Extraction, Isolation, Screening, and Preliminary Characterization of Polysaccharides with Anti–Oxidant Activities from Oudemansiella raphanipies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Crude ORPs
2.3. Experimental Design of Animals
2.4. Experimental Design of RSM
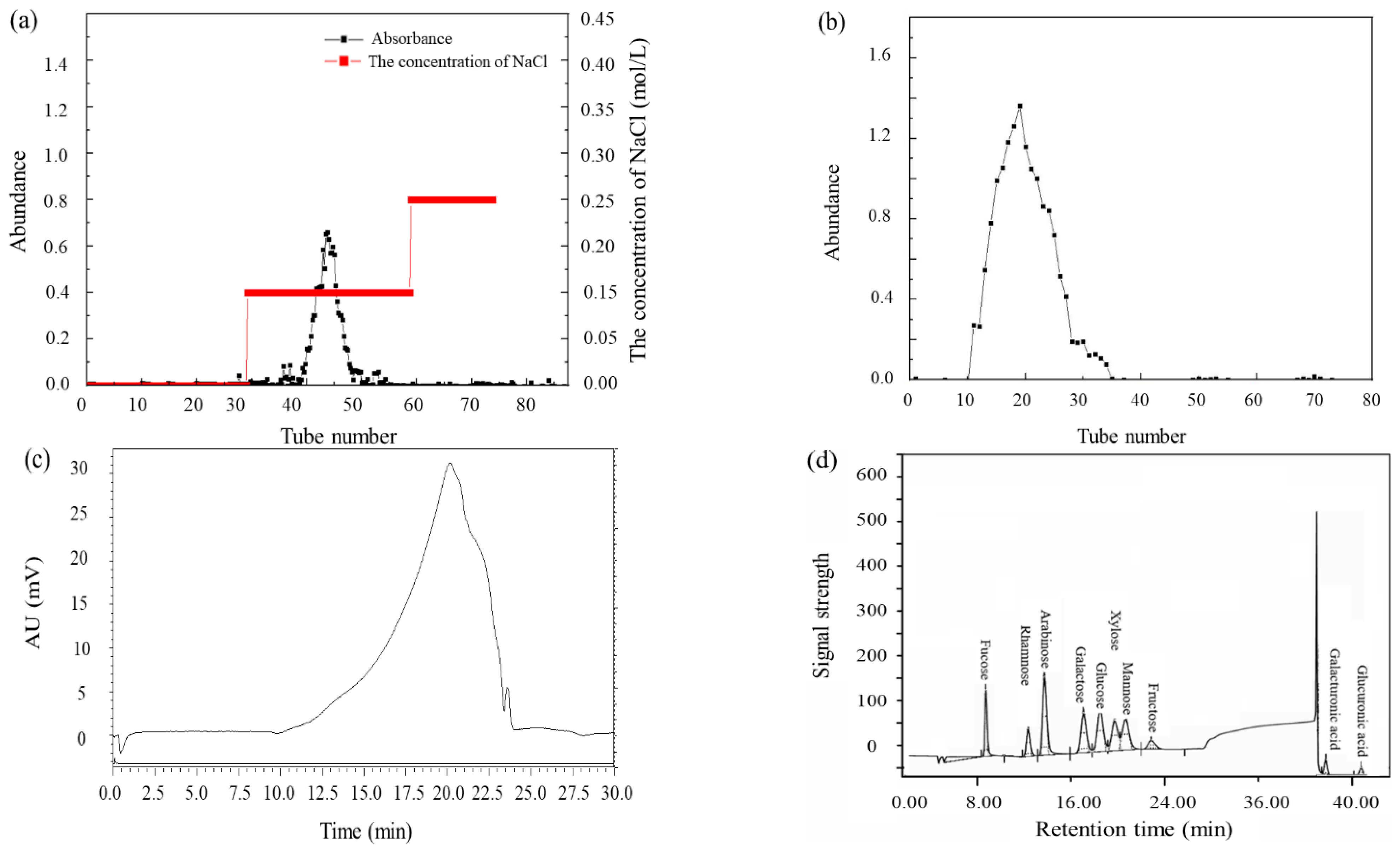
2.5. Purification of ORPs
2.6. Characterization of ORPs
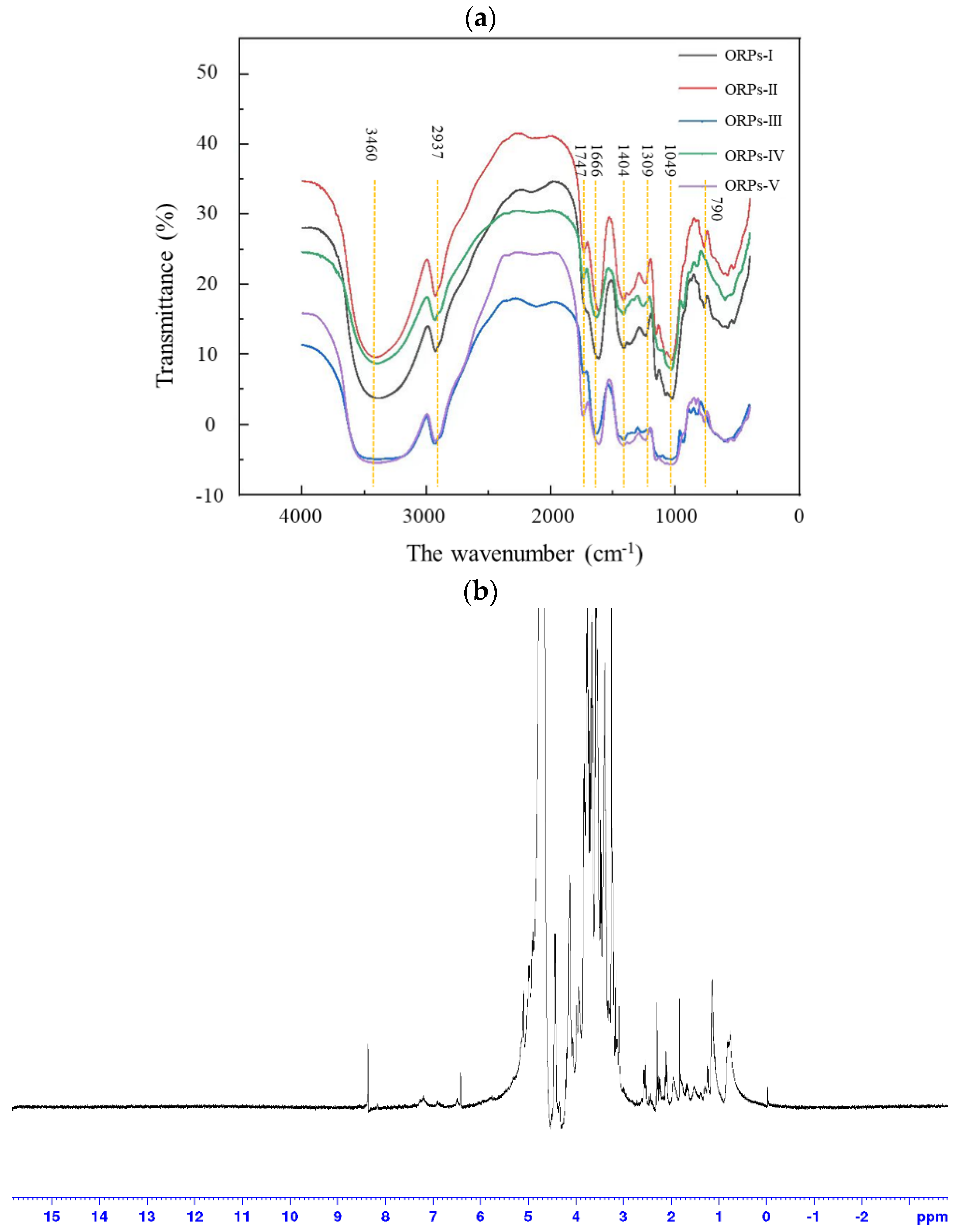
2.6.1. Fourier Transform Infrared (FT–IR) Analysis
2.6.2. Determination of Monosaccharide Composition
2.6.3. Determination of Molecular Weight
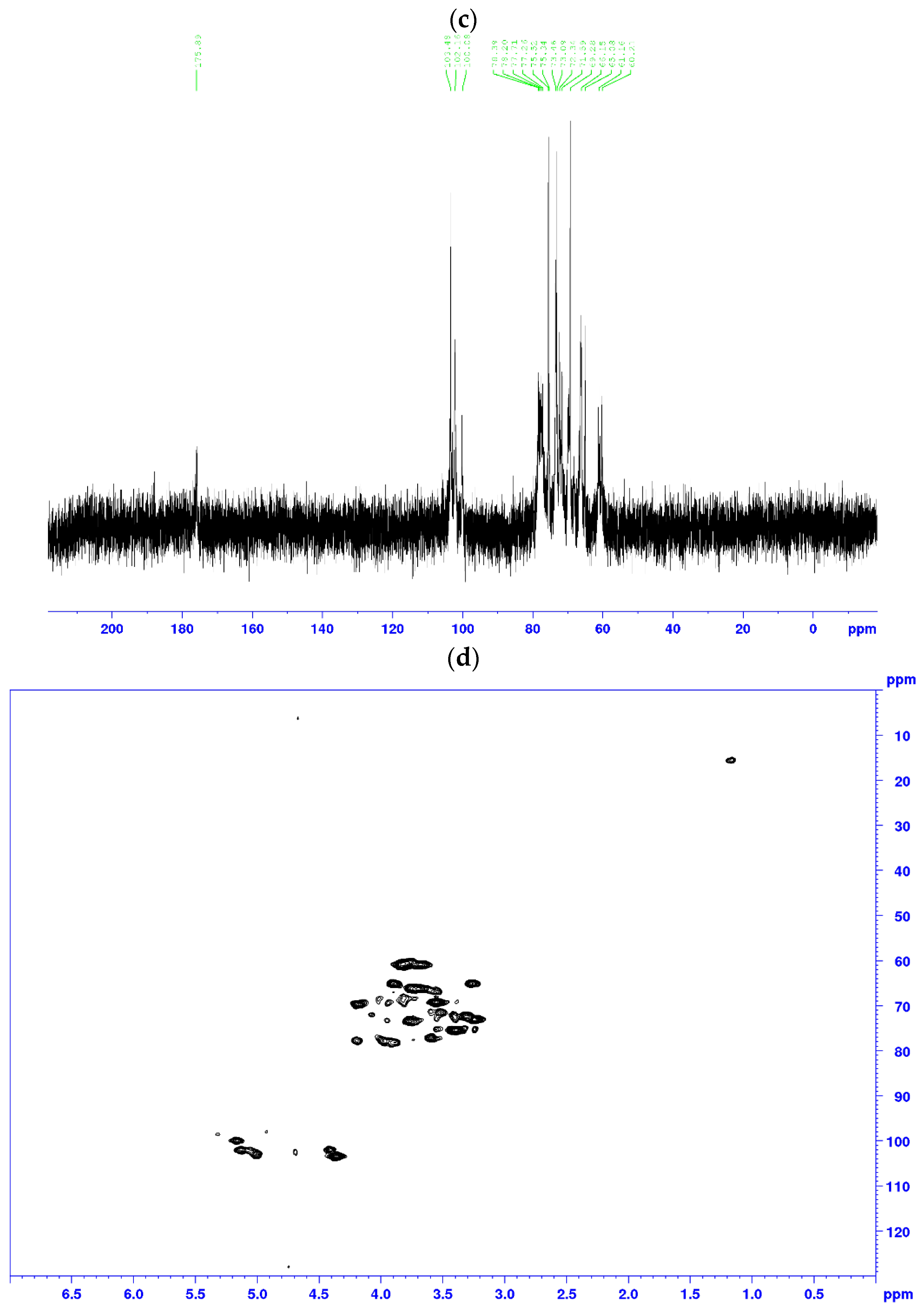
2.6.4. Nuclear Magnetic Resonance (NMR) Analysis
2.7. Anti–Oxidant Activities of ORPs
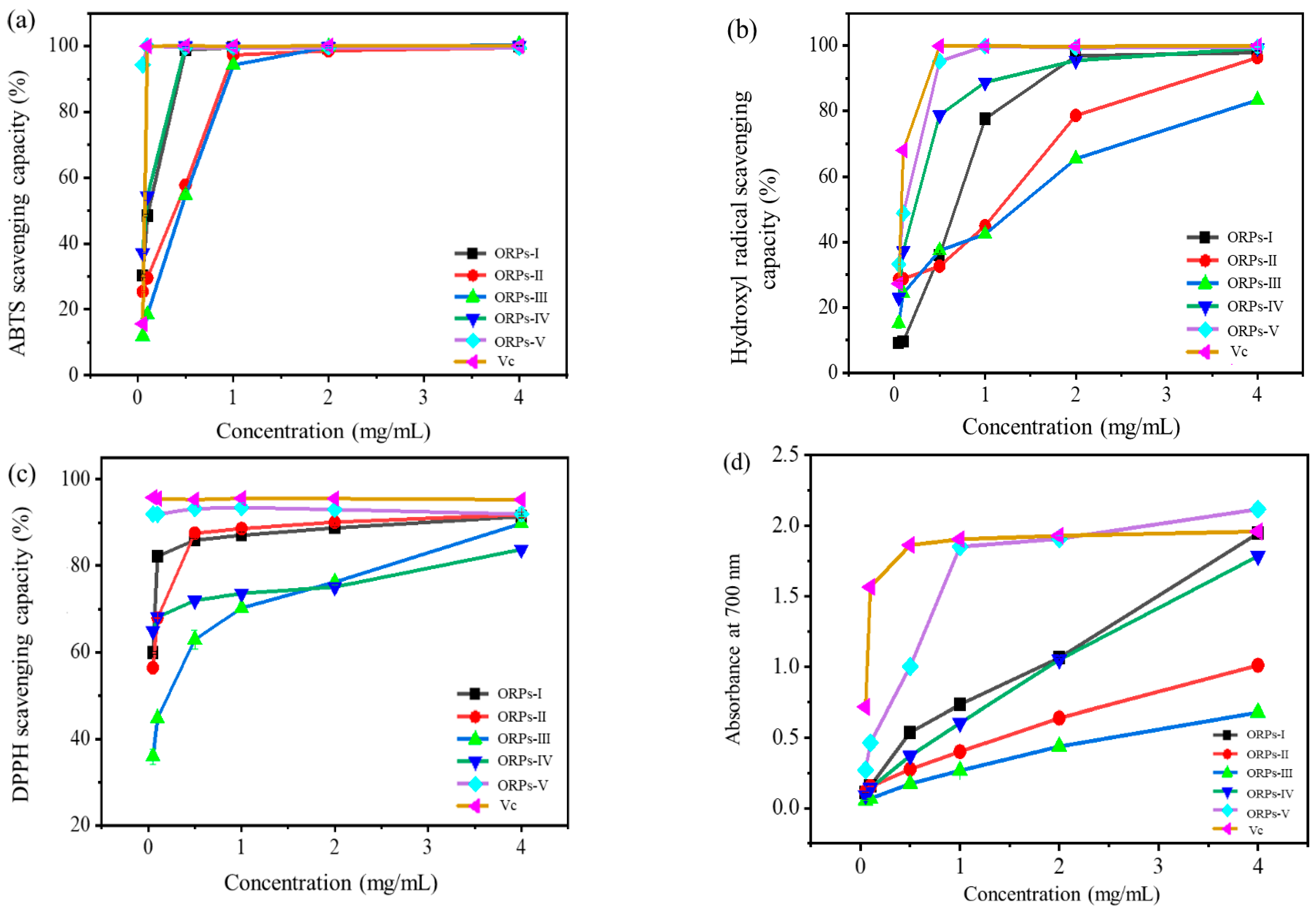
2.7.1. Anti–Oxidant Activity Assays In Vitro
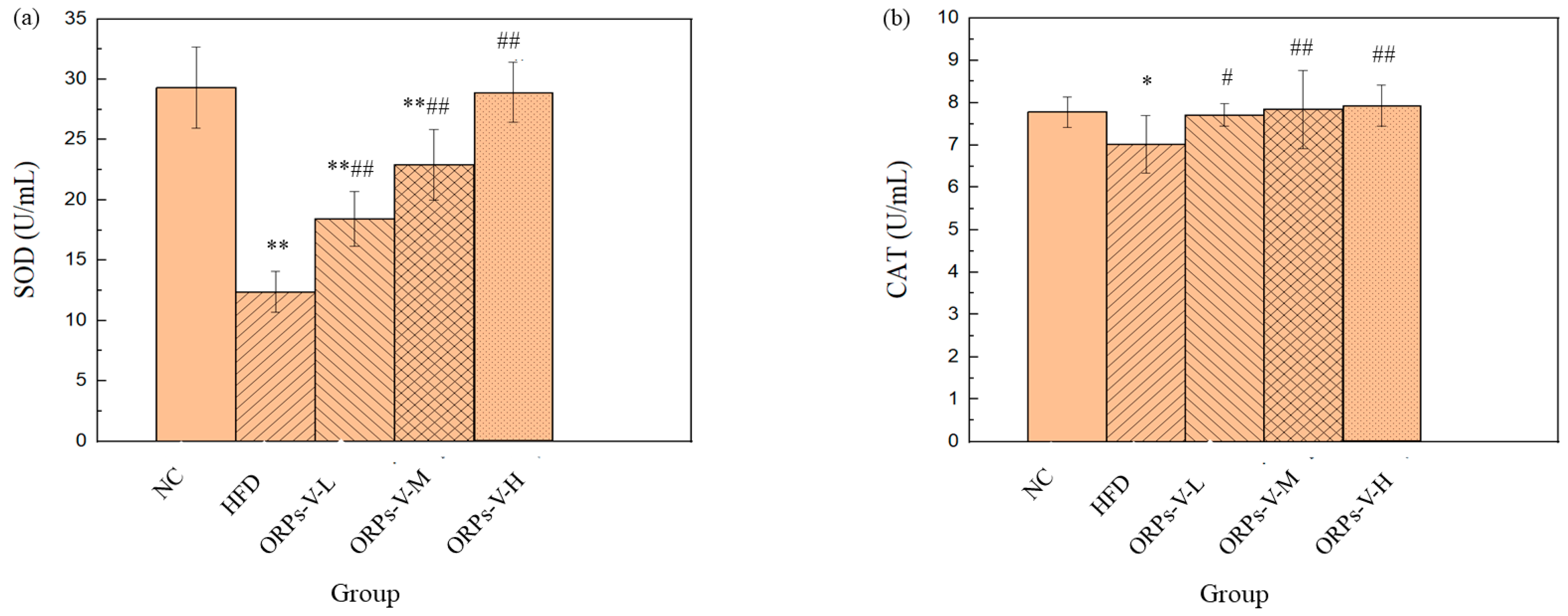
2.7.2. Anti–Oxidant Activity Assays In Vivo
2.8. Statistical Analysis
3. Results and Discussion
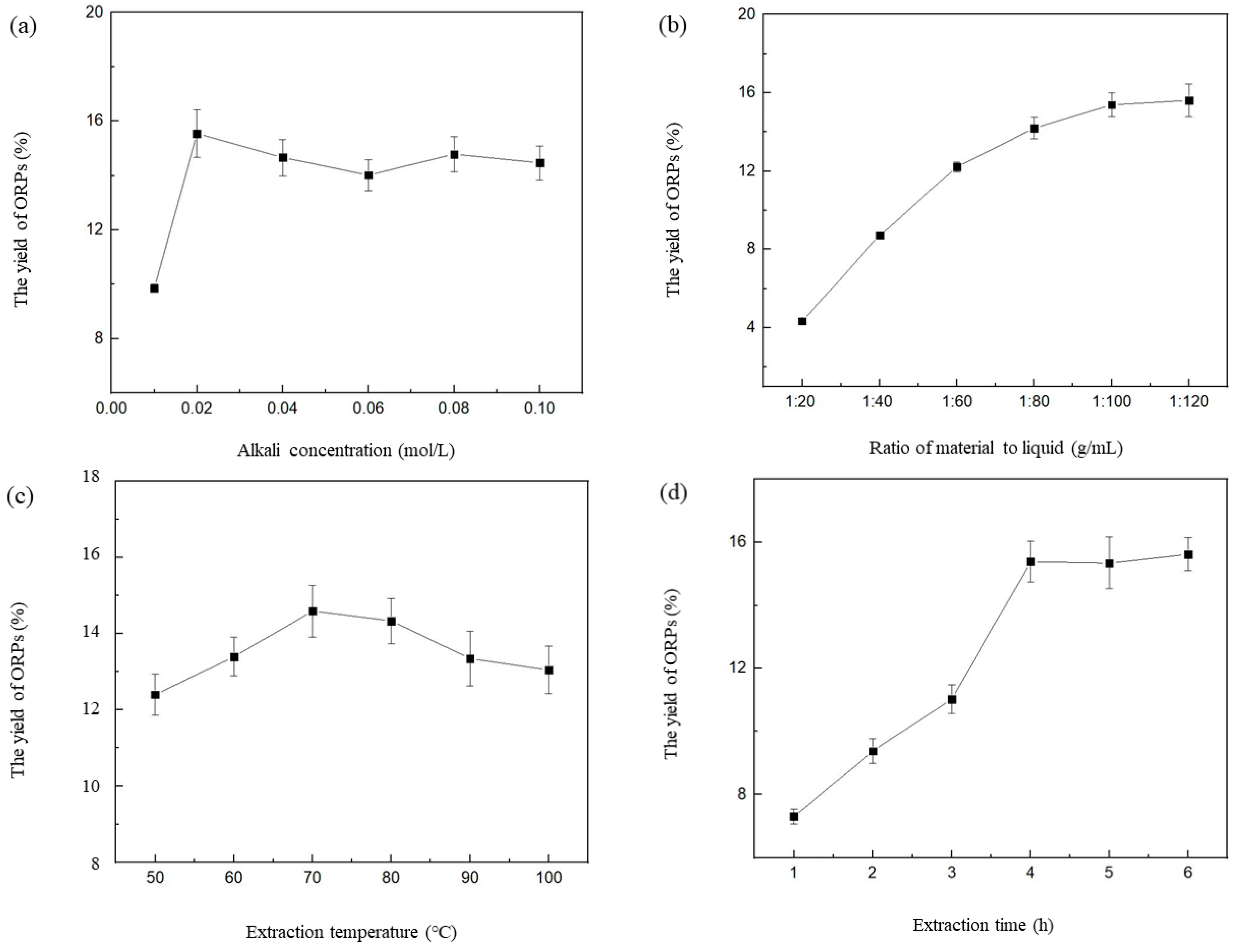
3.1. Influence of Different Extraction Parameters on the Extraction Rate of ORPs
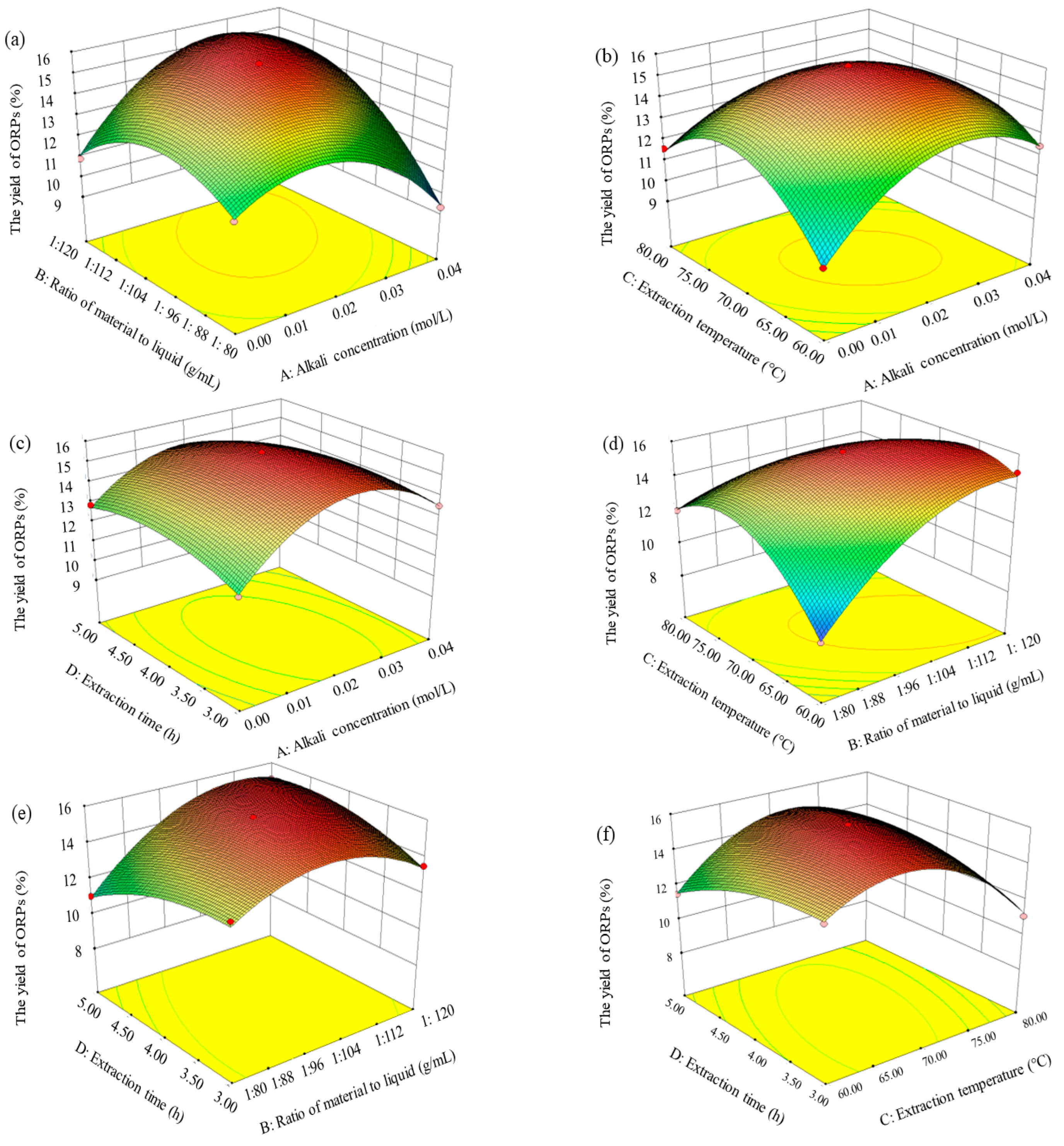
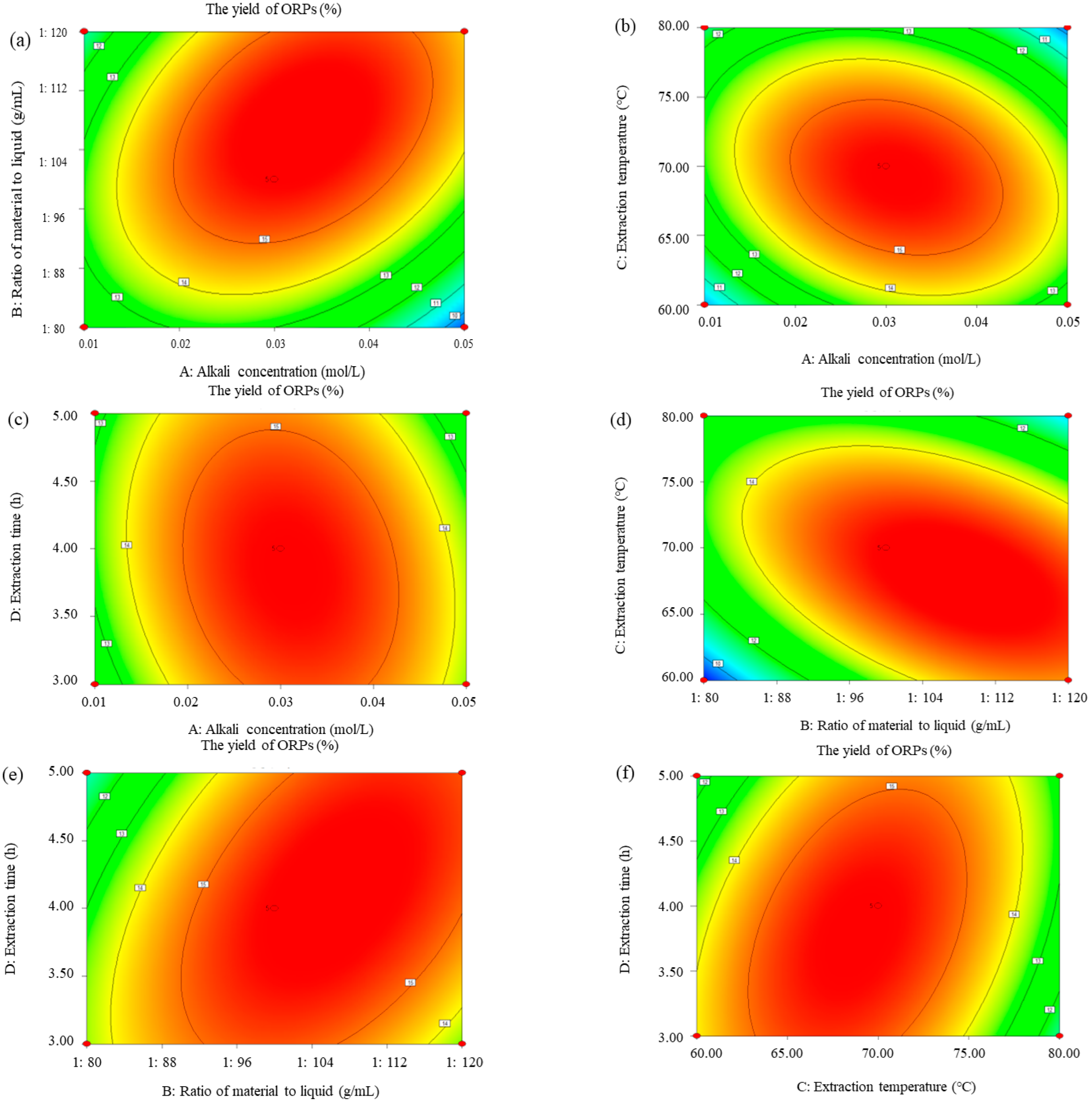
3.2. Optimization of Extraction Parameters of ORPs
3.2.1. Model Fitting and Adequacy Checking
3.2.2. Response Surface Analysis
3.2.3. Model Verification
3.3. Preparation and Physiochemical Characteristics
3.4. FT–IR Spectrometry
3.5. Monosaccharide Composition
3.6. NMR Analysis
3.7. Anti–Oxidant Activities In Vitro
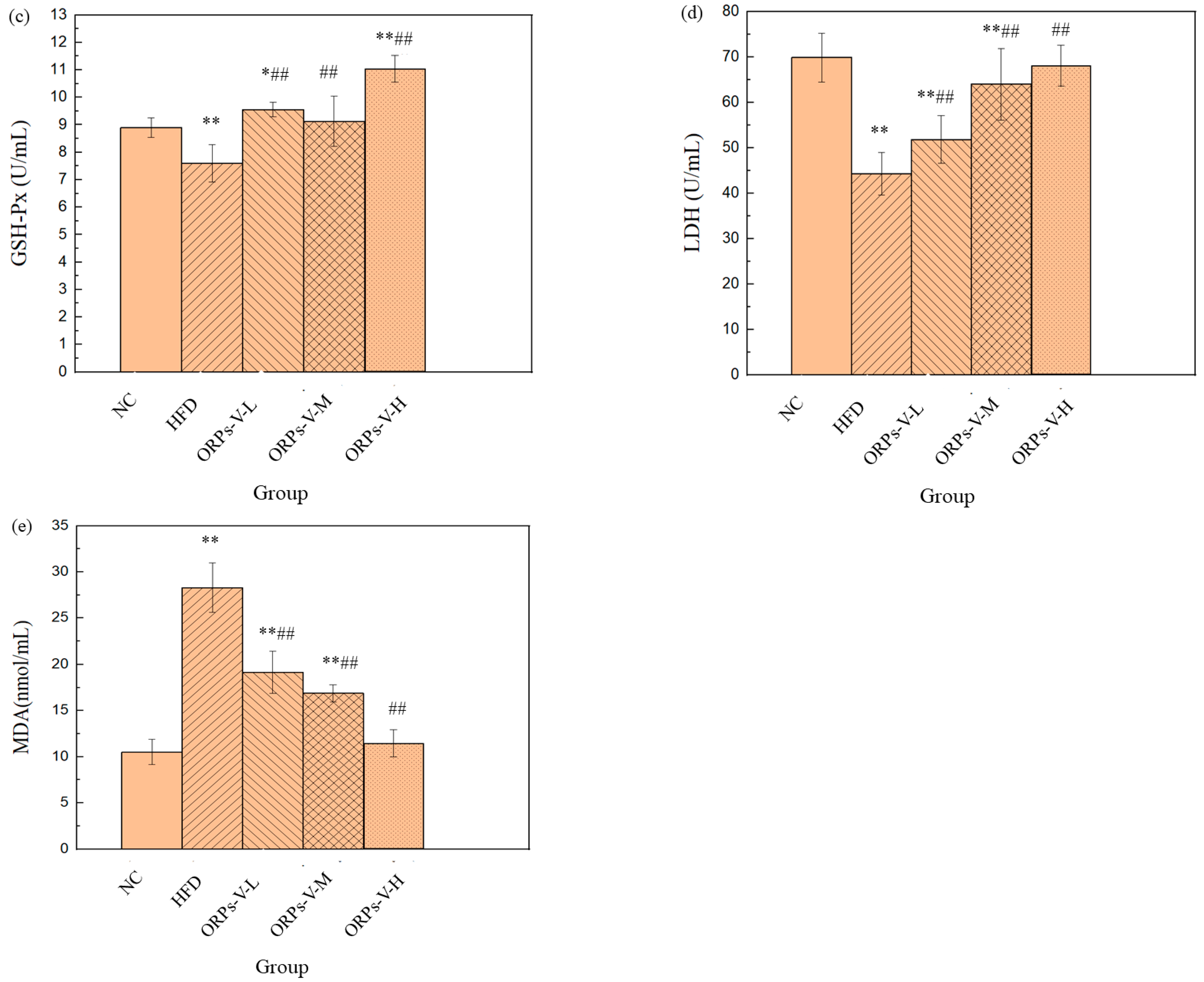
3.8. Anti–Oxidant Activities In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, R.R.; Wang, Z.C.; Xu, H.R.; Hou, Z.S.; Li, Y.T.; Wang, Y.F.; Feng, Y.; Zhang, X.; Xin, G. Cutting root treatment combined with low–temperature storage regimes on non-volatile and volatile compounds of Oudemansiella raphanipes. LWT–Food Sci. Technol. 2022, 166, 113754. [Google Scholar] [CrossRef]
- Jiang, J.; Qin, C.X.; Shu, X.Q.; Chen, R.; Song, H.H.; Li, Q.; Xu, H. Effects of copper on induction of thiol–compounds and antioxidant enzymes by the fruiting body of Oudemansiella radicata. Ecotoxicol. Environ. Saf. 2015, 111, 60–65. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, M.J.; Geng, X.R.; Wang, H.X.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the edible mushroom Oudemansiella radicata. Molecules 2017, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xu, W.; Wang, H.X.; Huang, F.; Wang, L.M. Natural polysaccharides experience physiochemical and functional changes during preparation: A review. Carbohydr. Polym. 2020, 234, 11596. [Google Scholar] [CrossRef] [PubMed]
