Smart Packaging Based on Polylactic Acid: The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical–Mechanical Properties, Colony Reduction, Perishable Food Shelf Life, and Future Prospective
Abstract
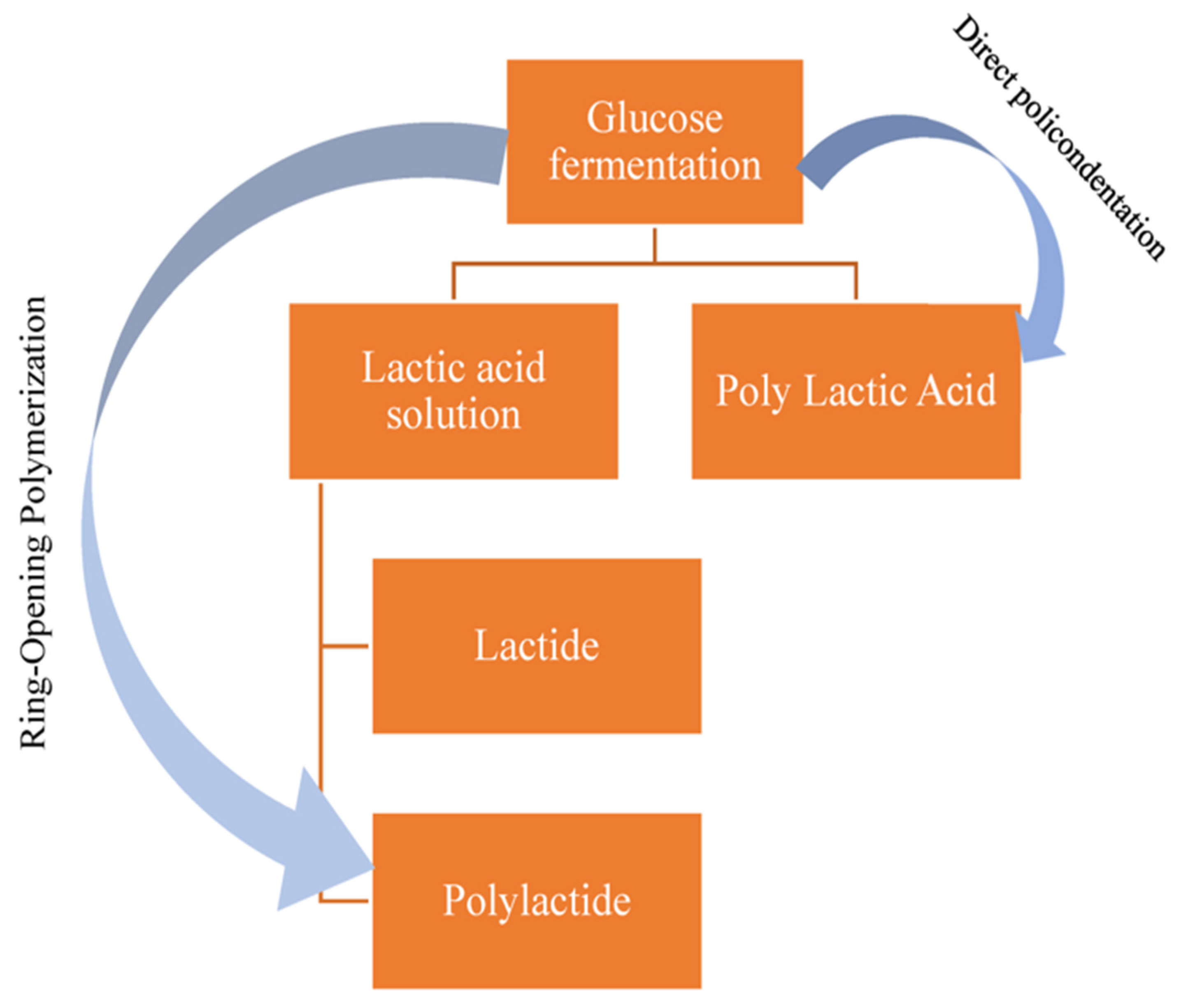
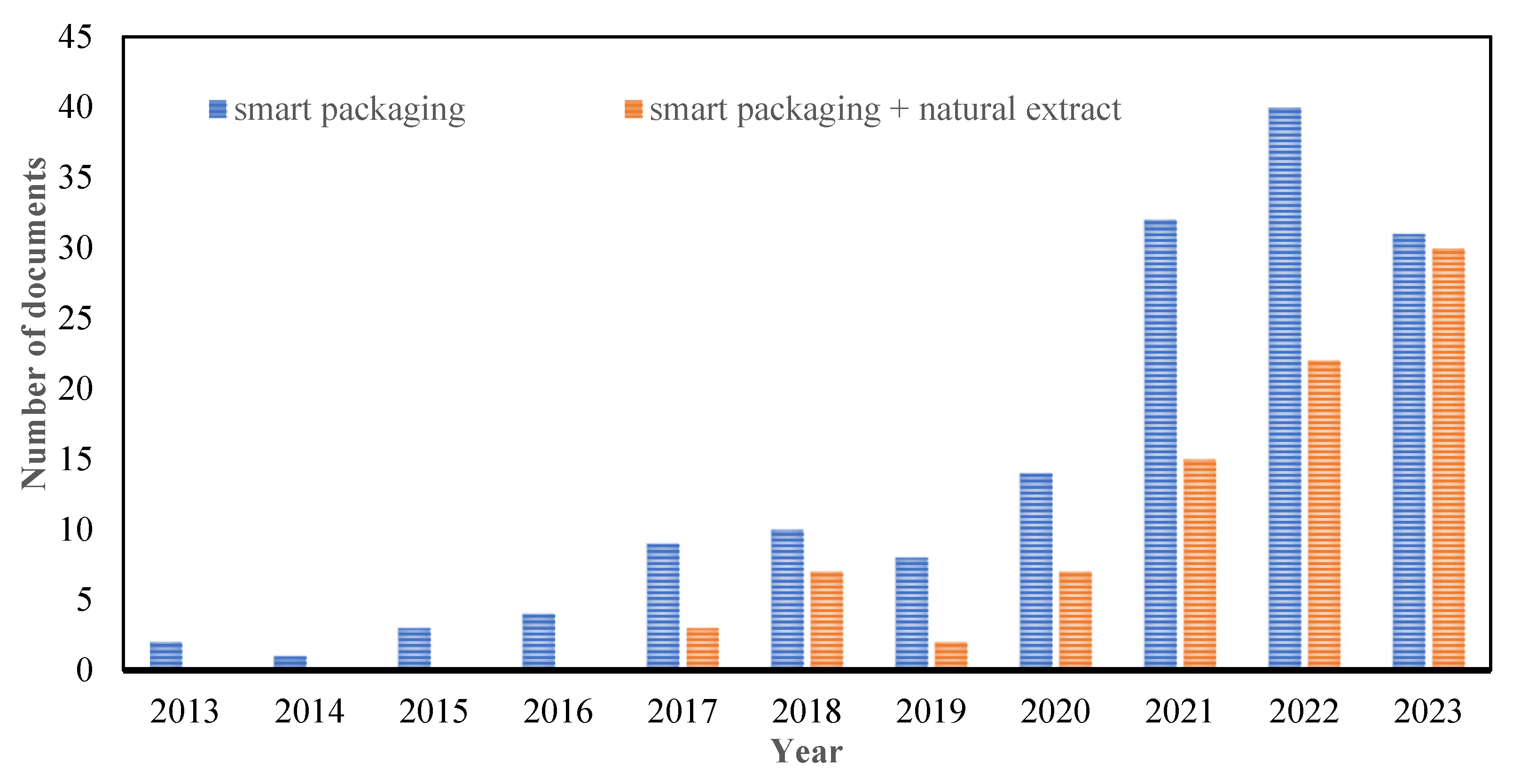
:1. Introduction
2. The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical–Mechanical Properties
2.1. Tensile Properties
2.2. Water Vapour Transmission Rate (WVTR)
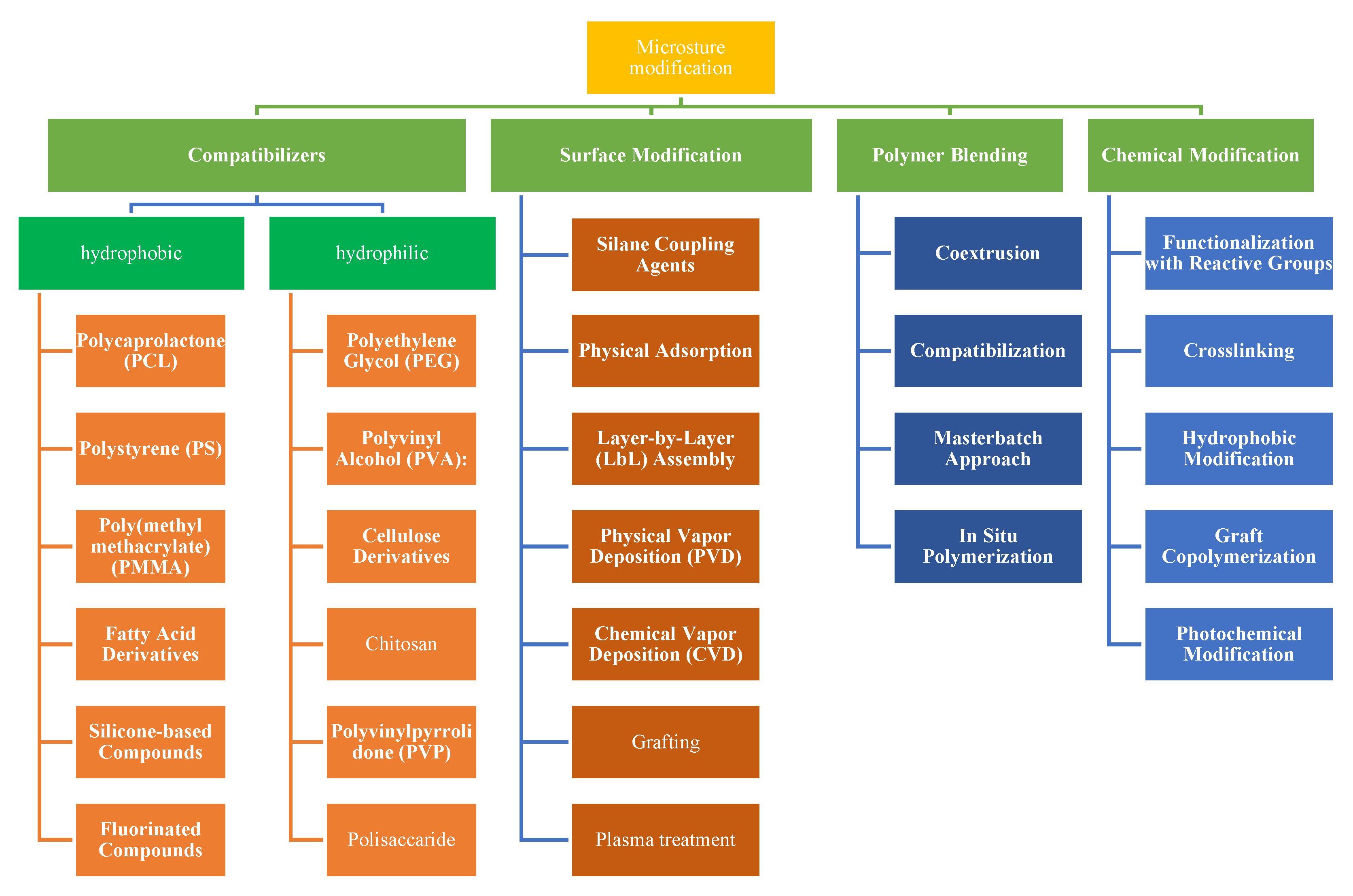
3. The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on the Microstructure of Smart Packaging
- Compatibilizers are additives used to improve the compatibility between two or more polymers with differing properties. In food packaging, where different polymers may need to work together, compatibilizers help create a cohesive structure and improve properties like adhesion, mechanical strength, and barrier performance. Compatibilizers achieve this by promoting interfacial interactions between polymers that would otherwise induce phase separation or have weak interactions.
- Surface modification encompasses the adjustment of material surface characteristics to amplify adhesion, wettability, and harmonization with additional substances. Surface modification assumes paramount significance in optimizing the interplay between packaging materials and the contents. Methodologies such as plasma treatment, layer-by-layer (LbL) assembly, and chemical grafting engender the introduction of functional groups onto the surface, fostering an augmented propensity for adhesion or coating. This in turn elevates the packaging material’s barrier properties, print quality, and holistic performance.
- Polymeric blending techniques offer effective ways to improve adhesion and compatibility between hydrophilic or hydrophobic polymer materials in smart packaging systems.
- Chemical modification involves changing the chemical structure of the polymer to achieve desired properties. Functional groups can be introduced to improve compatibility, adhesion, or specific interactions. In food packaging, chemical modification can adapt the properties of the packaging material to meet specific requirements.
4. The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Colony Reduction
5. The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Natural Perishable Food Shelf Life
6. The Future Trend of Smart Packaging Systems
- Cost
- Developing and incorporating smart packaging can be expensive, potentially increasing the overall cost of production and affecting product pricing.
- Compatibility: Ensuring compatibility between different components of smart packaging, such as sensors and communication systems, can be challenging.
- Data Security: Smart packaging often collects and transmits data, raising concerns about data security, privacy, and potential breaches.
- Regulations: Compliance with regulatory standards and certifications can be intricate, especially in industries like pharmaceuticals and food, where safety is crucial.
- Consumer Acceptance: Introducing new technology to consumers may require education and demonstration to ensure their understanding and willingness to use smart packaging.
- Sustainability: Balancing the integration of electronics with sustainable and recyclable packaging materials can be challenging.
