Multi-Spectroscopic Characterization of MgO/Nylon (6/6) Polymer: Evaluating the Potential of LIBS and Statistical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Sample Preparation
3. Results and Discussion
3.1. LIBS Spectra Analysis
3.2. Plasma Characterization
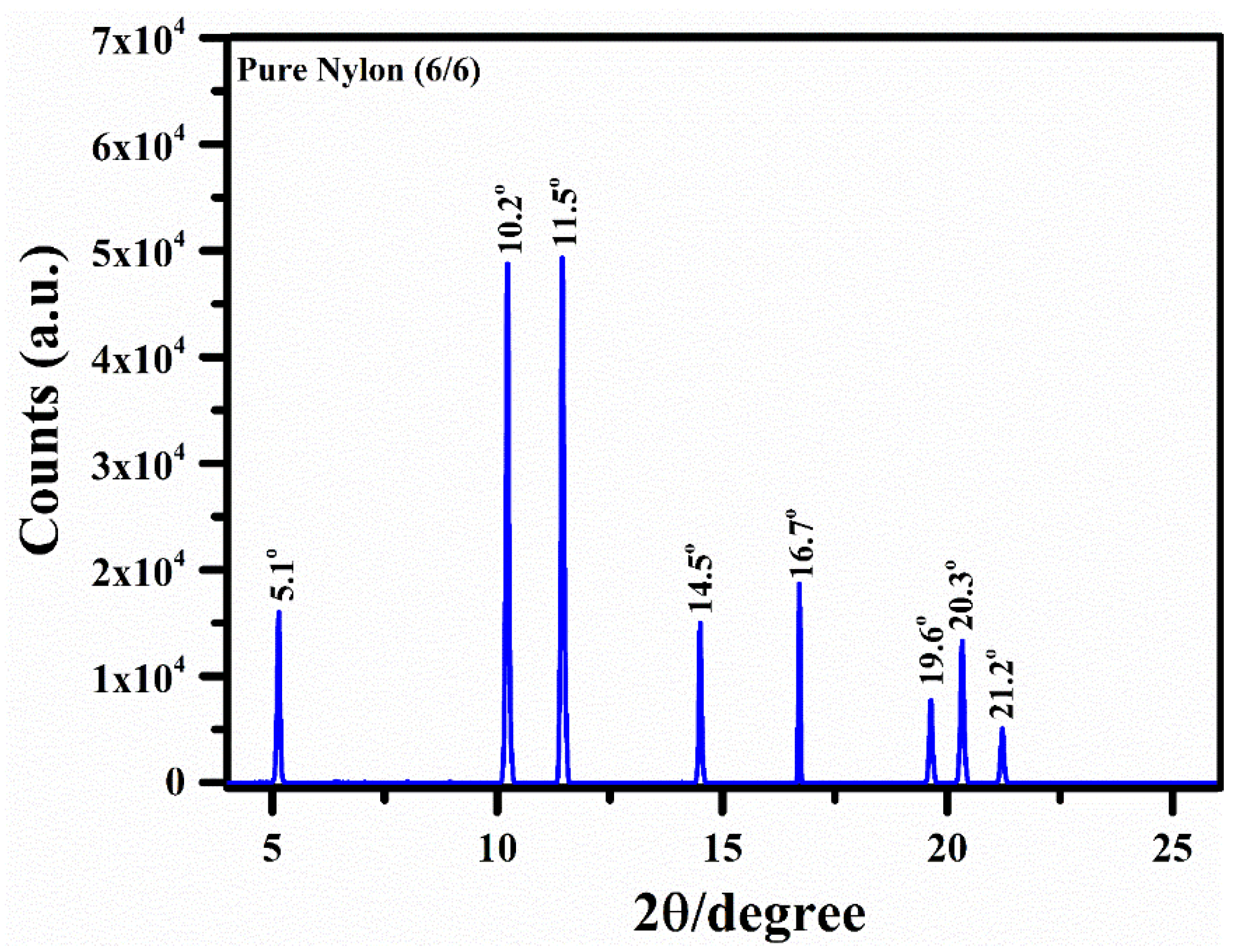
3.3. XRD Analysis
3.4. SEM-EDX Chemical Analysis
3.5. Validation of Nylon (6/6) Functional Groups Using FTIR
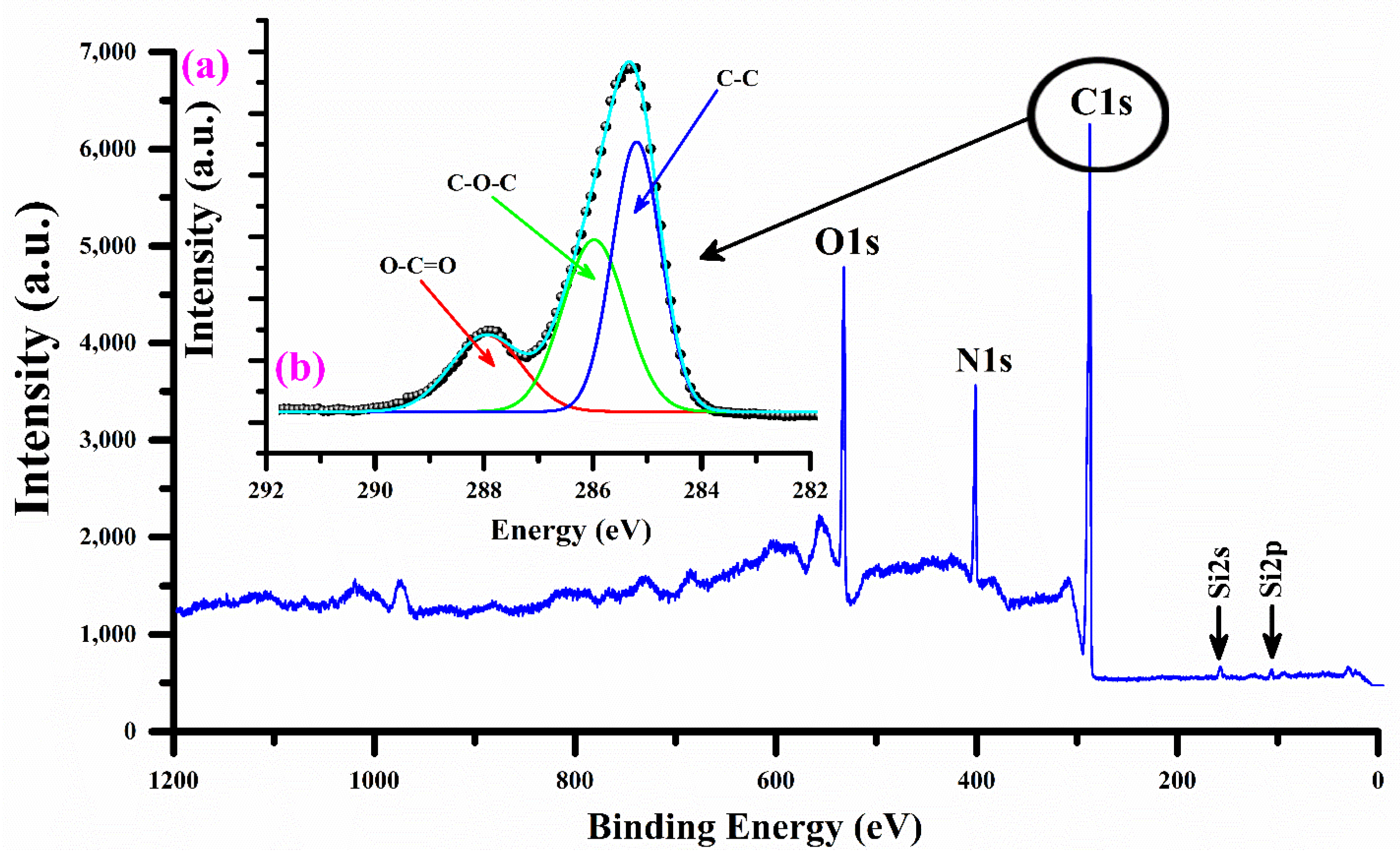
3.6. XPS Analysis
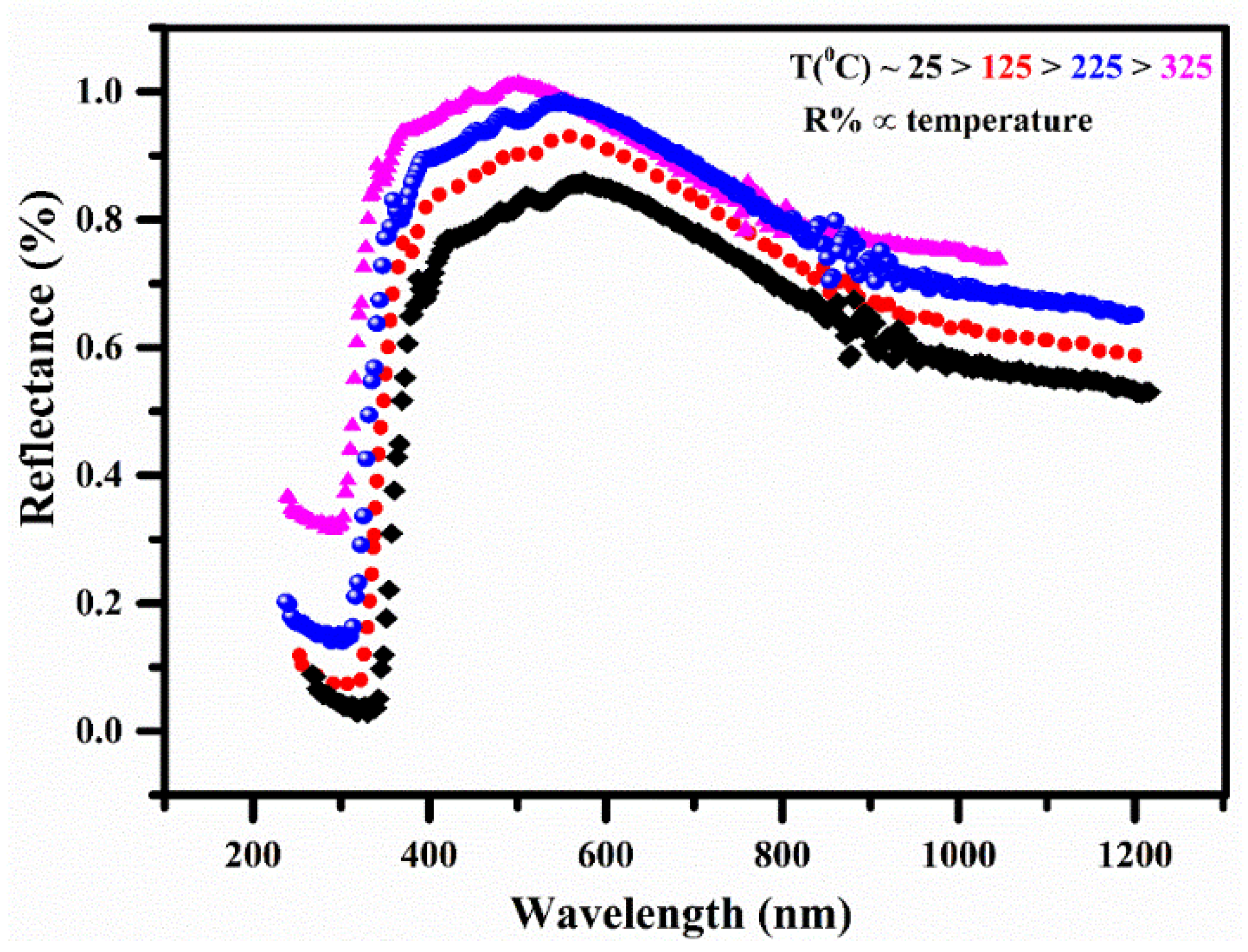
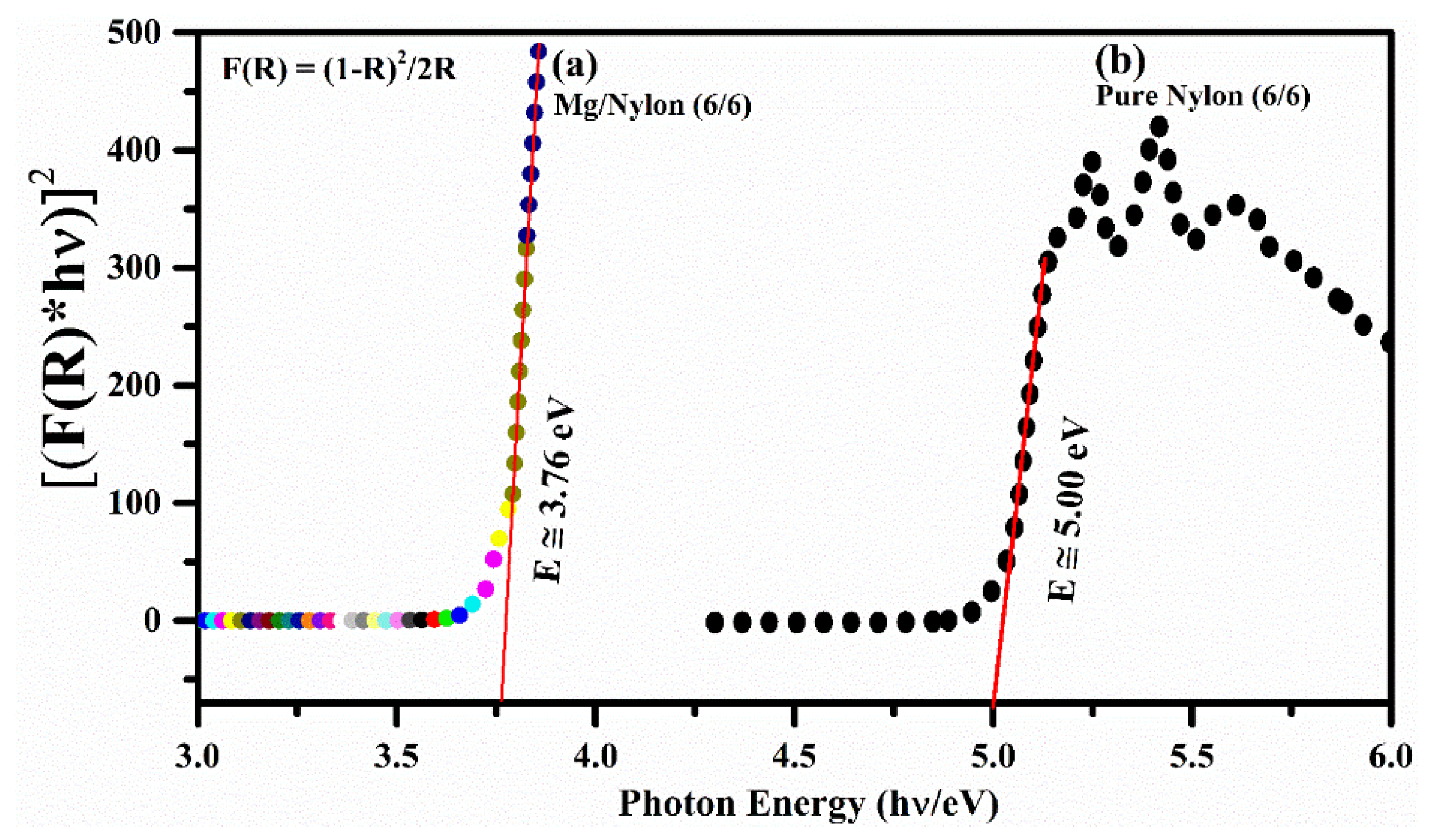
3.7. Diffuse Reflectance Spectroscopy (DRS)
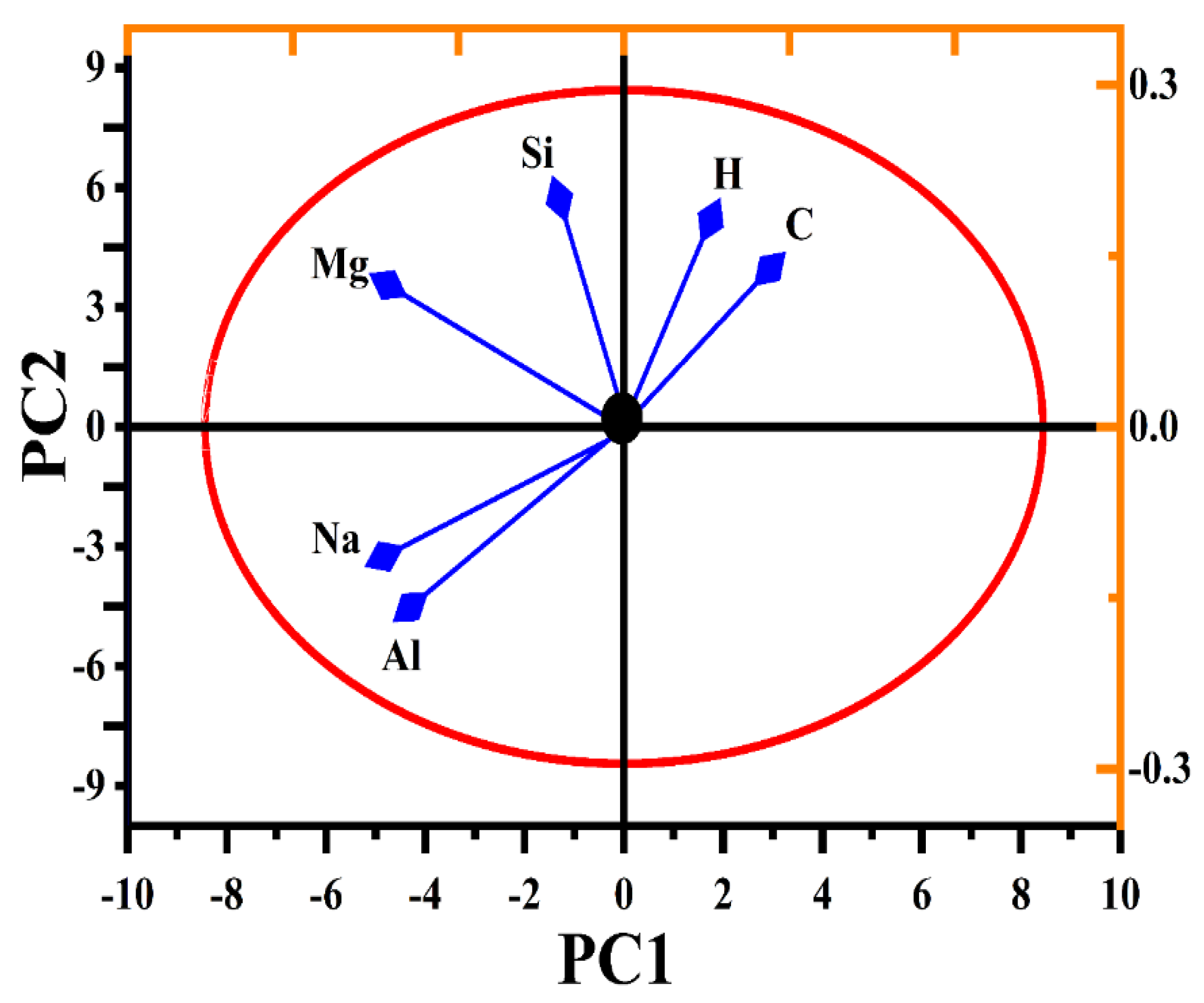
3.8. Prinicpal Component Analsysis
3.9. CF–LIBS Chemical Composition Analysis
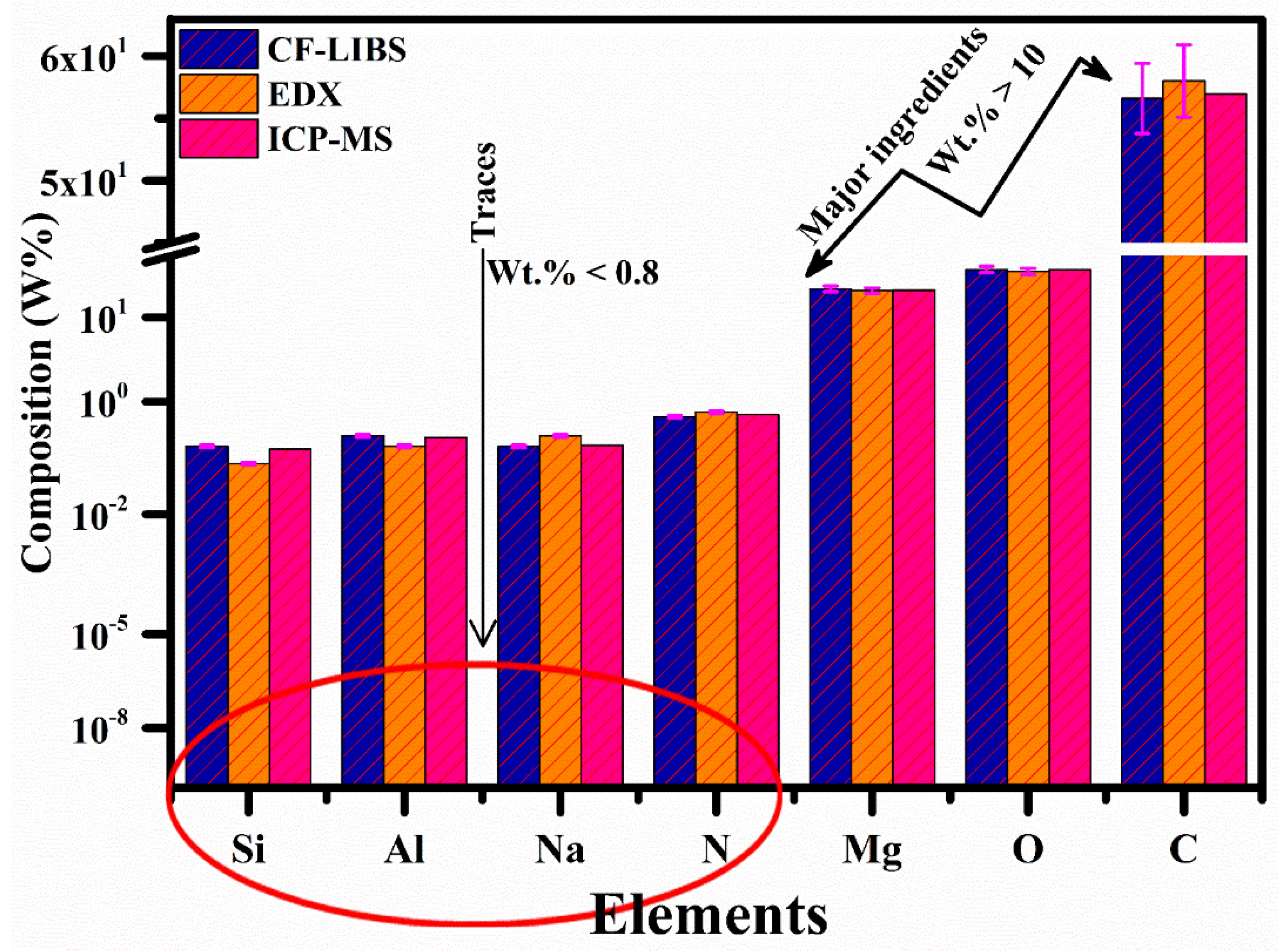
3.10. Comparative Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, Q.; Sirven, J.B.; Gabriel, J.C.P.; Tay, C.Y.; Lee, J.M. Laser induced breakdown spectroscopy for plastic analysis. TrAC Trends Anal. Chem. 2021, 140, 116280. [Google Scholar] [CrossRef]
- Fayyaz, A.; Liaqat, U.; Yaqoob, K.; Ahmed, R.; Umar, Z.A.; Baig, M.A. Combination of laser-induced breakdown spectroscopy, and time-of-flight mass spectrometry for the quantification of CoCrFeNiMo high entropy alloys. Spectrochim. Acta Part B At. Spectrosc. 2022, 198, 106562. [Google Scholar] [CrossRef]
- Sanghapi, H.K.; Jain, J.; Bol’shakov, A.; Lopano, C.; McIntyre, D.; Russo, R. Determination of elemental composition of shale rocks by laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2016, 122, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Luo, Y.; Zhang, Q. Laser-Induced Breakdown Spectroscopy-Based Coal-Rock Recognition: An in-Situ Sampling and Recognition Method. IEEE Access 2021, 9, 164732–164741. [Google Scholar] [CrossRef]