- Wusigale, L.; Liang, L.; Luo, Y.C. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Jiang, H.T.; Wang, R.L.; Zhou, F.; Wu, Y.L.; Li, S.J.; Huo, G.M.; Ye, J.C.; Hua, C.; Wang, Z.J. Preparation, physicochemical characterization, and cytotoxicity of selenium nanoparticles stabilized by Oudemansiella raphanipies polysaccharide. Int. J. Biol. Macromol. 2022, 211, 35–46. [Google Scholar] [CrossRef]
- Liu, Y.T.; Li, Y.W.; Ke, Y.; Li, C.; Zhang, Z.Q.; Wu, Y.L.; Hu, B.; Liu, A.P.; Luo, Q.Y.; Wu, W.J. In vitro saliva–gastrointestinal digestion and fecal fermentation of Oudemansiella radicata polysaccharides reveal its digestion profile and effect on the modulation of the gut microbiota. Carbohyd. Polym. 2021, 251, 117041. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, W.L.; Hu, S.J.; Kang, Y.C.; Zhang, Y.T.; Ng, T.B. Effects of Oudemansiella radicata polysaccharide on postharvest quality of oyster mushroom (Pleurotus ostreatus) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Technol. 2020, 166, 111207. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, C.; Tian, C.Y.; Ren, Z.Z.; Song, X.L.; Wang, X.X.; Xu, N.; Jing, H.J.; Li, S.S.; Liu, W.R.; et al. Characterization, antioxidation, anti–inflammation and renoprotection effects of selenized mycelia polysaccharides from Oudemansiella radicata. Carbohyd. Polym. 2018, 181, 1224–1234. [Google Scholar] [CrossRef]
- Gao, Z.; Li, J.; Song, X.L.; Zhang, J.J.; Wang, X.X.; Jing, H.J.; Ren, Z.Z.; Li, S.S.; Zhang, C.; Jia, L. Antioxidative, anti–inflammation and lung-protective effects of mycelia selenium polysaccharides from Oudemansiella radicata. Int. J. Biol. Macromol. 2017, 104 Pt A, 1158–1164. [Google Scholar] [CrossRef]
- Sun, Y.J.; Hou, S.T.; Song, S.; Zhang, B.; Ai, C.Q.; Chen, X.F.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Zhang, B.G.; Lu, C.; Xu, Z.C.; Guo, H.; Zhang, G.K.; Hao, Y.Q. Curculigo orchioides polysaccharides extraction, characterization, and their protective effects against femoral head necrosis. Arab. J. Chem. 2022, 15, 103414. [Google Scholar] [CrossRef]
- Kou, R.B.; Zuo, G.L.; Liu, J.F.; Di, D.L.; Guo, M. Structural properties and hypoglycaemic activity of polysaccharides extracted from the fruits of Lycium barbarum L. using various extraction media. Ind. Crop. Prod. 2022, 188 Pt B, 115725. [Google Scholar] [CrossRef]
- Zheng, Q.W.; Jia, R.B.; Luo, D.H.; Lin, L.Z.; Chen, C.; Zhao, M.M. The effect of extraction solution pH level on the physicochemical properties and α-glucosidase inhibitory potential of Fucus vesiculosus polysaccharide. LWT–Food Sci. Technol. 2022, 169, 114028. [Google Scholar] [CrossRef]
