Changes in the Gut Microbiota Composition during Implantation of Composite Scaffolds Based on Poly(3-hydroxybutyrate) and Alginate on the Large-Intestine Wall
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PHB and ALG
2.2. Isolation and Purification of PHB and ALG
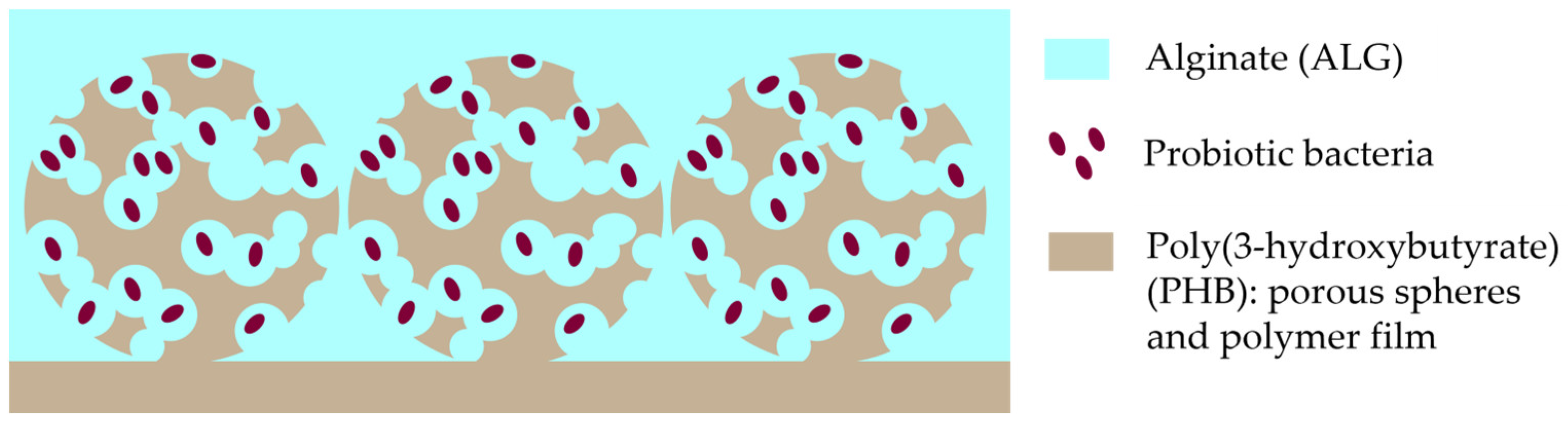
2.3. Design of PHB/ALG Scaffolds
2.4. Animal Experiments
2.5. Surgeries
2.6. Genomic DNA Isolation
2.7. Sequencing of 16S rRNA Gene Amplicon
2.8. Bioinformatics Analysis
2.9. Statistical Analysis
3. Results
3.1. Characterisation of the PHB/ALG Scaffolds
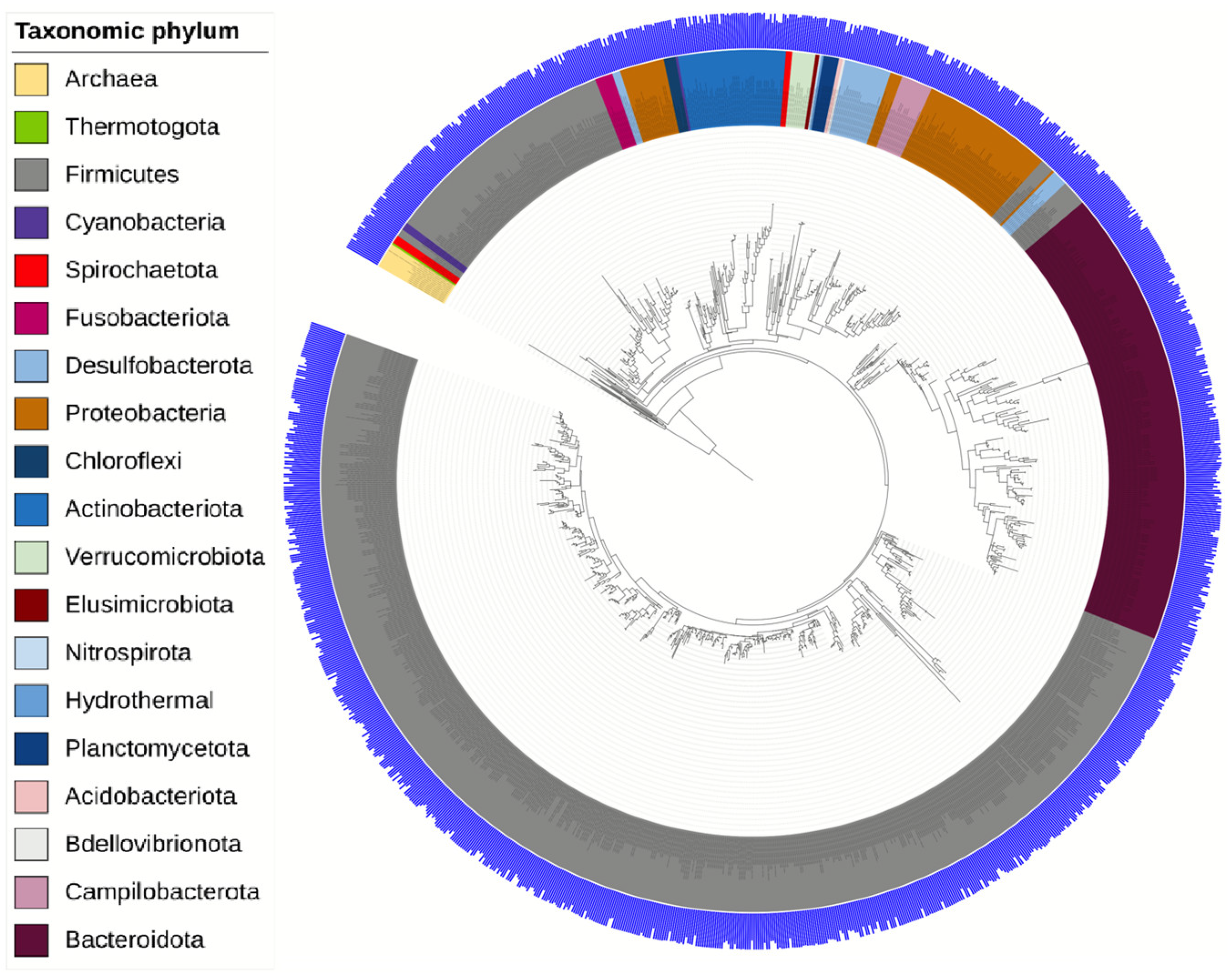
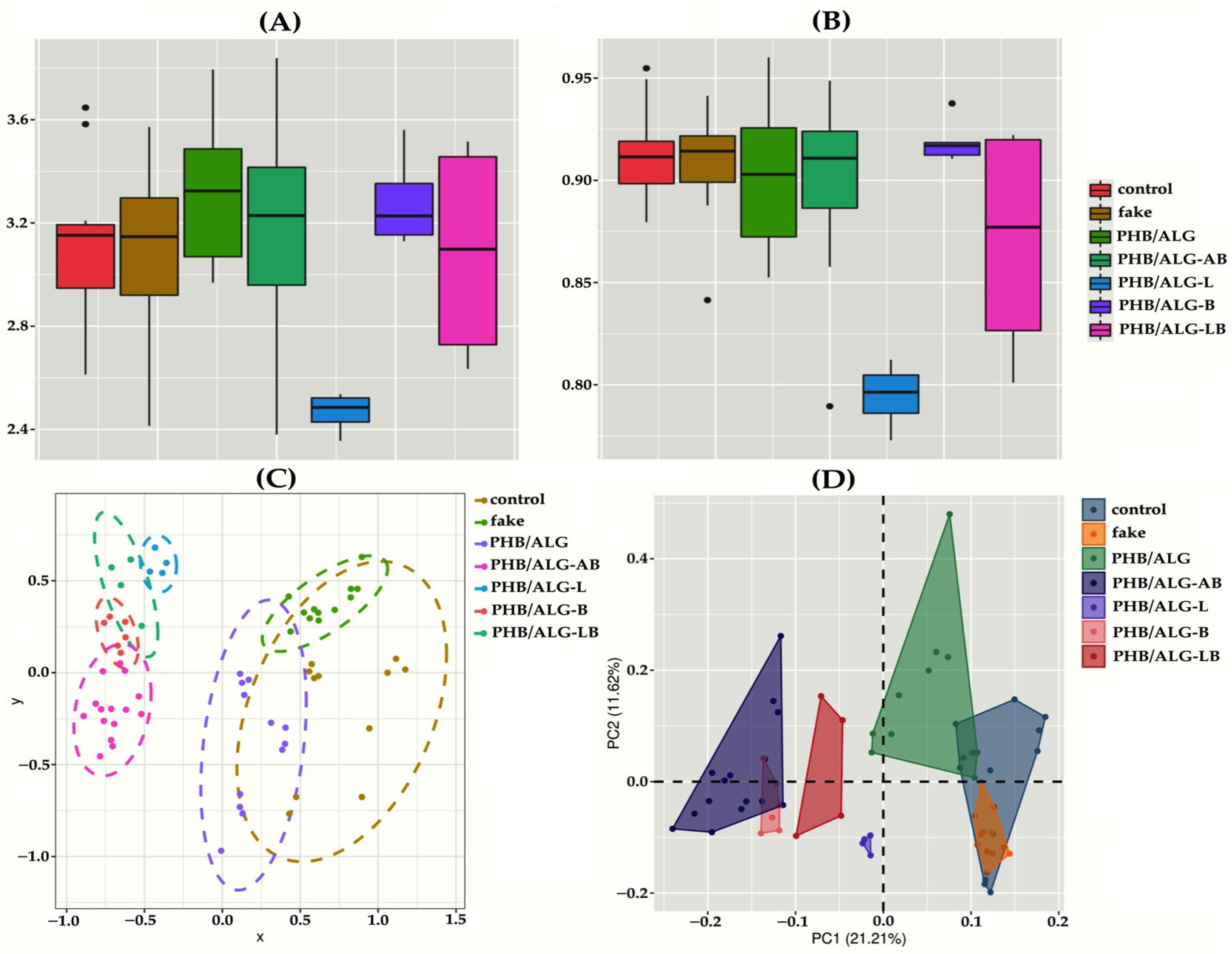
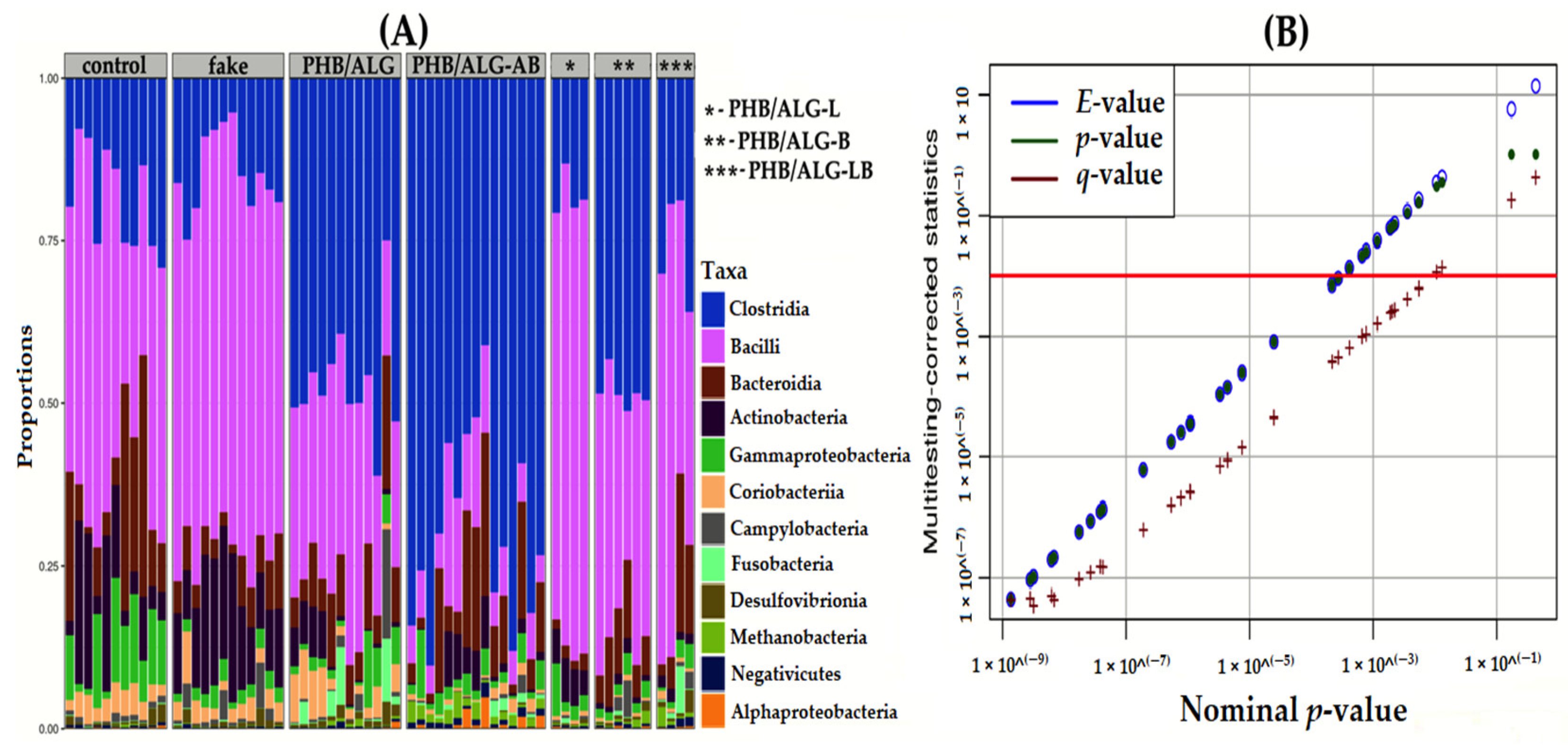
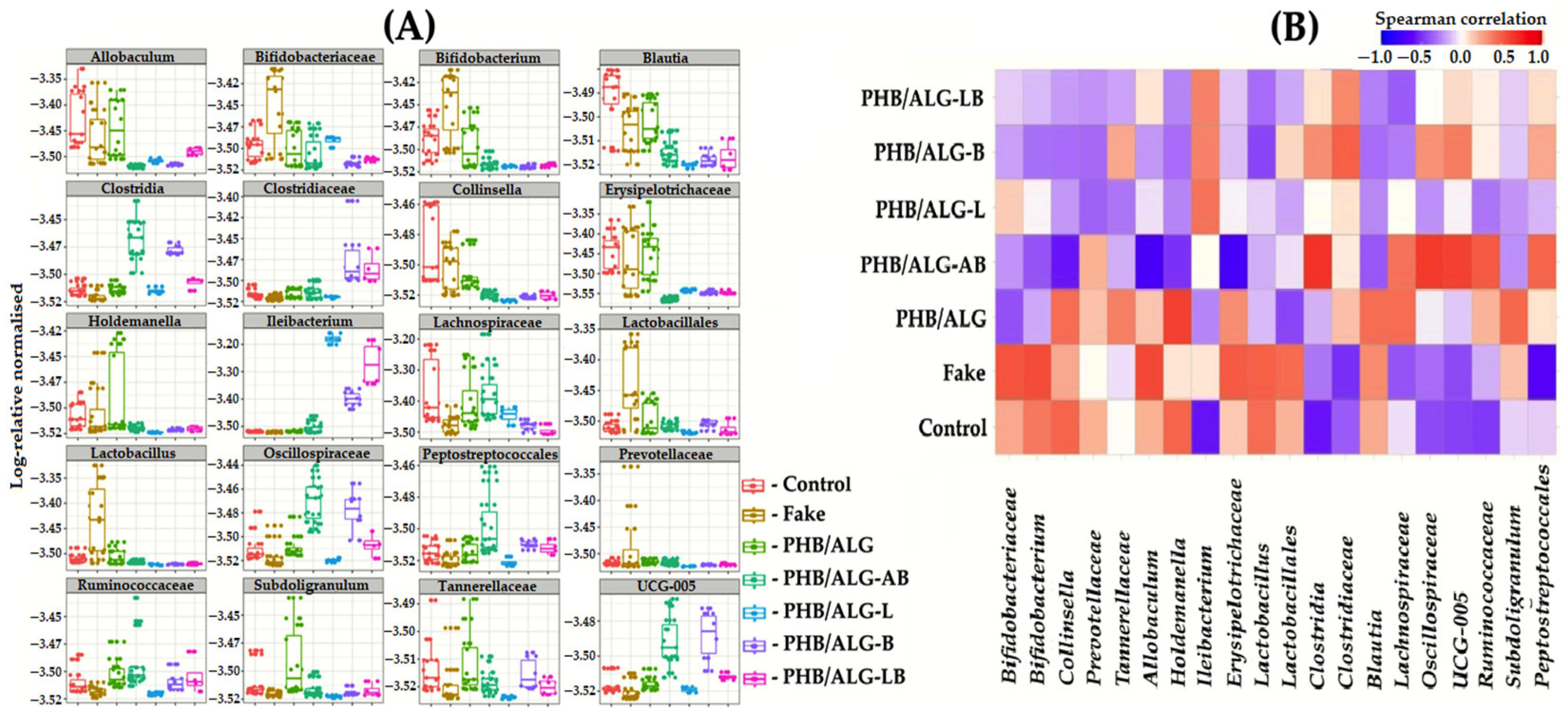
3.2. Analysis of the Gut Microbiota of Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Patel, T.; Bhattacharya, P.; Das, S. Gut microbiota: An Indicator to Gastrointestinal Tract Diseases. J. Gastrointest. Cancer 2016, 47, 232–238. [Google Scholar] [CrossRef]
- Imperatore, N.; Tortora, R.; Morisco, F.; Caporaso, N. Gut microbiota and functional diseases of the gastrointestinal tract. Minerva Gastroenterol. 2017, 63, 355–372. [Google Scholar] [CrossRef]
- Yu, S.; Xie, Y.; Qiu, Y.; Chen, Y.; Fang, J. Moderate alteration to gut microbiota brought by colorectal adenoma resection. J. Gastroenterol. Hepatol. 2019, 34, 1758–1765. [Google Scholar] [CrossRef]
- Tevis, S.E.; Kennedy, G.D. Postoperative Complications: Looking Forward to a Safer Future. Clin. Colon Rectal Surg. 2016, 29, 246–252. [Google Scholar] [CrossRef]
