded Multifunctional Hydrogel Accelerates Diabetic Wound Healing via Regulating the Continuously Abnormal Microenvironments. Adv. Healthc. Mater. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers. 2018, 10, 701. [Google Scholar] [CrossRef]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant Acitivity of Low Molecular Weight Hyaluronic Acid. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2670–2675. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds Compend. Clin. Res. Pract. 2016, 28, 78–88. [Google Scholar]
- Yang, H.; Song, L.; Zou, Y.; Sun, D.; Wang, L.; Yu, Z.; Guo, J. Role of Hyaluronic Acids and Potential as Regenerative Biomaterials in Wound Healing. ACS Appl. Bio Mater. 2021, 4, 311–324. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, W.K.V.; de Medeiros, W.R.D.B.; de Assis, C.F.; Dos Santos, E.S.; de Sousa Júnior, F.C. Physicochemical Characterization and in Vitro Antioxidant Activity of Hyaluronic Acid Produced by Streptococcus zooepidemicus CCT 7546. Prep. Biochem. Biotechnol. 2022, 52, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Katas, H.; Bukhari, S.N.A. Hyaluronic Acid-Based Biomaterials: A Versatile and Smart Approach to Tissue Regeneration and Treating Traumatic, Surgical, and Chronic Wounds. Polym. Rev. 2017, 57, 594–630. [Google Scholar] [CrossRef]
- Ebid, R.; Lichtnekert, J.; Anders, H.-J. Hyaluronan Is Not a Ligand but a Regulator of Toll-Like Receptor Signaling in Mesangial Cells: Role of Extracellular Matrix in Innate Immunity. ISRN Nephrol. 2014, 2014, 714081. [Google Scholar] [CrossRef]
- Ding, Y.-W.; Wang, Z.-Y.; Ren, Z.-W.; Zhang, X.-W.; Wei, D.-X. Advances in Modified Hyaluronic Acid-Based Hydrogels for Skin Wound Healing. Biomater. Sci. 2022, 10, 3393–3409. [Google Scholar] [CrossRef]
- Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef]
- Nyman, E.; Henricson, J.; Ghafouri, B.; Anderson, C.D.; Kratz, G. Hyaluronic Acid Accelerates Re-Epithelialization and Alters Protein Expression in a Human Wound Model. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2221. [Google Scholar] [CrossRef]
- Guan, S.; Li, Y.; Cheng, C.; Gao, X.; Gu, X.; Han, X.; Ye, H. Manufacture of PH- and HAase-Responsive Hydrogels with on-Demand and Continuous Antibacterial Activity for Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2020, 164, 2418–2431. [Google Scholar] [CrossRef]
- Deng, M.; Wu, Y.; Ren, Y.; Song, H.; Zheng, L.; Lin, G.; Wen, X.; Tao, Y.; Kong, Q.; Wang, Y. Clickable and Smart Drug Delivery Vehicles Accelerate the Healing of Infected Diabetic Wounds. J. Control. Release 2022, 350, 613–629. [Google Scholar] [CrossRef]
- Das, S.; Das, D. Rational Design of Peptide-Based Smart Hydrogels for Therapeutic Applications. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-Assembling Peptide-Based Hydrogels for Wound Tissue Repair. Adv. Sci. 2022, 9, 2104165. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, X. Self-Assemble Peptide Biomaterials and Their Biomedical Applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, H.; Luo, H.; Wang, S.; Zhou, Q.; Du, X.; Tang, C.; Chen, L.; Liu, J.; Shi, Y.-K.; et al. Temperature and PH Effects on Biophysical and Morphological Properties of Self-Assembling Peptide RADA16-I. J. Pept. Sci. 2008, 14, 152–162. [Google Scholar] [CrossRef]
- Ng, V.W.L.; Chan, J.M.W.; Sardon, H.; Ono, R.J.; García, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial Hydrogels: A New Weapon in the Arsenal against Multidrug-Resistant Infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Schnaider, L.; Brahmachari, S.; Schmidt, N.W.; Mensa, B.; Shaham-Niv, S.; Bychenko, D.; Adler-Abramovich, L.; Shimon, L.J.W.; Kolusheva, S.; DeGrado, W.F.; et al. Self-Assembling Dipeptide Antibacterial Nanostructures with Membrane Disrupting Activity. Nat. Commun. 2017, 8, 1365. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Velázquez, R.; Díez-Marqués, M.L.; Ruiz-Torres, M.P.; González-Rubio, M.; Rodríguez-Puyol, M.; Rodríguez Puyol, D. Arg-Gly-Asp-Ser (RGDS) Peptide Stimulates Transforming Growth Factor Beta1 Transcription and Secretion through Integrin Activation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 1529–1531. [Google Scholar] [CrossRef]
- Lee, S.; Trinh, T.H.T.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.B.; Ryou, C. Ryou Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850. [Google Scholar] [CrossRef]
- Sankar, S.; O’Neill, K.; Bagot D’Arc, M.; Rebeca, F.; Buffier, M.; Aleksi, E.; Fan, M.; Matsuda, N.; Gil, E.S.; Spirio, L. Clinical Use of the Self-Assembling Peptide RADA16: A Review of Current and Future Trends in Biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 679525. [Google Scholar] [CrossRef]
- PuraStat. Available online: https://3dmatrix.com/products/purastat/ (accessed on 30 March 2023).
