Pervaporation and Gas Separation Properties of High-Molecular Ladder-like Polyphenylsilsesquioxanes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Instrumentation
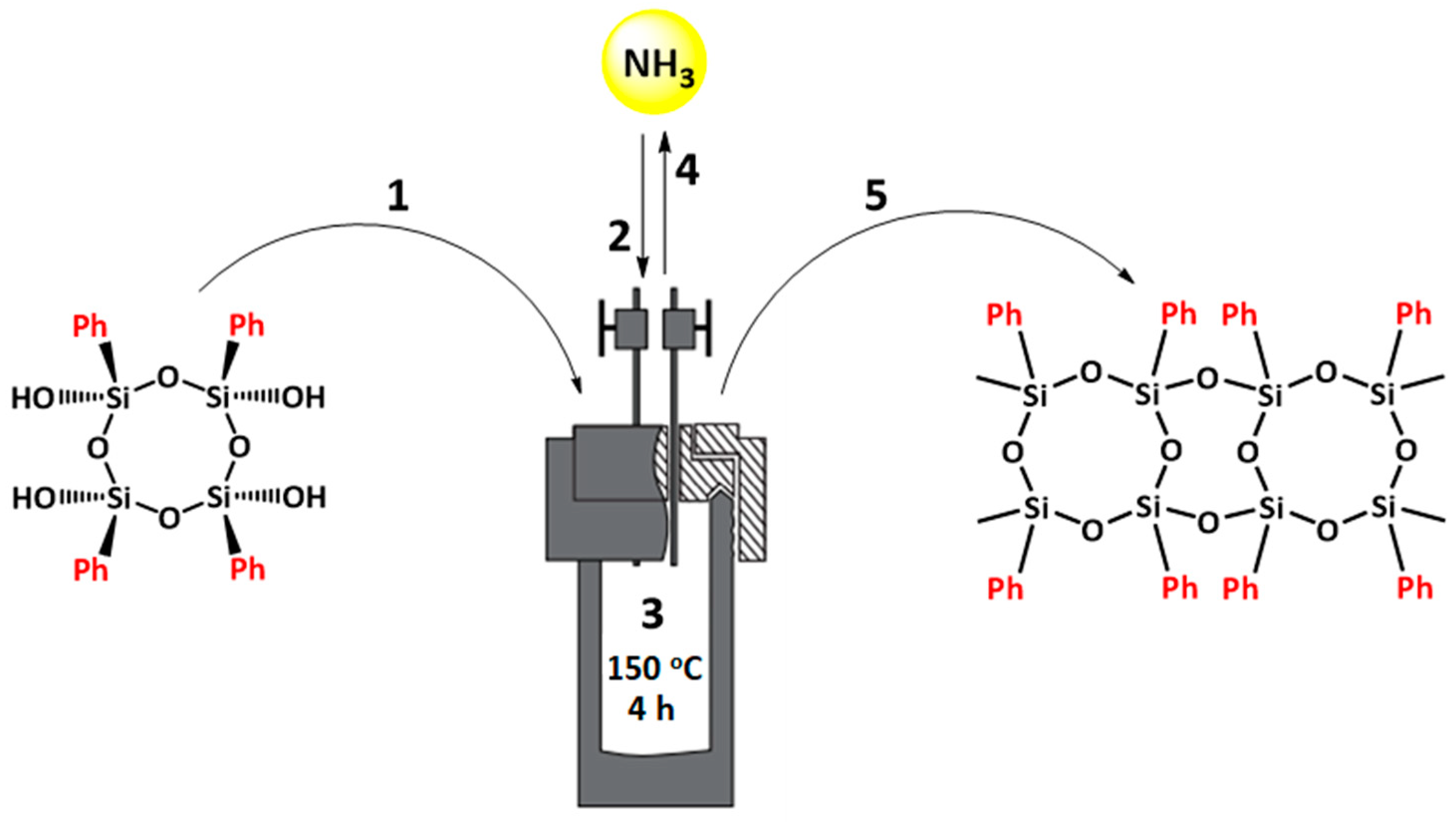
2.3. Synthetic Procedures
Synthesis of Ladder-like Polyphenylsilsesquioxane in Ammonia
2.4. Study of L-PPSQ Solubility
2.5. Preparation of L-PPSQ Films
2.6. Gas Transport Tests
2.7. Study of Pervaporative Properties
3. Results and Discussion
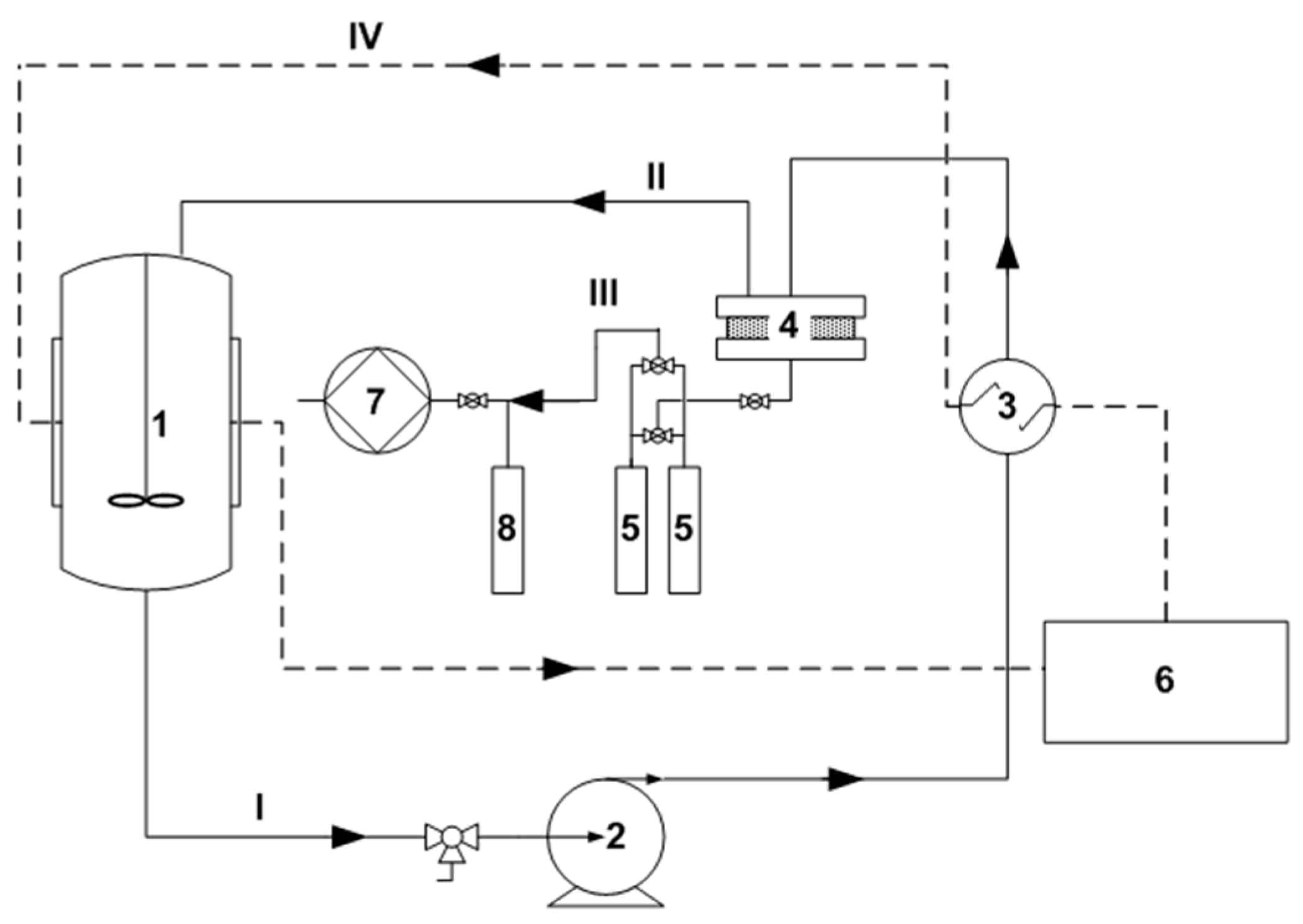
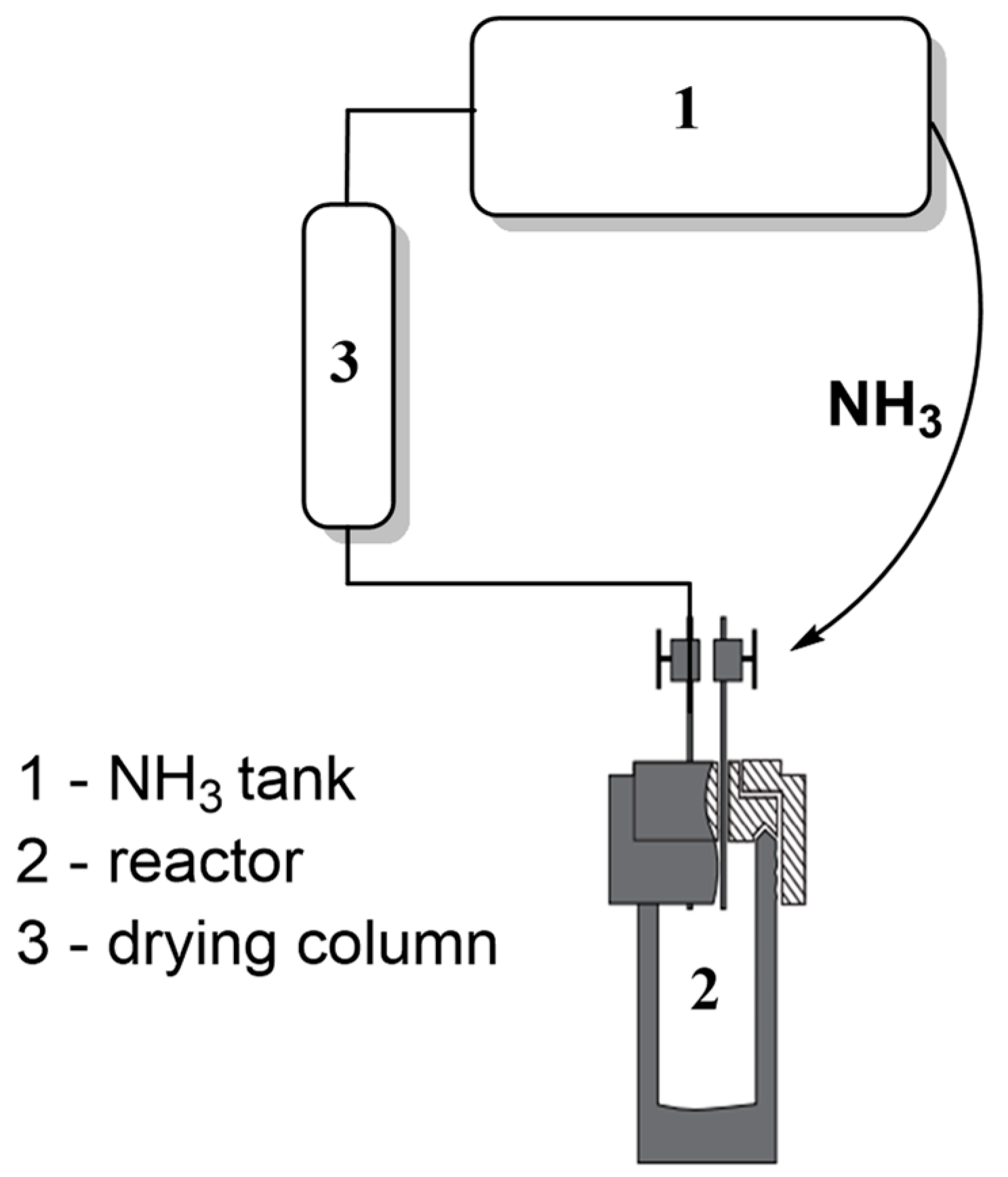
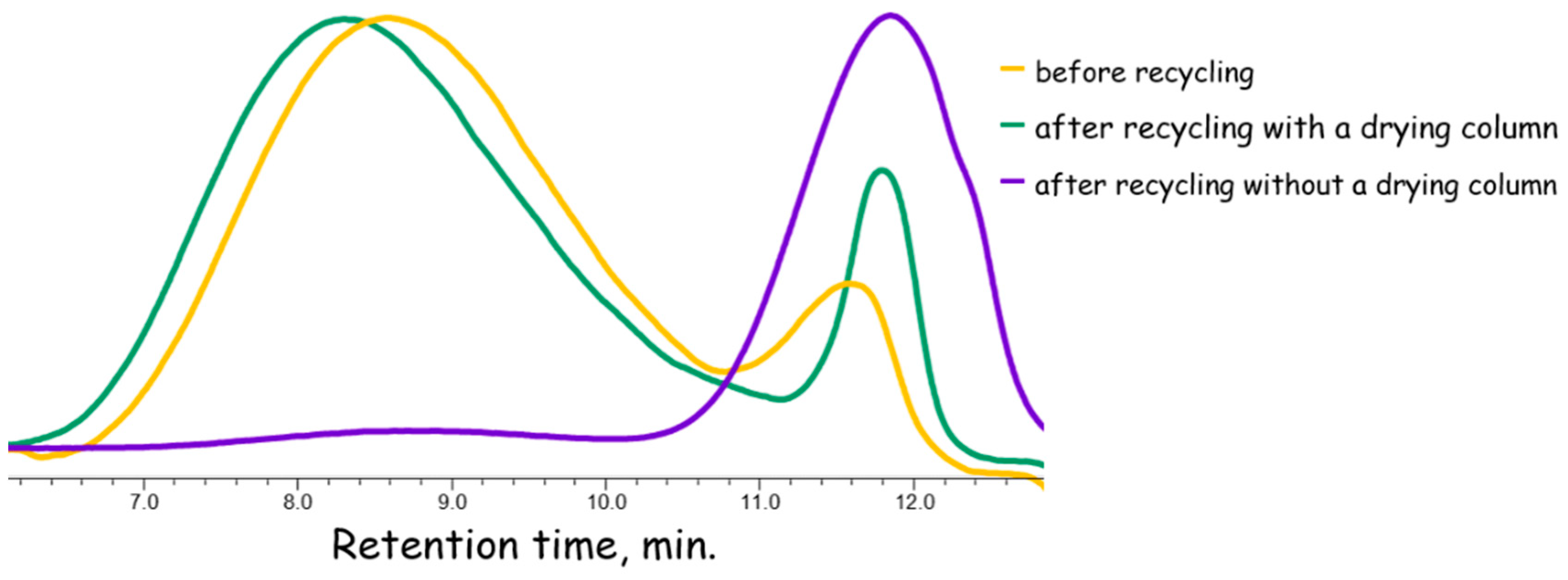
3.1. Ammonia Regeneration
3.2. Study of L-PPSQ Solubility
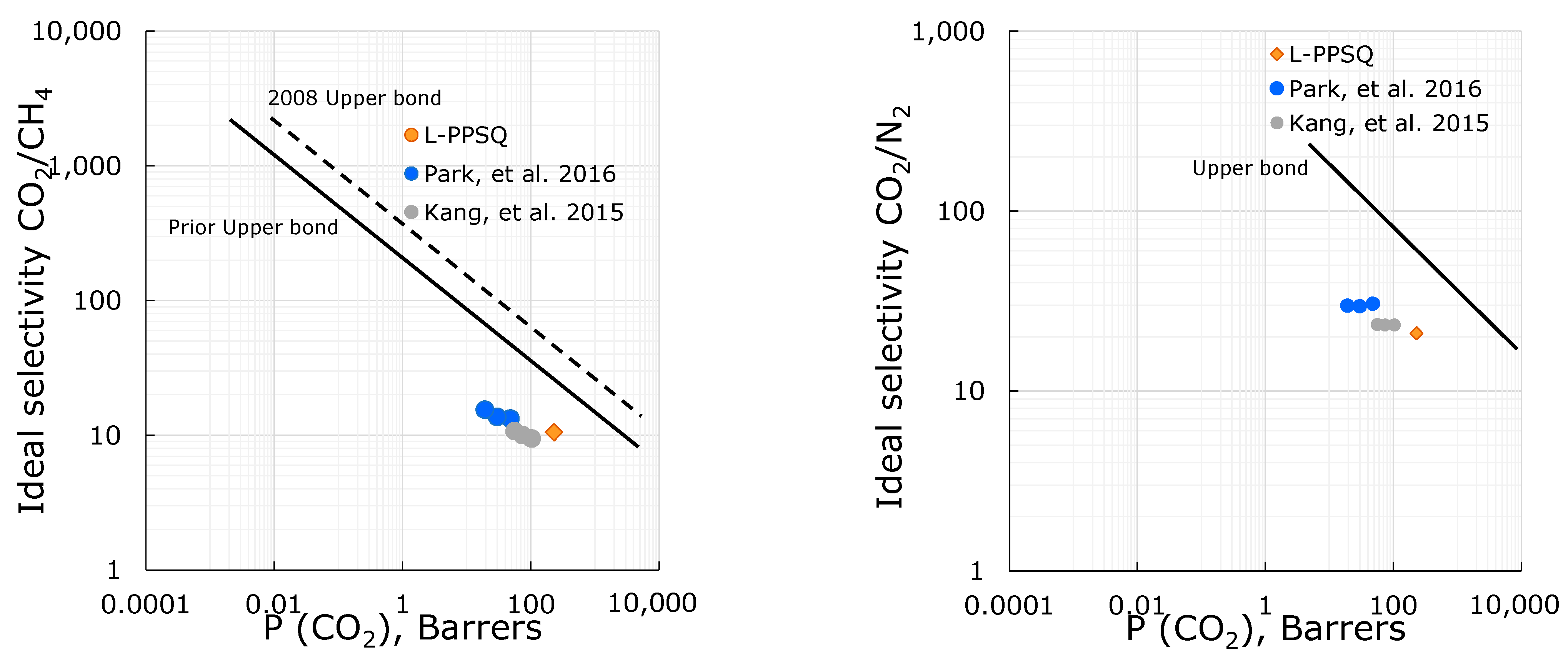
3.3. Gas Transport Properties
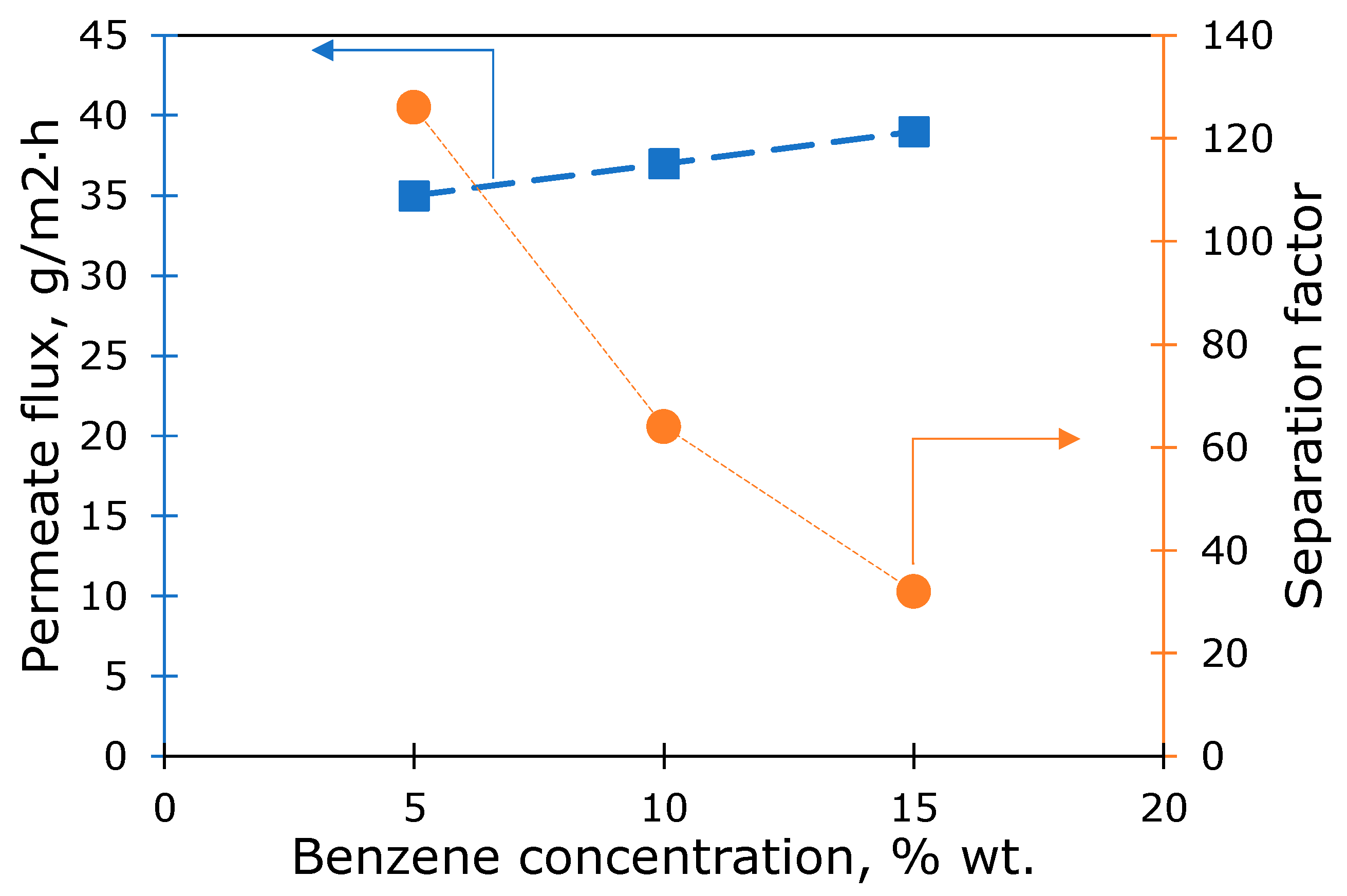
3.4. Vacuum Pervaporation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazhenov, S.; Chuboksarov, V.; Maximov, A.; Zhdaneev, O. Technical and economic prospects of CCUS projects in Russia. Sustain. Mater. Technol. 2022, 33, e00452. [Google Scholar] [CrossRef]
- Banerjee, A.; Ray, S.K. Synthesis of novel composite membranes by in-situ intercalative emulsion polymerization for separation of aromatic-aliphatic mixtures by pervaporation. J. Memb. Sci. 2020, 597, 117729. [Google Scholar] [CrossRef]
- Golubev, G.S.; Volkov, V.V.; Borisov, I.L. ScienceDirect High free volume polymers for pervaporation. Curr. Opin. Chem. Eng. 2022, 36, 100788. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane technology. Encycl. Polym. Sci. Technol. 2000, 3, 184–249. [Google Scholar] [CrossRef]
- Sidhikku, R.; Valappil, K.; Ghasem, N.; Al-marzouqi, M. Current and future trends in polymer membrane-based gas separation technology: A comprehensive review. J. Ind. Eng. Chem. 2021, 98, 103–129. [Google Scholar] [CrossRef]
- Hanford, W.E.; Joyce, R.M. Polytetrafluoroethylene. J. Am. Chem. Soc. 1946, 68, 2082–2085. [Google Scholar] [CrossRef]