- de Silva, S.; Ma, C.; Proulx, M.; Crespin, M.; Kaplan, B.S.; Hubbard, J.; Prusinkiewicz, M.; Fong, A.; Panaccione, R.; Ghosh, S.; et al. Postoperative Complications and Mortality Following Colectomy for Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Zakhem, E.; Raghavan, S.; Suhar, R.A.; Bitar, K.N. Bioengineering and Regeneration of Gastrointestinal Tissue: Where Are We Now and What Comes Next? Expert Opin. Biol. Ther. 2019, 19, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Zakhem, E.; Tamburrini, R.; Orlando, G.; Koch, K.L.; Bitar, K.N.; Piedrahita, J.A.; Williams, J.K.; Bansal, S.; Keah, N.M.; Neuwirth, A.L.; et al. Transplantation of a Human Tissue-Engineered Bowel in an Athymic Rat Model. Tissue Eng. Part C Methods 2017, 23, 652–660. [Google Scholar] [CrossRef]
- Nakase, Y.; Hagiwara, A.; Nakamura, T.; Nakashima, S.; Yoshikawa, T.; Fukuda, K.-I.; Kuriu, Y.; Miyagawa, K.; Sakakura, C.; Otsuji, E.; et al. Tissue Engineering of Small Intestinal Tissue Using Collagen Sponge Scaffolds Seeded with Smooth Muscle Cells. Tissue Eng. 2006, 12, 403–412. [Google Scholar] [CrossRef]
- Nikolaev, M.; Mitrofanova, O.; Broguiere, N.; Geraldo, S.; Dutta, D.; Tabata, Y.; Elci, B.; Brandenberg, N.; Kolotuev, I.; Gjorevski, N.; et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 2020, 585, 574–578. [Google Scholar] [CrossRef]
- Zheng, D.; Li, R.; An, J.; Xie, T.; Han, Z.; Xu, R.; Fang, Y.; Zhang, X. Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv. Mater. 2020, 32, e2004529. [Google Scholar] [CrossRef] [PubMed]