- Tian, T.; Li, Y.; Lin, Y. Prospects and Challenges of Dynamic DNA Nanostructures in Biomedical Applications. Bone Res. 2022, 10, 40. [Google Scholar] [CrossRef]
- Fu, L.; Li, P.; Zhu, J.; Liao, Z.; Gao, C.; Li, H.; Yang, Z.; Zhao, T.; Chen, W.; Peng, Y.; et al. Tetrahedral Framework Nucleic Acids Promote the Biological Functions and Related Mechanism of Synovium-Derived Mesenchymal Stem Cells and Show Improved Articular Cartilage Regeneration Activity in Situ. Bioact. Mater. 2022, 9, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, M.; Guo, X.; Yang, M.; Rasooly, A. Self-Assembled DNA-THPS Hydrogel as a Topical Antibacterial Agent for Wound Healing. ACS Appl. Bio Mater. 2019, 2, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Hutanu, D. Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod. Chem. Appl. 2014, 2, 2. [Google Scholar] [CrossRef]
- Handbook of Pharmaceutical Excipients 6th Edition|PDF|Magnesium|Tablet (Pharmacy). Available online: https://www.scribd.com/document/246196761/Handbook-of-Pharmaceutical-Excipients-6th-Edition (accessed on 26 March 2023).
- Chen, S.-L.; Fu, R.-H.; Liao, S.-F.; Liu, S.-P.; Lin, S.-Z.; Wang, Y.-C. A PEG-Based Hydrogel for Effective Wound Care Management. Cell Transplant. 2018, 27, 275–284. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and Properties of Poly(Vinyl Alcohol) Hydrogels with High Strength and Toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Bodratti, A.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Akiyama, H.; Tamaoki, N. Synthesis and Photoinduced Phase Transitions of Poly (N-Isopropylacrylamide) Derivative Functionalized with Terminal Azobenzene Units. Macromolecules 2007, 40, 5129–5132. [Google Scholar] [CrossRef]
- Duan, Q.; Miura, Y.; Narumi, A.; Shen, X.; Sato, S.-I.; Satoh, T.; Kakuchi, T. Synthesis and Thermoresponsive Property of End-Functionalized Poly (N-Isopropylacrylamide) with Pyrenyl Group. J. Polym. Sci. Part Polym. Chem. 2006, 44, 1117–1124. [Google Scholar] [CrossRef]
- Yu, B.; Chan, J.W.; Hoyle, C.E.; Lowe, A.B. Sequential Thiol-Ene/Thiol-Ene and Thiol-Ene/Thiol-Yne Reactions as a Route to Well-Defined Mono and Bis End-Functionalized Poly (N-Isopropylacrylamide). J. Polym. Sci. Part Polym. Chem. 2009, 47, 3544–3557. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef] [PubMed]
| Traditional Hydrogels | SHs | References |
|---|---|---|
| Protection of the damaged area to external factors | Enhanced protection of the damaged area due the self-adapting ability | [36,43] |
| Provide moisture of the wound bed depending on hydrogel composition without regarding on wound conditions (dry, wet) | Dynamic and interactive control of moisture, based on responsive behavior to wound condition (exudate, pH) or external triggers (pressure, light) | [42,62] |
| Frequent changing during treatment | The need of dressing changes may be reduced and adapted to patient and wound particularities | [12,61] |
| Strong adhesive properties with discomfort and secondary injuries when they are removed or lack of adhesive properties with inadequate contact with the damaged tissue | Excellent adhesive properties with intimate contact to the wound bed and easiness remove without damages or discomfort for patient | [63,64,65] |
| Low or no protection against microbial contamination | Efficient protection to microorganism growth through polymers characteristics, cross-linking methods, and manufacturing design | [60,64] |
