Active Cellulose Acetate/Chitosan Composite Films Prepared Using Solution Blow Spinning: Structure and Electrokinetic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Solutions for SBS
2.2.2. Solution Blow Spinning (SBS) of Porous CA/CS Films
2.2.3. Solution Casting of CA/CS Films
2.3. Characterization Techniques
2.3.1. Optical Microscopy—Profilometry
2.3.2. Porosity
2.3.3. Water Vapor Permeability
2.3.4. Wettability—Static Contact Angle Measurement
2.3.5. Structural Characterization—ATR-FTIR
2.3.6. Surface Charge Measurements and Adsorption Studies
3. Results and Discussion
3.1. Morphology and Physical Properties of CA/CS Films
3.2. Structural Characterization of CA/CS Films
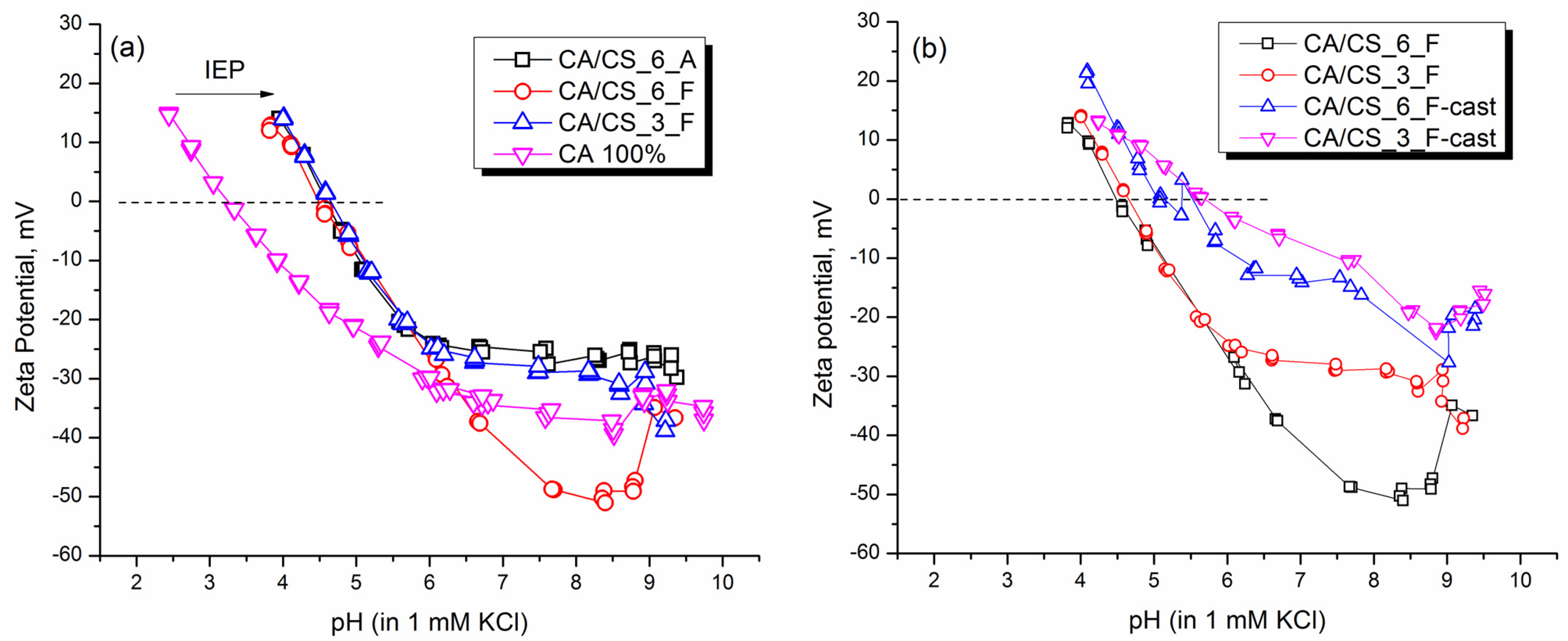
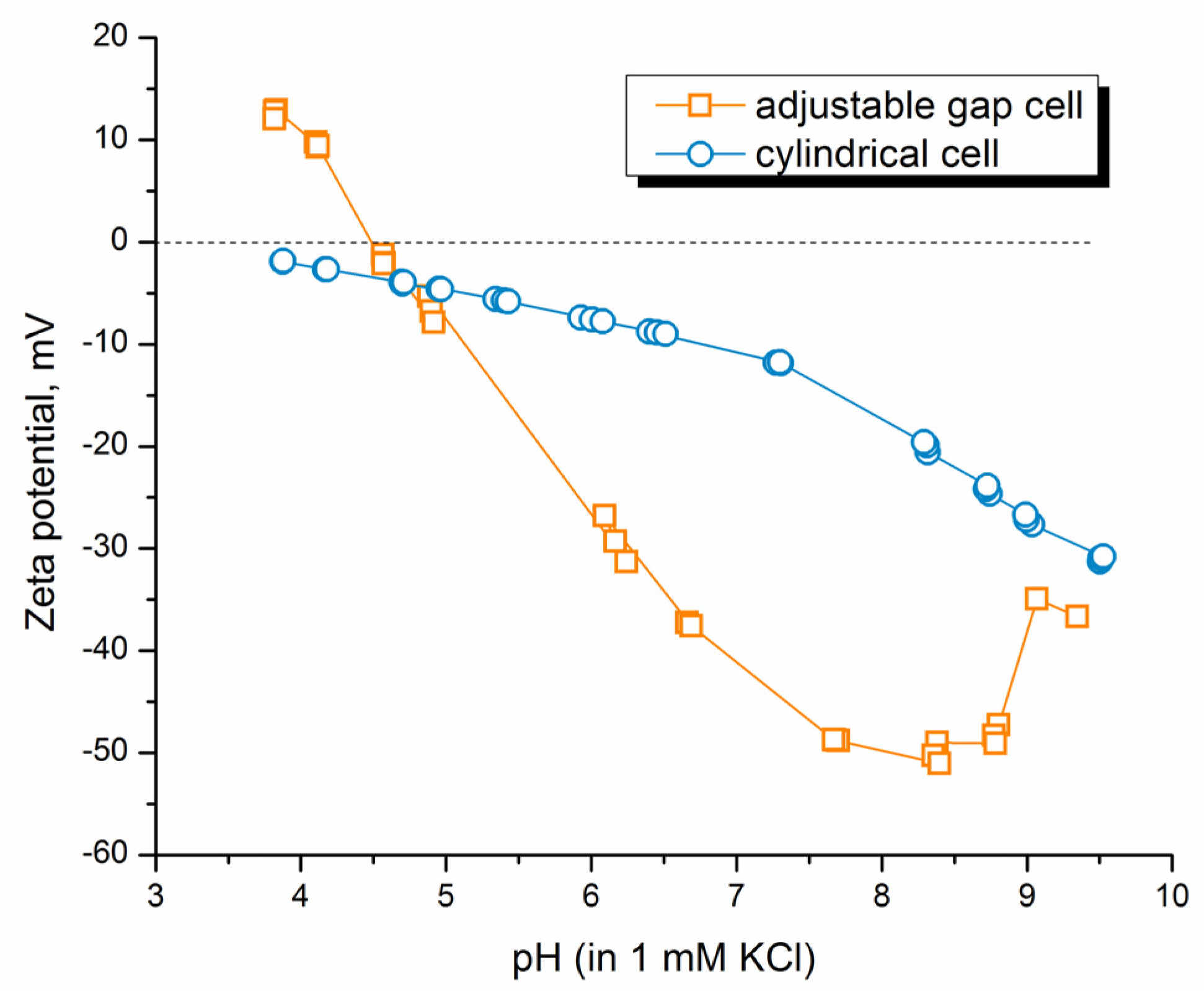
3.3. Surface Charge Analysis of CA/CS Films
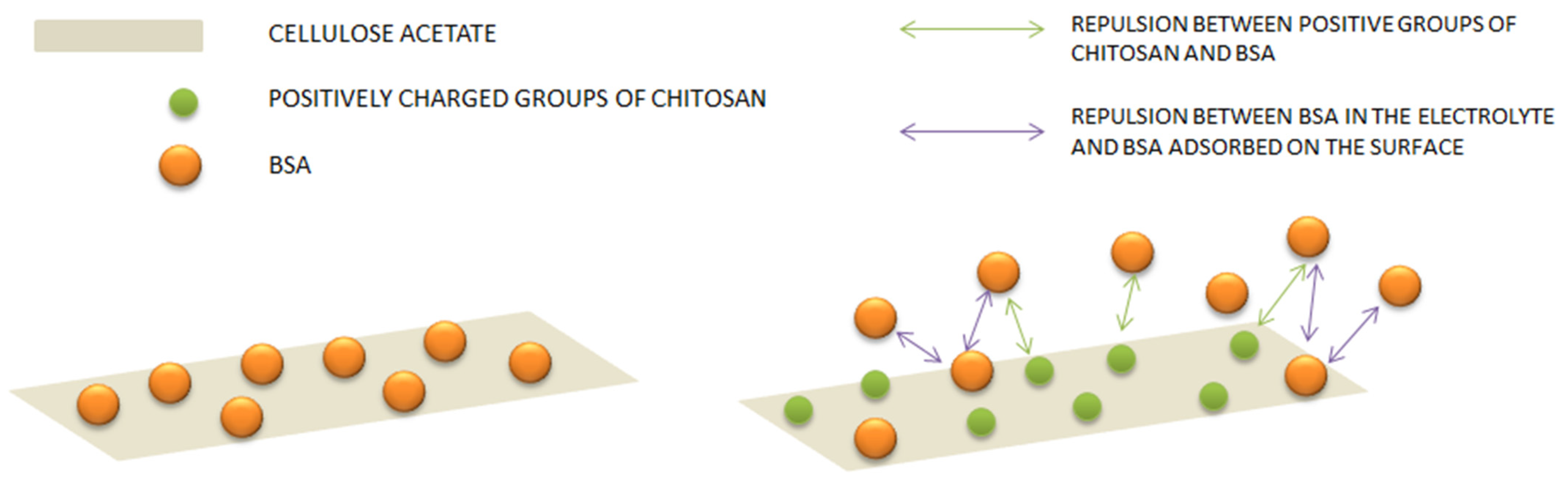
3.4. Adsorption Studies of Bovine Serum Albumin (BSA) onto CA/CS Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ (accessed on 1 February 2023).
- Food Waste and Loss. Available online: https://www.fao.org/platform-food-loss-waste/en/ (accessed on 1 February 2023).
- Rouf, T.B.; Kokini, J.L. Natural biopolymer-based nanocomposite films for packaging applications. In Bionanocomposites for Packaging Applications; Springer: Cham, Switerzland, 2017; pp. 149–177. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Paunonen, S. Strength and barrier enhancements of cellophane and cellulose derivative films: A review. BioResources 2013, 8, 3098–3121. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in food packaging: A review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef]
- Robertson, G.L. Legislative and Safety Aspects of Food Packaging. In Food Packaging, Principles and Practices; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013; ISBN 9781439862421. [Google Scholar]