- Eshrati, M.; Amadei, F.; Van de Wiele, T.; Veschgini, M.; Kaufmann, S.; Tanaka, M. Biopolymer-Based Minimal Formulations Boost Viability and Metabolic Functionality of Probiotics Lactobacillus rhamnosus GG through Gastrointestinal Passage. Langmuir 2018, 34, 11167–11175. [Google Scholar] [CrossRef] [PubMed]
- Akoulina, E.; Dudun, A.; Bonartsev, A.; Bonartseva, G.; Voinova, V. Effect of bacterial alginate on growth of mesenchymal stem cells. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 115–118. [Google Scholar] [CrossRef]
- Popletaeva, S.; Voinova, T.; Kartashov, M.; Pasechnik, T.; Arslanova, L.; Shcherbakova, L.; Statsyuk, N.; Bonartsev, A.; Zernov, A.; Bonartseva, G.; et al. Biodegradable Poly-3-Hydroxybutyrate as a Shielding Carrier for a Plant-Protecting MF3 Protein from Pseudomonas Fluorescens. Biointerface Res. Appl. Chem. 2018, 8, 3224–3231. [Google Scholar]
- Bonartsev, A.P.; Bonartseva, G.A.; Myshkina, V.L.; Voinova, V.V.; Mahina, T.K.; Zharkova, I.I.; Yakovlev, S.G.; Zernov, A.L.; Ivanova, E.V.; Akoulina, E.A.; et al. Biosynthesis of poly(3-hydroxybutyrateco-3-hydroxy-4-methylvalerate) by Strain Azotobacter chroococcum 7B. Acta Nat. 2016, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Bonartseva, G.A.; Akulina, E.A.; Myshkina, V.L.; Voinova, V.V.; Makhina, T.K.; Bonartsev, A.P. Alginate biosynthesis by Azotobacter bacteria. Appl. Biochem. Microbiol. 2017, 53, 52–59. [Google Scholar] [CrossRef]
- Dudun, A.A.; Akoulina, E.A.; Voinova, V.V.; Makhina, T.K.; Myshkina, V.L.; Zhuikov, V.A.; Bonartsev, A.P.; Bonartseva, G.A. Biosynthesis of Alginate and Poly(3-Hydroxybutyrate) by the Bacterial Strain Azotobacter agile 12. Appl. Biochem. Microbiol. 2019, 55, 654–659. [Google Scholar] [CrossRef]