- Diaz, D.; Hahn, D.W.; Panne, U. LIBS for aerosol analysis. In Laser-Induced Breakdown Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 499–535. [Google Scholar]
- Mansoori, A.; Roshanzadeh, B.; Khalaji, M.; Tavassoli, S.H. Quantitative analysis of cement powder by laser induced breakdown spectroscopy. Opt. Lasers Eng. 2011, 49, 318–323. [Google Scholar] [CrossRef]
- Lang, A.; Engelberg, D.; Smith, N.T.; Trivedi, D.; Horsfall, O.; Banford, A.; Martin, P.A.; Coffey, P.; Bower, W.R.; Walther, C.; et al. Analysis of contaminated nuclear plant steel by laser-induced breakdown spectroscopy. J. Hazard. Mater. 2018, 345, 114–122. [Google Scholar] [CrossRef]
- Bhatt, B.; Angeyo, K.H.; Dehayem-Kamadjeu, A. LIBS development methodology for forensic nuclear materials analysis. Anal. Methods 2018, 10, 791–798. [Google Scholar] [CrossRef]
- De Lucia, F.C.; Harmon, R.S.; McNesby, K.L.; Winkel, R.J.; Miziolek, A.W. Laser-induced breakdown spectroscopy analysis of energetic materials. Appl. Opt. 2003, 42, 6148–6152. [Google Scholar] [CrossRef]
- Diaz, D.; Hahn, D.W.; Molina, A. Polymer identification using laser induced breakdown spectroscopy (LIBS). Dyna 2009, 76, 217–228. [Google Scholar]
- Alrebdi, T.A.; Fayyaz, A.; Ben Gouider Trabelsi, A.; Asghar, H.; Alkallas, F.H.; Alshehri, A.M. Vibrational Emission Study of the CN and C2 in Nylon and ZnO/Nylon Polymer Using Laser-Induced Breakdown Spectroscopy (LIBS). Polymers 2022, 14, 3686. [Google Scholar] [CrossRef]
- Harmon, R.S.; Lawley, C.J.; Watts, J.; Harraden, C.L.; Somers, A.M.; Hark, R.R. Laser-induced breakdown spectroscopy—An emerging analytical tool for mineral exploration. Minerals 2019, 9, 718. [Google Scholar] [CrossRef] [Green Version]