- Zugenmaier, P. Crystalline Cellulose and Derivatives–Characterization and Structures; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540739333. [Google Scholar]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and applicaton. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Harini, K.; Sukumar, M. Development of cellulose-based migratory and nonmigratory active packaging films. Carbohydr. Polym. 2019, 204, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.M.; dos Santos, D.C.; Motta, J.F.G.; dos Santos, R.R.; Chávez, D.W.H.; de Melo, N.R. Structure and functional properties of cellulose acetate films incorporated with glycerol. Carbohydr. Polym. 2019, 209, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Marrez, D.A.; Abdelhamid, A.E.; Darwesh, O.M. Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf Life 2019, 20, 100302. [Google Scholar] [CrossRef]
- Santos, D.C.; Ribeiro-Santos, R.; Ventura, L.A.F.; Melo, N.R.; Costa, B.S.; Rojas, E.E.G.; Salgado, N.L. Antimicrobial activity studies and characterization of cellulose acetate films containing essential oils. Ital. J. Food Sci. 2016, 28, 248–257. [Google Scholar] [CrossRef]
- Xie, J.; Hung, Y.C. Methodology to evaluate the antimicrobial effectiveness of UV-activated TiO2 nanoparticle-embedded cellulose acetate film. Food Control 2019, 106, 106690. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Abarca, R.L.; Bruna, J.E.; Moya, P.E.; Galotto, M.J.; Guarda, A.; Padula, M. Effect of organoclay and preparation method on properties of antimicrobial cellulose acetate films. Polym. Compos. 2019, 40, 2311–2319. [Google Scholar] [CrossRef]
- Do Socorro Rocha Bastos, M.; Da Silva Laurentino, L.; Canuto, K.M.; Mendes, L.G.; Martins, C.M.; Silva, S.M.F.; Furtado, R.F.; Kim, S.; Biswas, A.; Cheng, H.N. Physical and mechanical testing of essential oil-embedded cellulose ester films. Polym. Test. 2016, 49, 156–161. [Google Scholar] [CrossRef]
- Cédric, D. Current Opinion on Chitosan and its Derivatives: Biological Impact in Antimicrobial Applications. Adv. Biotechnol. Microbiol. 2017, 6, 555684. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Celik, G.; Oksuz, A.U. Controlled release of ibuprofen from electrospun biocompatible nanofibers with in situ QCM measurements. J. Macromol. Sci. Part A Pure Appl. Chem. 2015, 52, 76–83. [Google Scholar] [CrossRef]