- Technical Reliability: Ensuring the reliability and accuracy of sensors and communication systems over the entire product lifecycle can be complex.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, S.; Budhwani, D.; Gurjar, P.; Telange, D.; Lambole, V. Pullulan based derivatives: Synthesis, enhanced physicochemical properties, and applications. Drug Deliv. 2022, 29, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Afridi, M.; Khan, N.A.; Sarwar, A. Quality deterioration of postharvest fruits and vegetables in developing country Pakistan: A mini overview. Asian J. Agric. Food Sci. 2021, 9, 83–90. [Google Scholar] [CrossRef]
- Ahmed, S.; Sameen, D.E.; Lu, R.; Li, R.; Dai, J.; Qin, W.; Liu, Y. Research progress on antimicrobial materials for food packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3088–3102. [Google Scholar] [CrossRef] [PubMed]
- Ailli, A.; Handaq, N.; Touijer, H.; Gourich, A.A.; Drioiche, A.; Zibouh, K.; Eddamsyry, B.; El Makhoukhi, F.; Mouradi, A.; Bin Jardan, Y.A.; et al. Phytochemistry and Biological Activities of Essential Oils from Six Aromatic Medicinal Plants with Cosmetic Properties. Antibiotics 2023, 12, 721. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Carocho, M.; Barros, D.; Velho, M.V.; Heleno, S.; Barros, L. Chemical composition and industrial applications of Maritime pine (Pinus pinaster Ait.) bark and other non-wood parts. Rev. Environ. Sci. Bio/Technol. 2022, 21, 583–633. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Mariño-Cortegoso, S.; Barbosa-Pereira, L.; Sendón, R.; Buonocore, G.G.; Stanzione, M.; Coelho, A.; Correia, C.B.; Saraiva, M.; et al. LDPE and PLA Active Food Packaging Incorporated with Lemon by-Products Extract: Preparation, Characterization and Effectiveness to Delay Lipid Oxidation in Almonds and Beef Meat. Foods 2023, 12, 2450. [Google Scholar] [CrossRef]
- Fontes, M.R.V.; Contessa, C.R.; Moraes, C.C.; Zavareze, E.D.R.; Dias, A.R.G. Antimicrobial properties of PLA membranes loaded with pink pepper (Schinus terebinthifolius Raddi) essential oil applied in simulated cream cheese packaging. Food Biophys. 2023, 18, 107–119. [Google Scholar] [CrossRef]
- Aziman, N.; Abdullah, N.; Bujang, A.; Mohd Noor, Z.; Abdul Aziz, A.; Ahmad, R. Phytochemicals of ethanolic extract and essential oil of Persicaria hydropiper and their potential as antibacterial agents for food packaging polylactic acid film. J. Food Saf. 2021, 41, e12864. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A sustainable material for food and medical applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly(lactic acid)(PLA) Nanocomposites: Incorporation of Various Nanofillers and their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar]
- Bonnenfant, C.; Gontard, N.; Aouf, C. Biobased and biodegradable polymers in a circular economy context: Understanding quercetin and gallic acid impacts on PHBV thermal properties. Polym. Degrad. Stab. 2022, 201, 109975. [Google Scholar] [CrossRef]
- Bouslamti, M.; El Barnossi, A.; Kara, M.; Alotaibi, B.S.; Al Kamaly, O.; Assouguem, A.; Lyoussi, B.; Benjelloun, A.S. Total polyphenols content, antioxidant and antimicrobial activities of leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 2022, 27, 4322. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Firoozjah, R.; Salim, S.A.; Hasanvand, S.; Assadpour, E.; Azizi-Lalabadi, M.; Prieto, M.A.; Jafari, S.M. Application of smart packaging for seafood: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1438–1461. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential oils and their application on active packaging systems: A review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Wang, X.; Wang, J.; Raposo, A. Microbial biofilms in the food industry—A comprehensive review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Chen, M.; Yan, X.; Cheng, M.; Zhao, P.; Wang, Y.; Zhang, R.; Chen, M. Preparation, characterization and application of poly(lactic acid)/corn starch/eucalyptus leaf essential oil microencapsulated active bilayer degradable film. Int. J. Biol. Macromol. 2022, 195, 264–273. [Google Scholar] [CrossRef]
- Chen, S.; He, S.; Xu, X.; Wang, H. Transcriptomic responses of foodborne pathogens to the food matrix. Curr. Opin. Food Sci. 2021, 42, 23–30. [Google Scholar] [CrossRef]
- Chowdhury, M.A.H.; Ashrafudoulla, M.; Mevo, S.I.U.; Mizan, M.F.R.; Park, S.H.; Ha, S.D. Current and future interventions for improving poultry health and poultry food safety and security: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1555–1596. [Google Scholar] [CrossRef]
- Cui, H.; Xu, R.; Hu, W.; Li, C.; Abdel-Samie, M.A.; Lin, L. Effect of soy protein isolate nanoparticles loaded with litsea cubeba essential oil on performance of lentinan edible films. Int. J. Biol. Macromol. 2023, 242, 124686. [Google Scholar] [CrossRef]
- Cvek, M.; Paul, U.C.; Zia, J.; Mancini, G.; Sedlarik, V.; Athanassiou, A. Biodegradable films of PLA/PPC and curcumin as packaging materials and smart indicators of food spoilage. ACS Appl. Mater. Interfaces 2022, 14, 14654–14667. [Google Scholar] [CrossRef]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2022, 80, 7247–7312. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Mishra, H.N. A comprehensive review of the spoilage of shrimp and advances in various indicators/sensors for shrimp spoilage monitoring. Food Res. Int. 2023, 173, 113270. [Google Scholar] [CrossRef] [PubMed]
- Desai, H.; Mehta, T.; Shah, N. Azeotropic dehydrative (solution) polycondensation of lactic acid to polylactic acid (PLA): A in-depth review of an overlooked method for manufacturing PLA. Polym.-Plast. Technol. Mater. 2023, 62, 1394–1402. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefin. 2022, 43, 1–22. [Google Scholar] [CrossRef]
