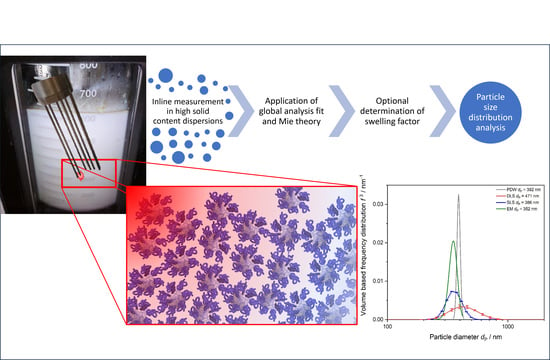
Advanced Particle Size Analysis in High-Solid-Content Polymer Dispersions Using Photon Density Wave Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. PS Seed Synthesis
2.1.2. PS-Seeded Emulsion Polymerization
2.1.3. Emulsion Polymerization of Polyvinyl Acetate Dispersions
2.2. Dispersion Analysis
2.3. PDW Spectroscopy Dispersion Analysis
3. Results and Discussion
3.1. Polystyrene—SDS Dispersions
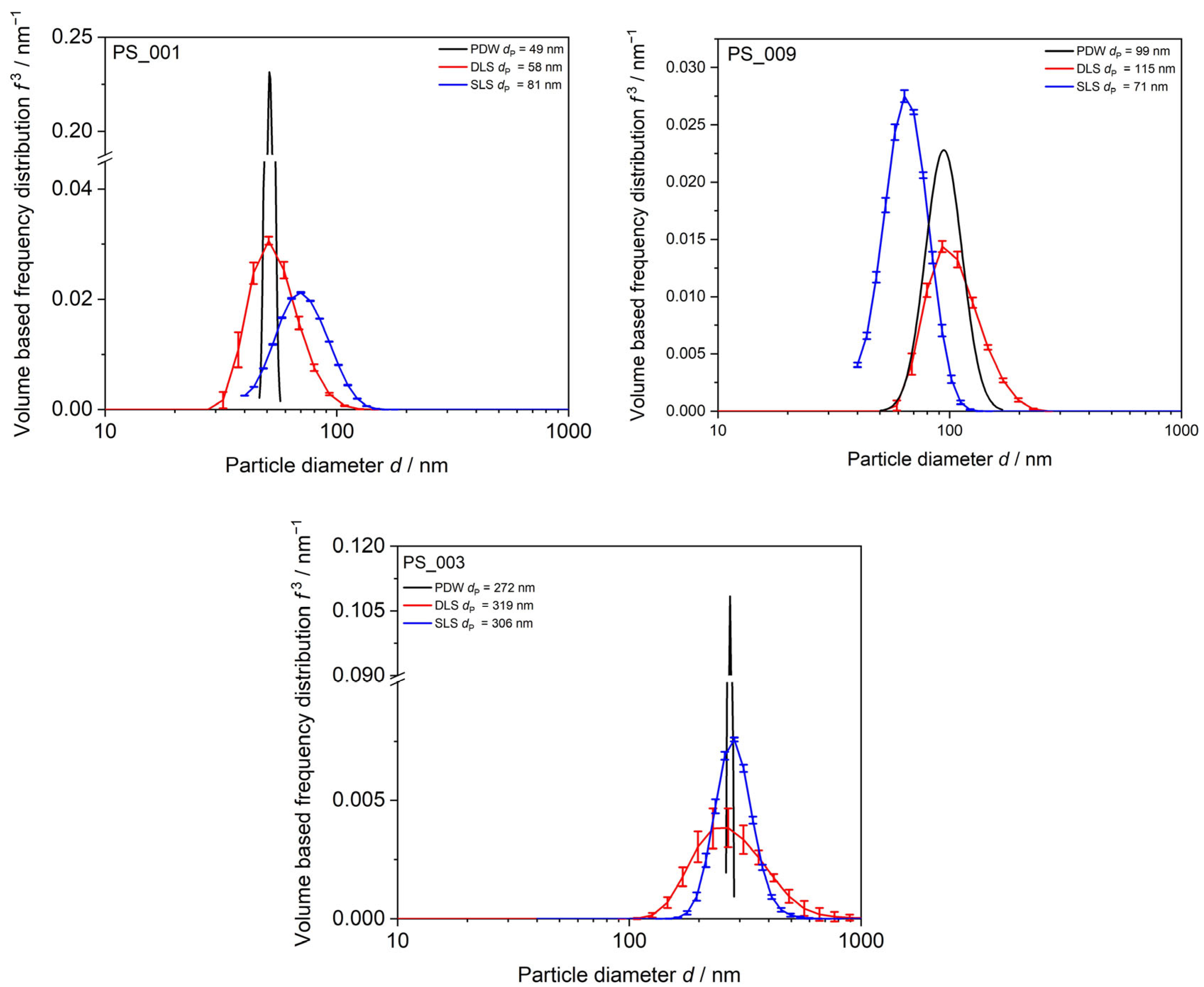
3.1.1. Reproducibility of Polystyrene Dispersions
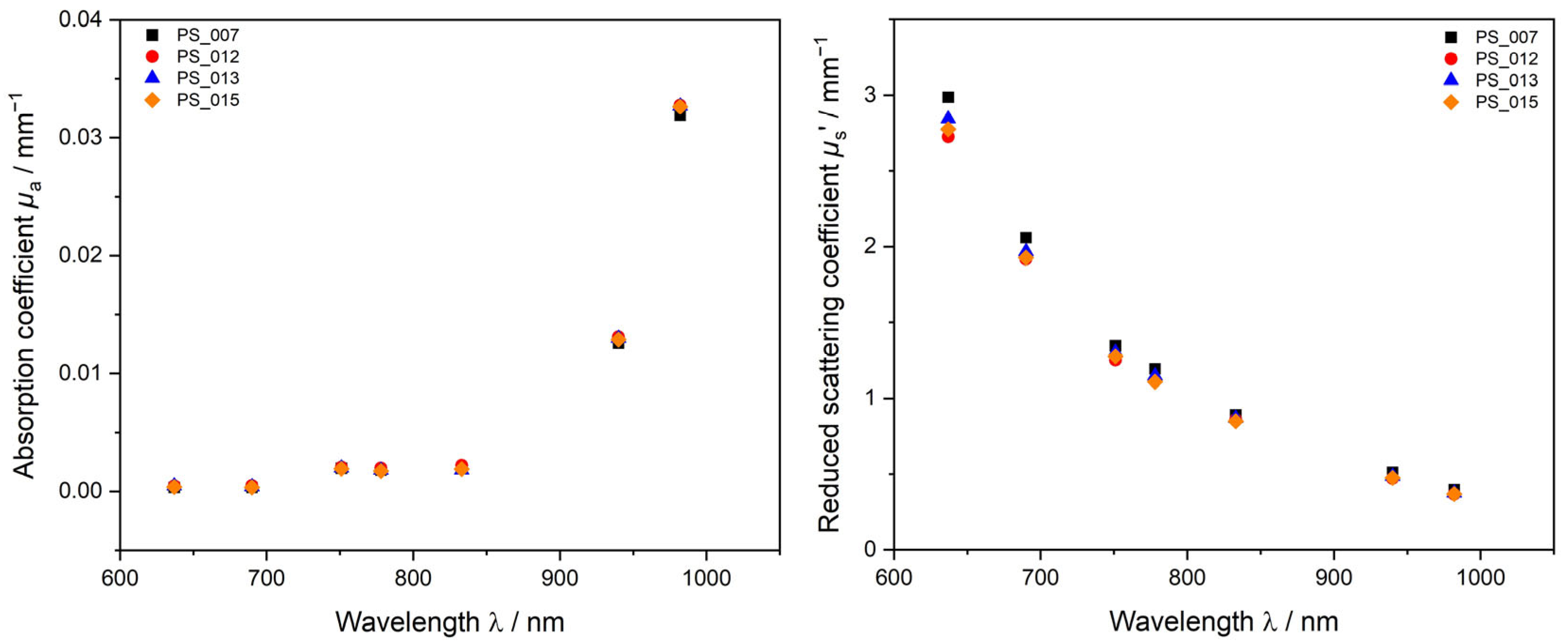
3.1.2. PDW Spectroscopy Analysis of Undiluted PS-SDS Dispersions
3.1.3. Method Comparison DLS, SLS, and PDW Spectroscopy
3.2. Polyvinyl Acetate—Polyvinyl Alcohol Dispersions
3.2.1. Reproducibility PVAc-PVA
3.2.2. PDW Spectroscopy Size Analysis of Undiluted PVAc-PVA Dispersions
3.2.3. Method Comparison DLS, SLS, EM, and PDW Spectroscopy
4. Conclusions
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample ID | Mean dP PDW | Mean dP SLS | Mean dP DLS | PDW/SLS | PDW/DLS |
|---|---|---|---|---|---|
| PS_001 * | 49.00 | 80.85 | 58.50 | 0.61 | 0.84 |
| PS_003 | 271.96 | 287.79 | 319.57 | 0.94 | 0.85 |
| PS_004 | 296.42 | 318.80 | 356.29 | 0.93 | 0.83 |
| PS_007 ** | 100.02 | 72.23 | 114.77 | 1.38 | 0.87 |
| PS_009_1 | 99.14 | 71.83 | 115.27 | 1.38 | 0.86 |
| PS_009_2 | 101.17 | 72.10 | 131.54 | 1.40 | 0.77 |
| PS_011 * | 75.87 | 82.99 | 78.06 | 0.91 | 0.97 |
| PS_012 ** | 97.32 | 71.07 | 109.13 | 1.37 | 0.89 |
| PS_013 ** | 99.00 | 71.65 | 116.47 | 1.38 | 0.85 |
| PS_014 | 251.77 | 250.56 | 254.23 | 1.00 | 0.99 |
| PS_015 ** | 96.46 | 72.09 | 113.04 | 1.34 | 0.85 |
| PS_016 | 274.34 | 321.80 | 283.50 | 0.85 | 0.97 |
| PS_018 | 259.82 | 288.54 | 278.27 | 0.90 | 0.93 |
| PS_019 | 259.48 | 315.30 | 271.33 | 0.82 | 0.96 |
| PS_020 * | 56.96 | 69.27 | 80.46 | 0.82 | 0.71 |
References
- Koltzenburg, S.; Maskos, M.; Nuyken, O. Polymere: Synthese, Eigenschaften und Anwendungen; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-34772-6/978-3-642-34773-3. [Google Scholar]
- Asua, J.M. On-line control of emulsion polymerization reactors: A perspective. Can. J. Chem. Eng. 2023, 1–7. [Google Scholar] [CrossRef]
- Lerche, D.; Miller, R.; Schaeffler, M. Dispersionseigenschaften 2D-Rheologie, 3D-Rheologie, Stabilitaet, 1st ed.; Eigenverlag: Berlin-Potsdam, Germany, 2015. [Google Scholar]