- Vartiainen, J.; Vähä-Nissi, M.; Harlin, A. Biopolymer Films and Coatings in Packaging Applications—A Review of Recent Developments. Mater. Sci. Appl. 2014, 5, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Claro, P.I.C.; Neto, A.R.S.; Bibbo, A.C.C.; Mattoso, L.H.C.; Bastos, M.S.R.; Marconcini, J.M. Biodegradable Blends with Potential Use in Packaging: A Comparison of PLA/Chitosan and PLA/Cellulose Acetate Films. J. Polym. Environ. 2016, 24, 363–371. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Peršin, Z.; Šauperl, O.; Rudolf, A.; Kostić, M. Medical textiles based on viscose rayon fabrics coated with chitosan-encapsulated iodine: Antibacterial and antioxidant properties. Text. Res. J. 2018, 88, 2519–2531. [Google Scholar] [CrossRef]
- Strnad, S.; Sauperl, O.; Fras-Zemljic, L. Cellulose Fibres Funcionalised by Chitosan: Characterization and Application. In Biopolymers; IntechOpen: London, UK, 2010. [Google Scholar] [CrossRef] [Green Version]
- Gopi, S.; Pius, A.; Kargl, R.; Kleinschek, K.S.; Thomas, S. Fabrication of cellulose acetate/chitosan blend films as efficient adsorbent for anionic water pollutants. Polym. Bull. 2019, 76, 1557–1571. [Google Scholar] [CrossRef]
- Zhou, H.; Tong, H.; Lu, J.; Cheng, Y.; Qian, F.; Tao, Y.; Wang, H. Preparation of bio-based cellulose acetate/chitosan composite film with oxygen and water resistant properties. Carbohydr. Polym. 2021, 270, 118381. [Google Scholar] [CrossRef]
- Cao, J.; Sun, X.; Lu, C.; Zhou, Z.; Zhang, X.; Yuan, G. Water-soluble cellulose acetate from waste cotton fabrics and the aqueous processing of all-cellulose composites. Carbohydr. Polym. 2016, 149, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Lin, X.; White, K.L.; Lin, S.; Wu, H.; Cao, S.; Huang, L.; Chen, L. Effect of the degree of substitution on the hydrophobicity of acetylated cellulose for production of liquid marbles. Cellulose 2016, 23, 811–821. [Google Scholar] [CrossRef]