| No effect on physiological mechanisms of healing process | Promote, accelerate, and modulate the endogenous mediators on wound site | [35,46] |
| A limited number of APIs (most water-stable) could be embedded into 3D matrix | A wide variety of APIs (chemical and bioactive molecules) could be embedded using versatile carriers (vesicles, liposomes, niosomes, nanoparticles) | [45,59] |
| Passive release of APIs. | Controllable release of API through endogenous or exogenous stimuli, depending on wound changes | [46,66] |
| No possibility to evaluate the wound condition during treatment | Possibilities to real-time monitor the healing of wound | [59,67] |
| Low cost of production, simple design, robust manufacturing at industrial scale | Challenging manufacturing process, difficulties to reproduce the quality attributes from batch to batch | [61,68] |
| Safe raw materials with well-established characteristics for human use | New synthetized raw materials or modified from traditional sources which may raise stability and safety issues | [39,55] |
| Simple to use, easy to apply | Requires training of the patient and/or medical staff | [38,42] |
| Polymer | Advantages | Challenges | Polymer-Based SHs (e.g.) | References |
|---|---|---|---|---|
| Gelatin | Haemostatic effect, promote migration of fibroblasts on injured area | Hypersensitivity reactions; 3D structure is destroyed through bacterial contamination | Antibacterial injectable self-healing hydrogel based on gelatine and poly(ethylene glicol) bis (benzandehide) loaded with clindamycin; Antibacterial self-healing hydrogel based on gelatine methacrylate, adenine acrylate and copper(II) chloride | [117,118,119] |
| Collagen | Haemostatic effect, promote cell migration, proliferation and differentiation; stimulate formation of granulation tissue and synthesis of ECM with reduced scars; anti-inflammatory and proangiogenic effects | High cost, enzymatic degradation, poor elasticity; no gelling properties (requires additional polymers) | pH- and ROS-responsive hydrogel based on recombined collagen and HA with controlled release of APIs; Collagen/xanthan gum-based hydrogels with antibacterial effect, and controlled release of ketorolac and methylene blue | [117,120,121] |
| Guaran | Haemostatic effects; lack of toxicity | Risk of bacterial contamination and of depolymerization | Self-healing injectable hydrogel based on oxidized quaternized guaran/carboxymethyl CS with hemostatic and antibacterial effects | [122] |
| Elastin | Chemotaxis to neutrophils, monocytes, macrophages, fibroblasts and endothelial cells, protease production (upregulation of matrix metalloprotease), mimics ECM | Insoluble in water, rapid degradation, low mechanical stability; cross-linking methods affect the elastin performance | Thermoresponsive hydrogels with shape-memory ability based on elastin and elastin-like polypeptides | [123,124,125,126] |
| Silk | Stimulates cell migration, proliferation, and collagen production, wound remodeling | Insoluble in water, immunogenic and inflammatory effects (due to sericin content), high cost for sericin free silk | Self-healing hydrogels based on silk-fibroin and β-cyclodextrin; Desferrioxamine-loaded injectable silk nanofibers hydrogels for diabetic wounds | [127,128,129,130] |
| Cellulose | Lack of toxicity, low cost | Insoluble in water or other common solvents; no bioactivity; allergic reactions | Magnetic responsive hydrogel based on cellulose and β-cyclodextrin to control drug release by swelling behavior | [131,132] |
