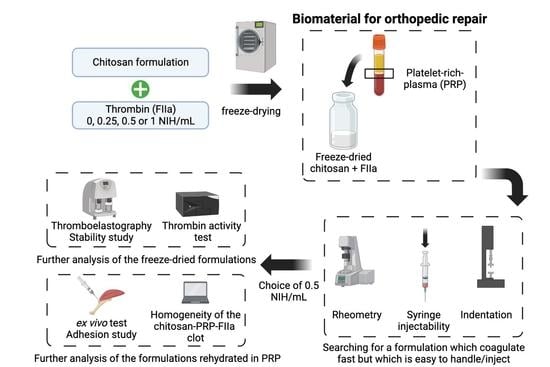
Injectable Lyophilized Chitosan-Thrombin-Platelet-Rich Plasma (CS-FIIa-PRP) Implant to Promote Tissue Regeneration: In Vitro and Ex Vivo Solidification Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Freeze-Dried Chitosan Formulations
2.2. Isolation of Platelet-Rich Plasma
2.3. Rehydration of the CS-FD Formulation
2.4. Assessment of Clotting Properties of CS-PRP Formulations by Thromboelastography
2.5. Assessment of Clotting Properties of CS-PRP Formulations through Rheology Measurements
2.6. Assessment of the Force Required to Eject the Biomaterial
2.7. Assessment of Hybrid-Clot Mechanical Properties through Indentation
2.8. Assessment of Hybrid-Clot Homogeneity through Histology and MASQH Algorithm
2.9. Assessment of CS-PRP Formulations Adhesion to Tendon Tissues Using an Ex Vivo Test
2.10. Assessment of Thrombin Activity in Freeze-Dried Chitosan Formulations
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chitosan Freeze-Dried with Thrombin Easily Solubilizes in PRP, Exhibits Thrombin Activity, and Maintains Stability for at Least 2 Months at Room Temperature
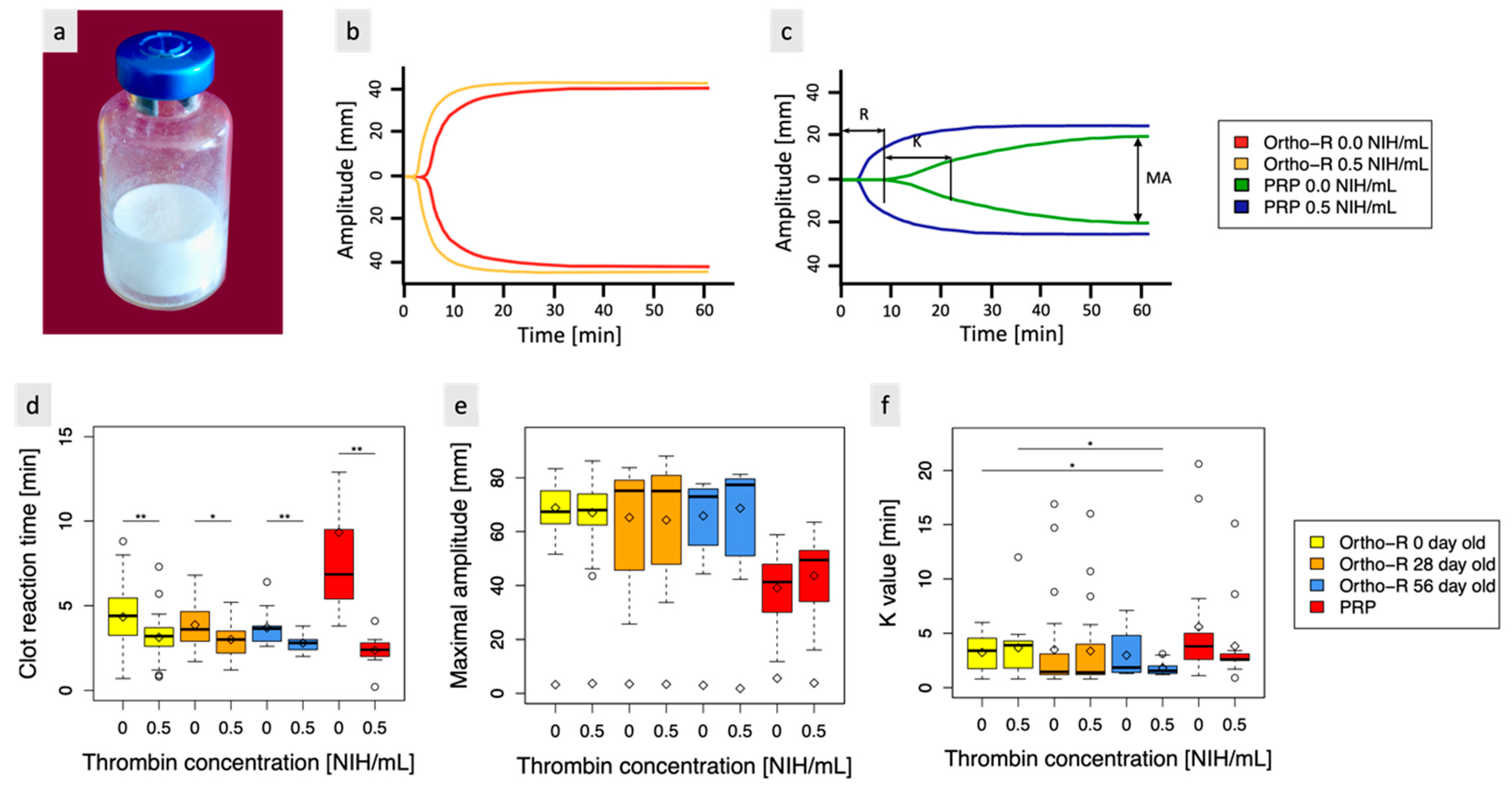
3.2. Biomaterial Containing 0.5 NIH/mL of Thrombin Is Easily Dispensed through an 18-Gauge Needle and 10-cc Syringe up to Six Min after Rehydration of the Lyophilized Formulation in PRP
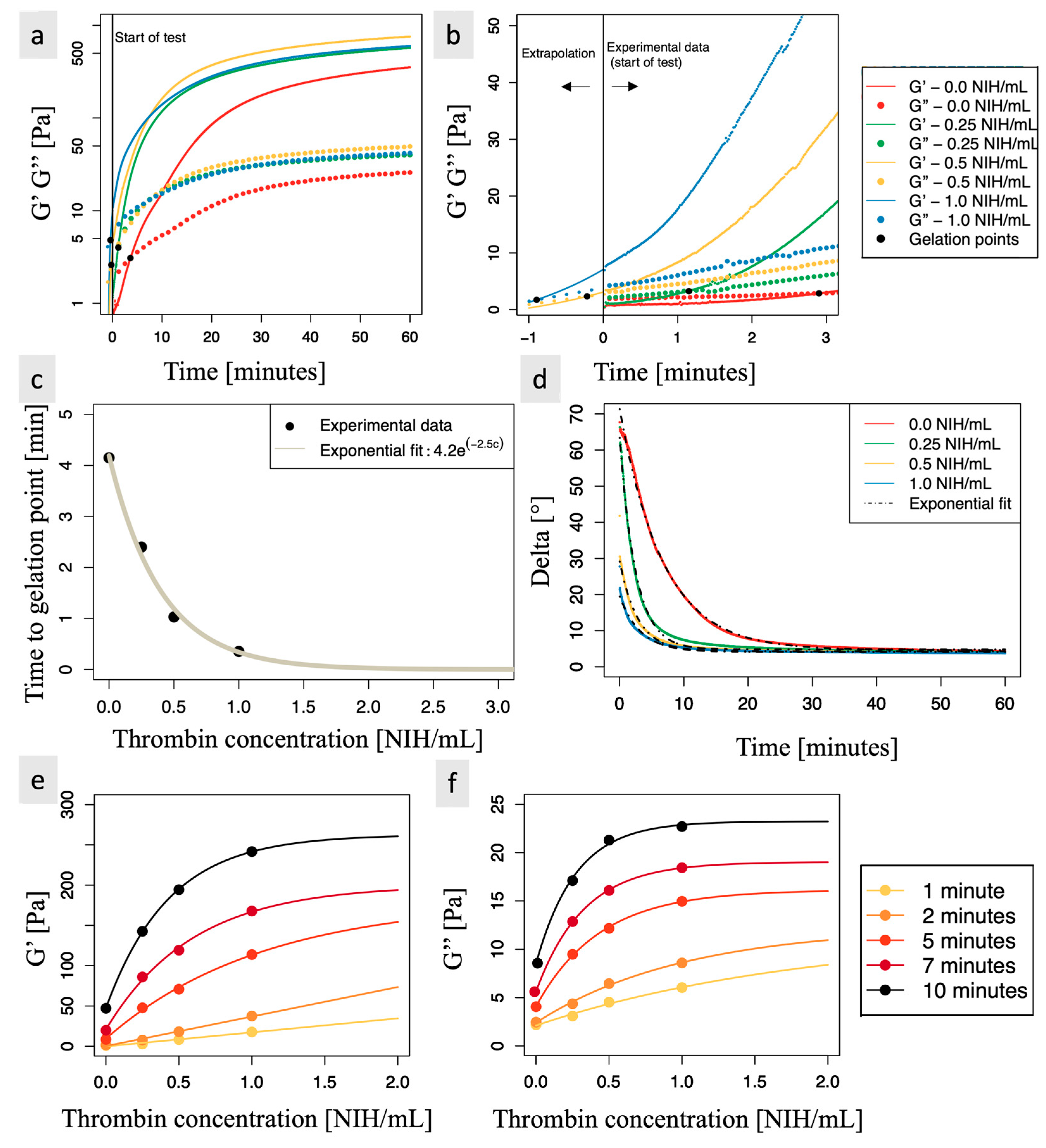
3.3. Rheometry Reveals That Thrombin Concentration Has a Significant Impact on Storage and Loss Modulus, as Well as on the Time to Gelation Point
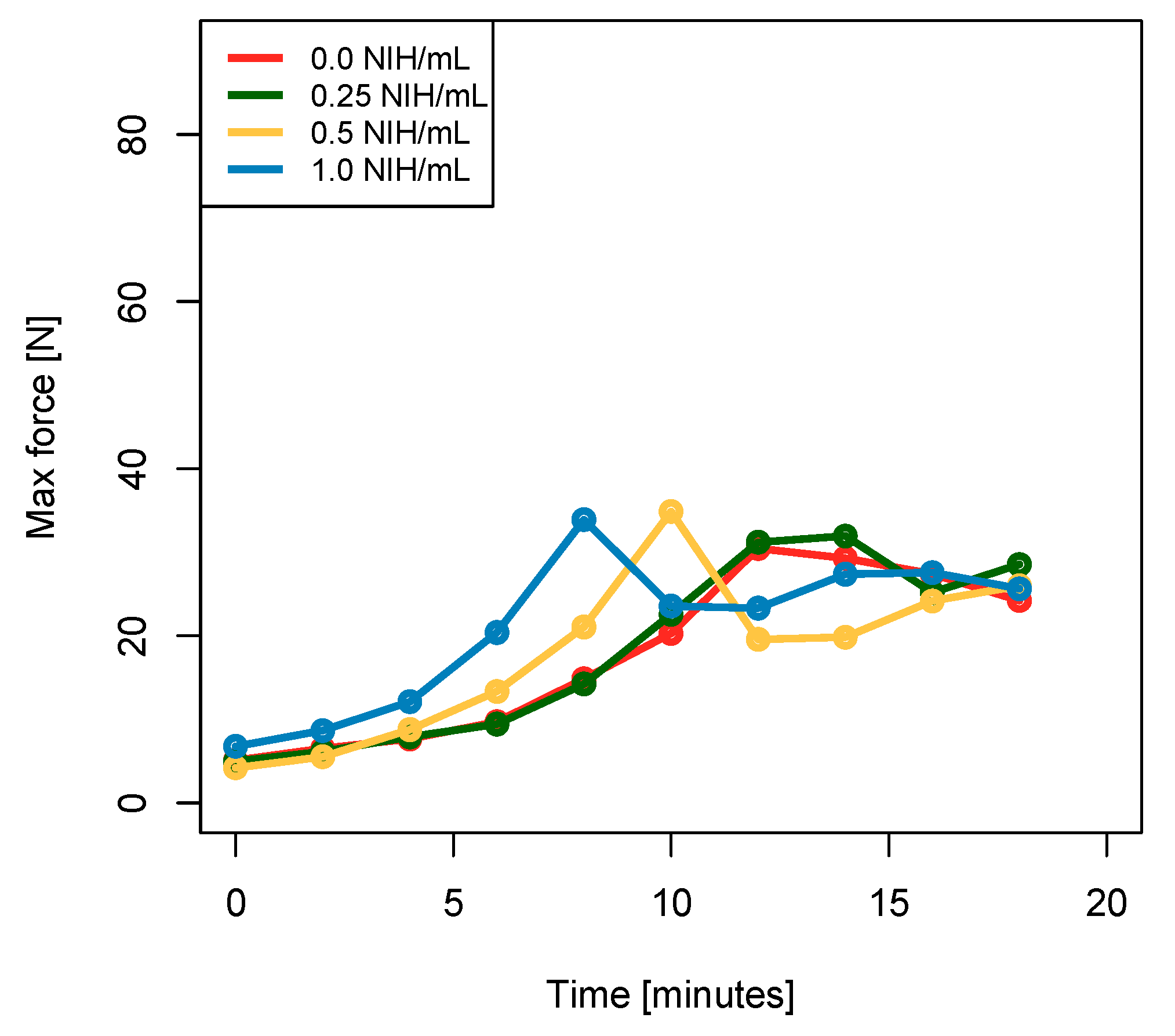