- Kramar, A.; González-Benito, J. Preparation of cellulose acetate film with dual hydrophobic-hydrophilic properties using solution blow spinning. Mater. Des. 2023, 227, 111788. [Google Scholar] [CrossRef]
- do Vale, D.A.; Vieira, C.B.; de Oliveria, J.M.; Vidal, M.F.; de Alcântara, L.O.; da Silva, A.I.M.; de Lima Silva, J.M.; Andrade, F.K.; Sousa, J.R.; Moreira Souza Filho, M.d.S.; et al. Determining the wetting capacity of the chitosan coatings from Ucides cordatus and evaluating the shelf-life quality of Scomberomorus brasiliensis fillets. Food Control 2020, 116, 107329. [Google Scholar] [CrossRef]
- Tanpichai, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Chitosan coating for the preparation of multilayer coated paper for food-contact packaging: Wettability, mechanical properties, and overall migration. Int. J. Biol. Macromol. 2022, 213, 534–545. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.D.F.F.; Botti, L.C.M.; Silva, W.A. Effect of essential oils in the properties of cellulosic active packaging. Macromol. Symp. 2011, 299–300, 199–205. [Google Scholar] [CrossRef]
- Vinodhini, P.A.; Sangeetha, K.; Thandapani, G.; Sudha, P.N.; Jayachandran, V.; Sukumaran, A. FTIR, XRD and DSC studies of nanochitosan, cellulose acetate and polyethylene glycol blend ultrafiltration membranes. Int. J. Biol. Macromol. 2017, 104, 1721–1729. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Fajardo, A.R.; Gerola, A.P.; Rodrigues, J.H.S.; Nakamura, C.V.; Muniz, E.C.; Hsieh, Y. Lo First report of electrospun cellulose acetate nanofibers mats with chitin and chitosan nanowhiskers: Fabrication, characterization, and antibacterial activity. Carbohydr. Polym. 2020, 250, 116954. [Google Scholar] [CrossRef]
- AL-Jbour, N.D.; Beg, M.D.; Gimbun, J.; Alam, A.K.M.M. An Overview of Chitosan Nanofibers and their Applications in the Drug Delivery Process. Curr. Drug Deliv. 2019, 16, 272–294. [Google Scholar] [CrossRef]