| Polymers | APIs | SHs Features | References |
|---|---|---|---|
| Oxidized dextran/quaternized CS | - | Stimulation of myoblast proliferation under electrical field; injectability | [141] |
| Oxidized dextran | Sulfadiazine and tobramycin | pH-responsive injectable hydrogel with controlled release of APIs | [142] |
| Oxidized dextran/peptide DP7 (VQWRIRVAVIRK) | Ceftazidime | pH-responsive hydrogel with scarless healing of multidrug-resistant infected wounds | [143] |
| Oxidized dextran/aminated gelatin | ZnO, paeoniflorin, norfloxacin | ROS-responsive hydrogel | [144] |
| Polymers | APIs | SHs Features | References |
|---|---|---|---|
| CS/oxidized konjac glucomannan | Silver (nanoparticles) | Self-healing, self-adapting | [71] |
| Oxidized CS/bacterial cellulose | - | Self-healing | [158] |
| N,O-carboxymethyl CS/oxidized dextran | - | Self- healing, injectability | [159] |
| Quaternized CS/oxidized dextran | Tobramycin | Self-healing | [160] |
| Carboxymethyl CS/oxidized quaternized guaran | - | Self-healing | [122] |
| CS/oxidized CS | Fusidic acid, alantoin, coenzyme Q10 | Self-healing, self-adapting | [161] |
| CS/polyvinylpyrrolidone/alginate/poly(ε-caprolactone) | - | Thermo-responsive | [162] |
| CS/poly(aspartic acid) | Amoxicilin | pH/thermo-responsive | [163] |
| Polymers | APIs | SHs Features | References |
|---|---|---|---|
| Alginate | Amikacin, naproxen | pH/ROS-responsive | [109] |
| Alginate/HA | Doxycycline | ROS–responsive | [60] |
| Oxidized alginate/gelatin | Ishophloroglucin A | Dynamic mechanical properties | [168] |
| Alginate/polyacrylamide | - | pH-responsive (color change) | [169] |
| Alginate/pluronic F127 | Vascular endothelial growth factor | Thermo-responsive | [170] |
| Alginate/polycaprolactone | Melatonin | Controlled release of APIs | [171] |
| Alginate/HA | Platelet rich plasma | ROS/glucose-responsive, self-healing, injectability | [172] |
| Polymers | APIs | SHs Features | References |
|---|---|---|---|
| Phenylboronic acid-modified HA | Tannic acid–silver (nanopartices) | pH/ROS-responsive, self-healing | [98] |
| Adipic acid dihydrazide grafted HA | Sisomicin sulfate | pH-responsive | [183] |
| Tannic acid grafted HA | Silver nanoclusters and deferoxamine | pH-responsive, injectability | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatarusanu, S.-M.; Lupascu, F.-G.; Profire, B.-S.; Szilagyi, A.; Gardikiotis, I.; Iacob, A.-T.; Caluian, I.; Herciu, L.; Giscă, T.-C.; Baican, M.-C.; et al. Modern Approaches in Wounds Management. Polymers 2023, 15, 3648. https://doi.org/10.3390/polym15173648
Tatarusanu S-M, Lupascu F-G, Profire B-S, Szilagyi A, Gardikiotis I, Iacob A-T, Caluian I, Herciu L, Giscă T-C, Baican M-C, et al. Modern Approaches in Wounds Management. Polymers. 2023; 15(17):3648. https://doi.org/10.3390/polym15173648
Chicago/Turabian StyleTatarusanu, Simona-Maria, Florentina-Geanina Lupascu, Bianca-Stefania Profire, Andrei Szilagyi, Ioannis Gardikiotis, Andreea-Teodora Iacob, Iulian Caluian, Lorena Herciu, Tudor-Catalin Giscă, Mihaela-Cristina Baican, and et al. 2023. "Modern Approaches in Wounds Management" Polymers 15, no. 17: 3648. https://doi.org/10.3390/polym15173648
APA StyleTatarusanu, S.-M., Lupascu, F.-G., Profire, B.-S., Szilagyi, A., Gardikiotis, I., Iacob, A.-T., Caluian, I., Herciu, L., Giscă, T.-C., Baican, M.-C., Crivoi, F., & Profire, L. (2023). Modern Approaches in Wounds Management. Polymers, 15(17), 3648. https://doi.org/10.3390/polym15173648