- Praneeth, K.; Bhargava, S.K.; James, T.; Sridhar, S. Design of novel ultrafiltration systems based on robust polyphenylsulfone hollow fiber membranes for treatment of contaminated surface water. Chem. Eng. J. 2014, 248, 297–306. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Jansen, J.C.; Tasselli, F.; Tocci, E.; Luis, P.; Degrève, J.; Drioli, E.; Bruggen, B. Van Der Novel polyphenylsulfone membrane for potential use in solvent nanofiltration. J. Memb. Sci. 2011, 379, 60–68. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Tasselli, F.; Jansen, J.C.; Tocci, E.; Bazzarelli, F.; Bernardo, P.; Luis, P.; Degrève, J.; Drioli, E.; Van Der Bruggen, B. Preparation of solvent stable polyphenylsulfone hollow fiber nanofiltration membranes. J. Memb. Sci. 2011, 384, 89–96. [Google Scholar] [CrossRef]
- Yang, J.; Vaidya, M.M.; Harrigan, D.J.; Duval, S.A.; Hamad, F.; Bahamdan, A.A. Separation and Puri fi cation Technology Modi fi ed rubbery siloxane membranes for enhanced C3+ hydrocarbon recovery from natural gas: Pure and multicomponent gas permeation evaluation. Sep. Purif. Technol. 2020, 242, 116774. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Volkov, A.V. High-Selectivity Polysiloxane Membranes for Gases and Liquids Separation (A Review). Pet. Chem. 2021, 61, 959–976. [Google Scholar] [CrossRef]
- Yampolskii, Y. Polymeric Gas Separation Membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Tan, X.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part II: Production Techniques with Polyethylene, Polydimethylsiloxane, Polypropylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.C.; Azevedo, M.A.; Coelhoso, I.M.; Brazinha, C.; Crespo, G. Steady-state and Transient Transport Studies of Gas Permeation Through Dense Membranes Using On-line Mass Spectrometry. Sep. Purif. Technol. 2017, 197, 18–26. [Google Scholar] [CrossRef]
- Petrusová, Z.; Machanová, K.; Stanovský, P.; Izák, P. Separation and Puri fi cation Technology Separation of organic compounds from gaseous mixtures by vapor permeation. Sep. Purif. Technol. 2019, 217, 95–107. [Google Scholar] [CrossRef]
