Efficient Biorenewable Membranes in Lithium-Oxygen Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
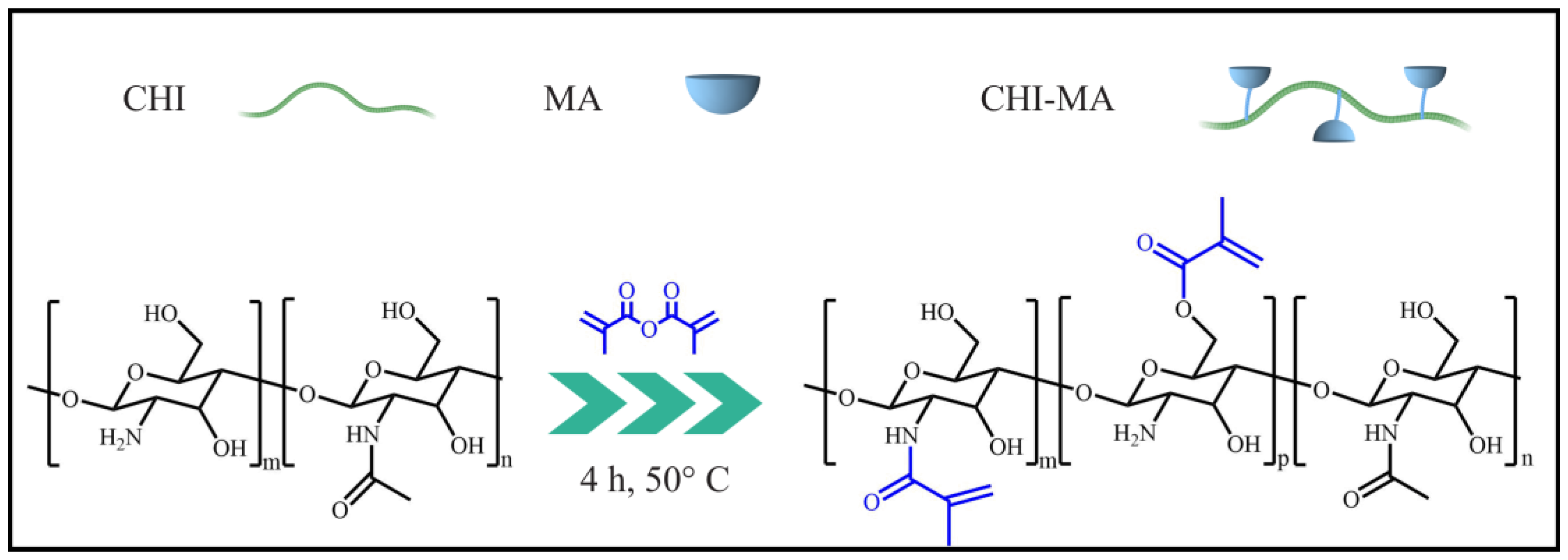
2.2. Synthesis of Methacrylated Chitosan
2.3. UV-Curing
2.4. Characterization Methods
3. Results and discussion
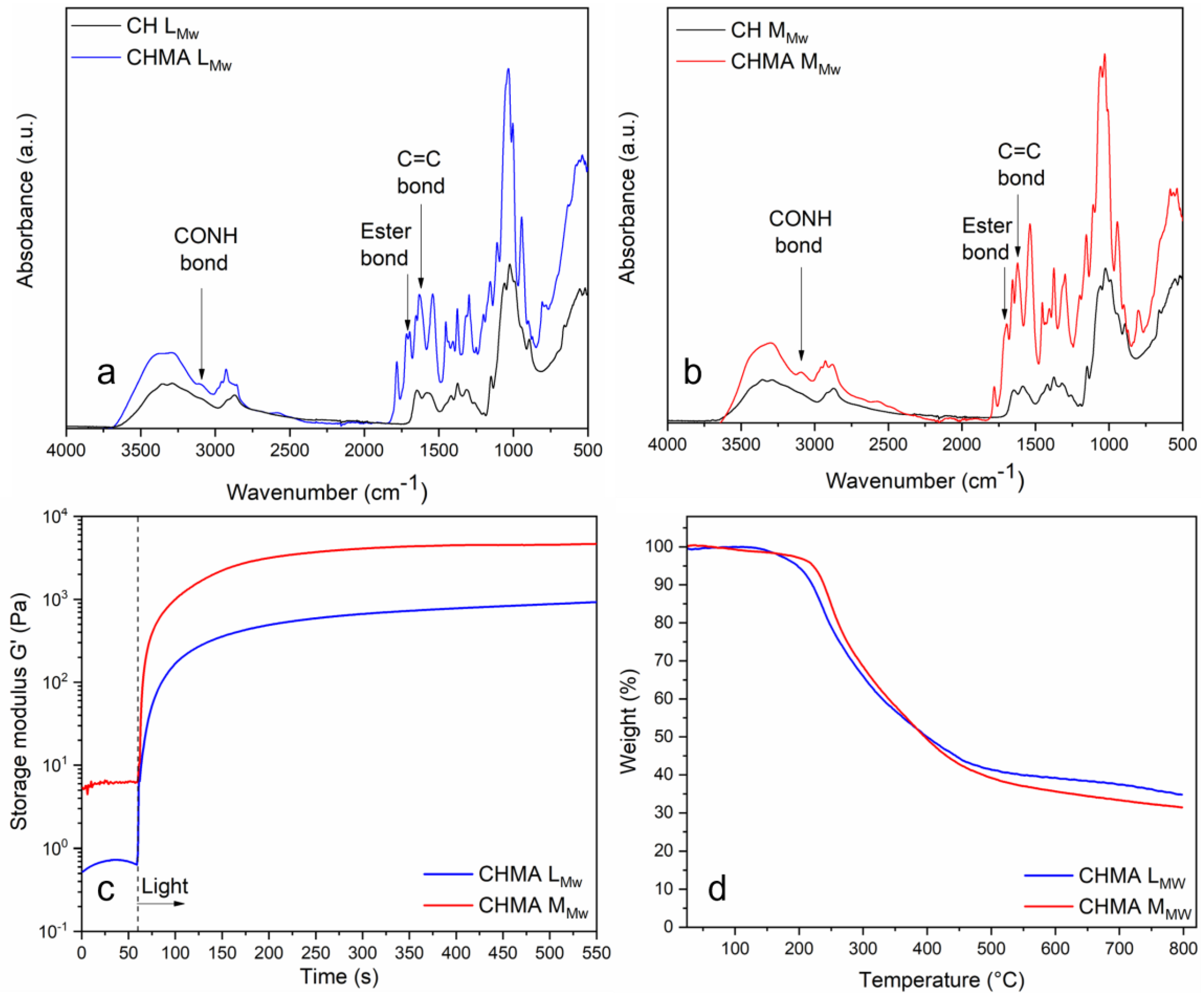
3.1. Physico-Chemical Characterizations
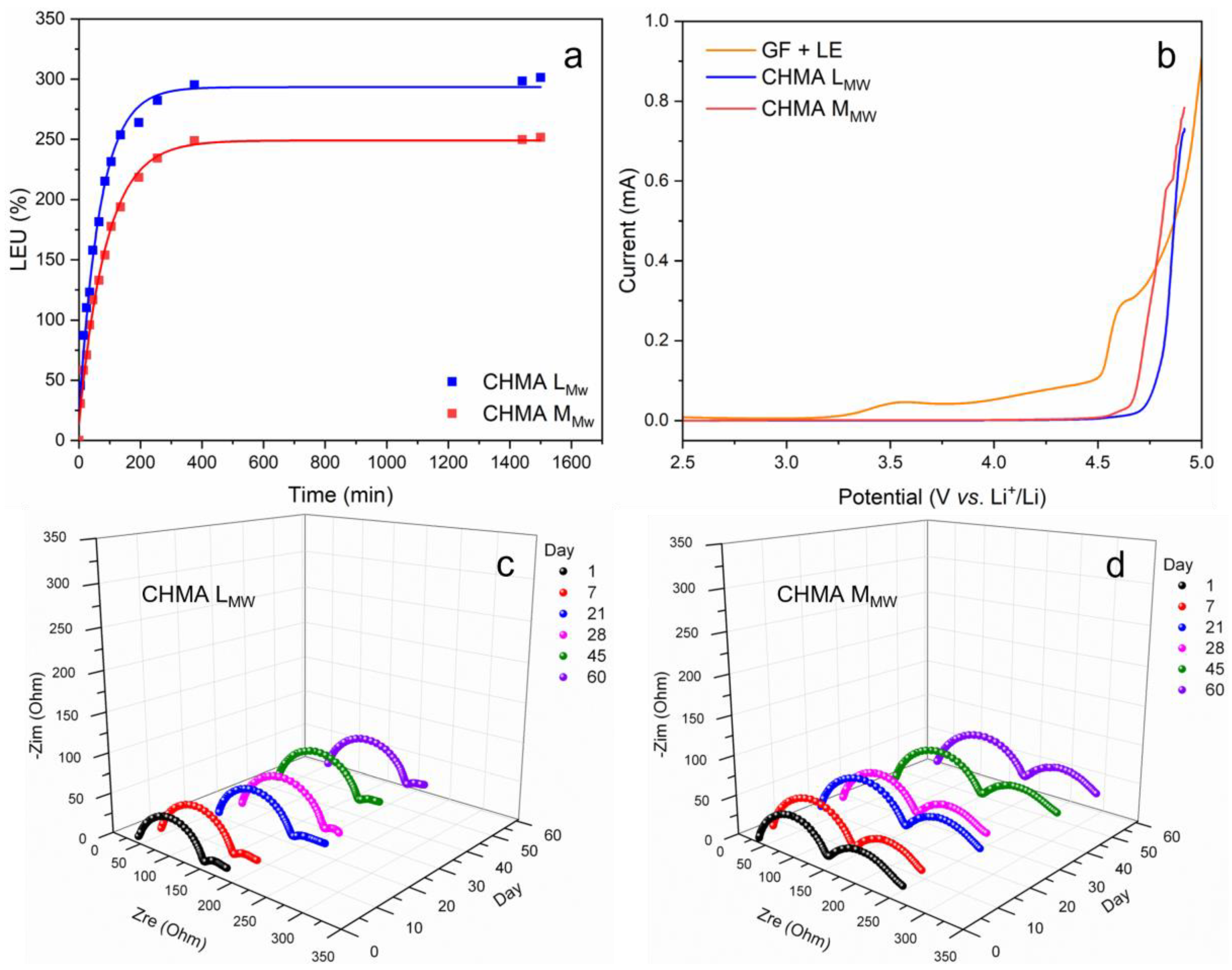
3.2. Electrochemical Characterizations
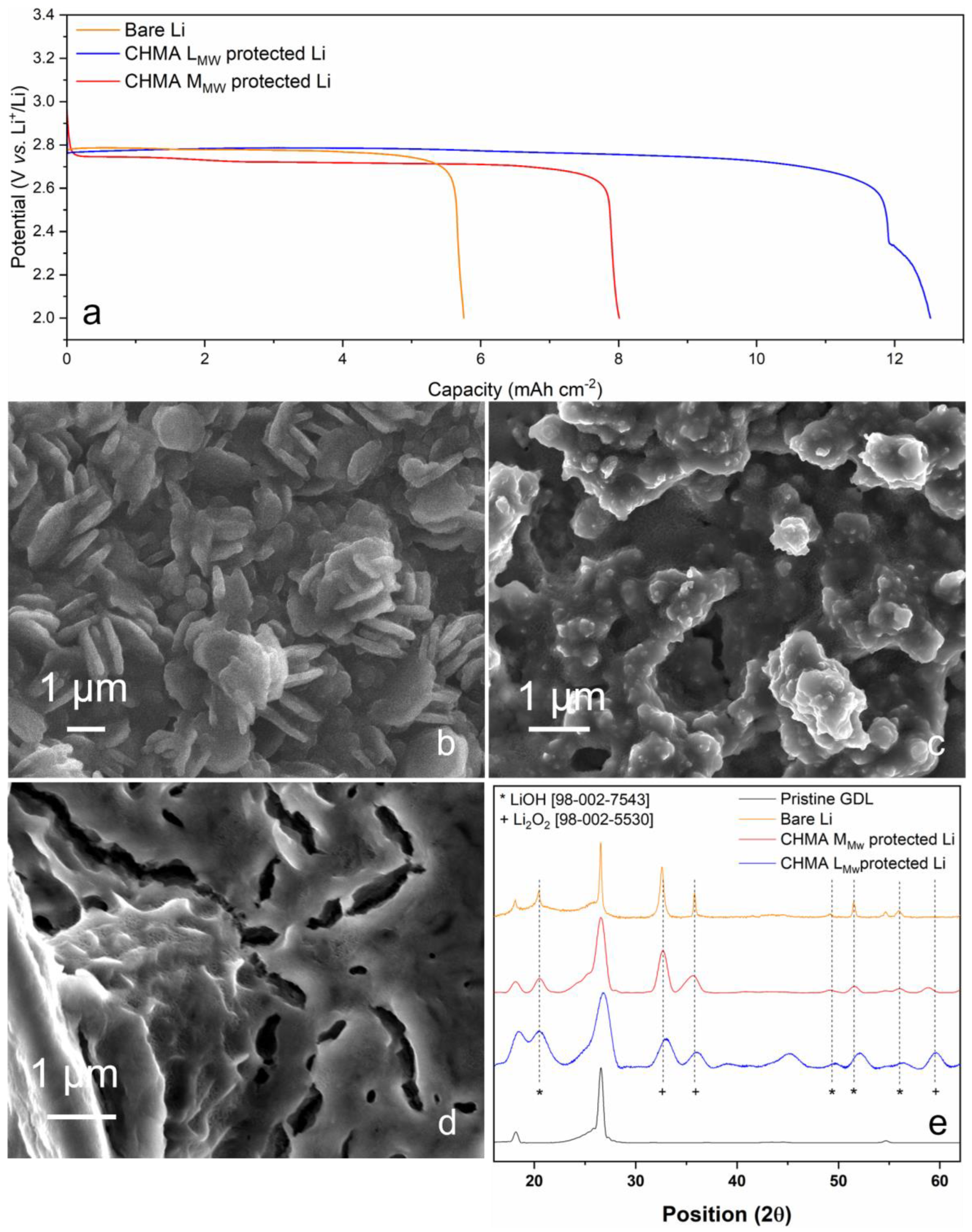
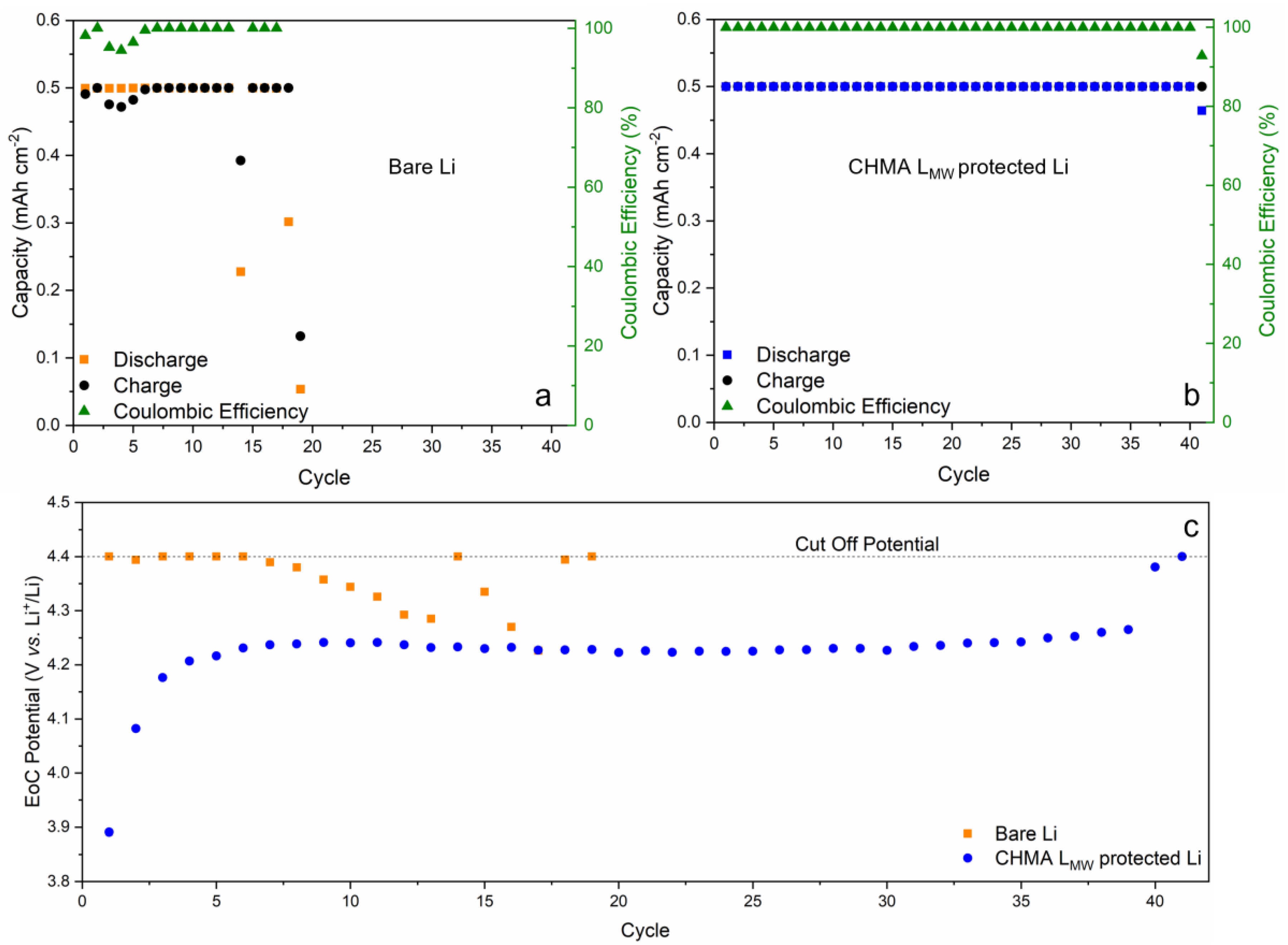
3.3. Lithium-Oxygen Cells Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-Ion Batteries. A Look into the Future. Energy Environ. Sci. 2011, 4, 3287. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S Batteries with High Energy Storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Min, K.-J.; Jeong, M.-G.; Jung, H.-G.; Sun, Y.-K. Resolving the Incomplete Charging Behavior of Redox-Mediated Li-O 2 Batteries via Sustainable Protection of Li Metal Anode. ACS Appl. Mater. Interfaces 2022, 14, 45945–45953. [Google Scholar] [CrossRef] [PubMed]
- Martinez Crespiera, S.; Amantia, D.; Knipping, E.; Aucher, C.; Aubouy, L.; Amici, J.; Zeng, J.; Francia, C.; Bodoardo, S. Electrospun Pd-Doped Mesoporous Carbon Nano Fibres as Catalysts for Rechargeable Li–O2 Batteries. RSC Adv. 2016, 6, 57335–57345. [Google Scholar] [CrossRef]
- Amici, J.; Romanin, S.; Alidoost, M.; Versaci, D.; Francia, C.; Smeacetto, F.; Bodoardo, S. UV-Cured Methacrylate Based Polymer Composite Electrolyte for Metallic Lithium Batteries. J. Electroanal. Chem. 2019, 837, 103–107. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, H.; Song, J.; Ryou, M.-H.; Lee, Y.M.; Kim, H.-T.; Park, J.-K. Composite Protective Layer for Li Metal Anode in High-Performance Lithium–Oxygen Batteries. Electrochem. Commun. 2014, 40, 45–48. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; He, P.; Zhou, H. A Versatile Halide Ester Enabling Li—Anode Stability and a High Rate Capability in Lithium–Oxygen Batteries. Angew. Chem. Int. Ed. 2019, 58, 2355–2359. [Google Scholar] [CrossRef]