- Aguirre, M.; Ballard, N.; Gonzalez, E.; Hamzehlou, S.; Sardon, H.; Calderon, M.; Paulis, M.; Tomovska, R.; Dupin, D.; Bean, R.H.; et al. Polymer Colloids: Current Challenges, Emerging Applications, and New Developments. Macromolecules 2023, 56, 2579–2607. [Google Scholar] [CrossRef]
- Ober, C.K.; Lok, K.P.; Hair, M.L. Monodispersed, micron-sized polystyrene particles by dispersion polymerization. J. Polym. Sci. Polym. Lett. Ed. 1985, 23, 103–108. [Google Scholar] [CrossRef]
- Chicoma, D.L.; Sayer, C.; Giudici, R. In-Line Monitoring of Particle Size during Emulsion Polymerization under Different Operational Conditions using NIR Spectroscopy: In-Line Monitoring of Particle Size during Emulsion Polymerization. Macromol. React. Eng. 2011, 5, 150–162. [Google Scholar] [CrossRef]
- Frauendorfer, E.; Wolf, A.; Hergeth, W.-D. Polymerization Online Monitoring. Chem. Eng. Technol. 2010, 33, 1767–1778. [Google Scholar] [CrossRef]
- Ito, K.; Kato, T.; Ona, T. Non-destructive method for the quantification of the average particle diameter of latex as water-based emulsions by near-infrared Fourier transform Raman spectroscopy. J. Raman Spectrosc. 2002, 33, 466–470. [Google Scholar] [CrossRef]
- Besseling, R.; Damen, M.; Wijgergangs, J.; Hermes, M.; Wynia, G.; Gerich, A. New unique PAT method and instrument for real-time inline size characterization of concentrated, flowing nanosuspensions. Eur. J. Pharm. Sci. 2019, 133, 205–213. [Google Scholar] [CrossRef]
- Hergeth, W.-D.; Frauendorfer, E. Online-Reaktionsverfolgung bei der Polymerisation. Chem. Ing. Tech. 2010, 82, 503–507. [Google Scholar] [CrossRef]
- de Kanter, M.; Meyer-Kirschner, J.; Viell, J.; Mitsos, A.; Kather, M.; Pich, A.; Janzen, C. Enabling the measurement of particle sizes in stirred colloidal suspensions by embedding dynamic light scattering into an automated probe head. Measurement 2016, 80, 92–98. [Google Scholar] [CrossRef]
- Calzolai, L.; Gilliland, D.; Rossi, F. Measuring nanoparticles size distribution in food and consumer products: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1183–1193. [Google Scholar] [CrossRef]
- Heiligtag, F.J.; Niederberger, M. The fascinating world of nanoparticle research. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, e1901556. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Vogel, R.; Savage, J.; Vella, G.; Law, A.; Della Camera, G.; Hannon, G.; Peacock, B.; Mehn, D.; Ponti, J.; et al. Measuring particle size distribution and mass concentration of nanoplastics and microplastics: Addressing some analytical challenges in the sub-micron size range. J. Colloid. Interface Sci. 2021, 588, 401–417. [Google Scholar] [CrossRef]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-71950-2. [Google Scholar]