- Çalhan, A.; Deniz, S.; Kujawski, W.; Kujawa, J. Silica Filled Polyphenylsulfone/Polydimethylsiloxane Composite Membranes for Pervaporation Separation of Biobutanol from ABE Mixtures. Chem. Eng. Process.-Process Intensif. 2020, 156, 108099. [Google Scholar] [CrossRef]
- Postel, S.; Schneider, C.; Wessling, M. Solvent dependent solute solubility governs retention in silicone based organic solvent nano fi ltration. J. Memb. Sci. 2016, 497, 47–54. [Google Scholar] [CrossRef]
- Van Eygen, G.; Van Der Bruggen, B.; Buekenhoudt, A.; Luis, P. Efficient membrane-based affinity separations for chemical applications: A review. Chem. Eng. Process.-Process Intensif. 2021, 169, 108613. [Google Scholar] [CrossRef]
- Tilahun, E.; Bayrakdar, A.; Sahinkaya, E. Performance of polydimethylsiloxane membrane contactor process for selective hydrogen sulfide removal from biogas. Waste Manag. 2017, 61, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Nidzhom, M.; Abidin, Z.; Mahmoud, M.; Veerman, J. Towards the development of new generation of ion exchange membranes for reverse electrodialysis: A review. Desalination 2022, 537, 115854. [Google Scholar] [CrossRef]
- Hwang, S.O.; Lee, J.Y.; Lee, J. Effect of the silsesquioxane structure on the mechanical properties of the silsesquioxane-reinforced polymer composite films. Prog. Org. Coat. 2019, 137, 105316. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Wu, L. Preparation of poly(phenylsilsesquioxane) (PPSQ) particles with ladder structure and the thermal stability of PP/PPSQ composites. Polym. Adv. Technol. 2011, 22, 2151–2156. [Google Scholar] [CrossRef]
- Ostanin, S.A.; Kalinin, A.V.; Bratsyhin, Y.Y.; Saprykina, N.N.; Zuev, V.V. Linear/Ladder-Like Polysiloxane Block Copolymers with Methyl-, Trifluoropropyl- and Phenyl- Siloxane Units for Surface Modification. Polymers 2021, 13, 2063. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cheng, Y.; Xiao, F. Thermal stable superhydrophobic polyphenylsilsesquioxane/nanosilica composite coatings. Appl. Surf. Sci. 2011, 258, 1572–1580. [Google Scholar] [CrossRef]