3.4. Indentation Experiments Indicate That There Is No Significant Difference in Stiffness and Equilibrium Force between the Coagulated Biomaterials Containing Thrombin and the Control Samples
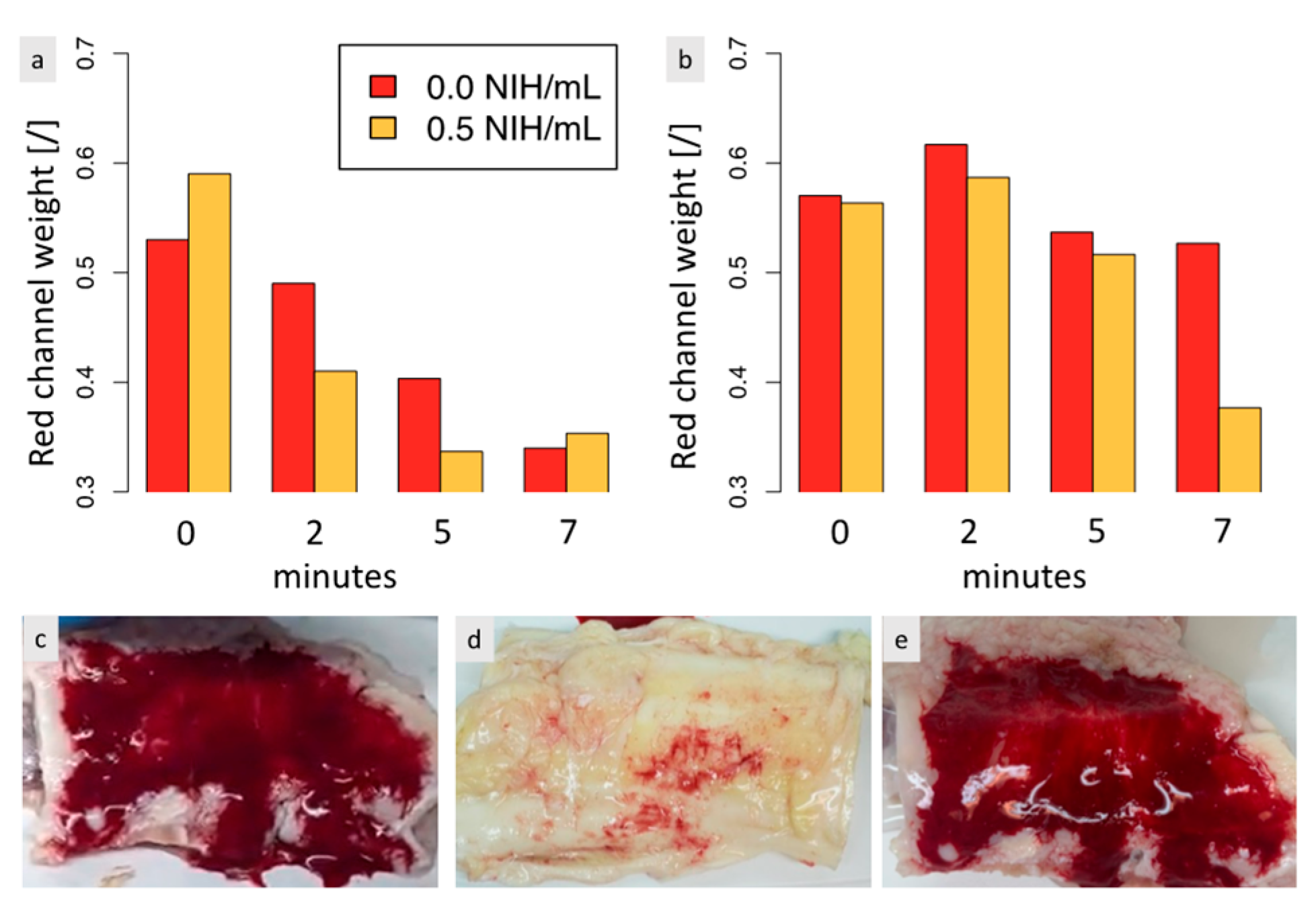
3.5. The Adherence of the Biomaterial to Tendon Tissues Is Impacted by the Biomaterial-Tendon Contact Duration and Increases Faster When Thrombin Is Present
3.6. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Assessment of Clotting Properties of CS-PRP Formulations through Rheology Measurements
References
- Chevrier, A.; Darras, V.; Picard, G.; Nelea, M.; Veilleux, D.; Lavertu, M.; Hoemann, C.D.; Buschman, M.D. Injectable chitosan-platelet-rich plasma implants to promote tissue regeneration: In vitro properties, in vivo residence, degradation, cell recruitment and vascularization. J. Tissue Eng. Regen. Med. 2018, 12, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, H.; Pan, S.; Fu, C.; Chang, Y.; Li, H.; Yang, X.; Qi, Z. Evaluation of New Film Based on Chitosan/Gold Nanocomposites on Antibacterial Property and Wound-Healing Efficacy. Adv. Mater. Sci. Eng. 2020, 2020, 6212540. [Google Scholar] [CrossRef]
- Duceac, I.A.; Vereștiuc, L.; Coroaba, A.; Arotăriței, D.; Coseri, S. All-polysaccharide hydrogels for drug delivery applications: Tunable chitosan beads surfaces via physical or chemical interactions, using oxidized pullulan. Int. J. Biol. Macromol. 2021, 181, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.; Fong, D. Immunological responses to chitosan for biomedical applications. In Chitosan Based Biomaterials Volume 1; Elsevier: Amsterdam, The Netherlands, 2017; pp. 45–79. [Google Scholar]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Lai, H.; Chen, G.; Zhang, W.; Wu, G.; Xia, Z. Research trends on platelet-rich plasma in the treatment of wounds during 2002–2021: A 20-year bibliometric analysis. Int. Wound J. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet rich plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Thu, A.C. The use of platelet-rich plasma in management of musculoskeletal pain: A narrative review. J. Yeungnam Med. Sci. 2022, 39, 206–215. [Google Scholar] [CrossRef]
- Popescu, M.N.; Iliescu, M.G.; Beiu, C.; Popa, L.G.; Mihai, M.M.; Berteanu, M.; Ionescu, A.M. Autologous platelet-rich plasma efficacy in the field of regenerative medicine: Product and quality control. BioMed Res. Int. 2021, 2021, 4672959. [Google Scholar] [CrossRef]
- Depres-Tremblay, G.; Chevrier, A.; Snow, M.; Rodeo, S.; Buschmann, M.D. Freeze-dried chitosan-platelet-rich plasma implants improve supraspinatus tendon attachment in a transosseous rotator cuff repair model in the rabbit. J. Biomater. Appl. 2019, 33, 792–807. [Google Scholar] [CrossRef]
- Deprés-Tremblay, G.; Chevrier, A.; Tran-Khanh, N.; Nelea, M.; Buschmann, M.D. Chitosan inhibits platelet-mediated clot retraction, increases platelet-derived growth factor release, and increases residence time and bioactivity of platelet-rich plasma in vivo. Biomed. Mater. 2017, 13, 015005. [Google Scholar] [CrossRef]
- Chevrier, A.; Hurtig, M.; Lacasse, F.; Lavertu, M.; Potter, H.; Pownder, S.; Rodeo, S.; Buschmann, M. Freeze-dried chitosan solubilized in platelet-rich plasma in a sheep model of rotator cuff repair. Orthop. Proc. 2020, 102-B, 57. [Google Scholar]