- Pecora, R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanoparticle Res. 2020, 2, 9. [Google Scholar] [CrossRef]
- Takahashi, K.; Kato, H.; Saito, T.; Matsuyama, S.; Kinugasa, S. Precise Measurement of the Size of Nanoparticles by Dynamic Light Scattering with Uncertainty Analysis. Part Part. Syst. Charact. 2008, 25, 31–38. [Google Scholar] [CrossRef]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Schlappa, S.; Brenker, L.J.; Bressel, L.; Hass, R.; Münzberg, M. Process Characterization of Polyvinyl Acetate Emulsions Applying Inline Photon Density Wave Spectroscopy at High Solid Contents. Polymers 2021, 13, 669. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.I.; Pauer, W. In-line monitoring of latex-particle size during emulsion polymerizations with a high polymer content of more than 60. RSC Adv. 2020, 10, 26528–26534. [Google Scholar] [CrossRef]
- Bressel, L.; Hass, R.; Reich, O. Particle sizing in highly turbid dispersions by Photon Density Wave spectroscopy. J. Quant. Spectrosc. Radiat. Transf. 2013, 126, 122–129. [Google Scholar] [CrossRef]
- Schiewe, T.; Gutschmann, B.; Santolin, L.; Waldburger, S.; Neubauer, P.; Hass, R.; Riedel, S.L. Real-time monitoring of biomass during Escherichia coli high-cell-density cultivations by in-line photon density wave spectroscopy. Biotechnol. Bioeng. 2023, 1–10. [Google Scholar] [CrossRef]
- Li, W.; Xue, F.; Cheng, R. States of water in partially swollen poly(vinyl alcohol) hydrogels. Polymer 2005, 46, 12026–12031. [Google Scholar] [CrossRef]
- Hodge, R.M.; Edward, G.H.; Simon, G.P. Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 1996, 37, 1371–1376. [Google Scholar] [CrossRef]
- Higuchi, A.; Iijima, T. DSC investigation of the states of water in poly(vinyl alcohol) membranes. Polymer 1985, 26, 1207–1211. [Google Scholar] [CrossRef]
- Ochigbo, S.; Suleiman, M. Comparisons between Polyvinyl Alcohol (PVOH) and Sodium Dodecyl Sulphate (SDS) as Surfactants in Natural Rubber (NR)/Polyvinyl Acetate (PVAc) Latex Blends. IRJPAC 2015, 5, 99–105. [Google Scholar] [CrossRef]