- Dudun, A.A.; Akoulina, E.A.; Zhuikov, V.A.; Makhina, T.K.; Voinova, V.V.; Belishev, N.V.; Khaydapova, D.D.; Shaitan, K.V.; Bonartseva, G.A.; Bonartsev, A.P. Competitive Biosynthesis of Bacterial Alginate Using Azotobacter vinelandii 12 for Tissue Engineering Applications. Polymers 2021, 14, 131. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Burch, J.M.; Franciose, R.J.; Moore, E.E.; Biffl, W.L.; Offner, P.J. Single-Layer Continuous versus Two-Layer Interrupted Intestinal Anastomosis: A Prospective Randomized Trial. Ann. Surg. 2000, 231, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Nickkholgh, A.; Contin, P.; Abu-Elmagd, K.; Golriz, M.; Gotthardt, D.; Morath, C.; Schemmer, P.; Mehrabi, A. Intestinal transplantation: Review of operative techniques. Clin. Transplant. 2013, 27, 56–65. [Google Scholar] [CrossRef]
- Bosmans, J.W.A.M.; Jongen, A.C.H.M.; Boonen, B.T.C.; van Rijn, S.; Scognamiglio, F.; Stucchi, L.; Gijbels, M.J.J.; Marsich, E.; Bouvy, N.D. Comparison of three different application routes of butyrate to improve colonic anastomotic strength in rats. Int. J. Color. Dis. 2016, 32, 305–313. [Google Scholar] [CrossRef]
- Renaud, G.; Stenzel, U.; Maricic, T.; Wiebe, V.; Kelso, J. deML: Robust demultiplexing of Illumina sequences using a likelihood-based approach. Bioinformatics 2014, 31, 770–772. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Fung, C.; Rusling, M.; Lampeter, T.; Love, C.; Karim, A.; Bongiorno, C.; Yuan, L. Automation of QIIME2 Metagenomic Analysis Platform. Curr. Protoc. 