- Loh, T.C.; Ng, C.M.; Kumar, R.N.; Ismail, H.; Ahmad, Z. Improvement of thermal ageing and transparency of methacrylate based poly (siloxane-silsesquioxane) for optoelectronic application. J. Appl. Polym. Sci. 2017, 134, 45285. [Google Scholar] [CrossRef]
- Li, Z.; Kong, J.; Wang, F.; He, C. Polyhedral Oligomeric Silsesquioxanes (POSSs): An Important Building Block for Organic Optoelectronic Materials. J. Mater. Chem. 2017, 5, 5283–5298. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, J.; Zhuo, J.; Chen, H.; Zhang, X. Characterization of sol-gel ORMOSIL antireflective coatings from phenyltriethoxysilane and tetraethoxysilane: Microstructure control and application. Surf. Coat. Technol. 2018, 345, 177–182. [Google Scholar] [CrossRef]
- Temnikov, M.N.; Muzafarov, A.M. Polyphenylsilsesquioxanes. New structures–new properties. RSC Adv. 2020, 10, 43129–43152. [Google Scholar] [CrossRef]
- Temnikov, M.N.; Vasil’ev, V.G.; Buzin, M.I.; Muzafarov, A.M. Synthesis and comparison of the rheological and thermal properties of acyclic and polycyclic forms of polyphenylsilsesquioxane. Eur. Polym. J. 2020, 130, 109676. [Google Scholar] [CrossRef]
- Temnikov, M.N.; Buzin, M.I.; Demchenko, N.V.; Cherkaev, G.V.; Vasilenko, N.G.; Muzafarov, A.M. Acyclic polyphenylsilsesquioxane: Synthesis and properties. Mendeleev Commun. 2016, 26, 121–123. [Google Scholar] [CrossRef]
- Brown, J.F.; Vogt, L.H.; Katchman, A.; Eustance, J.W.; Kiser, K.M.; Krantz, K.W. Double chain polymers of phenylsilsesquioxane. J. Am. Chem. Soc. 1960, 82, 6194–6195. [Google Scholar] [CrossRef]
- Andrianov, K.A.; Bushin, S.V.; Vitovskaya, M.G.; Yemelyanov, V.N.; Lavrenko, P.N.; Makarova, N.N.; Muzafarov, A.M.; Nikolaev, V.Y.; Kolbina, G.F.; Shtennikov, I.N.; et al. Synthesis and conformational characteristics of some ladder-like polyphenylsilsesquioxanes. Vysok. Soedin. Seriya A 1977, 19, 469–474. [Google Scholar]
- Andrianov, K.A.; Makarova, N.N. Polymerization of phenyltrichlorosilane hydrolysis products. Vysok. Soedin. Seriya A 1970, 12, 663–700. [Google Scholar]