- Guan, L.; Xu, H.; Huang, D. The investigation on states of water in different hydrophilic polymers by DSC and FTIR. J. Polym. Res. 2011, 18, 681–689. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Nakamura, K.; Hatakeyama, H. Determination of bound water content in polymers by DTA, DSC and TG. Thermochim. Acta 1988, 123, 153–161. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Q.; Li, L. Evaporation behaviour of water and its plasticizing effect in modified poly(vinyl alcohol) systems: Evaporation of water and plasticizing effect in modified PVA. Polym. Int. 2003, 52, 1820–1826. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Saini, R. Preparation and characterization of novel biocompatible cryogels of poly (vinyl alcohol) and egg-albumin and their water sorption study. J. Mater. Sci. Mater. Med. 2006, 17, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Hodge, R.M.; Bastow, T.J.; Edward, G.H.; Simon, G.P.; Hill, A.J. Free Volume and the Mechanism of Plasticization in Water-Swollen Poly(vinyl alcohol). Macromolecules 1996, 29, 8137–8143. [Google Scholar] [CrossRef]
- Miyagi, Z.; Tanaka, K. Sorption of water vapor by poly(vinyl acetate). Colloid. Polym. Sci. 1979, 257, 259–265. [Google Scholar] [CrossRef]
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Gonzlez, G.S.M.; Dimonie, V.L.; Sudol, E.D.; Yue, H.J.; Klein, A.; El-Aasser, M.S. Characterization of poly(vinyl alcohol) during the emulsion polymerization of vinyl acetate using poly(vinyl alcohol) as emulsifier. J. Polym. Sci. Part. A Polym. Chem. 1996, 34, 849–862. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Yamaura, K. Preparation of theophylline hydrogels of atactic poly(vinyl alcohol)/NaCl/H2O system for drug delivery system. J. Control. Release 2002, 81, 367–377. [Google Scholar] [CrossRef]
- Lepizzera, S.M.; Hamielec, A.E. Nucleation of particles in seeded emulsion polymerization of vinyl acetate with poly(vinyl alcohol) as emulsifier. Macromol. Chem. Phys. 1994, 195, 3103–3115. [Google Scholar] [CrossRef]
- Capek, I.; Lin, S.-Y.; Hsu, T.-J.; Chern, C.-S. Effect of temperature on styrene emulsion polymerization in the presence of sodium dodecyl sulfate. II. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1477–1486. [Google Scholar] [CrossRef]
- Mariz, I.d.F.; de La Cal, J.C.; Leiza, J.R. Control of particle size distribution for the synthesis of small particle size high solids content latexes. Polymer 2010, 51, 4044–4052. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Liu, Y.; Tan, Z.; Zhou, C.; Zhang, H. Particle Nucleation and Growth in the Emulsion Polymerization of Styrene: Effect of Monomer/Water Ratio and Electrolyte Concentration. J. Macromol. Sci. Part A 2015, 52, 147–154. [Google Scholar] [CrossRef]