2021, 1, e254. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Liland, K.H.; Vinje, H.; Snipen, L. microclass: An R-package for 16S taxonomy classification. BMC Bioinform. 2017, 18, 172. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, D.; Law, H.K.-W.; Wu, Y.; Zhu, G.-H.; Huang, W.-Y.; Kang, Y. Integrative Analysis of Gut Microbiota and Fecal Metabolites in Rats after Prednisone Treatment. Microbiol. Spectr. 2021, 9, e0065021. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, T.; Wu, F.; Li, F.; Liu, Y.; Li, W.; Dai, N.; Tan, L.; Li, T.; Song, Y. Correlation of gut microbiota and neurotransmitters in a rat model of post-traumatic stress disorder. J. Tradit. Chin. Med Sci. 2020, 7, 375–385. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int. J. Food Microbiol. 2011, 149, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.I.; Efimova, D.A.; Nikogosov, D.A.; Osipenko, D.A.; et al. Microbiome Responses to an Uncontrolled Short-Term Diet Intervention in the Frame of the Citizen Science Project. Nutrients 2018, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Athrey, G. Cloacal Swabs Are Unreliable Sources for Estimating Lower Gastro-Intestinal Tract Microbiota Membership and Structure in Broiler Chickens. Microorganisms 2020, 8, 718. [Google Scholar] [CrossRef]
- Moraes, L.C.; Lang, P.M.; Arcanjo, R.A.; Rampelotto, P.H.; Fatturi-Parolo, C.C.; Ferreira, M.B.C.; Montagner, F. Microbial ecology and predicted metabolic pathways in various oral environments from patients with acute endodontic infections. Int. Endod. J. 2020, 53, 1603–1617. [Google Scholar] [CrossRef]