- Andrianov, K.A.; Zhdanov, A.A.; Levin, V.Y. Some Physical Properties of Organosilicon Ladder Polymers. Annu. Rev. Mater. Sci. 1978, 8, 313–326. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Andrianov, K.A.; Ryumtsev, E.I.; Shtennikova, I.N.; Vitovskaya, M.G.; Makarova, N.N.; Kurasheva, N.A. Molecular conformations, hydrodynamics and optics of ladder-like polymers. Vysok. Soedin. Seriya A 1973, 15, 400–414. [Google Scholar]
- Yang, X.F.; Cao, C.; Chen, Z.H.; Liu, J.; Luo, M.X.; Lai, G.Q. Synthesis of ladder-like polyphenylsilsesquioxanes with fairly high regularity using 1,2-ethylenediamine as endo-template. Chin. J. Polym. Sci. Engl. Ed. 2015, 33, 1305–1312. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Chen, Z.; Liu, J.; Bassindale, A.R.; Lai, G. Preparation and characterization of a type of ladder-like poly(phenyl silsesquioxane) based hybrid star-shaped copolymer of ε-caprolactone. J. Appl. Polym. Sci. 2015, 132, 42335. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, A.S.; Hwang, S.S.; Baek, K.Y. Structural Control of Fully Condensed Polysilsesquioxanes: Ladderlike vs Cage Structured Polyphenylsilsesquioxanes. Macromolecules 2015, 48, 6063–6070. [Google Scholar] [CrossRef]
- Ershova, T.; Anisimov, A.; Krylov, F.; Polshchikova, N.; Temnikov, M.; Shchegolikhina, O.; Muzafarov, A. A new highly efficient method for the preparation of phenyl-containing siloxanes by condensation of phenylsilanols in liquid ammonia. Chem. Eng. Sci. 2022, 247, 116916. [Google Scholar] [CrossRef]
- Ershova, T.O.; Anisimov, A.A.; Temnikov, M.N.; Novikov, M.A.; Buzin, M.I.; Nikiforova, G.G.; Dyuzhikova, Y.S.; Ushakov, I.E.; Shchegolikhina, O.I.; Muzafarov, A.M. A Versatile Equilibrium Method for the Synthesis of High-Strength, Ladder-like Polyphenylsilsesquioxanes with Finely Tunable Molecular Parameters. Polymers 2021, 13, 4452. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion; Wiley: New York, NY, USA, 1972. [Google Scholar]