- Hsia, T.-N.; Lu, H.-C.; Hsueh, Y.-C.; Rajesh Kumar, S.; Yen, C.-S.; Yang, C.-C.; Jessie Lue, S. Superdry Poly(Vinylidene Fluoride-Co-Hexafluoropropylene) Coating on a Lithium Anode as a Protective Layer and Separator for a High-Performance Lithium-Oxygen Battery. J. Colloid. Interface Sci. 2022, 626, 524–534. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Tang, P.; Wang, H.; Li, K.; Yin, Y.; Yang, S. A Novel Strategy for Improving Performance of Lithium-Oxygen Batteries. J. Colloid. Interface Sci. 2021, 584, 246–252. [Google Scholar] [CrossRef]
- Liang, W.; Lian, F.; Meng, N.; Lu, J.; Ma, L.; Zhao, C.-Z.; Zhang, Q. Adaptive Formed Dual-Phase Interface for Highly Durable Lithium Metal Anode in Lithium–Air Batteries. Energy Storage Mater. 2020, 28, 350–356. [Google Scholar] [CrossRef]
- Yu, Y.; Yin, Y.-B.; Ma, J.-L.; Chang, Z.-W.; Sun, T.; Zhu, Y.-H.; Yang, X.-Y.; Liu, T.; Zhang, X.-B. Designing a Self-Healing Protective Film on a Lithium Metal Anode for Long-Cycle-Life Lithium-Oxygen Batteries. Energy Storage Mater. 2019, 18, 382–388. [Google Scholar] [CrossRef]
- Elia, G.A.; Hassoun, J. A Gel Polymer Membrane for Lithium-Ion Oxygen Battery. Solid. State Ion. 2016, 287, 22–27. [Google Scholar] [CrossRef]
- Wu, X.; Cui, S.; Fei, M.; Liu, S.; Gao, X.; Li, G. Inverse-Opal Structured TiO2 Regulating Electrodeposition Behavior to Enable Stable Lithium Metal Electrodes. Green. Energy Environ. 2022, in press. [Google Scholar] [CrossRef]
- Kwak, W.-J.; Park, J.; Nguyen, T.T.; Kim, H.; Byon, H.R.; Jang, M.; Sun, Y.-K. A Dendrite- and Oxygen-Proof Protective Layer for Lithium Metal in Lithium–Oxygen Batteries. J. Mater. Chem. A Mater. 2019, 7, 3857–3862. [Google Scholar] [CrossRef]