- Dwivedi, G.; Chevrier, A.; Hoemann, C.D.; Buschmann, M.D. Injectable freeze-dried chitosan-platelet-rich-plasma implants improve marrow-stimulated cartilage repair in a chronic-defect rabbit model. J. Tissue Eng. Regen. Med. 2019, 13, 599–611. [Google Scholar] [CrossRef]
- Marchand, C.; Rivard, G.E.; Sun, J.; Hoemann, C.D. Solidification mechanisms of chitosan–glycerol phosphate/blood implant for articular cartilage repair. Osteoarthr. Cartil. 2009, 17, 953–960. [Google Scholar] [CrossRef]
- Sung, Y.K.; Lee, D.R.; Chung, D.J. Advances in the development of hemostatic biomaterials for medical application. Biomater. Res. 2021, 25, 37. [Google Scholar] [CrossRef]
- Zamora, M.; Robles, J.P.; Aguilar, M.B.; Romero-Gómez, S.d.J.; Bertsch, T.; Martinez de la Escalera, G.; Triebel, J.; Clapp, C. Thrombin cleaves prolactin into a potent 5.6-kDa vasoinhibin: Implication for tissue repair. Endocrinology 2021, 162, bqab177. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Nguyen, S.; Winnik, F.M.; Buschmann, M.D. Improved reproducibility in the determination of the molecular weight of chitosan by analytical size exclusion chromatography. Carbohydr. Polym. 2009, 75, 528–533. [Google Scholar] [CrossRef]
- Zheng, Y.; Mak, A.F.; Lue, B. Objective assessment of limb tissue elasticity: Development of a manual indentation procedure. J. Rehabil. Res. Dev. 1999, 36, 71–85. [Google Scholar] [PubMed]
- Forte, A.E.; Galvan, S.; Manieri, F.; y Baena, F.R.; Dini, D. A composite hydrogel for brain tissue phantoms. Mater. Des. 2016, 112, 227–238. [Google Scholar] [CrossRef]