- Bellie, V.; Gokulraju, R.; Rajasekar, C.; Vinoth, S.; Mohankumar, V.; Gunapriya, B. Laser induced Breakdown Spectroscopy for new product development in mining industry. Mater. Today Proc. 2021, 45, 8157–8161. [Google Scholar] [CrossRef]
- Gaft, M. Laser-Induced Breakdown Spectroscopy (LIBS) for On-line Control in Mining Industry. In Applied Industrial Optics: Spectroscopy, Imaging and Metrology; Optica Publishing Group: Washington, DC, USA, 2011; p. AITuA2. [Google Scholar]
- McLaughlin, R.P.; Mason, G.S.; Miller, A.L.; Stipe, C.B.; Kearns, J.D.; Prier, M.W.; Rarick, J.D. Note: A portable laser induced breakdown spectroscopy instrument for rapid sampling and analysis of silicon-containing aerosols. Rev. Sci. Instrum. 2016, 87, 056103. [Google Scholar] [CrossRef] [Green Version]
- Rifai, K.; Özcan, L.; Doucet, F.; Vidal, F. Quantification of copper, nickel and other elements in copper-nickel ore samples using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2020, 165, 105766. [Google Scholar] [CrossRef]
- Gottfried, J.L.; Harmon, R.S.; La Pointe, A. Progress in LIBS for landmine detection. In Detection and Sensing of Mines, Explosive Objects, and Obscured Targets XIV; SPIE: Bellingham, WA, USA, 2009; Volume 7303, pp. 442–452. [Google Scholar]
- Kim, E.; Choi, W.Z. Real-time identification of plastics by types using laser-induced breakdown spectroscopy. J. Mater. Cycles Waste Manag. 2019, 21, 176–180. [Google Scholar] [CrossRef]
- Vahid Dastjerdi, M.; Mousavi, S.J.; Soltanolkotabi, M.; Nezarati Zadeh, A. Identification and sorting of PVC polymer in recycling process by laser-induced breakdown spectroscopy (LIBS) combined with support vector machine (SVM) model. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 959–965. [Google Scholar] [CrossRef]