- Jacob, L.I.; Pauer, W.; Schröter, B. Influence of redox initiator component ratios on the emulsion copolymerisation of vinyl acetate and neodecanoic acid vinyl ester. RSC Adv. 2022, 12, 14197–14208. [Google Scholar] [CrossRef]
- Zecha, H.; Weitzel, H.-P. Method for Producing Vinyl Ester Polymers Having Specifically Settable Dispersity and Low Polydispersity. U.S. Patent US 9,650,507, 2 April 2013. [Google Scholar]
- Hass, R.; Münzberg, M.; Bressel, L.; Reich, O. Industrial applications of photon density wave spectroscopy for in-line particle sizing Invited. Appl. Opt. 2013, 52, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Munzke, D.; Ruiz, S.V.; Tippmann, J.; Reich, O. Optical monitoring of chemical processes in turbid biogenic liquid dispersions by Photon Density Wave spectroscopy. Anal. Bioanal. Chem. 2015, 407, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Bressel, K.; Müller, W.; Leser, M.E.; Reich, O.; Hass, R.; Wooster, T.J. Depletion-Induced Flocculation of Concentrated Emulsions Probed by Photon Density Wave Spectroscopy. Langmuir 2020, 36, 3504–3513. [Google Scholar] [CrossRef]
- Münzberg, M.; Hass, R.; Reich, O. In-line characterization of phase inversion temperature emulsification by photon density wave spectroscopy. SOFW J. 2013, 4, 38–46. [Google Scholar]
- Häne, J.; Brühwiler, D.; Ecker, A.; Hass, R. Real-time inline monitoring of zeolite synthesis by Photon Density Wave spectroscopy. Microporous Mesoporous Mater. 2019, 288, 109580. [Google Scholar] [CrossRef]
- Jacob, L.I.; Pauer, W. Scale-up of Emulsion Polymerisation up to 100 L and with a Polymer Content of up to 67 wt%, Monitored by Photon Density Wave Spectroscopy. Polymers 2022, 14, 1574. [Google Scholar] [CrossRef] [PubMed]
- Bressel, L. Bedeutung Der Abhaengigen Streuung Für Die Optischen Eigenschaften Hochkonzentrierter Dispersionen. Ph.D. Thesis, University of Potsdam, Potsdam, Germany, 2016. [Google Scholar]
- Reich, O. Photonendichtewellenspektroskopie Mit Intensitaetsmodulierten Diodenlasern. Ph.D. Thesis, University of Potsdam, Potsdam, Germany, 2005. [Google Scholar]
- Hass, R. Angewandte Photonendichtewellen Spektroskopie. Ph.D. Thesis, University of Potsdam, Potsdam, Germany, 2011. [Google Scholar]
- What Is the Z-Average Size Determined by DLS. Available online: https://www.horiba.com/en_en/dynamic-light-scattering-dls-z-average/ (accessed on 28 December 2021).