- Shi, X.D.; Feng, J.W.; Wang, S.Y.; Huang, J.J.; Yu, M.L. Primary structure, physicochemical properties, and digestive properties of four sequentially extracted polysaccharides from Tremella fuciformis. J. Food Compos. Anal. 2023, 115, 105005. [Google Scholar] [CrossRef]
- Luo, D.H.; Wang, Z.J. Study on extraction optimization, structure features, and bioactivities of an Oudemansiella raphanipies polysaccharide. Process Biochem. 2023, 126, 136–146. [Google Scholar] [CrossRef]
- Jiang, H.T.; Zhu, H.; Huo, G.M.; Li, S.J.; Wu, Y.L.; Zhou, F.; Hua, C.; Hu, Q.H. Oudemansiella raphanipies polysaccharides improve lipid metabolism disorders in murine high-fat diet-induced non-alcoholic fatty liver disease. Nutrients 2022, 14, 4092. [Google Scholar] [CrossRef]
- Pan, W.J.; Shi, L.L.; Ren, Y.R.; Yao, C.Y.; Lu, Y.M.; Chen, Y. Polysaccharide ORP-1 isolated from Oudemansiella raphanipes ameliorates age-associated intestinal epithelial barrier dysfunction in Caco-2 cells monolayer. Food Res. Int. 2022, 162 Pt A, 112038. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wang, Z.M.; Hu, D.; Xiao, K.J.; Wu, J.Y. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018, 79, 189–196. [Google Scholar] [CrossRef]
- Hu, Q.B.; Bae, M.; Fleming, E.; Lee, J.Y.; Luo, Y.C. Biocompatible polymeric nanoparticles with exceptional gastrointestinal stability as oral delivery vehicles for lipophilic bioactives. Food Hydrocoll. 2019, 89, 386–395. [Google Scholar] [CrossRef]
- Feng, L.; Yin, J.Y.; Nie, S.P.; Wan, Y.Q.; Xie, M.Y. Fractionation, physicochemical property and immunological activity of polysaccharides from Cassia obtusifolia. Int. J. Biol. Macromol. 2015, 91, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.K.; Xu, J.Q.; Zhang, J.L.; Wei, Y.N.; Cai, X.; Tan, J.Q. Modeling, optimization, purification, and characterization of polysaccharides from Lilium lancifolium Thunb. LWT-Food Sci. Technol. 2022, 162, 113491. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, P.Z.; Shen, J.W.; Qian, Y.Y.; Liu, M.; Ruan, Y.; Wang, X.G.; Zhang, S.N.; Ma, B.J. Physicochemical properties and bioactivities of original and Se-enriched polysaccharides with different molecular weights extracted from Pleurotus ostreatus. Int. J. Biol. Macromol. 2019, 141, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Jia, J.X.; Ren, X.J.; Li, B.H.; Zhang, Q.; Jia, L. Extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from Oudemansiella radicata mushroom. Int. J. Biol. Macromol. 2018, 120, 1760–1769. [Google Scholar] [CrossRef]
- Chen, S.H.; Huang, H.L.; Huang, G.L. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int. J. Biol. Macromol. 2019, 140, 1047–1053. [Google Scholar] [CrossRef]