- Huber, N.; Eschlböck-Fuchs, S.; Scherndl, H.; Freimund, A.; Heitz, J.; Pedarnig, J.D. In-line measurements of chlorine containing polymers in an industrial waste sorting plant by laser-induced breakdown spectroscopy. Appl. Surf. Sci. 2014, 302, 280–285. [Google Scholar] [CrossRef]
- Aquino, F.W.; Paranhos, C.M.; Pereira-Filho, E.R. Method for the production of acrylonitrile-butadiene-styrene (ABS) and polycarbonate (PC)/ABS standards for direct Sb determination in plastics from e-waste using laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2016, 31, 1228–1233. [Google Scholar] [CrossRef]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part II: Review of instrumental and methodological approaches to material analysis and applications to different fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nair, L.S.; Bhattacharyya, S.; Laurencin, C.T. Polymeric nanofibers as novel carriers for the delivery of therapeutic molecules. J. Nanosci. Nanotechnol. 2006, 6, 2591–2607. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar] [CrossRef] [Green Version]
- Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Sambudi, N.S.; Mohd Yusoff, A.R. Improving performance of electrospun nylon 6, 6 nanofiber membrane for produced water filtration via solvent vapor treatment. Polymers 2019, 11, 2117. [Google Scholar] [CrossRef] [Green Version]
- Senthilvelan, S.; Gnanamoorthy, R. Damping characteristics of unreinforced, glass and carbon fiber reinforced nylon 6/6 spur gears. Polym. Test. 2006, 25, 56–62. [Google Scholar] [CrossRef]