- Bai, P.; Li, J.; Brushett, F.R.; Bazant, M.Z. Transition of Lithium Growth Mechanisms in Liquid Electrolytes. Energy Environ. Sci. 2016, 9, 3221–3229. [Google Scholar] [CrossRef] [Green Version]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Cai, Z.; Huang, T.; Zhang, H.; Yu, A. A Modified Natural Polysaccharide as a High-Performance Binder for Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 4311–4317. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R. Enabling Green Manufacture of Polymer Products via Vegetable Oil Epoxides. Ind. Eng. Chem. Res. 2023, 62, 1725–1735. [Google Scholar] [CrossRef]
- Liu, J.; Chu, Z.; Li, P.; Lin, J.; Yang, Y.; Yang, Z. A Reactive Eco–Vegetable Oil–Based Binder for High–Performance Lithium–Sulfur Batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131526. [Google Scholar] [CrossRef]
- Raviolo, S.; Bracamonte, M.V.; Calderón, C.A.; Cometto, F.P.; Luque, G.L. A Green Solution to Energy Storage: Brewers’ Spent Grains Biocarbon–Silica Composites as High--Performance Lithium—Ion Batteries Anodes. Energy Technol. 2023, 2300342. [Google Scholar] [CrossRef]
- Lucherelli, M.A.; Duval, A.; Avérous, L. Biobased Vitrimers: Towards Sustainable and Adaptable Performing Polymer Materials. Prog. Polym. Sci. 2022, 127, 101515. [Google Scholar] [CrossRef]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of Crystalline Structural Differences Between α- and β-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial Activity, Interactions with Food Components and Applicability as a Coating on Fruit and Vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/Chitosan: Modifications and Their Unlimited Application Potential—An Overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Morinval, A.; Averous, L. Systems Based on Biobased Thermoplastics: From Bioresources to Biodegradable Packaging Applications. Polym. Rev. 2022, 62, 653–721. [Google Scholar] [CrossRef]