- Zeng, L.; Zhong, F.W.; Chen, Z.L.; Li, G.X.; Zhu, Q. Polygonatum sibiricum polysaccharides protect against obesity and non–alcoholic fatty liver disease in rats fed a high-fat diet. Food Sci. Hum. Wellness 2022, 11, 1045–1052. [Google Scholar] [CrossRef]
- Zhou, L.J.; Luo, S.Y.; Li, J.J.; Zhou, Y.L.; Wang, X.J.; Kong, Q.B.; Chen, T.; Feng, S.L.; Yuan, M.; Ding, C.B. Optimization of the extraction of polysaccharides from the shells of Camellia oleifera and evaluation on the antioxidant potential in vitro and in vivo. J. Funct. Foods 2021, 86, 104678. [Google Scholar] [CrossRef]
- Hu, W.C.; Jiang, Y.Y.; Xue, Q.; Sun, F.T.; Zhang, J.M.; Zhou, J.; Niu, Z.Q.; Li, Q.T.; Li, F.; Shen, T. Structural characterisation and immunomodulatory activity of a polysaccharide isolated from lotus (Nelumbo nucifera Gaertn.) root residues. J. Funct. Foods 2019, 60, 103457. [Google Scholar] [CrossRef]
- He, J.Q.; Lu, J.W.; Zhan, L.M.; Zheng, D.P.; Wang, Y.D.; Meng, J.X.; Li, P.; Zhao, J.Z.; Zhang, W.X. An alkali–extracted polysaccharide from Poriacocos activates RAW 264.7 macrophages via NF–κB signaling pathway. Arab. J. Chem. 2023, 16, 104592. [Google Scholar] [CrossRef]
- Bai, L.L.; Zhu, P.L.; Wang, W.B.; Wang, M.C. The influence of extraction pH on the chemical compositions, macromolecular characteristics, and rheological properties of polysaccharide: The case of okra polysaccharide. Food Hydrocoll. 2020, 102, 105586. [Google Scholar] [CrossRef]
- Shi, L.F.; Hao, G.X.; Chen, J.; Wang, J.L.; Weng, W.Y. Structural characterization and immunostimulatory activity of a water–soluble polysaccharide from abalone (Haliotis discus hannai Ino) muscle. Food Sci. Hum. Wellness 2023, 12, 495–502. [Google Scholar] [CrossRef]
- Tian, J.Q.; Cui, P.S.; Ge, S.Q.; Cai, X.; Li, Q.; Xue, H.K. Ultrasound assisted aqueous two–phase extraction of polysaccharides from Cornus offcinalis fruit: Modeling, optimization, purifcation, and characterization. Ultrason. Sonochem. 2022, 84, 105966. [Google Scholar]
- Guo, L.N.; Kong, N.; Zhang, X.Y.; Ma, H.L. Multimode ultrasonic extraction of polysaccharides from maca (Lepidium meyenii): Optimization, purification, and in vitro immunoregulatory activity. Ultrason. Sonochem. 2022, 88, 106062. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yu, H.Y.; Liu, Y.Z.; Qin, Z.; Liu, H.M.; Ma, Y.X.; Wang, X.D. Isolation and structural characterization of cell wall polysaccharides from sesame kernel. LWT–Food Sci. Technol. 2022, 163, 113574. [Google Scholar] [CrossRef]
- Guo, Y.D.; Wang, L.L.; Li, L.; Zhang, Z.Y.; Zhang, J.Q.; Zhang, J.; Wang, J. Characterization of polysaccharide fractions from Allii macrostemonis bulbus and assessment of their antioxidant. LWT-Food Sci. Technol. 2022, 165, 113687. [Google Scholar] [CrossRef]