- Pistiki, A.; Ryabchykov, O.; Bocklitz, T.W.; Rösch, P.; Popp, J. Use of polymers as wavenumber calibration standards in deep-UVRR. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122062. [Google Scholar] [CrossRef] [PubMed]
- Guirado-Moreno, J.C.; González-Ceballos, L.; Carreira-Barral, I.; Ibeas, S.; Fernández-Muiño, M.A.; Sancho, M.T.; García, J.M.; Vallejos, S. Smart sensory polymer for straightforward Zn(II) detection in pet food samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 284, 121820. [Google Scholar] [CrossRef]
- Boueri, M.; Motto-Ros, V.; Lei, W.Q.; Ma, Q.L.; Zheng, L.J.; Zeng, H.P.; Yu, J. Identification of polymer materials using laser-induced breakdown spectroscopy combined with artificial neural networks. Appl. Spectrosc. 2011, 65, 307–314. [Google Scholar] [CrossRef]
- Farooq, W.A.; Al-Johani, A.S.; Alsalhi, M.S.; Tawfik, W.; Qindeel, R. Analysis of polystyrene and polycarbonate used in manufacturing of water and food containers using laser induced breakdown spectroscopy. J. Mol. Struct. 2020, 1201, 127152. [Google Scholar] [CrossRef]
- Trautner, S.; Jasik, J.; Parigger, C.G.; Pedarnig, J.D.; Spendelhofer, W.; Lackner, J.; Veis, P.; Heitz, J. Laser-induced optical breakdown spectroscopy of polymer materials based on evaluation of molecular emission bands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 174, 331–338. [Google Scholar] [CrossRef]
- Junjuri, R.; Zhang, C.; Barman, I.; Gundawar, M.K. Identification of post-consumer plastics using laser-induced breakdown spectroscopy. Polym. Test. 2019, 76, 101–108. [Google Scholar] [CrossRef]
- Costa, V.C.; Aquino, F.W.B.; Paranhos, C.M.; Pereira-Filho, E.R. Identification and classification of polymer e-waste using laser-induced breakdown spectroscopy (LIBS) and chemometric tools. Polym. Test. 2017, 59, 390–395. [Google Scholar] [CrossRef]