- Thomas, J.C. The determination of log normal particle size distributions by dynamic light scattering. J. Colloid. Interface Sci. 1987, 117, 187–192. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, L.; Labrie, D.; Chylek, P. Refractive indices of water and ice in the 0.65- to 2.5-µm spectral range. Appl. Opt. 1993, 32, 3531–3540. [Google Scholar] [CrossRef] [Green Version]
- Pope, R.M.; Fry, E.S. Absorption spectrum (380–700 nm) of pure water. II. Integrating cavity measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef]
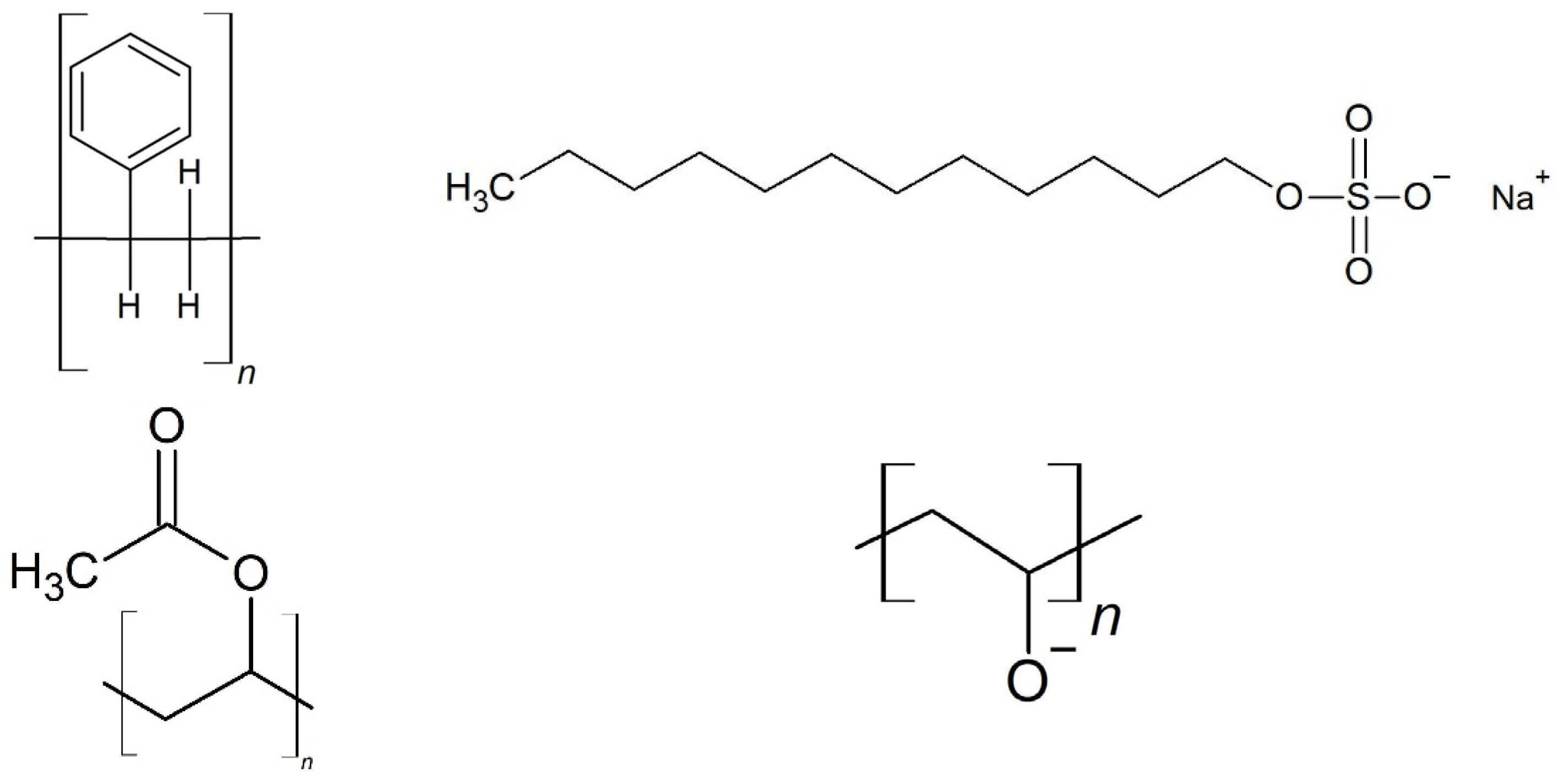





| Chemical | Amount [g] | |
|---|---|---|
| Medium | Milli-Q® water | 530 |
| Monomer | Styrene | 135 |
| Stabilizer | SDS | 6.2 a |
| Buffer | Na-Tetra Borate | 0.125 |
| Initiator | Potassium peroxodisulfate | 5.0 b |
| Chemical | Amount [g] | Feedrate [mL min−1] | |
|---|---|---|---|
| Medium | Milli-Q® water | 308.6 a | |
| Seed | PS Seed | 31.5 b | |
| Monomer | Styrene | 337 c | 2.1 |
| Stabilizer | 10% SDS solution d | 164 | |
| Buffer | Na-Tetra Borate | 0.25 | |
| Initiator | 5% potassium peroxodisulfate solution | 100 |