- Xing, R.; Yu, H.; Liu, S.; Zhang, W.; Zhang, Q.; Li, Z.; Li, P. Antioxidant Activity of Differently Regioselective Chitosan Sulfates in Vitro. Bioorg Med. Chem. 2005, 13, 1387–1392. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. The Synthesis and Antioxidant Activity of the Schiff Bases of Chitosan and Carboxymethyl Chitosan. Bioorg Med. Chem. Lett. 2005, 15, 4600–4603. [Google Scholar] [CrossRef]
- Zhong, Z.; Ji, X.; Xing, R.; Liu, S.; Guo, Z.; Chen, X.; Li, P. The Preparation and Antioxidant Activity of the Sulfanilamide Derivatives of Chitosan and Chitosan Sulfates. Bioorg Med. Chem. 2007, 15, 3775–3782. [Google Scholar] [CrossRef]
- XUE, C.; YU, G.; HIRATA, T.; TERAO, J.; LIN, H. Antioxidative Activities of Several Marine Polysaccharides Evaluated in a Phosphatidylcholine-Liposomal Suspension and Organic Solvents. Biosci. Biotechnol. Biochem. 1998, 62, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Du, Y.; Li, J.; Hu, Y.; Kennedy, J.F. Enhancement of Antioxidant Activity of Chitosan by Irradiation. Carbohydr. Polym. 2008, 73, 126–132. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, D.; Liu, Z.; Wang, Z.; Gu, Z.; Wang, D.; Yao, X. Cellulose Acetate-Based High-Electrolyte-Uptake Gel Polymer Electrolyte for Semi-Solid-State Lithium-Oxygen Batteries with Long-Cycling Stability. Chem. Asian J. 2022, 17, e202200712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fu, J.; Du, Z.; Jia, X.; Qu, Y.; Yu, F.; Du, J.; Chen, Y. High-Strength and Flexible Cellulose/PEG Based Gel Polymer Electrolyte with High Performance for Lithium Ion Batteries. J. Memb. Sci. 2020, 593, 117428. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, L.; Tufail, M.K.; Yang, L.; Zhai, P.; Chen, R.; Yang, W. In-Situ Synthesized Non-Flammable Gel Polymer Electrolyte Enable Highly Safe and Dendrite-Free Lithium Metal Batteries. Chem. Eng. J. 2021, 415, 128846. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. Electrochemical Measurement of Transference Numbers in Polymer Electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar]