- Domínguez, J.E.; Olivos, E.; Vázquez, C.; Rivera, J.M.; Hernández-Cortes, R.; González-Benito, J. Automated low-cost device to produce sub-micrometric polymer fibers based on blow spun method. HardwareX 2021, 10, e00218. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Mattoso, L.H.C.; Orts, W.J.; Medeiros, E.S. Structural and morphological characterization of micro and nanofibers produced by electrospinning and solution blow spinning: A comparative study. Adv. Mater. Sci. Eng. 2013, 2013, 409572. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Kwon, O.H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Homayoni, H.; Ravandi, S.A.H.; Valizadeh, M. Electrospinning of chitosan nanofibers: Processing optimization. Carbohydr. Polym. 2009, 77, 656–661. [Google Scholar] [CrossRef]
- Salazar-Brann, S.A.; Patiño-Herrera, R.; Navarrete-Damián, J.; Louvier-Hernández, J.F. Electrospinning of chitosan from different acid solutions. AIMS Bioeng. 2021, 8, 112–129. [Google Scholar] [CrossRef]
- Menachem, L. (Ed.) Handbook of Fiber Chemistry; CRC Press Taylor& Francis Group: Boca Raton, FL, USA, 2007; ISBN 9780824725655. [Google Scholar]
- Bračič, M.; Fras-Zemljič, L.; Pérez, L.; Kogej, K.; Stana-Kleinschek, K.; Kargl, R.; Mohan, T. Protein-repellent and antimicrobial nanoparticle coatings from hyaluronic acid and a lysine-derived biocompatible surfactant. J. Mater. Chem. B 2017, 5, 3888–3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, T.; Čas, A.; Bračič, M.; Plohl, O.; Vesel, A.; Rupnik, M.; Zemljič, L.F.; Rebol, J. Highly Protein Repellent and Antiadhesive Polysaccharide Biomaterial Coating for Urinary Catheter Applications. ACS Biomater. Sci. Eng. 2019, 5, 5825–5832. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [Green Version]
- Kramar, A.; Rodríguez Ortega, I.; González-Gaitano, G.; Gonzalez-Benito, J. Solution casting of cellulose acetate films: Influence of surface substrate and sumidity on wettability, morphology and optical properties. Cellulose 2023, 30, 2037–2052. [Google Scholar] [CrossRef]
- ISO 4288:1997; Surface Texture: Profile Method-Rules and Procedures for the Assessment of Surface Texture. International Organization for Standardization: London, UK, 1997.
