A Review on Biopolymer-Based Biodegradable Film for Food Packaging: Trends over the Last Decade and Future Research
Abstract
1. Introduction
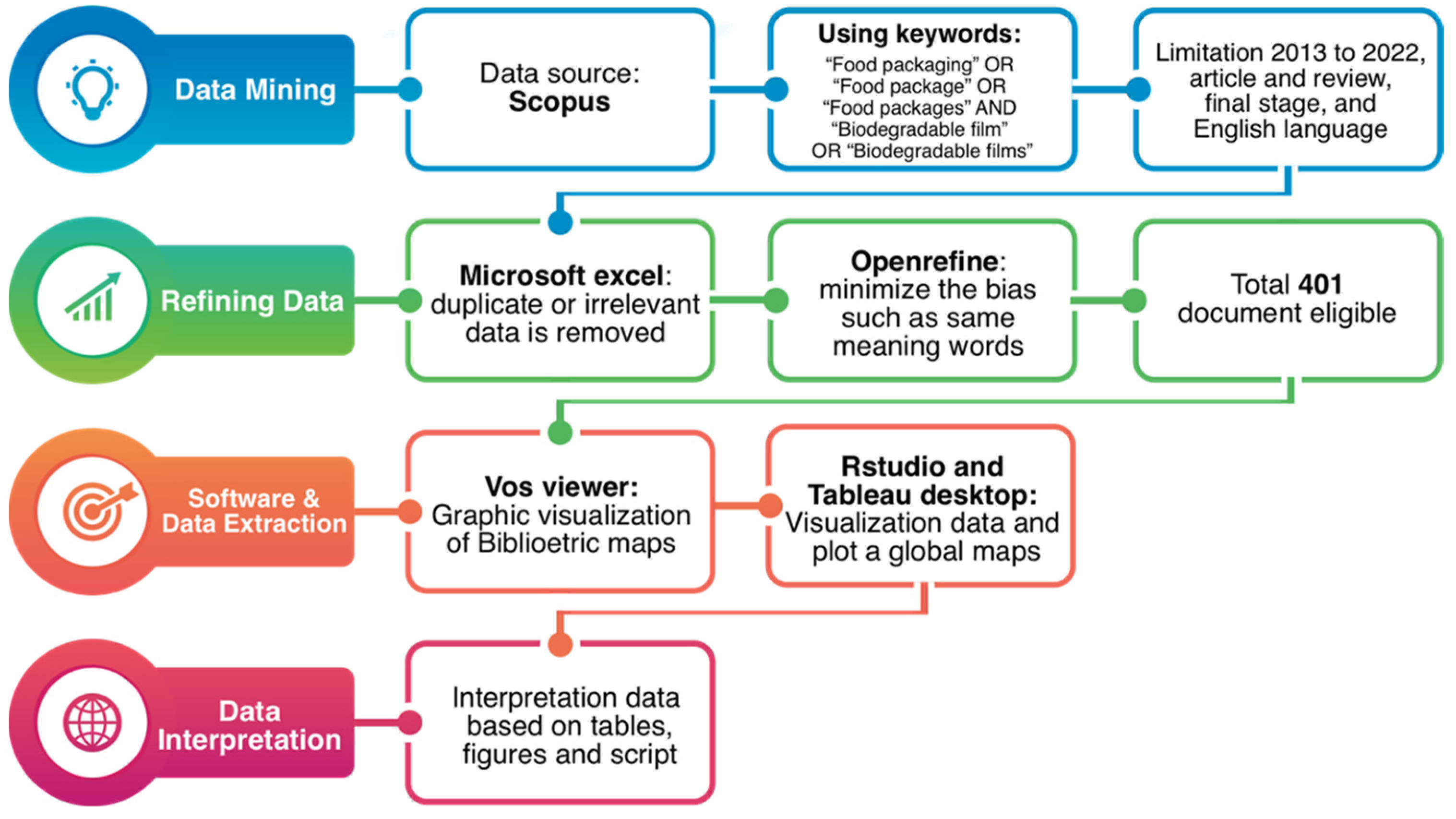
2. Materials and Methods
2.1. Data Sources
2.2. Data Analysis
- -
- Inclusion criteria: Study period between 2013 and 2022; studies in the final publication phase; publications in the English language; document types: article and review
- -
- Exclusion criteria: Publications in languages other than English; theses, dissertations, books, book chapter, and conference papers; and gray literature.