- Feng, Z.; Hakkarainen, M.; Grützmacher, H.; Chiappone, A.; Sangermano, M. Photocrosslinked Chitosan Hydrogels Reinforced with Chitosan--Derived Nano--Graphene Oxide. Macromol. Chem. Phys. 2019, 220, 1900174. [Google Scholar] [CrossRef]
- Sesia, R.; Ferraris, S.; Sangermano, M.; Spriano, S. UV-Cured Chitosan-Based Hydrogels Strengthened by Tannic Acid for the Removal of Copper Ions from Water. Polymer 2022, 14, 4645. [Google Scholar] [CrossRef]
- Zanon, M.; Chiappone, A.; Garino, N.; Canta, M.; Frascella, F.; Hakkarainen, M.; Pirri, C.F.; Sangermano, M. Microwave-Assisted Methacrylation of Chitosan for 3D Printable Hydrogels in Tissue Engineering. Mater. Adv. 2022, 3, 514–525. [Google Scholar] [CrossRef]
- Noè, C.; Zanon, M.; Arencibia, A.; López-Muñoz, M.-J.; Fernández de Paz, N.; Calza, P.; Sangermano, M. UV-Cured Chitosan and Gelatin Hydrogels for the Removal of As(V) and Pb(II) from Water. Polymer 2022, 14, 1268. [Google Scholar] [CrossRef]
- Zanon, M.; Cue-López, R.; Martínez-Campos, E.; Bosch, P.; Versace, D.-L.; Hayek, H.; Garino, N.; Pirri, C.F.; Sangermano, M.; Chiappone, A. Bioderived Dyes-Mediated Vat Photopolymerization 3D Printing of Chitosan Hydrogels for Tissue Engineering. Addit. Manuf. 2023, 69, 103553. [Google Scholar] [CrossRef]
- Jurado-López, B.; Vieira, R.S.; Rabelo, R.B.; Beppu, M.M.; Casado, J.; Rodríguez-Castellón, E. Formation of Complexes between Functionalized Chitosan Membranes and Copper: A Study by Angle Resolved XPS. Mater. Chem. Phys. 2017, 185, 152–161. [Google Scholar] [CrossRef]
- Woo, H.S.; Son, H.; Min, J.Y.; Rhee, J.; Lee, H.T.; Kim, D.W. Ionic Liquid-Based Gel Polymer Electrolyte Containing Zwitterion for Lithium-Oxygen Batteries. Electrochim. Acta 2020, 345, 136248. [Google Scholar] [CrossRef]