- Van Dommelen, J.; Van der Sande, T.; Hrapko, M.; Peters, G. Mechanical properties of brain tissue by indentation: Interregional variation. J. Mech. Behav. Biomed. Mater. 2010, 3, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Rossomacha, E.; Hoemann, C.D.; Shive, M.S. Simple methods for staining chitosan in biotechnological applications. J. Histotechnol. 2004, 27, 31–36. [Google Scholar] [CrossRef]
- Milano, F.; Chevrier, A.; Crescenzo, G.D.; Lavertu, M. Robust Segmentation-Free Algorithm for Homogeneity Quantification in Images. IEEE Trans. Image Process. 2021, 30, 5533–5544. [Google Scholar] [CrossRef]
- Memon, M.; Kay, J.; Gholami, A.; Simunovic, N.; Ayeni, O.R. Fluid Extravasation in Shoulder Arthroscopic Surgery: A Systematic Review. Orthop. J. Sport. Med. 2018, 6, 2325967118771616. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Liu, W.; Zhang, J.; Cao, Z.; Xu, F.; Yao, K. A chitosan-arginine conjugate as a novel anticoagulation biomaterial. J. Mater. Sci. Mater. Med. 2004, 15, 1199–1203. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Cheng, N.; Cao, Z.; Yao, K. Anticoagulation activity of crosslinked N-sulfofurfuryl chitosan membranes. J. Appl. Polym. Sci. 2004, 94, 53–56. [Google Scholar] [CrossRef]
- Aleksakhina, E.; Parfenov, A.; Priyatkin, D.; Fomina, N.; Tomilova, I. Spectral and structural properties of clotting factor proteins under mechanical stress. J. Phys. Conf. Ser. 2021, 2094, 022044. [Google Scholar] [CrossRef]
- Cone, S.J.; Fuquay, A.T.; Litofsky, J.M.; Dement, T.C.; Carolan, C.A.; Hudson, N.E. Inherent fibrin fiber tension propels mechanisms of network clearance during fibrinolysis. Acta Biomater. 2020, 107, 164–177. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Prince, E.; Weitz, J.I.; Panyukov, S.; Ramachandran, A.; Rubinstein, M.; Kumacheva, E. Fibrous hydrogels under biaxial confinement. Nat. Commun. 2022, 13, 3264. [Google Scholar] [CrossRef]