- Mukherjee, S.; Joardar, N.; Sengupta, S.; Babu, S.P.S. Gut microbes as future therapeutics in treating inflammatory and infectious diseases: Lessons from recent findings. J. Nutr. Biochem. 2018, 61, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.Y.; Yoon, S.S. Disruption of the Gut Ecosystem by Antibiotics. Yonsei Med J. 2018, 59, 4–12. [Google Scholar] [CrossRef]
- Feng, P.; Ye, Z.; Kakade, A.; Virk, A.K.; Li, X.; Liu, P. A Review on Gut Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics and Gut Microbiota. Nutrients 2018, 11, 22. [Google Scholar] [CrossRef]
- Burke, K.E.; Lamont, J.T. Clostridium difficile Infection: A Worldwide Disease. Gut Liver 2014, 8, 1–6. [Google Scholar] [CrossRef]
- Shen, A.; Edwards, A.N.; Sarker, M.R.; Paredes-Sabja, D. Sporulation and Germination in Clostridial Pathogens. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van De Wiele, T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef] [PubMed]
- Lepage, P.; Häsler, R.; Spehlmann, M.E.; Rehman, A.; Zvirbliene, A.; Begun, A.; Ott, S.; Kupcinskas, L.; Doré, J.; Raedler, A.; et al. Twin Study Indicates Loss of Interaction Between Microbiota and Mucosa of Patients With Ulcerative Colitis. Gastroenterology 2011, 141, 227–236. [Google Scholar] [CrossRef]
- Cox, L.M.; Sohn, J.; Tyrrell, K.L.; Citron, D.M.; Lawson, P.A.; Patel, N.B.; Iizumi, T.; Perez-Perez, G.I.; Goldstein, E.J.C.; Blaser, M.J. Corrigendum: Description of two novel members of the family Erysipelotrichaceae: Ileibacterium valens gen. nov., sp. nov. and Dubosiella newyorkensis, gen. nov., sp. nov., from the murine intestine, and emendation to the description of Faecalibacterium rodentium. Int. J. Syst. Evol. Microbiol. 2017, 67, 4289. [Google Scholar] [CrossRef]