| Sample ID | PVAiC [g] | Milli-Q®iC [g] | c (PVAiC) [g L−1] | Solid Content ω [%] | Density ρP [g cm−3] |
|---|---|---|---|---|---|
| PVAc-PVA 0 g L−1 | 0 | 302 | 0 | 41.07 | 1.216 |
| PVAc-PVA 1 g L−1 | 0.299 | 302 | 0.99 | 41.61 | 1.214 |
| PVAc-PVA 2 g L−1 | 0.599 | 302 | 1.98 | 40.92 | 1.216 |
| PVAc-PVA 5.5 g L−1 | 1.66 | 302 | 5.50 | 41.57 | 1.212 |
| PVAc-PVA 11 g L−1 | 3.31 | 302 | 10.96 | 40.77 | 1.214 |
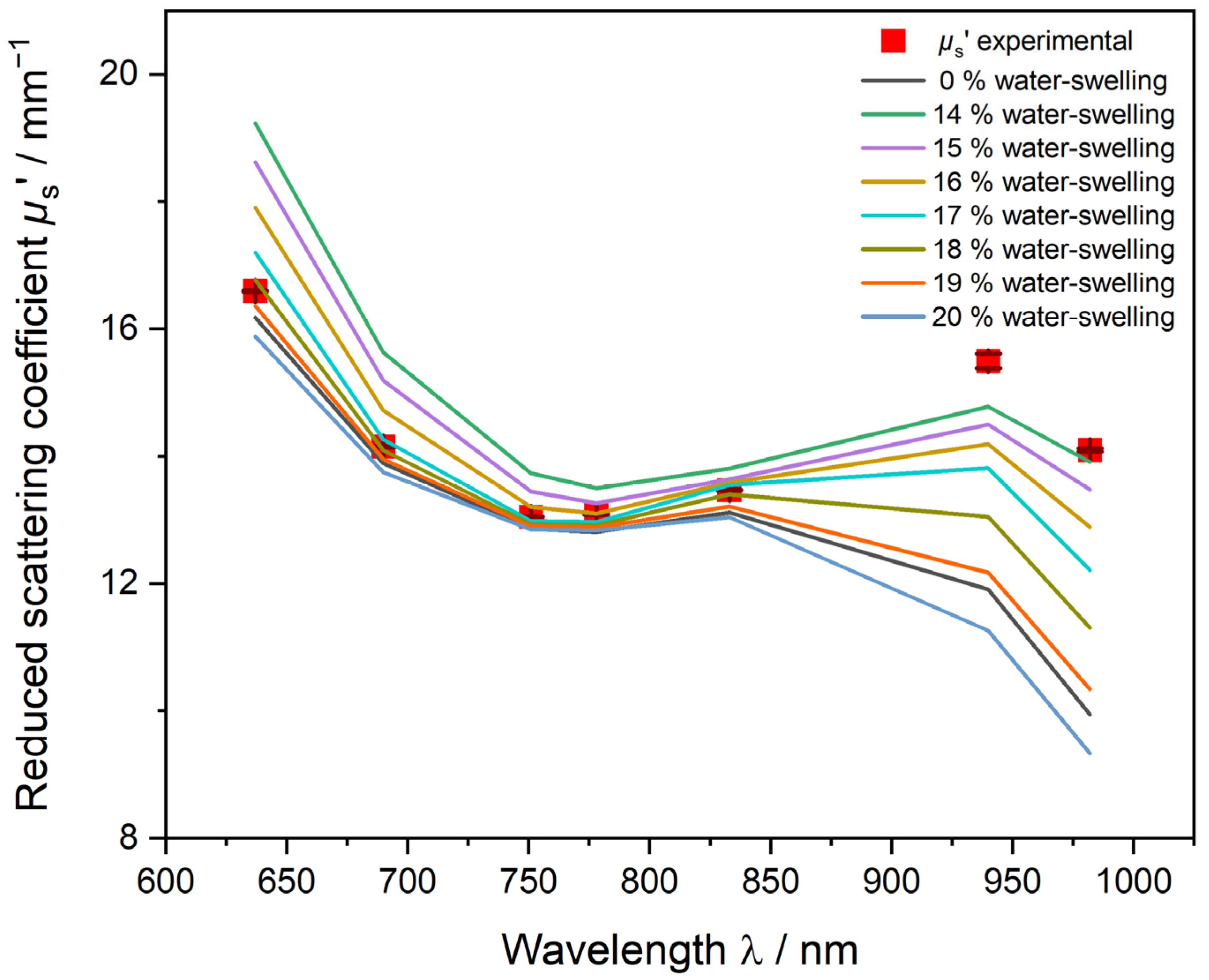
| Sample | Degree of Swelling θswollen/% | Χ2 | Χ2 Improvement Factor |
|---|---|---|---|
| PVAc-PVA 0 g L−1 | 19.29 | 87 | 13.7 |
| PVAc-PVA 1 g L−1 | 22.00 | 250 | 7.2 |
| PVAc-PVA 2 g L−1 | 17.07 | 1352 | 4.06 |
| PVAc-PVA 5.5 g L−1 | 12.38 | 2,5944 | 3.38 |
| PVAc-PVA 11 g L−1 | 38.41 | 4084 | 12.1 |
| Sample ID | Mean dP PDW | Mean dP SLS | Mean dP DLS | PDW/SLS | PDW/DLS |
|---|---|---|---|---|---|
| PVAc-PVA 0 g L−1 | 507.91 | 438.51 | 579.78 | 1.16 | 0.88 |
| PVAc-PVA 1 g L−1_1 * | 412.34 | 401.17 | 483.30 | 1.03 | 0.85 |
| PVAc-PVA 1 g L−1_2 * | 409.86 | 403.54 | 493.21 | 1.02 | 0.83 |
| PVAc-PVA 2 g L−1 | 391.57 | 385.50 | 470.77 | 1.02 | 0.83 |