- Shchegolikhina, O.I.; Pozdnyakova, Y.A.; Molodtsova, Y.A.; Korkin, S.D.; Bukalov, S.S.; Leites, L.A.; Lyssenko, K.A.; Peregudov, A.S.; Auner, N.; Katsoulis, D.E. Synthesis and Properties of Stereoregular Cyclic Polysilanols: cis-[PhSi(O)OH]4, cis-[PhSi(O)OH]6, and Tris-cis-tris-trans-[PhSi(O)OH]12. Inorg. Chem. 2002, 41, 6892–6904. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.F. Double Chain Polymers and Nonrandom Crosslinking. J. Polym. Sci. Part C Polym. Symp. 1963, 97, 83–97. [Google Scholar] [CrossRef]
- Shishatskii, A.M.; Yampol’skii, Y.P.; Peinemann, K.-V. Effects of film thickness on density and gas permeation parameters of glassy polymers. J. Membr. Sci. 1996, 112, 275–285. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Podtynnikov, I.A.; Golubev, G.S.; Volkov, V.V.; Borisov, I.L. Polyheptylmethylsiloxane—A novel material for removal of oxygenates from water by pervaporation. Petr. Chem. 2018, 58, 941–948. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Park, S.; Lee, A.S.; Do, Y.S.; Kim, J.F.; Hwang, S.S.; Lee, Y.M.; Lee, J.-H.; Lee, J.S. Side-chain engineering of ladder-structured polysilsesquioxane membranes for gas separations. J. Membr. Sci. 2016, 516, 202–214. [Google Scholar] [CrossRef]
- Mi, Y.; Stern, S.A. Gas Permeability of a New Silicone Ring Polymer: Lsotactic Poly(phenylsilsesquioxane). J. Polym. Sci. Part B Polym. Phys. 1991, 29, 389–393. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Kang, W.R.; Lee, A.S.; Park, S.; Park, S.H.; Baek, K.Y.; Lee, K.B.; Lee, S.H.; Lee, J.H.; Hwang, S.S.; Lee, J.S. Free-standing, polysilsesquioxane-based inorganic/organic hybrid membranes for gas separations. J. Membr. Sci. 2015, 475, 384–394. [Google Scholar] [CrossRef]
- Kim, Y.H. Energy saving of benzene separation process for environmentally friendly gasoline using an extended DWC (divided wall column). Energy 2016, 100, 58–65. [Google Scholar] [CrossRef]
- Pulyalina, A.Y.; Polotskaya, G.A.; Toikka, A.M. Membrane materials based on polyheteroarylenes and their application for pervaporation. Russ. Chem. Rev. 2016, 85, 81. [Google Scholar] [CrossRef]