- Dimic-Misic, K.; Kostic, M.M.; Bratislav, O.; Kuraica, M.; Kramar, A.; Imani, M.; Gane, P. Iso- and Anisotropic Etching of Micro Nanofibrillated Cellulose Films by Sequential Oxygen and Nitrogen Gas Plasma Exposure for Tunable Wettability on Crystalline and Amorphous Regions. Materials 2021, 14, 3571. [Google Scholar] [CrossRef]
- Tam, D.K.Y.; Ruan, S.; Gao, P.; Yu, T. 10—High-performance ballistic protection using polymer nanocomposites. In Advances in Military Textiles and Personal Equipment; Woodhead Publishing Series in Textiles; Sparks, E., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 213–237. ISBN 978-1-84569-699-3. [Google Scholar]
- Kim, U.J.; Lee, Y.R.; Kang, T.H.; Choi, J.W.; Kimura, S.; Wada, M. Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohydr. Polym. 2017, 163, 34–42. [Google Scholar] [CrossRef]
- Kilic-Akyilmaz, M.; Gülsünoğlu Konuşkan, Z. Additives and preservatives. In Handbook of Vegetable Preservation and Processing; CRC Press: Boca Raton, FL, USA, 2015; pp. 301–318. [Google Scholar]
- Wu, S.; Qin, X.; Li, M. The structure and properties of cellulose acetate materials: A comparative study on electrospun membranes and casted films. J. Ind. Text. 2014, 44, 85–98. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Domínguez, J.E.; Kasiri, A.; González-Benito, J. Wettability behavior of solution blow spun polysulfone by controlling morphology. J. Appl. Polym. Sci. 2021, 138, 50200. [Google Scholar] [CrossRef]
- Figueiredo, A.S.; Garcia, A.R.; Minhalma, M.; Ilharco, L.; De Pinho, M.N. The ultrafiltration performance of cellulose acetate asymmetric membranes: A new perspective on the correlation with the infrared spectra. J. Membr. Sci. Res. 2020, 6, 70–80. [Google Scholar] [CrossRef]