- Dsouza, A.; Constantinidou, C.; Arvanitis, T.N.; Haddleton, D.M.; Charmet, J.; Hand, R.A. Multifunctional composite hydrogels for bacterial capture, growth/elimination, and sensing applications. ACS Appl. Mater. Interfaces 2022, 14, 47323–47344. [Google Scholar] [CrossRef]
- Filipini, G.; Romani, V.P.; Guimarães Martins, V. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. [Google Scholar] [CrossRef]
- Akshaykranth, A.; Jayarambabu, N.; Rao, T.V.; Kumar, R.R.; Rao, L.S. Novel nanocomposite polylactic acid films with Curcumin-ZnO: Structural, thermal, optical and antibacterial properties. Curr. Res. Green Sustain. Chem. 2022, 5, 100332. [Google Scholar] [CrossRef]
- Fiore, A.; Park, S.; Volpe, S.; Torrieri, E.; Masi, P. Active packaging based on PLA and chitosan-caseinate enriched rosemary essential oil coating for fresh minced chicken breast application. Food Packag. Shelf Life 2021, 29, 100708. [Google Scholar] [CrossRef]
- Freeland, B.; McCarthy, E.; Balakrishnan, R.; Fahy, S.; Boland, A.; Rochfort, K.D.; Dabros, M.; Marti, R.; Kelleher, S.M.; Gaughran, J. A review of polylactic acid as a replacement material for single-use laboratory components. Materials 2022, 15, 2989. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Sudheer, S.M.; Bhat, R. Biopolymers as sustainable alternatives in the food packaging industry. In Sustainable Development and Pathways for Food Ecosystems; Academic Press: Cambridge, MA, USA, 2023; pp. 227–258. [Google Scholar]
- Gui, H.; Zhao, M.; Zhang, S.; Yin, R.; Hu, C.; Fan, M.; Li, L. Active antioxidant packaging from essential oils incorporated polylactic acid/poly (butylene adipate-co-terephthalate)/thermoplastic starch for preserving straw mushroom. Foods 2022, 11, 2252. [Google Scholar] [CrossRef]
- Gunny, A.A.N.; Leem, S.J.; Makhtar, M.M.Z.; Zainuddin, N.; Roslim, M.H.M.; Hashim, R.H.R.; Pusphanathan, K.; Siddiqui, M.R.; Alam, M.; Rafatullah, M. The Use of Essential Oil Embedded in Polylactic Acid/Chitosan-Based Film for Mango Post-Harvest Application against Pathogenic Fungi. Polymers 2023, 15, 2722. [Google Scholar] [CrossRef]
- He, X.; Pu, Y.; Chen, L.; Jiang, H.; Xu, Y.; Cao, J.; Jiang, W. A comprehensive review of intelligent packaging for fruits and vegetables: Target responders, classification, applications, and future challenges. Compr. Rev. Food Sci. Food Saf. 2023, 22, 842–881. [Google Scholar] [CrossRef] [PubMed]
- Kamuni, M.S.; Devasani, T.P.; Nalla, L.R.; Liyakat, K.K.S. Fruit Quality Detection using Thermometer. J. Image Process. Intell. Remote Sens. 2022, 2, 20–27. [Google Scholar] [CrossRef]
- He, Z.; Zhang, X.; Song, Z.; Li, L.; Chang, H.; Li, S.; Zhou, W. Quercetin inhibits virulence properties of Porphyromas gingivalis in periodontal disease. Sci. Rep. 2020, 10, 18313. [Google Scholar] [CrossRef]
- Hojatoleslami, M.; Ahari, H.; Larijani, K.; Sharifan, A. Preservation effect of Lippia citriodora and Laurus nobilis nanoemulsions incorporated with polylactic acid composite film for rainbow trout fillet packaging. Food Sci. Technol. 2022, 42, e83921. [Google Scholar] [CrossRef]
- Imade, E.E.; Ajiboye, T.O.; Fadiji, A.E.; Onwudiwe, D.C.; Babalola, O.O. Green synthesis of zinc oxide nanoparticles using plantain peel extracts and the evaluation of their antibacterial activity. Sci. Afr. 2022, 16, e01152. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Suppakul, P. Active and intelligent packaging: The indication of quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef]
- Javaherzadeh, R.; Bafroee, A.T.; Kanjari, A. Preservation effect of Polylophium involucratum essential oil incorporated poly lactic acid/nanochitosan composite film on shelf life and sensory properties of chicken fillets at refrigeration temperature. LWT 2020, 118, 108783. [Google Scholar] [CrossRef]
- Jeong, E.W.; Baek, Y.; Lee, H.G. Development of propolis extract-loaded nanoparticles with chitosan and hyaluronic acid for improving solubility and stability. LWT 2023, 181, 114738. [Google Scholar]
- Khan, S.; Monteiro, J.K.; Prasad, A.; Filipe, C.D.; Li, Y.; Didar, T.F. Material Breakthroughs in Smart Food Monitoring: Intelligent Packaging and On-Site Testing Technologies for Spoilage and Contamination Detection. Adv. Mater. 2023, 22, 2300875. [Google Scholar] [CrossRef]
- Khanna, A.; Jain, S.; Burgio, A.; Bolshev, V.; Panchenko, V. Blockchain-enabled supply chain platform for Indian dairy industry: Safety and traceability. Foods 2022, 11, 2716. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Recent advances and future prospects of cellulose, starch, chitosan, polylactic acid and polyhydroxyalkanoates for sustainable food packaging applications. Int. J. Biol. Macromol. 2022, 221, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and α-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, H.; Jiang, L.; Chuan, Y.; Yuan, M.; Chen, H. Characterization of active packaging films made from poly(lactic acid)/poly(trimethylene carbonate) incorporated with oregano essential oil. Molecules 2016, 21, 695. [Google Scholar] [CrossRef] [PubMed]
- Loest, D.; Uhland, F.C.; Young, K.M.; Li, X.-Z.; Mulvey, M.R.; Reid-Smith, R.; Sherk, L.M.; Carson, C.A. Carbapenem-resistant Escherichia coli from shrimp and salmon available for purchase by consumers in Canada: A risk profile using the Codex framework. Epidemiol. Infect. 2022, 150, e148. [Google Scholar] [CrossRef] [PubMed]