- Cunha, V.S.; Paredes, M.L.L.; Borges, C.P.; Habert, A.C.; Nobrega, R. Removal of aromatics from multicomponent organic mixtures by pervaporation using polyurethane membranes: Experimental and modeling. J. Membr. Sci. 2002, 206, 277–290. [Google Scholar] [CrossRef]
- Cunhar, V.S.; Nobrega, R.; Habert, A.C. Fractionation of benzene/n-hexane mixtures by pervaporation using polyurethane membranes. Braz. J. Chem. Eng. 1999, 16, 297–308. [Google Scholar] [CrossRef]
- Yamasaki, A.; Shinbo, T.; Mizoguchi, K. Pervaporation of benzene/cyclohexane and benzene/n-hexane mixtures through PVA membranes. J. Appl. Polym. Sci. 1997, 64, 1061–1065. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Fan, B.C.; Liu, Q.L.; Zhu, A.M.; Shi, F.F. A novel poly(dimethyl siloxane)/poly(oligosilsesquioxanes) composite membrane for pervaporation desulfurization. J. Membr. Sci. 2011, 366, 335–341. [Google Scholar] [CrossRef]

| Mp, kDa | Mw, kDa | Mn, kDa | Td5%, °C (Air) | Td5%, °C (Argon) | E, MPa | σ, MPa | ε, % |
|---|---|---|---|---|---|---|---|
| 577.9 | 540.4 | 238.1 | 537 | 587 | 1700 | 44 | 6 |
| Compound | Solubility |
|---|---|
| L-PPSQ | − |
| Chloroform | + |
| Benzene | + |
| NMP | + |
| DMAA | + |
| Hexane | − |
| Acetone | − |
| Ethanol | − |
| Benzene/hexane 10/90 | − |
| Benzene/hexane 30/70 | Strong swelling |
| Gas | P, Barrer | D·108, cm2/s | S·102, cm3/(cm3 × cmHg) |
|---|---|---|---|
| He | 60 | 2200 | 0.03 |
| H2 | 98 | 1000 | 0.10 |
| N2 | 11 | 44 | 0.25 |
| O2 | 33 | 81 | 0.41 |
| CO2 | 230 | 60 | 3.8 |
| CH4 | 22 | 17 | 1.3 |
| C2H6 | 29 | 2.8 | 10 |
| C4H10 | 25 | 0.5 | 53 |
| Gas | αP | αD | αS |
|---|---|---|---|
| He/CH4 | 2.7 | 129 | 0.02 |
| H2/CH4 | 4.5 | 58.8 | 0.08 |
| CO2/N2 | 20.9 | 1.4 | 15.2 |
| O2/N2 | 3 | 1.8 | 1.64 |
| CO2/CH4 | 10.5 | 3.5 | 2.92 |
| C2H6/CH4 | 1.3 | 0.2 | 7.7 |
| C4H10/CH4 | 1.1 | 0.03 | 40.8 |
| Membrane | Conditions | Specific Permeate Flux, g·µm/(m2·h) | Separation Factor | Source |
|---|---|---|---|---|
| L-PPSQ | Benzene/hexane, 5/95%, T = 20 °C | 1050 | 126 | This study |
| L-PPSQ | Benzene/hexane, 10/90%, T = 20 °C | 1110 | 64 | This study |