| PVAc-PVA 5.5 g L−1 * | 306.82 | 324.11 | 359.60 | 0.95 | 0.85 |
| PVAc-PVA 11 g L−1 | 271.37 | 213.27 | 284.80 | 1.27 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlappa, S.; Bressel, L.; Reich, O.; Münzberg, M. Advanced Particle Size Analysis in High-Solid-Content Polymer Dispersions Using Photon Density Wave Spectroscopy. Polymers 2023, 15, 3181. https://doi.org/10.3390/polym15153181
Schlappa S, Bressel L, Reich O, Münzberg M. Advanced Particle Size Analysis in High-Solid-Content Polymer Dispersions Using Photon Density Wave Spectroscopy. Polymers. 2023; 15(15):3181. https://doi.org/10.3390/polym15153181
Chicago/Turabian StyleSchlappa, Stephanie, Lena Bressel, Oliver Reich, and Marvin Münzberg. 2023. "Advanced Particle Size Analysis in High-Solid-Content Polymer Dispersions Using Photon Density Wave Spectroscopy" Polymers 15, no. 15: 3181. https://doi.org/10.3390/polym15153181
APA StyleSchlappa, S., Bressel, L., Reich, O., & Münzberg, M. (2023). Advanced Particle Size Analysis in High-Solid-Content Polymer Dispersions Using Photon Density Wave Spectroscopy. Polymers, 15(15), 3181. https://doi.org/10.3390/polym15153181