3. Overview of Biodegradable Polymers
3.1. Natural Biopolymers
3.2. Synthetic Biopolymers
3.3. Microbial Biopolymers
4. Results and Discussion
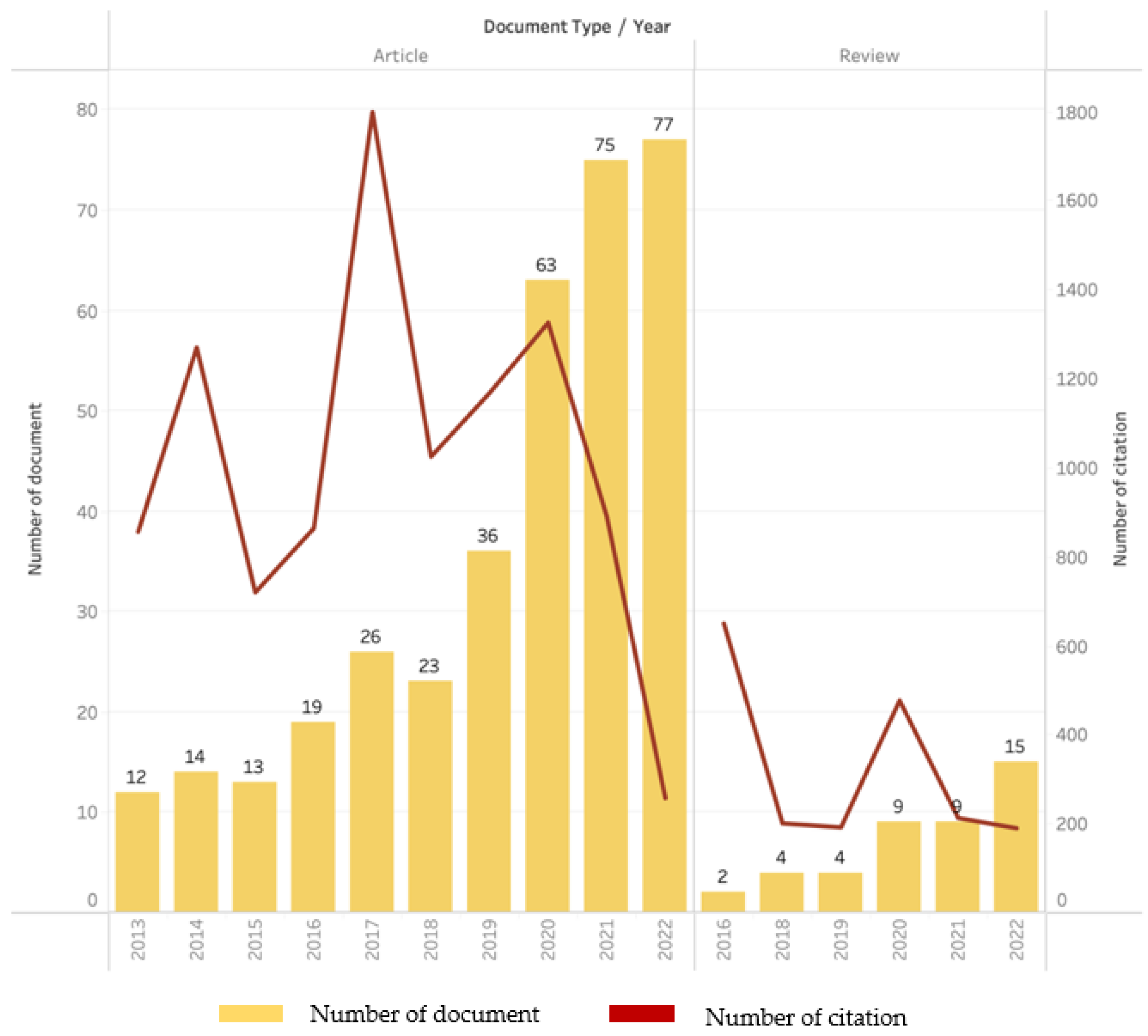
4.1. Bibliometric Analysis and Scientific Performance
4.2. Bibliometric Analysis of Country Performance
4.3. Bibliometric Analysis of the Most Relationship between the Journals, Countries, and Keywords
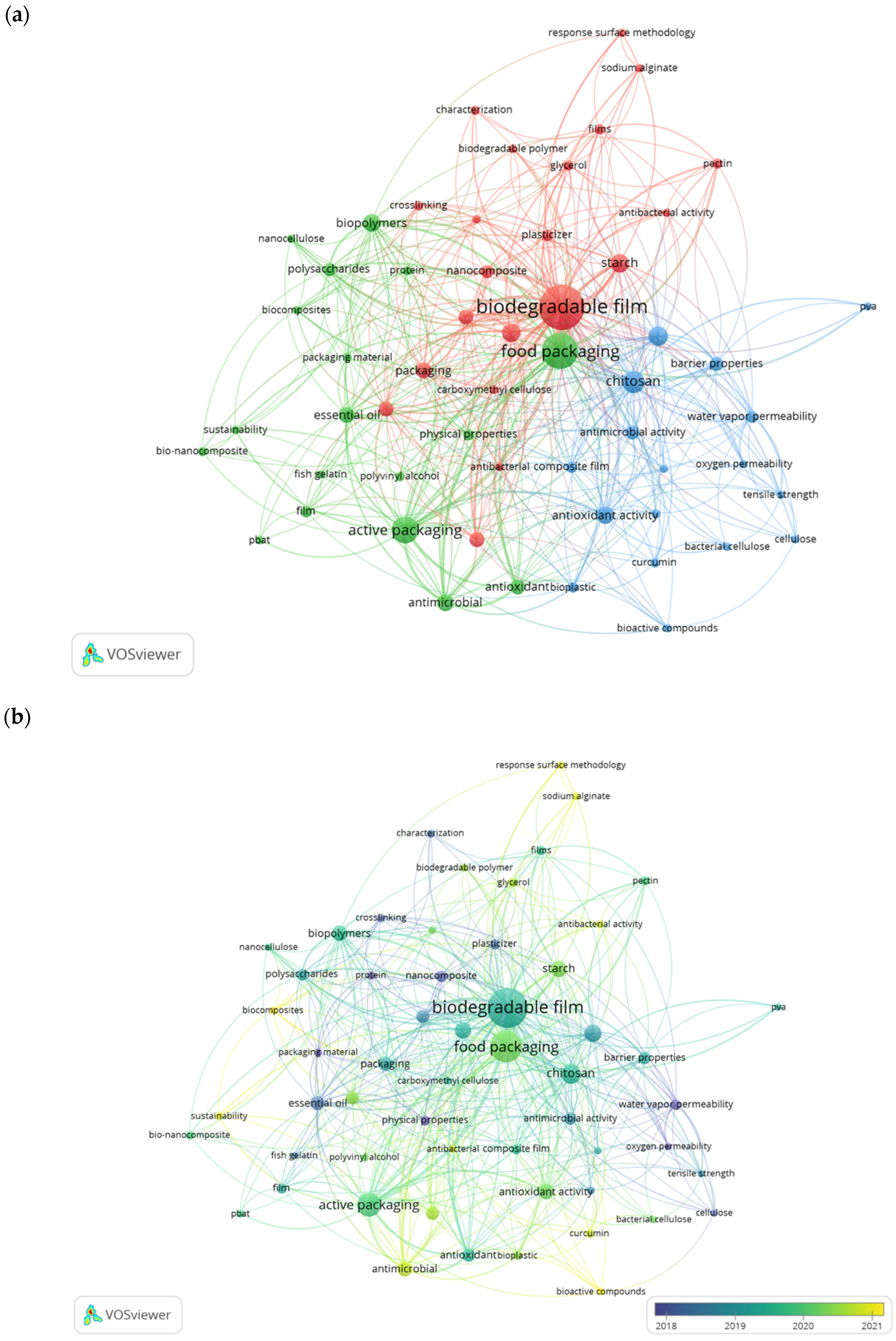
4.4. Analysis of Keywords
5. Application of Biodegradable Films Packaging for Food
| Type of Polymers | Application | Active/Antioxidant Compound | Characteristic Packaging/ Improved Features | Ref. |
|---|---|---|---|---|
| CS | Chicken fillet | Garlic essential oil |
| [78] |
| Cellulose/potato peel (PP) | Fresh pork | Curcumin |
| [73] |
| PLA/PBAT | Bakery | Carvacrol |
| [44] |
| ZNO/CS | Fresh poultry and minced meat | Zinc oxide nanoparticles |
| [86] |
| Corn starch | Strawberry and ricotta | Chitosan oligomers |
| [67] |
| PBAT/PLA | Shrimps | Carvacrol, citral and α-terpineol |
| [42] |
| PBAT/thermoplastic starch (TPS) | Fresh noodles | Sorbate and benzoate |
| [87] |
| Skate skin gelatin (SSG) | Chicken tenderloin | Thyme essential oil |
| [80] |
| Purple yam starch (PYS)/CS/glycerol | Apples | Chitosan |
| [88] |
| PVA | Strawberry and cherry tomatoes | Citric acid (CA)/carboxymethyl chitosan (CMCS) |
| [68] |
| Gelatin | Fresh durian cut | Durian leaf extract |
| [69] |
| Triticale flour | Cheese | Natamycin |
| [89] |
| Fish skin gelatin (FSG) | Cheese | Moringa oleifera Lam. leaf extract (ME) |
| [90] |
| PLA | Beef | Nisin/ε-poly lysine (ε-PL) |
| [91] |
| Rice flour/PBAT | Pasta | Potassium sorbate |
| [92] |
| PVA, cyclodextrin, and gelatin | Chicken meat | Mango peel (MP) |
| [75] |
| Rye starch | Chicken breast | Rosehip extract (RHE) |
| [74] |
| Curdlan | Fresh pork | Nanocellulose (NC) |
| [93] |
| U. pinnatifida protein (UPP)/gelatin | Smoked chicken breast | Vanillin |
| [94] |
| CS/pullulan | Goat meat | Carvacrol |
| [95] |
| PBAT | Strawberry | Moringa oleifera (MO) |
| [72] |
| CS/bacterial cellulose (BC) | Grass carp | Tea polyphenol (TP) |