| L-PPSQ | Benzene/hexane, 15/85%, T = 20 °C | 1170 | 32 | This study |
| 3,39,4,49-diphenylsulfone-tetracarboxylic dianhydride-2,8(6)-dimethyl-3,7- diaminobenzothiophene-5,5-dioxide polyimide (DSDA-DDBT) | Benzene/hexane, 17/83%, T = 60 °C | 1033 | 10.6 | [54] |
| Polyurethane | Benzene/hexane, 8/92%, T = 40 °C | 600 | 9.5 | [55] |
| Polyurethane | Benzene/hexane, 50/50%, T = 25 °C | 388 | 5.6 | [55] |
| polyurethane | Benzene/hexane, 10/90%, T = 25 °C | 1000 | 25 | [56] |
| Poly(ethylene oxide imide) | Benzene/hexane, 40/60%, T = 60 °C | 28 | 7.5 | [54] |
| Poly(vinylalcohol) | Benzene/hexane, 20/80%, T = 50 °C | 168 | 3 | [57] |
| Polydimethylsiloxane with 6% wt. poly(oligosilsesquioxanes) * | Benzene/heptane, 0.6/99.4%, T = 70 °C | 12 825 | 3.5 | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anokhina, T.S.; Ershova, T.O.; Anisimov, A.A.; Temnikov, M.N.; Grushevenko, E.A.; Borisov, I.L.; Volkov, A.V.; Muzafarov, A.M. Pervaporation and Gas Separation Properties of High-Molecular Ladder-like Polyphenylsilsesquioxanes. Polymers 2023, 15, 3277. https://doi.org/10.3390/polym15153277
Anokhina TS, Ershova TO, Anisimov AA, Temnikov MN, Grushevenko EA, Borisov IL, Volkov AV, Muzafarov AM. Pervaporation and Gas Separation Properties of High-Molecular Ladder-like Polyphenylsilsesquioxanes. Polymers. 2023; 15(15):3277. https://doi.org/10.3390/polym15153277
Chicago/Turabian StyleAnokhina, Tatiana S., Tatyana O. Ershova, Anton A. Anisimov, Maxim N. Temnikov, Evgenia A. Grushevenko, Ilya L. Borisov, Alexey V. Volkov, and Aziz M. Muzafarov. 2023. "Pervaporation and Gas Separation Properties of High-Molecular Ladder-like Polyphenylsilsesquioxanes" Polymers 15, no. 15: 3277. https://doi.org/10.3390/polym15153277
APA StyleAnokhina, T. S., Ershova, T. O., Anisimov, A. A., Temnikov, M. N., Grushevenko, E. A., Borisov, I. L., Volkov, A. V., & Muzafarov, A. M. (2023). Pervaporation and Gas Separation Properties of High-Molecular Ladder-like Polyphenylsilsesquioxanes. Polymers, 15(15), 3277. https://doi.org/10.3390/polym15153277