- Han, J.; Huang, Y.; Chen, Y.; Song, A.; Deng, X.; Liu, B.; Li, X.; Wang, M. High--Performance Gel Polymer Electrolyte Based on Chitosan–Lignocellulose for Lithium--Ion Batteries. ChemElectroChem 2020, 7, 1213–1224. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Chi, Z.; Chen, S.; Li, S.; Guo, Z.; Wang, L. Designing a New-Type PMMA Based Gel Polymer Electrolyte Incorporating Ionic Liquid for Lithium Oxygen Batteries with Ru-Based Binder-Free Cathode. Appl. Surf. Sci. 2021, 565, 150612. [Google Scholar] [CrossRef]
- Qiu, Z.; Shi, L.; Wang, Z.; Mindemark, J.; Zhu, J.; Edström, K.; Zhao, Y.; Yuan, S. Surface Activated Polyethylene Separator Promoting Li+ Ion Transport in Gel Polymer Electrolytes and Cycling Stability of Li-Metal Anode. Chem. Eng. J. 2019, 368, 321–330. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, L.; Deng, S.; Cui, P.; Yao, X. 10 Μm--Thick High--Strength Solid Polymer Electrolytes with Excellent Interface Compatibility for Flexible All--Solid--State Lithium--Metal Batteries. Adv. Mater. 2021, 33, 2100353. [Google Scholar] [CrossRef]
- Wood, K.N.; Kazyak, E.; Chadwick, A.F.; Chen, K.H.; Zhang, J.G.; Thornton, K.; Dasgupta, N.P. Dendrites and Pits: Untangling the Complex Behavior of Lithium Metal Anodes through Operando Video Microscopy. ACS Cent. Sci. 2016, 2, 790–801. [Google Scholar] [CrossRef]
- Drvarič Talian, S.; Bobnar, J.; Sinigoj, A.R.; Humar, I.; Gaberšček, M. Transmission Line Model for Description of the Impedance Response of Li Electrodes with Dendritic Growth. J. Phys. Chem. C 2019, 123, 27997–28007. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.-X.; Yin, Y.-X.; Li, N.-W.; Du, W.-C.; Guo, Y.-G.; Wan, L.-J. Reshaping Lithium Plating/Stripping Behavior via Bifunctional Polymer Electrolyte for Room-Temperature Solid Li Metal Batteries. J. Am. Chem. Soc. 2016, 138, 15825–15828. [Google Scholar] [CrossRef] [PubMed]
- Gittleson, F.S.; Ryu, W.-H.; Schwab, M.; Tong, X.; Taylor, A.D. Pt and Pd Catalyzed Oxidation of Li 2 O 2 and DMSO during Li–O 2 Battery Charging. Chem. Commun. 2016, 52, 6605–6608. [Google Scholar] [CrossRef]