- Banaee, M.; Tavassoli, S.H. Discrimination of polymers by laser induced breakdown spectroscopy together with the DFA method. Polym. Test. 2012, 31, 759–764. [Google Scholar] [CrossRef]
- Sommer, C.; Nguyen, J.; Menzel, T.; Prume, J.A.; Ruckdäschel, H.; Koch, M. Weathering-induced oxidation: An investigation of artificially aged polystyrene samples using Laser-induced Breakdown Spectroscopy. Polym. Test. 2022, 112, 107623. [Google Scholar] [CrossRef]
- Nie, J.; Wen, X.; Niu, X.; Chu, Y.; Chen, F.; Wang, W.; Zhang, D.; Hu, Z.; Xiao, J.; Guo, L. Identification of different colored plastics by laser-induced breakdown spectroscopy combined with neighborhood component analysis and support vector machine. Polym. Test. 2022, 112, 107624. [Google Scholar] [CrossRef]
- Hanif, I.M.; Syuhaili, M.N.; Hasmori, M.F.; Shahmi, S.M. Effect of nylon fiber on mechanical properties of cement based mortar. In Proceedings of the Global Congress on Construction, Material and Structural Engineering 2017 (GCoMSE2017), Johor Bahru, Malaysia, 28–29 August 2017; IOP Publishing: Bristol, UK, 2017; Volume 271, p. 012080. [Google Scholar]
- Yanilmaz, M.; Dirican, M.; Zhang, X. Evaluation of electrospun SiO2/nylon 6, 6 nanofiber membranes as a thermally-stable separator for lithium-ion batteries. Electrochim. Acta 2014, 133, 501–508. [Google Scholar] [CrossRef]
- NIST. NIST Standard Reference Database 78; NIST: Gaithersburg, MD, USA, 1995.
- Zhang, S.; Wang, X.; He, M.; Jiang, Y.; Zhang, B.; Hang, W.; Huang, B. Laser-induced plasma temperature. Spectrochim. Acta Part B At. Spectrosc. 2014, 97, 13–33. [Google Scholar] [CrossRef]