- Zhu, Y.J.; He, Z.X.; Bao, X.Y.; Wang, M.; Yin, S.; Song, L.J.; Peng, Q. Purification, in–depth structure analysis and antioxidant stress activity of a novel pectin–type polysaccharide from Ziziphus Jujuba cv. Muzaoresidue. J. Funct Foods 2021, 80, 104439. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Yaseen, F.X.A.; Chen, B.; Zhang, G.L.; Wang, M.K.; Shen, X.F.; Li, F. A polysaccharide TKP–2–1 from Tamarindus indica L: Purification, structural characterization and immunomodulating activity. J. Funct. Foods 2021, 78, 104384. [Google Scholar] [CrossRef]
- Peng, X.W.; Hu, X.; Zhang, Y.J.; Xu, H.; Tang, J.R.; Zhang, G.L.; Deng, J.; Kan, H.; Zhao, P.; Liu, Y. Extraction, characterization, antioxidant and anti–tumor activities of polysaccharides from Camellia fascicularis leaves. Int. J. Biol. Macromol. 2022, 222 Pt A, 373–384. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ni, Z.J.; Zhang, F.; Thakur, K.; Zhang, J.G.; Khan, M.R.; Busquets, R.; Wei, Z.J. Physicochemical and antioxidant properties of Lycium barbarum seed dreg polysaccharides prepared by continuous extraction. Food Chem. X 2022, 14, 100282. [Google Scholar] [CrossRef]
- Huo, J.Y.; Wu, Z.Y.; Zhao, H.; Sun, W.Z.; Wu, J.H.; Huang, M.Q.; Zhang, J.L.; Wang, Z.H.; Sun, B.G. Structure–activity relationship of antioxidant polysaccharides from Huangshui based on the HPLC fingerprint combined with chemometrics methods. LWT–Food Sci. Technol. 2022, 159, 113201. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhao, Y.; Zhang, M.K.; Yang, X.F.; Yue, P.X.; Tang, D.K.; Wei, X.L. Structural characterization and antioxidant activity of a new polysaccharide from Bletilla striata fibrous roots. Carbohyd. Polym. 2020, 227, 115362. [Google Scholar] [CrossRef]
- Qiu, J.Q.; Zhang, H.; Wang, Z.Y.; Liu, S.M.; Regenstein, J.M. Response surface methodology for the synthesis of an Auricularia auriculajudae polysaccharides-CDDP complex. Int. J. Biol. Macromol. 2016, 93 Pt A, 333–343. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, S.S.; Li, M.Y.; Gao, B.; Zhang, L.T. Structural characterization and tartary buckwheat polysaccharides alleviate insulin resistance by suppressing SOCS3–induced IRS1 protein degradation. J. Funct. Foods 2022, 89, 104961. [Google Scholar] [CrossRef]
- Chen, J.C.; Li, L.; Zhang, X.; Wan, L.T.; Zheng, Q.S.; Xu, D.; Li, Y.T.; Liang, Y.; Chen, M.S.; Li, B.; et al. Structural characterization of polysaccharide from Centipeda minima and its hypoglycemic activity through alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2021, 82, 104478. [Google Scholar] [CrossRef]