- Madureira, J.; Melgar, B.; Alves, V.D.; Moldão-Martins, M.; Margaça, F.M.A.; Santos-Buelga, C.; Barros, L.; Verde, S.C. Effect of Olive Pomace Extract Application and Packaging Material on the Preservation of Fresh-Cut Royal Gala Apples. Foods 2023, 12, 1926. [Google Scholar] [CrossRef]
- Lv, S.; Liu, C.; Li, H.; Zhang, Y. Assessment of structural modification and time-dependent behavior of poly (lactic acid) based composites upon hydrolytic degradation. Eur. Polym. J. 2022, 166, 111058. [Google Scholar] [CrossRef]
- Mao, L.; Bai, Z.; Yao, J.; Liu, Y. Development of novel poly (lactic acid) active multilayer composite films by incorporating catechol-functionalized layered clay into chitosan/poly(vinyl alcohol) coatings. Prog. Org. Coat. 2022, 170, 107000. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the barrier properties of PLA: A state-of-the-art review for food packaging applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Martínez-Aguilar, V.; Peña-Juárez, M.G.; Carrillo-Sanchez, P.C.; López-Zamora, L.; Delgado-Alvarado, E.; Gutierrez-Castañeda, E.J.; Flores-Martínez, N.L.; Herrera-May, A.L.; Gonzalez-Calderon, J.A. Evaluation of the Antioxidant and Antimicrobial Potential of SiO2 Modified with Cinnamon Essential Oil (Cinnamomum Verum) for Its Use as a Nanofiller in Active Packaging PLA Films. Antioxidants 2023, 12, 1090. [Google Scholar] [CrossRef]
- Martins, C.; Vilarinho, F.; Silva, A.S.; Andrade, M.; Machado, A.V.; Castilho, M.C.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active polylactic acid film incorporated with green tea extract: Development, characterization and effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Cerqueira, M.A.; Azevedo, A.G.; Barros, C.; Machado, A.V.; Coelho, A.; Furtado, R.; Correia, C.B.; Saraiva, M.; et al. PLA films loaded with green tea and rosemary polyphenolic extracts as an active packaging for almond and beef. Food Packag. Shelf Life 2023, 36, 101041. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly(lactic acid) and poly (butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Lu, W.; Cui, R.; Zhu, B.; Qin, Y.; Cheng, G.; Li, L.; Yuan, M. Influence of clove essential oil immobilized in mesoporous silica nanoparticles on the functional properties of poly(lactic acid) biocomposite food packaging film. J. Mater. Res. Technol. 2021, 11, 1152–1161. [Google Scholar] [CrossRef]
- Cheng, J.; Lin, X.; Wu, X.; Liu, Q.; Wan, S.; Zhang, Y. Preparation of a multifunctional silver nanoparticles polylactic acid food packaging film using mango peel extract. Int. J. Biol. Macromol. 2021, 188, 678–688. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Ghorbani, M.; Mohammadi, M.; Pezeshki, A. Improvement of the physico-mechanical properties of antibacterial electrospun poly lactic acid nanofibers by incorporation of guar gum and thyme essential oil. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126659. [Google Scholar] [CrossRef]
- Ardjoum, N.; Chibani, N.; Shankar, S.; Fadhel, Y.B.; Djidjelli, H.; Lacroix, M. Development of antimicrobial films based on poly (lactic acid) incorporated with Thymus vulgaris essential oil and ethanolic extract of Mediterranean propolis. Int. J. Biol. Macromol. 2021, 185, 535–542. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly(lactic acid)/poly(butylene-succinate-co-adipate)(PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packag. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Freitas, P.A.; Gil, N.J.B.; González-Martínez, C.; Chiralt, A. Antioxidant poly (lactic acid) films with rice straw extract for food packaging applications. Food Packag. Shelf Life 2022, 34, 101003. [Google Scholar] [CrossRef]
- Dai, L.; Li, R.; Liang, Y.; Liu, Y.; Zhang, W.; Shi, S. Development of Pomegranate Peel Extract and Nano ZnO Co-Reinforced Polylactic Acid Film for Active Food Packaging. Membranes 2022, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Huang, X.; Zhou, L.; Wang, Y. Essential oil-loaded antimicrobial and antioxidant zein/poly (lactic acid) film as active food packaging. Food Packag. Shelf Life 2022, 34, 100977. [Google Scholar] [CrossRef]
- Melo, J.; Quintas, C. Minimally processed fruits as vehicles for foodborne pathogens. AIMS Microbiol. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Rahman, R.; Mohammed, A.M.; Adam, T.; Betar, B.O.; Osman, A.F.; Dahham, O.S. Surface treatment to improve water repellence and compatibility of natural fiber with polymer matrix: Recent advancement. Polym. Test. 2022, 115, 107707. [Google Scholar] [CrossRef]
- Safitri, A.; Sinaga PS, D.; Nasution, H.; Harahap, H.; Masyithah, Z.; Hasibuan, R. The role of various plastisizers and fillers additions in improving tensile strength of starch-based bioplastics: A mini review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1115, 012076. [Google Scholar] [CrossRef]
- Mohan, S.; Panneerselvam, K. Development of polylactic acid based functional films reinforced with ginger essential oil and curcumin for food packaging applications. J. Food Meas. Charact. 2022, 16, 4703–4715. [Google Scholar] [CrossRef]
- Mohd Aris, Z.F.; Bavishi, V.; Sharma, R.; Nagarajan, R. Barrier properties and abrasion resistance of biopolymer-based coatings on biodegradable poly(lactic acid) films. Polym. Eng. Sci. 2019, 59, 1874–1881. [Google Scholar] [CrossRef]
- Moreno-Serna, V.; Oyarzún, C.; Ulloa-Flores, M.T.; Rivas, L.; Sepúlveda, F.A.; Loyo, C.; Toro, E.L.; Zapata, P.A. Venus antiqua Clamshell-Derived Calcium Oxide Nanoparticles for the Preparation of PLA/d-Limonene/CaO Nanocomposites with Antimicrobial Properties. ACS Sustain. Chem. Eng. 2023, 11, 10755–10766. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Ghalamchi, L.; Vatanpour, V.; Khataee, A.; Yousefpoor, M. Novel polymeric additives in the preparation and modification of polymeric membranes: A comprehensive review. J. Ind. Eng. Chem. 2022, 109, 100–124. [Google Scholar] [CrossRef]