| [96] |
| Alginate/TiO2 | Beef | Cumin essential oil |
| [81] |
| PBAT | Mozzarella cheese | Origanum vulgare oil (EOE) |
| [82] |
| CS/Chickpea flour (CF) | Chicken breast | Citric acid/Curcumin (CUR) |
| [85] |
| PBAT/PLA | Strawberry | Cinnamon essential oil (EO) |
| [43] |
| PLA | Avocado fresh cut | Natural olive wastewater extract (OWE) |
| [76] |
6. Biodegradable Film as a Current Trend in the Food Sector
6.1. Soil Burial
6.2. Compost Environment
6.3. Water Environment
| Type of Polymers | Type of Degradation | Degradation Parameters | Degradation Characteristics | Ref. | ||
|---|---|---|---|---|---|---|
| Temperatures | Degradation Period (Days) | Test Method | ||||
| CS/thyme essential oil (TEO) | Soil burial | NA | 28 | Weight loss |
| [97] |
| Cellulose/carboxymethyl cellulose/snail mucus extracted | Soil burial | NA | 30 | Weight loss |
| [110] |
| PVA | Soil burial | NA | 5 | Weight loss |
| [111] |
| Starch/PBAT | Composting | 58 °C | 18 | Percent biodegradation |
| [107] |
| Starch/PVA | Soil burial | 27 ± 5 °C | 21 | Weight loss |
| [112] |
| Starch/carrageenan | Seawater | NA | 70 | Visualization (digital camera) |
| [108] |
| Composting | NA | 30 | Visualization (digital camera) |
| ||
| Hemicelullose/celullose nanocrystal (CNC)/cellulose nanofibril (CNF) | Soil burial | NA | 10 | Visualization (digital camera) |
| [113] |
| PVA/starch/pectin | Soil burial | 30–37 °C | 90 | Weight loss |
| [114] |
| PVA/carboxymethyl CS (CMCS)/citric acid (CA) films | Soil burial | NA | 48 | Visualization (digital camera) |
| [68] |
| Cellulose/CS/castor oil | Compost | 25 °C | 20 | Visualization (digital camera) |
| [115] |
| PVA/chitin | Soil burial | NA | 30 | Weight loss |
| [116] |
| Hake protein/gluten/zein | Soil burial | NA | 60 | Weight loss |
| [117] |
| Polyhydroxyalkanoate (PHA) | Soil burial | 23 °C | 80 | Weight loss |
| [118] |
| CS/polyurethane (PU) | Soil burial | Room temperature | 28 | Weight loss |
| [100] |
| CS/PVA | Soil burial | NA | 30 | Weight loss |
| [99] |
| CS/PVA/guar gum | Soil burial | NA | 7 | Weight loss |
| [62] |