- Yang, J.; Kwon, G.J.; Hwang, K.; Kim, D.Y. Cellulose-chitosan antibacterial composite films prepared from LiBr solution. Polymers 2018, 10, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korica, M.; Peršin, Z.; Trifunovic, S.; Mihajlovski, K.; Nikolic, T.; Maletic, S.; Zemljic, L.F.; Kostic, M.M. Influence of different pretreatments on the antibacterial properties of chitosan functionalized viscose fabric: TEMPO oxidation and coating with TEMPO oxidized cellulose nanofibrils. Materials 2019, 12, 3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramar, A.D.; Ilic-Tomic, T.R.; Lađarević, J.M.; Nikodinovic-Runic, J.B.; Kostic, M.M. Halochromic cellulose textile obtained via dyeing with biocolorant isolated from Streptomyces sp. strain NP4. Cellulose 2021, 28, 8771–8784. [Google Scholar] [CrossRef]
- Monvisade, P.; Siriphannon, P. Chitosan intercalated montmorillonite: Preparation, characterization and cationic dye adsorption. Appl. Clay Sci. 2009, 42, 427–431. [Google Scholar] [CrossRef]
- Chandra Dey, S.; Al–Amin, M.; Ur Rashid, T.; Zakir Sultan, M.; Ashaduzzaman, M.; Sarker, M.; Md Shamsuddin, S. Preparation, Characterization and Performance Evaluation of Chitosan as an Adsorbent for Remazol Red. Int. J. Latest Res. Eng. Technol. 2016, 2, 52–62. [Google Scholar]
- Atay, H.Y. Antibacterial Activity of Chitosan-Based Systems. In Fuctional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. ISBN 9789811502637. [Google Scholar]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- González-Benito, J.; Torres, D.; Ballesteros, C.; Ruiz, V.M.; Teno, J. PVDF based nanocomposites produced by solution blow spinning, structure and morphology induced by the presence of MWCNT and their consequences on some properties. Colloid Polym. Sci. 2019, 297, 1105–1118. [Google Scholar] [CrossRef]
- Teno, J.; González-Gaitano, G.; González-Benito, J. Poly (ethylene-co-vinyl acetate) films prepared by solution blow spinning: Surface characterization and its relation with E. coli adhesion. Polym. Test. 2017, 60, 140–148. [Google Scholar] [CrossRef]
- Stana-Kleinschek, K.; Ribitsch, V.; Kreze, T. Determination of the adsorption character of cellulose fibres using surface tension and surface charge. Mater. Res. Innov. 2002, 6, 13–18. [Google Scholar] [CrossRef]