- Liu, H.M.; Liu, X.Y.; Yan, Y.Y.; Gao, J.H.; Qin, Z.; Wang, X.D. Structural properties and antioxidant activities of polysaccharides isolated from sunflower meal after oil extraction. Arab. J. Chem. 2021, 14, 103420. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.L.; He, Y.; Li, C.L.; Wang, J.M.; Ma, X. Study on the structure characterization and moisturizing effect of Tremella polysaccharide fermented from GCMCC5.39. Food Sci. Hum. Wellness 2021, 10, 471–479. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Chen, G.Q.; Wang, F.; Zhao, H.; Wei, Y.X.; Liu, L.J.; Zhang, H.B. Extraction, characterization, antioxidant activity and rheological behavior of a polysaccharide produced by the extremely salt tolerant Bacillus subtilis LR-1. LWT–Food Sci. Technol. 2022, 162, 113413. [Google Scholar] [CrossRef]
- Li, M.F.; Zhang, X.N.; Zhang, Y.F.; Shao, X.Q.; Liu, H.L.; Guo, L.; Wang, C.F. Study on the characterization of polysaccharide from Tuber sinense and its desensitization effect to β–lactoglobulin in vivo. J. Funct. Foods 2022, 91, 105028. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Chen, J.; Chen, Y.; Ma, Y.T.; Yang, Q.S.; Fan, Y.Q.; Fu, C.M.; Limsila, B.; Li, R.; Liao, W. Extraction, structural characterization and antioxidant activity of turmeric polysaccharides. LWT–Food Sci. Technol. 2022, 154, 112805. [Google Scholar] [CrossRef]
- You, Y.; Song, H.R.; Wang, L.L.; Peng, H.R.; Sun, Y.J.; Ai, C.Q.; Wen, C.R.; Zhu, B.W.; Song, S. Structural characterization and SARS-CoV-2 inhibitory activity of a sulphated polysaccharide from Caulerpa lentillifera. Carbohyd. Polym. 2022, 280, 119006. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Wang, Y.H.; Xiang, D.; Zhang, Z.K. Structural properties, antioxidant and hypoglycemic activities of polysaccharides purified from pepper leaves by high-speed counter–current chromatography. J. Funct. Foods 2022, 89, 104916. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis andtherapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Xu, Z.X.; Hu, J.R.; Zhang, Y.F.; Bai, L.R. Evaluation of largemouth bass (Micropterus salmoide) fed selenium yeast diets: Growth, histopathology, antioxidant ability, and apoptosis. Aquac. Rep. 2023, 29, 101505. [Google Scholar] [CrossRef]
- Xiang, H.; Sun–Waterhouse, D.X.; Cui, C. Hypoglycemic polysaccharides from Auricularia auricula and Auricularia polytricha inhibit oxidative stress, NF-κB signaling and proinflammatory cytokine production in streptozotocin-induced diabetic mice. Food Sci. Hum. Wellness 2021, 10, 87–93. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.W.; Zhao, H.Q.; Liu, M.; Lin, C.Y.; Li, L.Z.; Ma, B.J. Pectin polysaccharide from Flos Magnoliae (Xin Yi, Magnolia biondii Pamp. flower buds): Hot–compressed water extraction, purification and partial structural characterization. Food Hydrocoll. 2022, 122, 107061. [Google Scholar] [CrossRef]