- Olonisakin, K.; Wen, A.; He, S.; Lin, H.; Tao, W.; Chen, S.; Lin, W.; Li, R.; Zhang, X.-X.; Yang, W. The Development of Biodegradable PBAT-Lignin-Tannic Acid Composite Film: Properties, Biodegradability, and Potential Barrier Application in Food Packaging. Food Bioprocess Technol. 2023, 16, 1525–1540. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Biodegradable active materials containing phenolic acids for food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3910–3930. [Google Scholar] [CrossRef]
- Osmólska, E.; Stoma, M.; Starek-Wójcicka, A. Application of Biosensors, Sensors, and Tags in Intelligent Packaging Used for Food Products—A Review. Sensors 2022, 22, 9956. [Google Scholar] [CrossRef] [PubMed]
- Oyom, W.; Zhang, Z.; Bi, Y.; Tahergorabi, R. Application of starch-based coatings incorporated with antimicrobial agents for preservation of fruits and vegetables: A review. Prog. Org. Coat. 2022, 166, 106800. [Google Scholar] [CrossRef]
- Pabon, K.S.M.; Aponte, A.A.A.; Duque, J.F.S.; Villada, H.S. Characterization and antimicrobial efficacy of active biocomposite containing polylactic acid, oregano essential oil and nisin for pork storage. Food Sci. Technol. 2021, 42, e67420. [Google Scholar] [CrossRef]
- Palai, B.; Mohanty, S.; Nayak, S.K. Synergistic effect of polylactic acid (PLA) and Poly(butylene succinate-co-adipate)(PBSA) based sustainable, reactive, super toughened eco-composite blown films for flexible packaging applications. Polym. Test. 2020, 83, 106130. [Google Scholar] [CrossRef]
- Partovi, R.; Talebi, F.; Babaei, A.; Sharifzadeh, A. Antimicrobial Activity of Polylactic Acid Film Incorporated with Marjoram and Clove Essential Oils on Microbial and Chemical Properties of Minced Beef during Refrigerated Storage. Int. J. Enteric Pathog. 2020, 8, 25–31. [Google Scholar] [CrossRef]
- Moldovan, A.; Cuc, S.; Prodan, D.; Rusu, M.; Popa, D.; Taut, A.C.; Petean, I.; Bomboş, D.; Doukeh, R.; Nemes, O. Development and Characterization of Polylactic Acid (PLA)-Based Nanocomposites Used for Food Packaging. Polymers 2023, 15, 2855. [Google Scholar] [CrossRef]
- Pirsa, S.; Sani, I.K.; Mirtalebi, S.S. Nano-biocomposite based color sensors: Investigation of structure, function, and applications in intelligent food packaging. Food Packag. Shelf Life 2022, 31, 100789. [Google Scholar] [CrossRef]
- Popescu, P.-A.; Palade, L.M.; Nicolae, I.-C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-Based Edible Coatings Containing Essential Oils to Preserve the Shelf Life and Postharvest Quality Parameters of Organic Strawberries and Apples during Cold Storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef]
- Porta, R.; Sabbah, M.; Di Pierro, P. Biopolymers as food packaging materials. Int. J. Mol. Sci. 2020, 21, 4942. [Google Scholar] [CrossRef]
- Priyanka, S.; Namasivayam, S.K.R.; Bharani, R.A.; John, A. Biocompatible green technology principles for the fabrication of food packaging material with noteworthy mechanical and antimicrobial properties A sustainable developmental goal towards the effective, safe food preservation strategy. Chemosphere 2023, 336, 139240. [Google Scholar] [CrossRef] [PubMed]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and incorporation of functional ingredients in edible films and coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; Andrade, E.H.d.A.; de Oliveira, M.S. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Wemyss, A.M.; Iacovidou, E.; Wan, C. Design and control of compostability in synthetic biopolyesters. ACS Sustain. Chem. Eng. 2021, 9, 9151–9164. [Google Scholar] [CrossRef]
- Salimnejhad, Z.; Hassanzadazar, H.; Aminzare, M. Epinecidin-1 (an active marine antimicrobial peptide): Effects on the survival of inoculated Escherichia coli O157: H7 and Staphylococcus aureus bacteria, antioxidant, and sensory attributes in raw milk. Food Sci. Nutr. Process 2023, 11, 5573–5581. [Google Scholar] [CrossRef]
- Sangeetha, G.; Vijayalakshmi, M. Role of smart sensors in minimizing food deficit by prediction of shelf-life in agricultural supply chain. In Principles of Internet of Things (IoT) Ecosystem: Insight Paradigm; Springer: Berlin/Heidelberg, Germany, 2020; pp. 153–175. [Google Scholar]
- Sedarat, Z.; Taylor-Robinson, A.W. Biofilm formation by pathogenic bacteria: Applying a staphylococcus aureus model to appraise potential targets for therapeutic intervention. Pathogens 2022, 11, 388. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and antimicrobial activity of biodegradable active packaging enriched with clove and thyme essential oil for food packaging application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, V.; Mudgal, D. Current trends, applications, and challenges of coatings on additive manufacturing based biopolymers: A state of art review. Polym. Compos. 2022, 43, 6749–6781. [Google Scholar] [CrossRef]
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for food packaging. Trends Food Sci. Technol. 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Silva, F.V.M. Pasteurization of Food and Beverages by High Pressure Processing (HPP) at Room Temperature: Inactivation of Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Salmonella, and Other Microbial Pathogens. Appl. Sci. 2023, 13, 1193. [Google Scholar] [CrossRef]
- Silva, L.N.; Da Hora, G.C.A.; Soares, T.A.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.D.S. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci. Rep. 2017, 7, 2823. [Google Scholar] [CrossRef] [PubMed]