- Younesi, R.; Hahlin, M.; Roberts, M.; Edström, K. The SEI Layer Formed on Lithium Metal in the Presence of Oxygen: A Seldom Considered Component in the Development of the Li–O2 Battery. J. Power Sources 2013, 225, 40–45. [Google Scholar] [CrossRef]
- Amici, J.; Torchio, C.; Versaci, D.; Dessantis, D.; Marchisio, A.; Caldera, F.; Bella, F.; Francia, C.; Bodoardo, S. Nanosponge-Based Composite Gel Polymer Electrolyte for Safer Li-O<inf>2</Inf> Batteries. Polymer 2021, 13, 1625. [Google Scholar] [CrossRef]
- Versaci, D.; Kastrinaki, G.; Ganas, G.; Zarvalis, D.; Karagiannakis, G.; Amici, J.; Francia, C.; Bodoardo, S. Influence of Electrode Fabrication Process on Nanocrystalline Tin Oxide Electrochemical Behaviour for High Voltage SnO2/LNMO Full Cell Li-Ion Battery. J. Energy Storage 2023, 65, 107357. [Google Scholar] [CrossRef]
- Jiao, W.; Su, Q.; Ge, J.; Dong, S.; Wang, D.; Zhang, M.; Ding, S.; Du, G.; Xu, B. Mo2C Quantum Dots Decorated Ultrathin Carbon Nanosheets Self-Assembled into Nanoflowers toward Highly Catalytic Cathodes for Li-O2 Batteries. Mater. Res. Bull. 2021, 133, 111020. [Google Scholar] [CrossRef]
- Aksel, S.; Eder, D. Catalytic Effect of Metal Oxides on the Oxidation Resistance in Carbon Nanotube–Inorganic Hybrids. J. Mater. Chem. 2010, 20, 9149. [Google Scholar] [CrossRef]
- Sun, G.; Zhao, Q.; Wu, T.; Lu, W.; Bao, M.; Sun, L.; Xie, H.; Liu, J. 3D Foam-Like Composites of Mo 2 C Nanorods Coated by N-Doped Carbon: A Novel Self-Standing and Binder-Free O2 Electrode for Li–O2 Batteries. ACS Appl. Mater. Interfaces 2018, 10, 6327–6335. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dinh, K.N.; Sun, Y.; Fan, H.; Wang, Y.; Jing, Y.; Li, S.; Srinivasan, M.; Yan, Q. Performance-Improved Li-O2 Batteries by Tailoring the Phases of Mo x C Porous Nanorods as an Efficient Cathode. Nanoscale 2018, 10, 14877–14884. [Google Scholar] [CrossRef]