- Phan, H.T.M.; Bartelt-Hunt, S.; Rodenhausen, K.B.; Schubert, M.; Bartz, J.C. Investigation of Bovine Serum Albumin (BSA) Attachment onto Self-Assembled Monolayers (SAMs) Using Combinatorial Quartz Crystal Microbalance with Dissipation (QCM-D) and Spectroscopic Ellipsometry (SE). PLoS ONE 2015, 10, e0141282. [Google Scholar] [CrossRef] [PubMed]
- Ajdnik, U.; Luxbacher, T.; Fras-Zemljič, L. Proteins at polysaccharide-based biointerfaces: A comparative study of QCM-D and electrokinetic measurements. Colloids Surf. B Biointerfaces 2023, 221, 113011. [Google Scholar] [CrossRef] [PubMed]

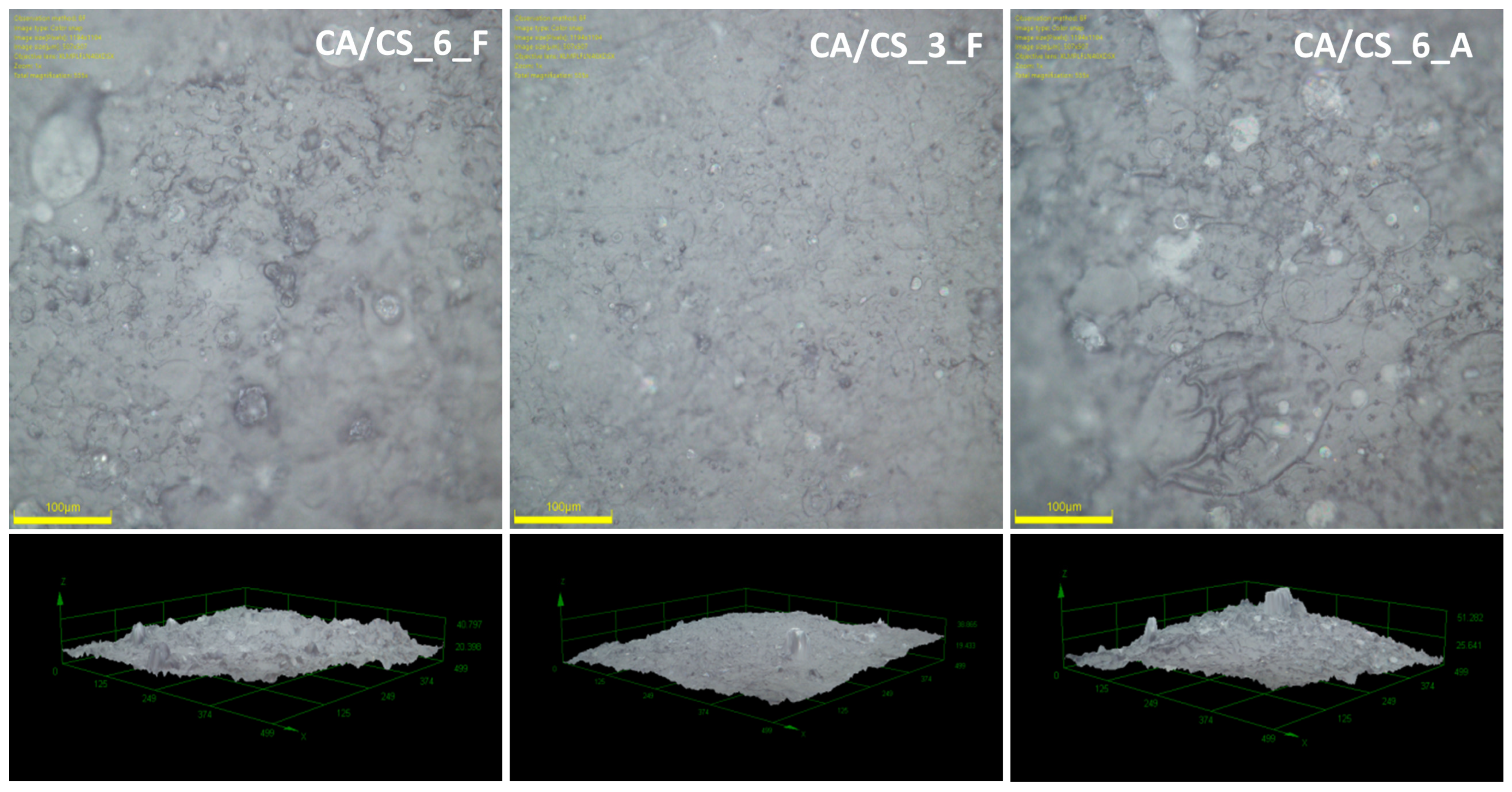


| Sample Code | Surface Roughness (Ra), µm | Porosity, % | Water Vapor Permeability, % |
|---|---|---|---|
| CA/CS_3_F | 1.9 ± 0.4 | 63 | 78.5 |
| CA/CS_6_F | 3.2 ± 0.5 | 58 | 76.3 |
| CA/CS_6_A | 3.2 ± 0.6 | 62 | 76.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramar, A.; Luxbacher, T.; Moshfeghi Far, N.; González-Benito, J. Active Cellulose Acetate/Chitosan Composite Films Prepared Using Solution Blow Spinning: Structure and Electrokinetic Properties. Polymers 2023, 15, 3276. https://doi.org/10.3390/polym15153276
Kramar A, Luxbacher T, Moshfeghi Far N, González-Benito J. Active Cellulose Acetate/Chitosan Composite Films Prepared Using Solution Blow Spinning: Structure and Electrokinetic Properties. Polymers. 2023; 15(15):3276. https://doi.org/10.3390/polym15153276
Chicago/Turabian StyleKramar, Ana, Thomas Luxbacher, Nasrin Moshfeghi Far, and Javier González-Benito. 2023. "Active Cellulose Acetate/Chitosan Composite Films Prepared Using Solution Blow Spinning: Structure and Electrokinetic Properties" Polymers 15, no. 15: 3276. https://doi.org/10.3390/polym15153276
APA StyleKramar, A., Luxbacher, T., Moshfeghi Far, N., & González-Benito, J. (2023). Active Cellulose Acetate/Chitosan Composite Films Prepared Using Solution Blow Spinning: Structure and Electrokinetic Properties. Polymers, 15(15), 3276. https://doi.org/10.3390/polym15153276