- McWhirter, R.W.P. Plasma Diagnostic Techniques; Library of Congress Catalog Card Number 65-22763; Huddlestone, R.H., Leonard, L.S., Eds.; Academic Press: Cambridge, MA, USA, 1965. [Google Scholar]
- Konjević, N.; Ivković, M.; Sakan, N. Hydrogen Balmer lines for low electron number density plasma diagnostics. Spectrochim. Acta Part B At. Spectrosc. 2012, 76, 16–26. [Google Scholar] [CrossRef]
- Sun, L.; Yu, H. Correction of self-absorption effect in calibration-free laser-induced breakdown spectroscopy by an internal reference method. Talanta 2009, 79, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson Education Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Alrebdi, T.A.; Fayyaz, A.; Asghar, H.; Kamal, A.; Iqbal, J.; Piracha, N.K. Chemometrics and Spectroscopic Analyses of Peganum harmala Plant’s Seeds by Laser-Induced Breakdown Spectroscopy. Appl. Sci. 2023, 13, 2780. [Google Scholar] [CrossRef]
- Umar, Z.A.; Ahmed, R.; Asghar, H.; Liaqat, U.; Fayyaz, A.; Baig, M.A. VO2 thin film based highly responsive and fast VIS/IR photodetector. Mater. Chem. Phys. 2022, 290, 126655. [Google Scholar] [CrossRef]
- Fayyaz, A.; Asghar, H.; Alshehri, A.M.; Alrebdi, T.A. LIBS assisted PCA analysis of multiple rare-earth elements (La, Ce, Nd, Sm, and Yb) in phosphorite deposits. Heliyon 2023, 9, e13957. [Google Scholar] [CrossRef]
- Beattie, J.R.; Esmonde-White, F.W. Exploration of principal component analysis: Deriving principal component analysis visually using spectra. Appl. Spectrosc. 2021, 75, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, A.; Corsi, M.; Palleschi, V.; Rastelli, S.; Salvetti, A.; Tognoni, E. New procedure for quantitative elemental analysis by laser-induced plasma spectroscopy. Appl. Spectrosc. 1999, 53, 960–964. [Google Scholar] [CrossRef]