- Srisa, A.; Harnkarnsujarit, N. Antifungal films from trans-cinnamaldehyde incorporated poly(lactic acid) and poly(butylene adipate-co-terephthalate) for bread packaging. Food Chem. 2020, 333, 127537. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; John, A.; Pušnik Črešnar, K.; Fras Zemljič, L.; Lambropoulou, D.A.; Bikiaris, D.N. Active Agents Incorporated in Polymeric Substrates to Enhance Antibacterial and Antioxidant Properties in Food Packaging Applications. Macromol 2022, 3, 1–27. [Google Scholar] [CrossRef]
- Stoleru, E.; Vasile, C.; Irimia, A.; Brebu, M. Towards a bioactive food packaging: Poly(lactic acid) surface functionalized by chitosan coating embedding clove and argan oils. Molecules 2021, 26, 4500. [Google Scholar] [CrossRef]
- Sundar, N.; Stanley, S.J.; Kumar, S.A.; Keerthana, P.; Kumar, G.A. Development of dual purpose, industrially important PLA–PEG based coated abrasives and packaging materials. J. Appl. Polym. Sci. 2021, 138, 50495. [Google Scholar] [CrossRef]
- Tagrida, M.; Gulzar, S.; Nilsuwan, K.; Prodpran, T.; Zhang, B.; Benjakul, S. Polylactic acid film coated with electrospun gelatin/chitosan nanofibers containing betel leaf ethanolic extract: Properties, bioactivities, and use for shelf-life extension of Tilapia slices. Molecules 2022, 27, 5877. [Google Scholar] [CrossRef]
- Taib, N.A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Thakur, N.; Raposo, A. Development and application of fruit and vegetable based green films with natural bio-actives in meat and dairy products: A review. J. Sci. Food Agric. 2023, 103, 6167–6179. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Z.; Sun, J.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of polylactic acid nanofiber films loading Perilla essential oil for antibacterial packaging of chilled chicken. Int. J. Biol. Macromol. 2021, 192, 379–388. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, F.; Huang, J.; Li, Q.; Yang, Q.; Ju, J. Application of essential oils as slow-release antimicrobial agents in food preservation: Preparation strategies, release mechanisms and application cases. Crit. Rev. Food Sci. Nutr. 2023, 12, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 2601–2621. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Julianti, E.; Dalimunthe, N.F.; Wulandari, G. Tensile properties and antimicrobial activity of bioplastic based on sago starch utilized piper betel leaf. AIP Conf. Proc. 2022, 2493, 1. [Google Scholar]
- Khuntia, A.; Kumar, R.; Premjit, Y.; Mitra, J. Release behavior of vitamin C nanoliposomes from starch–vitamin C active packaging films. J. Food Process Eng. 2022, 45, e14075. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable biopolymers for active packaging: Demand, development and directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Rihayat, T.; Aidy, N.; Safitri, A.; Aida, A. Synthesis of poly lactic acid (PLA)/nanochitosan–based for bioscaffold materials with the addition of Zn-curcumin. Mater. Today Proc. 2022, 63, S526–S531. [Google Scholar] [CrossRef]
- Wang, D.; Sun, J.; Li, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/zein nanofiber films loaded with perillaldehyde, thymol, or ɛ-polylysine and evaluation of their effects on the preservation of chilled chicken breast. Food Chem. 2022, 373, 131439. [Google Scholar] [CrossRef]
- Wu, W.; Liu, L.; Goksen, G.; Demir, D.; Shao, P. Multidimensional (0D-3D) nanofillers: Fascinating materials in the field of bio-based food active packaging. Food Res. Int. 2022, 157, 111446. [Google Scholar] [CrossRef]
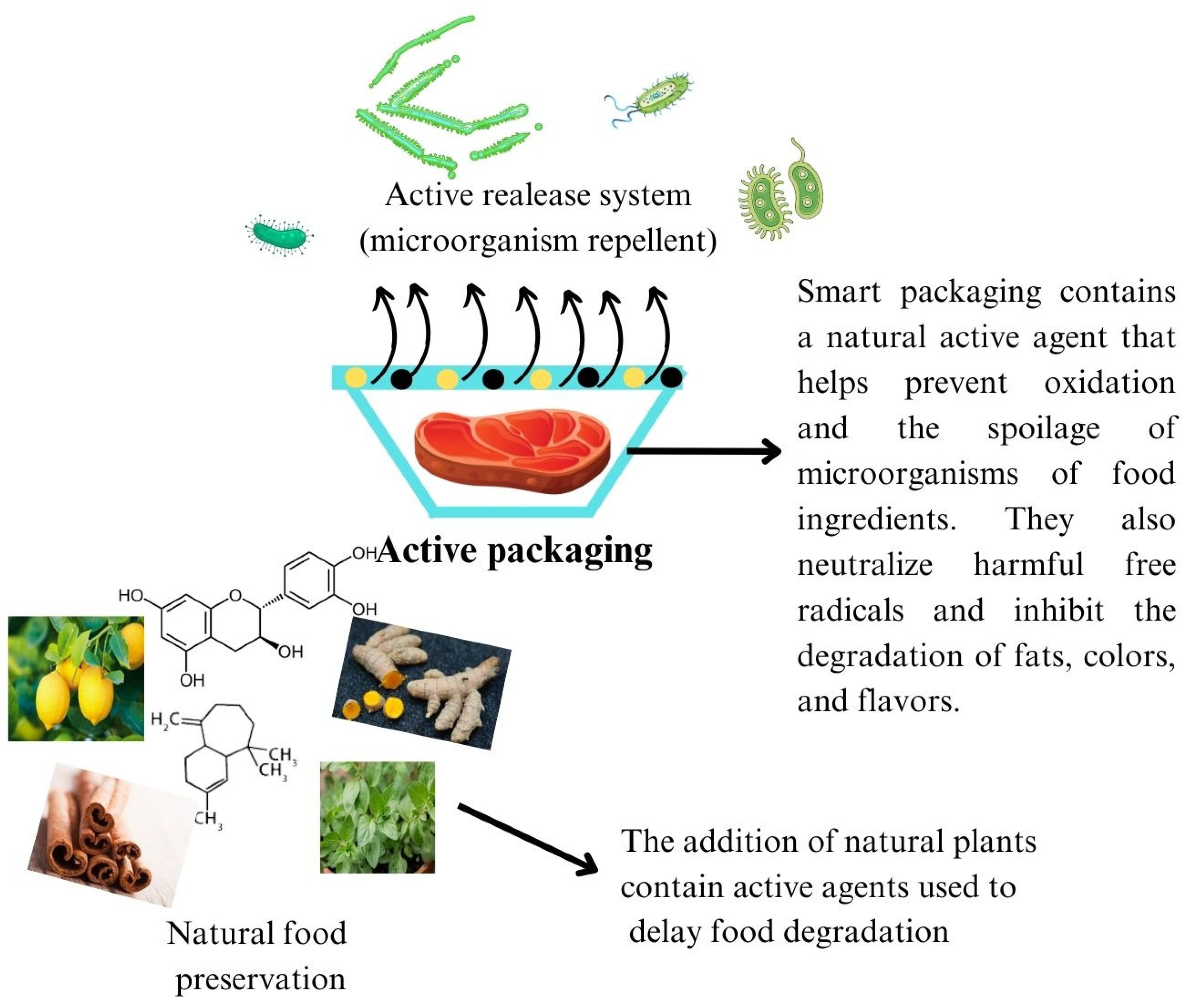

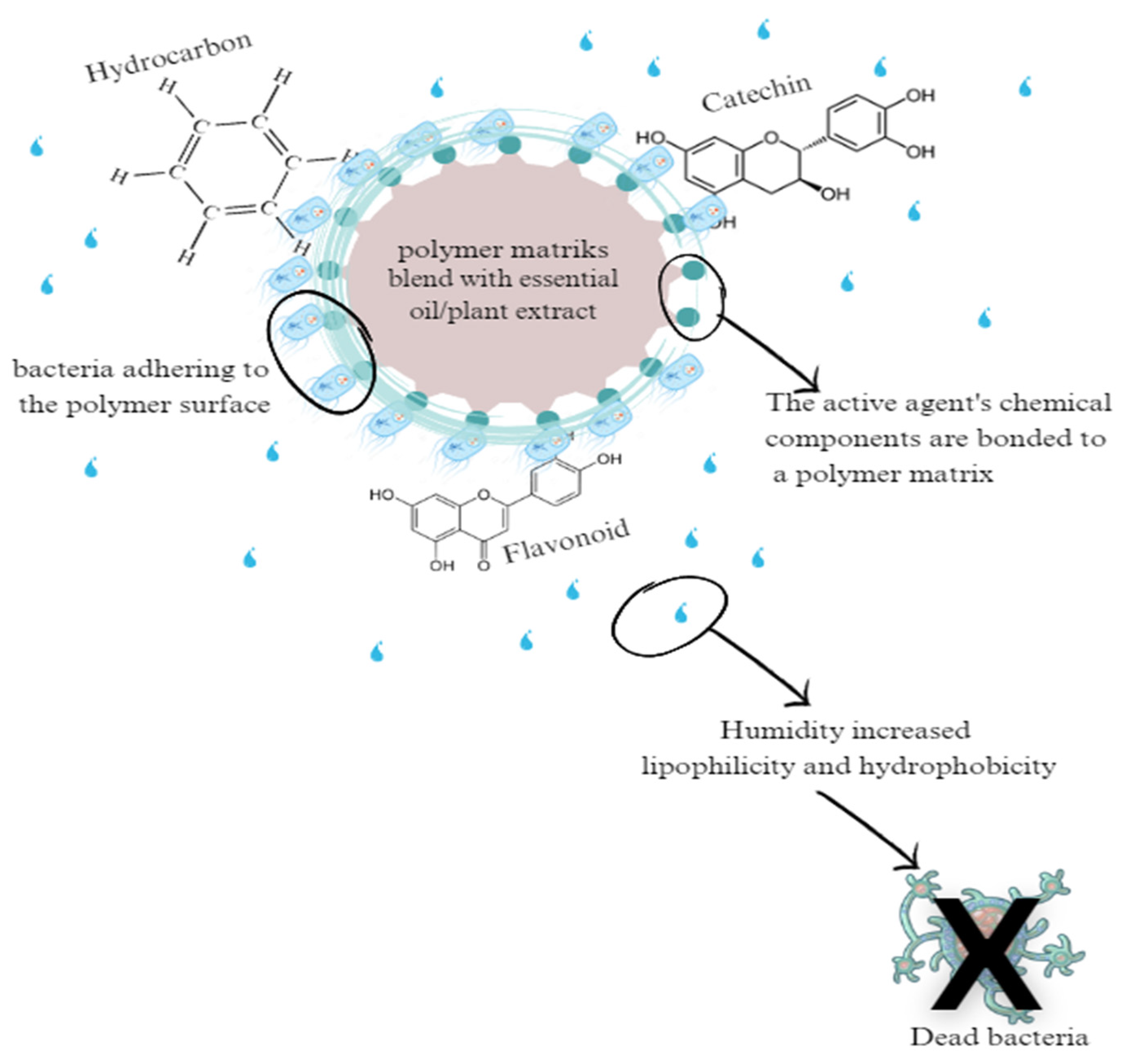
| Plant | Active Components |
|---|---|
| Jamun | β-humulen, α-guaiene, Caryophyllene, α-humulene, β-elemene |
| Propolis | dihydrochrysin, pinostrobin, caryophyllene and chrysin |
| Green tea | epigallo-catechin gallate |
| Clove | eugenol, eugenyl acetate and caryophyllene |
| Turmeric | α-turmerone, β-turmerone and ar-turmerone |
| Cinnamon | cinnamaldehyde, camphor, cinnamyl-acetate, caryophyllene, trans α-bergamotene, caryophillene oxide, linalool, geraniol, bornyl acetate, eugenol, γ-elemene, α-copaene, guaiol, and α-cubebene |
| Lemon | limonene, p-mentha-3,8-diene, β-pinene, γ-terpinene, myrcene, sabinene, myrcene, and geranial |
| Cymbopogon | myrcene, limonene, citral, geraniol, citronellol, geranyl acetate, neral, and nerol |
| Thymol | p-cymene, γ-terpinene and thymol |
| Eucalyptus | 1,8-cineol and α-pinene |
| Oregano | rosmarinic acid, linalool, thymol, carvacrol, tannins, flavonoids, triterpenes, phenol carvacrol, and thymol. |
| Syzygium aromaticum | eugenyl acetate, eugenol, and β-caryophyllene |
| PLA/Active Agent Composition (%) | Active Agent | Tensile Strength (MPa) | Elongation at Breaks (%) | Reference | ||
|---|---|---|---|---|---|---|
| a | b | a | b | |||
| 98/2 | green tea extract | 12.52 | 10.29 | 260.11 | 121.95 | [52] |
| 95/5 | carvacrol | 26.8 | 16.4 | 267.3 | 194.9 | [53] |
| 97/3 | clove essential oil | 43.30 | 11.8 | 2.60 | 30.7 | [54] |
| 98/2 | mango peel extract | 57.77 | 46.48 | 6.77 | 14.31 | [55] |
| 99/1 | thyme essential oil | 2.90 | 3.90 | 11.33 | 23.19 | [56] |
| 95/5 | mediterranean propolis extract | 36.80 | 26.2 | 2.9 | 3.0 | [57] |
| 91/9 | thyme essential oil | 64.16 | 49.81 | 3.08 | 175.99 | [58] |
| 95/5 | thymol | 29.6 | 4.02 | 176.4 | 76.61 | [59] |
| 98/2 | rice straw extract | 34 | 34 | 6 | 3.4 | [60] |
| 99.5/0.5 | pomegranate peel extract | 88.7 | 67.92 | 47.3 | 69.04 | [61] |