- Floch, M.H. The Role of Prebiotics and Probiotics in Gastrointestinal Disease. Gastroenterol. Clin. North Am. 2018, 47, 179–191. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’Homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. Int. Rev. J. 2017, 8, 484–494. [Google Scholar] [CrossRef]
- Greetham, H.L.; Gibson, G.R.; Giffard, C.; Hippe, H.; Merkhoffer, B.; Steiner, U.; Falsen, E.; Collins, M.D. Allobaculum stercoricanis gen. nov., sp. nov., isolated from canine feces. Anaerobe 2004, 10, 301–307. [Google Scholar] [CrossRef]
- Shin, N.-R.; Kang, W.; Tak, E.J.; Hyun, D.-W.; Kim, P.S.; Kim, H.S.; Lee, J.-Y.; Sung, H.; Whon, T.W.; Bae, J.-W. Blautia hominis sp. nov., Isolated from Human Faeces. Int. J. Syst. Evol. Micr. 2018, 68, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Paek, J.; Shin, Y.; Kook, J.-K.; Chang, Y.-H. Blautia argi sp. nov., a New Anaerobic Bacterium Isolated from Dog Faeces. Int. J. Syst. Evol. Micr. 2019, 69, 33–38. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. mSystems 2017, 2, e00130-17. [Google Scholar] [CrossRef] [PubMed]
- Yutin, N.; Galperin, M.Y. A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. Environ. Microbiol. 2013, 15, 2631–2641. [Google Scholar] [CrossRef] [PubMed]
- Fomenky, B.E.; Do, D.N.; Talbot, G.; Chiquette, J.; Bissonnette, N.; Chouinard, Y.P.; Lessard, M.; Ibeagha-Awemu, E.M. Direct-fed microbial supplementation influences the bacteria community composition of the gastrointestinal tract of pre- and post-weaned calves. Sci. Rep. 2018, 8, 14147. [Google Scholar] [CrossRef]