- Amici, J.; Marquez, P.; Mangini, A.; Torchio, C.; Dessantis, D.; Versaci, D.; Francia, C.; Aguirre, M.J.; Bodoardo, S. Sustainable, Economic, and Simple Preparation of an Efficient Catalyst for Li-O2 Batteries. J. Power Sources 2022, 546, 231942. [Google Scholar] [CrossRef]
- Fu, J.; Guo, X.; Huo, H.; Chen, Y.; Zhang, T. Easily Decomposed Discharge Products Induced by Cathode Construction for Highly Energy-Efficient Lithium–Oxygen Batteries. ACS Appl. Mater. Interfaces 2019, 11, 14803–14809. [Google Scholar] [CrossRef]
- Tamer, T.; Valachová, K.; Mohyeldin, M.; Soltes, L. Free Radical Scavenger Activity of Chitosan and Its Aminated Derivative. J. Appl. Pharm. Sci. 2016, 6, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Wang, P.; Li, C.; Li, Z.; Li, P. Relevance of Molecular Weight of Chitosan and Its Derivatives and Their Antioxidant Activities in Vitro. Bioorg Med. Chem. 2005, 13, 1573–1577. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amici, J.; Banaudi, G.; Longo, M.; Gandolfo, M.; Zanon, M.; Francia, C.; Bodoardo, S.; Sangermano, M. Efficient Biorenewable Membranes in Lithium-Oxygen Batteries. Polymers 2023, 15, 3182. https://doi.org/10.3390/polym15153182
Amici J, Banaudi G, Longo M, Gandolfo M, Zanon M, Francia C, Bodoardo S, Sangermano M. Efficient Biorenewable Membranes in Lithium-Oxygen Batteries. Polymers. 2023; 15(15):3182. https://doi.org/10.3390/polym15153182
Chicago/Turabian StyleAmici, Julia, Giorgio Banaudi, Mattia Longo, Matteo Gandolfo, Michael Zanon, Carlotta Francia, Silvia Bodoardo, and Marco Sangermano. 2023. "Efficient Biorenewable Membranes in Lithium-Oxygen Batteries" Polymers 15, no. 15: 3182. https://doi.org/10.3390/polym15153182
APA StyleAmici, J., Banaudi, G., Longo, M., Gandolfo, M., Zanon, M., Francia, C., Bodoardo, S., & Sangermano, M. (2023). Efficient Biorenewable Membranes in Lithium-Oxygen Batteries. Polymers, 15(15), 3182. https://doi.org/10.3390/polym15153182