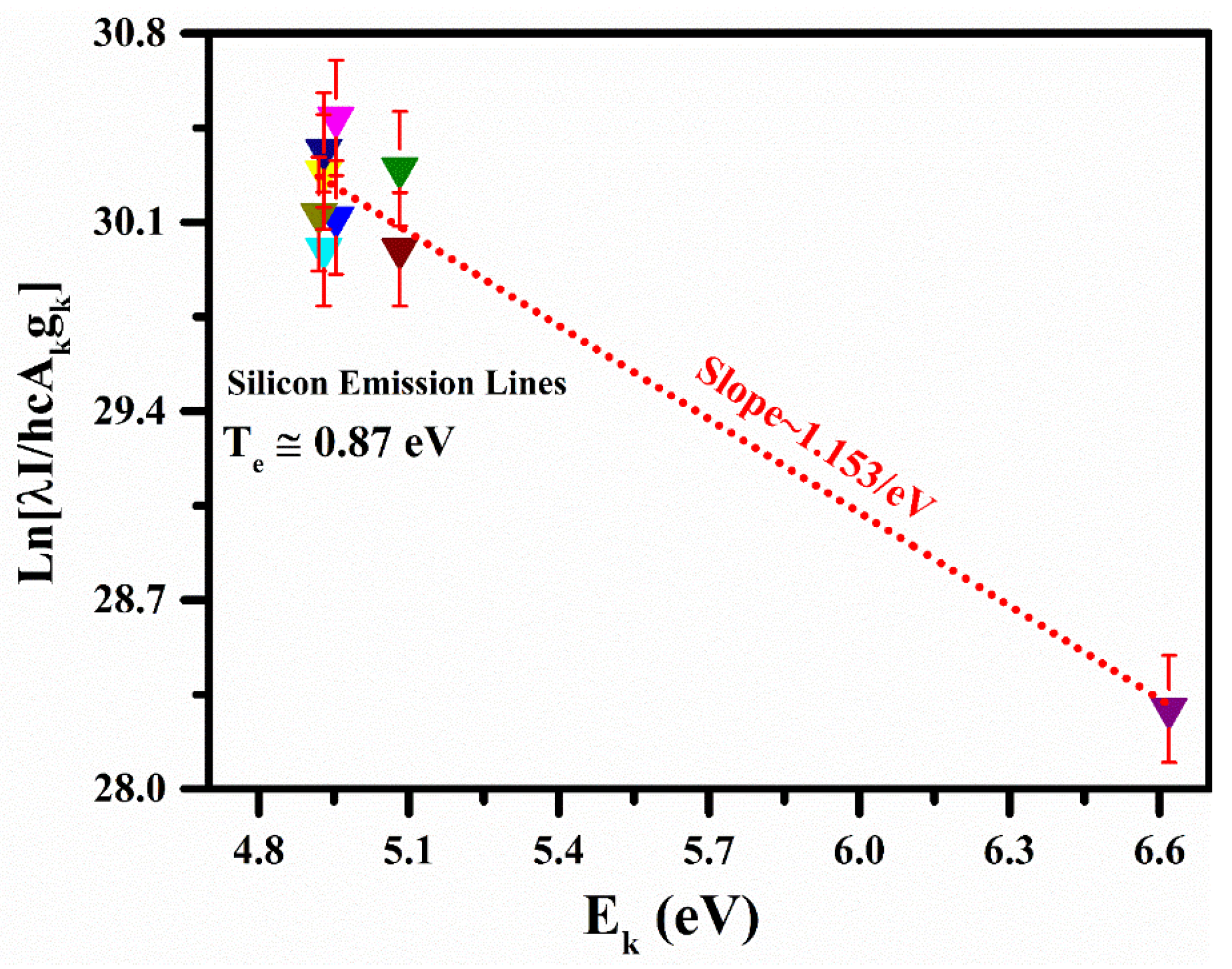



| Element | Wavelength (nm) | Ak (s−1) | gk | Ek (eV) |
|---|---|---|---|---|
| Silicon (I) | 250.69 | 5.47 × 107 | 5 | 4.95 |
| 251.43 | 7.39 × 107 | 3 | 4.93 | |
| 251.61 | 1.68 × 108 | 5 | 4.95 | |
| 251.92 | 5.49 × 107 | 3 | 4.93 | |
| 252.41 | 2.22 × 108 | 1 | 4.92 | |
| 252.85 | 9.04 × 107 | 3 | 4.93 | |
| 263.13 | 1.06 × 108 | 3 | 6.62 | |
| 288.16 | 2.17 × 108 | 3 | 5.08 | |
| 390.55 | 1.33 × 107 | 3 | 5.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayyaz, A.; Asghar, H.; Waqas, M.; Kamal, A.; Al-Onazi, W.A.; Al-Mohaimeed, A.M. Multi-Spectroscopic Characterization of MgO/Nylon (6/6) Polymer: Evaluating the Potential of LIBS and Statistical Methods. Polymers 2023, 15, 3156. https://doi.org/10.3390/polym15153156
Fayyaz A, Asghar H, Waqas M, Kamal A, Al-Onazi WA, Al-Mohaimeed AM. Multi-Spectroscopic Characterization of MgO/Nylon (6/6) Polymer: Evaluating the Potential of LIBS and Statistical Methods. Polymers. 2023; 15(15):3156. https://doi.org/10.3390/polym15153156
Chicago/Turabian StyleFayyaz, Amir, Haroon Asghar, Muhammad Waqas, Asif Kamal, Wedad A. Al-Onazi, and Amal M. Al-Mohaimeed. 2023. "Multi-Spectroscopic Characterization of MgO/Nylon (6/6) Polymer: Evaluating the Potential of LIBS and Statistical Methods" Polymers 15, no. 15: 3156. https://doi.org/10.3390/polym15153156
APA StyleFayyaz, A., Asghar, H., Waqas, M., Kamal, A., Al-Onazi, W. A., & Al-Mohaimeed, A. M. (2023). Multi-Spectroscopic Characterization of MgO/Nylon (6/6) Polymer: Evaluating the Potential of LIBS and Statistical Methods. Polymers, 15(15), 3156. https://doi.org/10.3390/polym15153156