- Weber, C. Commensal bacteria and intestinal surgery complications. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 371. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudun, A.A.; Chesnokova, D.V.; Voinova, V.V.; Bonartsev, A.P.; Bonartseva, G.A. Changes in the Gut Microbiota Composition during Implantation of Composite Scaffolds Based on Poly(3-hydroxybutyrate) and Alginate on the Large-Intestine Wall. Polymers 2023, 15, 3649. https://doi.org/10.3390/polym15173649
Dudun AA, Chesnokova DV, Voinova VV, Bonartsev AP, Bonartseva GA. Changes in the Gut Microbiota Composition during Implantation of Composite Scaffolds Based on Poly(3-hydroxybutyrate) and Alginate on the Large-Intestine Wall. Polymers. 2023; 15(17):3649. https://doi.org/10.3390/polym15173649
Chicago/Turabian StyleDudun, Andrei A., Dariana V. Chesnokova, Vera V. Voinova, Anton P. Bonartsev, and Garina A. Bonartseva. 2023. "Changes in the Gut Microbiota Composition during Implantation of Composite Scaffolds Based on Poly(3-hydroxybutyrate) and Alginate on the Large-Intestine Wall" Polymers 15, no. 17: 3649. https://doi.org/10.3390/polym15173649
APA StyleDudun, A. A., Chesnokova, D. V., Voinova, V. V., Bonartsev, A. P., & Bonartseva, G. A. (2023). Changes in the Gut Microbiota Composition during Implantation of Composite Scaffolds Based on Poly(3-hydroxybutyrate) and Alginate on the Large-Intestine Wall. Polymers, 15(17), 3649. https://doi.org/10.3390/polym15173649