- Cui, C.; Lu, J.H.; Sun–Waterhouse, D.X.; Mu, L.X.; Sun, W.Z.; Zhao, M.M.; Zhao, H.F. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. LWT–Food Sci. Technol. 2016, 73, 602–608. [Google Scholar] [CrossRef]
| Run | Alkali Concentration (mol/L) X1 | Ratio of Material to Liquid (g/mL) X2 | Extraction Temperature (°C) X3 | Extraction Time (h) X4 | ORPs Yield (%) |
|---|---|---|---|---|---|
| 1 | 0.02 | 1:120 | 80 | 4 | 10.4 |
| 2 | 0.02 | 1:100 | 70 | 4 | 15.7 |
| 3 | 0.02 | 1:80 | 70 | 3 | 13.8 |
| 4 | 0.02 | 1:120 | 70 | 5 | 15.1 |
| 5 | 0.02 | 1:100 | 60 | 3 | 14.0 |
| 6 | 0.03 | 1:100 | 70 | 5 | 12.4 |
| 7 | 0.02 | 1:120 | 60 | 4 | 15.0 |
| 8 | 0.03 | 1:120 | 70 | 4 | 14.2 |
| 9 | 0.03 | 1:100 | 80 | 4 | 9.8 |
| 10 | 0.02 | 1:120 | 70 | 3 | 13.5 |
| 11 | 0.02 | 1:100 | 70 | 4 | 15.7 |
| 12 | 0.02 | 100 | 70 | 4 | 15.7 |
| 13 | 0.02 | 80 | 60 | 4 | 8.8 |
| 14 | 0.01 | 100 | 80 | 4 | 11.6 |
| 15 | 0.02 | 80 | 80 | 4 | 12.0 |
| 16 | 0.01 | 100 | 70 | 3 | 12.2 |
| 17 | 0.03 | 100 | 60 | 4 | 12.5 |
| 18 | 0.02 | 80 | 70 | 5 | 11.0 |
| 19 | 0.01 | 100 | 70 | 5 | 12.9 |
| 20 | 0.03 | 80 | 70 | 4 | 9.3 |
| 21 | 0.01 | 80 | 70 | 4 | 11.9 |
| 22 | 0.02 | 100 | 80 | 5 | 12.8 |
| 23 | 0.01 | 100 | 60 | 4 | 10.0 |
| 24 | 0.03 | 100 | 70 | 3 | 13.5 |
| 25 | 0.01 | 120 | 70 | 4 | 10.9 |
| 26 | 0.02 | 100 | 60 | 5 | 11.5 |
| 27 | 0.02 | 100 | 80 | 3 | 11.1 |
| 28 | 0.02 | 100 | 70 | 4 | 15.7 |
| 29 | 0.02 | 100 | 70 | 4 | 15.7 |
| Parameter | Sum of Square | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 123.25 | 14 | 8.80 | 270.65 | <0.0001 | ** |
| X1 | 0.400 | 1 | 0.40 | 12.4 | 0.0034 | ** |
| X2 | 12.36 | 1 | 12.36 | 380.08 | <0.0001 | ** |
| X3 | 1.39 | 1 | 1.39 | 42.65 | <0.0001 | ** |
| X4 | 0.52 | 1 | 8.097 | 16.01 | 0.0013 | ** |
| X1X2 | 8.44 | 1 | 8.44 | 259.45 | <0.0001 | ** |
| X1X3 | 12.1452 | 1 | 4.49 | 138.18 | <0.0001 | ** |
| X1X4 | 0.3306 | 1 | 0.82 | 25.18 | 0.0002 | ** |
| X2X3 | 64.2402 | 1 | 14.78 | 454.53 | <0.0001 | ** |
| X2X4 | 35.1056 | 1 | 4.71 | 144.77 | <0.0001 | ** |
| X3X4 | 15.4056 | 1 | 4.47 | 137.53 | <0.0001 | ** |
| X12 | 34.89 | 1 | 34.89 | 1072.61 | <0.0001 | ** |
| X22 | 19.23 | 1 | 19.23 | 591.12 | <0.0001 | ** |
| X32 | 41.66 | 1 | 41.66 | 1280.70 | <0.0001 | ** |
| X42 | 3.47 | 1 | 3.47 | 106.76 | <0.0001 | ** |
| Residual | 0.46 | 14 | 0.033 | |||
| Lack of fit | 0.46 | 10 | 0.46 | 0.4931 | 0.6515 | - |
| Pure error | 0.00 | 4 | 0.00 | CV% = 16.16 | ||
| R-Squared | 0.9963 | Adi.R-squared | 0.9926 |