- Watt, R.P.; Khatri, H.; Dibble, A.R.G. Injectability as a function of viscosity and dosing materials for subcutaneous administration. Int. J. Pharm. 2019, 554, 376–386. [Google Scholar] [CrossRef]
- Lechner, R.; Helm, M.; Mueller, M.; Wille, T.; Riesner, H.-J.; Friemert, B. In-vitro study of species-specific coagulation differences in animals and humans using rotational thromboelastometry (ROTEM). BMJ Mil. Health 2019, 165, 356–359. [Google Scholar] [CrossRef]
- Seegers, W.H.; Smith, H. Factors which influence the activity of purified thrombin. Am. J. Physiol.-Leg. Content 1942, 137, 348–354. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Plasenzotti, R.; Spiel, A.; Quehenberger, P.; Jilma, B. Interspecies differences in coagulation profile. Thromb. Haemost. 2008, 100, 397–404. [Google Scholar] [CrossRef]
- Doolittle, R.F.; Oncley, J.L.; Surgenor, D.M. Species differences in the interaction of thrombin and fibrinogen. J. Biol. Chem. 1962, 237, 3123–3127. [Google Scholar] [CrossRef]
- Evans, P.A.; Hawkins, K.; Williams, P.R. Rheometry for blood coagulation studies. Rheol. Rev. 2006, 2006, 255–291. [Google Scholar]
- Tomaiuolo, G.; Carciati, A.; Caserta, S.; Guido, S. Blood linear viscoelasticity by small amplitude oscillatory flow. Rheol. Acta 2016, 55, 485–495. [Google Scholar] [CrossRef]
- Alves, M.M.; Rocha, C.; Gonçalves, M.P. Study of the rheological behaviour of human blood using a controlled stress rheometer. Clin. Hemorheol. Microcirc. 2013, 53, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Kar, A.; Chaudhury, K.; Maiti, T.K.; Chakraborty, S. Formation of blood droplets: Influence of the plasma proteins. ACS Omega 2018, 3, 10967–10973. [Google Scholar] [CrossRef] [PubMed]