| Film Composition | WVTR (g/m2/s × 10−4) | Effect on Film Properties | Reference | |
|---|---|---|---|---|
| a | b | |||
| PLA-Cinnamon essential oil | 0.345 | 0.793 |
| [66] |
| PLA-Betel leaf ethanolic extract | 0.410 | 0.30 |
| [67] |
| PLA/PBAT-Peppermint essential oil | 0.916 | 1.036 |
| [68] |
| PLA-Rosemary essential oil | 1.70 | 1.58 |
| [69] |
| PLA-Carvacrol essential oil | 0.045 | 0.043 |
| [70] |
| PLA-PEG | 6.28 | 6.44 |
| [71] |
| PLA/PBSA | 0.175 | 0.129 |
| [72] |
| PLA/PBAT-Trans-cinnamaldehyde | 0.154 | 0.169 |
| [73] |
| PLA-Pea Starch | 0.22 | 0.27 |
| [74] |
| PLA-Chitosan | 3.75 | 0.085 |
| [75] |
| PLA/PHB-Cinnamaldehyde | 0.26 | 0.69 |
| [76] |
| PLA-Oregano Essential Oil | 0.112 | 0.135 |
| [77] |
| Polymers | Inhibitory Effect | Reference |
|---|---|---|
| PLA-pink pepper essential oil | Pink pepper essential oil contains myrcene, which has antimicrobial action against S. aureus and L. monocytogenes, resulting in an inhibitory effect of 30 and 62%for L. monocytogenes and S. aureus, respectively, on day 21 of storage. | [86] |
| PLA-d-Limonene essential oil | Regardless of irradiation source or d-limonene loading, PLA/limonene films demonstrated 99.99% efficiency against Escherichia coli. | [87] |
| PLA-Polyphenols quercetin | The antibacterial level of reducing bacterial colonies against Escherichia coli films based on PLA increased to 87.8% with the addition of the polyphenol quercetin. | [88] |
| PLA-Ginger Essential Oil | The bacterial growth of the PLA/Ginger Essential Oil composite film was gradually stopped because of the presence of α-zingiberene and β-sesquiphellandrene. | [89] |
| PLA-Carvacrol essential oil | Carvacrol-containing films inhibited the growth of Rhizopus sp. and Penicillium sp. | [90] |
| PLA-Argan essential oil | The addition of argan essential oil was able to reduce the bacterial colonies of E. coli (86.5%), L. monocytogenes (72.2%) and S. Typhimurium (81.9%). | [91] |
| PLA-Persicaria hydropiper extract | The antibacterial activity of the ethanol extract of Persicaria hydropiper was able to reduce the growth of S. aureus (12.5%) but was unable to reduce the growth of E. coli and S. Typhimurium. | [92] |
| PLA-Oregano essential oil | The growth inhibition of S. Typhimurium, E. coli, and L. monocytogenes was up to 99%, after the addition of oregano oil stopped the growth of pathogenic bacteria in vitro. | [93] |
| PLA-Thyme essential oil | E. coli growth was slightly inhibited by thyme oil film (2.76%). | [94] |
| PLA-Allium ursinum extract | The antimicrobial activity of allium ursinum extract reduced colony growth of S. aureus (53%) and E. coli (100%) | [95] |
| Polymers | Methodology | Activity | References |
|---|---|---|---|
| PLA-Lemon extract | Lipid Oxidation Assays of almond including the following: Thiobarbituric acid-reactive substances (TBARS), Fat extraction, Peroxide value, p-Anisidine value. |
| [99] |
| PLA-Olive Pomace Extract | Physicochemical parameters (hardness, weight loss, and color) were evaluated after 12 days of storage at 4 °C. |
| [100] |
| PLA-Lippia citriodora essential oil | The Quality Index Method (QIM) was used to perform sensory analysis on the rainbow trout fillet skin appearance (shiny to dull), the color of the fillets (pink to dark pink), the odor (freshness, seaweed, sour and rancid), and the texture (firm, elastic, soft, and very soft). |
| [101] |
| PLA-Perilla essential oil | Kjeldahl distillation was used to determine the TVB-N content of chicken breast fillets. |
| [102] |
| PLA-Marjoram essential oil | The total volatile base nitrogen (TVB-N) content of meat samples was determined using the AOAC (Association of Official Analytical Chemists) method. |
| [103] |
| PLA-Oregano essential oil | TVC was calculated to track when minced fish began to deteriorate microbiologically (TVC > 7 log cfu/g). Thiobarbituric acid (TBA) based on Malondialdehyde (MDA) value and Sensory evaluation (acceptability test) was performed using a hedonic scale point from 9 (most liked) to 1 (least liked) for minced fish. |
| [104] |
| PLA-Green tea extract | Smoked salmon was tested based on fat extraction to examine its peroxide value, p-Anisidine value and TBARS. |
| [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasution, H.; Harahap, H.; Julianti, E.; Safitri, A.; Jaafar, M. Smart Packaging Based on Polylactic Acid: The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical–Mechanical Properties, Colony Reduction, Perishable Food Shelf Life, and Future Prospective. Polymers 2023, 15, 4103. https://doi.org/10.3390/polym15204103
Nasution H, Harahap H, Julianti E, Safitri A, Jaafar M. Smart Packaging Based on Polylactic Acid: The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical–Mechanical Properties, Colony Reduction, Perishable Food Shelf Life, and Future Prospective. Polymers. 2023; 15(20):4103. https://doi.org/10.3390/polym15204103
Chicago/Turabian StyleNasution, Halimatuddahliana, Hamidah Harahap, Elisa Julianti, Aida Safitri, and Mariatti Jaafar. 2023. "Smart Packaging Based on Polylactic Acid: The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical–Mechanical Properties, Colony Reduction, Perishable Food Shelf Life, and Future Prospective" Polymers 15, no. 20: 4103. https://doi.org/10.3390/polym15204103
APA StyleNasution, H., Harahap, H., Julianti, E., Safitri, A., & Jaafar, M. (2023). Smart Packaging Based on Polylactic Acid: The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical–Mechanical Properties, Colony Reduction, Perishable Food Shelf Life, and Future Prospective. Polymers, 15(20), 4103. https://doi.org/10.3390/polym15204103