| Samples | Fuc | Rha | Ara | Glc | Gal | Man | Xyl | Fru | GalA | GlcA |
|---|---|---|---|---|---|---|---|---|---|---|
| ORPs–I | 3.55 | 0.69 | 1.43 | 4.70 | 6.81 | 0.14 | 5.27 | 2.78 | – | 3.91 |
| ORPs–II | 4.00 | 0.64 | 1.61 | 7.85 | 8.63 | 2.45 | 3.17 | 1.96 | – | 1.79 |
| ORPs–III | 0.02 | – | 0.19 | 0.29 | 0.35 | – | – | 1.07 | – | – |
| ORPs–IV | – | – | 0.01 | 0.01 | 0.03 | 0.01 | 0.02 | 0.10 | 0.01 | 14.73 |
| ORPs–V | 1.73 | 1.20 | 1.13 | 2.87 | 8.71 | 2.89 | 1.42 | 0.81 | 0.60 | 0.90 |
| Residues | Sugar Linkage | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 |
|---|---|---|---|---|---|---|---|
| Gal | →3, 6)–β–Galp–(1→ | 4.37 102.71 | 3.43 71.07 | 3.64 78.23 | 3.51 71.54 | 3.24 71.26 | 3.93 69.19 |
| →4, 6)–β–Galp–(1→ | 4.41 102.71 | 3.24 71.26 | 3.51 71.44 | 3.94 73.24 | 3.59 70.97 | 4.03 69.67 | |
| Ara | T–Araf–(1→ | 5.16 100.45 | 4.17 79.34 | 3.72 73.45 | 3.78 73.39 | 3.63 71.14 | 3.53 67.67 |
| 1, 3, 5–Araf–(1→ | 5.23 100.45 | 4.25 79.34 | 3.77 73.39 | 3.63 71.14 | 3.51 67.67 | 3.41 n.d. | |
| Man | →6)–β–Manp–(1→ | 4.41 103.20 | 3.55 70.80 | 3.82 71.50 | 3.98 68.30 | 3.96 72.60 | 4.09 68.00 |
| 1, 2–β–Manp–(1→ | 5.14 102.00 | 4.16 79.00 | 3.97 71.30 | 3.73 69.40 | 3.94 74.50 | 3.93 64.50 | |
| Xyl | T–β–Xylp–(1→ | 4.45 102.16 | 3.30 73.09 | 3.48 75.52 | 3.51 73.46 | n.d. n.d. | n.d. n.d. |
| 4.74 104.10 | 3.33 73.22 | 3.25 76.23 | 3.63 69.21 | 3.74 67.30 | n.d. n.d. | ||
| Glc | 1, 6–β–Glcp–(1→ | 4.48 102.81 | 3.29 73.10 | 3.45 75.64 | n.d. n.d. | n.d. n.d. | n.d. n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Shi, W.; Miao, J.; Hu, H.; Gao, Y. Extraction, Isolation, Screening, and Preliminary Characterization of Polysaccharides with Anti–Oxidant Activities from Oudemansiella raphanipies. Polymers 2023, 15, 2917. https://doi.org/10.3390/polym15132917
Qiu J, Shi W, Miao J, Hu H, Gao Y. Extraction, Isolation, Screening, and Preliminary Characterization of Polysaccharides with Anti–Oxidant Activities from Oudemansiella raphanipies. Polymers. 2023; 15(13):2917. https://doi.org/10.3390/polym15132917
Chicago/Turabian StyleQiu, Junqiang, Wang Shi, Jingnan Miao, Hui Hu, and Yanan Gao. 2023. "Extraction, Isolation, Screening, and Preliminary Characterization of Polysaccharides with Anti–Oxidant Activities from Oudemansiella raphanipies" Polymers 15, no. 13: 2917. https://doi.org/10.3390/polym15132917
APA StyleQiu, J., Shi, W., Miao, J., Hu, H., & Gao, Y. (2023). Extraction, Isolation, Screening, and Preliminary Characterization of Polysaccharides with Anti–Oxidant Activities from Oudemansiella raphanipies. Polymers, 15(13), 2917. https://doi.org/10.3390/polym15132917