| Gelatin/dialdehyde xanthan gum (DXG) | Soil burial | Room temperature | 30 | Weight loss |
| [119] |
| Gelatin | Soil burial | NA | 15 | Weight loss |
| [120] |
| Starch | Soil burial | NA | 15 | Weight loss |
| [121] |
| Starch/glycerol | Compost | NA | 12 | Visualization (digital camera) |
| [106] |
| Cellulose/S. urens short fiber (SUSF) | Compost | 30 ± 2 °C | 40 | Weight loss |
| [122] |
| CS/PVA | Soil burial | NA | 15 | Weight loss |
| [123] |
| Gelatin | Soil burial | NA | 49 | Weight loss |
| [124] |
7. Commercial Application of Biopolymer-Based Biodegradable Film
| Suppliers | Materials | Brand Names | Application | Properties | Ref. |
|---|---|---|---|---|---|
| Innovia Films (Wingston, UK) | Cellulose | Propafilm TM RC30 | Biscuits, cookies, crackers, bakery |
| [128] |
| Propafilm TM FFF | Candy and confectionery |
| |||
| Propafilm™ Strata SL | Coffee |
| |||
| RayoForm™ Propafilm™ | Dairy products |
| |||
| Propafilm™ Strata | Granola (nutritional bars) |
| |||
| Propafilm™ QLD Propafilm™ QID | Ice cream and frozen novelties |
| |||
| RayoWrap™ | Juice and sports drinks |
| |||
| Propafilm™ QLD Propafilm™ MPM 17 Propafilm™ GPD 17 | Snacks |
| |||
| PropafilmTM | Tea and infusions |
| |||
| Amcor (Zutphen, Netherlands) | Cellulose, starch, and PLA | HeatFlex™ | Shelf-stable ready meals, juices, smoothies, and sports and energy drinks |
| [126] |
| PrimeSeal™ | Fresh meat and poultry |
| |||
| DairySeal™ | Cheese |
| |||
| AmPrima™ PE Plus | Dry baby food, milk formula, coffee, cereals, nuts and dried fruits; liquid pouches: yogurt and fresh cheese, juices and smoothies, and other dairy products |
| |||
| AmPrima™ | Fresh fruits and vegetables, frozen fruits and vegetables, cereals, snack bars, cheese, frozen meat, poultry, and coffee |
| |||
| Bio4Pack (Haaksbergen, Netherlands) | Starch and PLA | Bio4Pack | Rice, grain, cookies, perishable products such as meat, and crisps |
| [130] |
| Novamont (Italy) | Starch | Mater-Bi | Fresh and dry foodstuffs |
| [127] |
| Plantic Technologies Ltd. (Jena, German) | Starch | Plantic™ | Meat, snack, coffee, and dairy products |
| [131] |