| Time [min] | 0.0 NIH/mL | 0.25 NIH/mL | 0.5 NIH/mL | 1.0 NIH/mL | ||||
|---|---|---|---|---|---|---|---|---|
| G′ | G″ | G′ | G″ | G′ | G″ | G′ | G″ | |
| 5 | 5% | 25% | 25% | 58% | 40% | 76% | 63% | 93% |
| 7 | 10% | 30% | 41% | 67% | 61% | 85% | 83% | 97% |
| 10 | 18% | 37% | 54% | 75% | 74% | 90% | 92% | 98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milano, F.; Chevrier, A.; De Crescenzo, G.; Lavertu, M. Injectable Lyophilized Chitosan-Thrombin-Platelet-Rich Plasma (CS-FIIa-PRP) Implant to Promote Tissue Regeneration: In Vitro and Ex Vivo Solidification Properties. Polymers 2023, 15, 2919. https://doi.org/10.3390/polym15132919
Milano F, Chevrier A, De Crescenzo G, Lavertu M. Injectable Lyophilized Chitosan-Thrombin-Platelet-Rich Plasma (CS-FIIa-PRP) Implant to Promote Tissue Regeneration: In Vitro and Ex Vivo Solidification Properties. Polymers. 2023; 15(13):2919. https://doi.org/10.3390/polym15132919
Chicago/Turabian StyleMilano, Fiona, Anik Chevrier, Gregory De Crescenzo, and Marc Lavertu. 2023. "Injectable Lyophilized Chitosan-Thrombin-Platelet-Rich Plasma (CS-FIIa-PRP) Implant to Promote Tissue Regeneration: In Vitro and Ex Vivo Solidification Properties" Polymers 15, no. 13: 2919. https://doi.org/10.3390/polym15132919
APA StyleMilano, F., Chevrier, A., De Crescenzo, G., & Lavertu, M. (2023). Injectable Lyophilized Chitosan-Thrombin-Platelet-Rich Plasma (CS-FIIa-PRP) Implant to Promote Tissue Regeneration: In Vitro and Ex Vivo Solidification Properties. Polymers, 15(13), 2919. https://doi.org/10.3390/polym15132919