| Taghleef Industries (Koblenz, German) | PLA | Extendo® | Bakery, coffee, snacks, ice cream, and freshly cut produce |
| [132] |
| Cellulose and PLA | Nativia® | Fresh products, bakery, dairy-perishable, snacks, and confectionery |
| ||
| Sidaplax (Ghent, Belgium) | PLA | Earthfirst® Biopolymer Films | Fresh and dry products |
| [133] |
| Clondalkin group (Wieringerwerf, NL) | PLA | Wentus (Wentopro®) | Fresh and dry products |
| [134] |
8. Overview of Social, Environmental, and Economic Aspects
9. Conclusions: Limitation, Challenges, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Africa 2023, 6, 117–144. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- The International Institute for Sustainable Development. Global Plastic Outlook Calls for More Circularity, National Roadmaps. Available online: https://sdg.iisd.org/news/global-plastic-outlook-calls-for-more-circularity-national-roadmaps/ (accessed on 12 January 2023).
- OECD. Plastic Leakage and Greenhouse Gas Emissions Are Increasing. Available online: https://www.oecd.org/environment/plastics/increased-plastic-leakage-and-greenhouse-gas-emissions.htm (accessed on 12 January 2023).
- Sandhu, R.S.; Shakya, M.; Durgavati Vishwavidyalaya, R. Comparative Study of Synthetic Plastics and Biodegradable Plastics. Glob. J. Biosci. Biotechnol. 2019, 8, 107–112. [Google Scholar]
- Galati, A.; Scalenghe, R. Plastic End-of-Life Alternatives, with a Focus on the Agricultural Sector. Curr. Opin. Chem. Eng. 2021, 32, 100681. [Google Scholar] [CrossRef]
- Ford, H.V.; Jones, N.H.; Davies, A.J.; Godley, B.J.; Jambeck, J.R.; Napper, I.E.; Suckling, C.C.; Williams, G.J.; Woodall, L.C.; Koldewey, H.J. The Fundamental Links between Climate Change and Marine Plastic Pollution. Sci. Total Environ. 2022, 806, 150392. [Google Scholar] [CrossRef]
- Buijzen, F.; Corbion, T. End-of-Life Options for Bioplastics the Role of PLA in the Circular Economy. Available online: https://www.totalenergies-corbion.com/media/bm1p2dwl/totalcorbionpla_whitepaper_end-of-life-201127.pdf (accessed on 13 June 2023).
- Gioia, C.; Giacobazzi, G.; Vannini, M.; Totaro, G.; Sisti, L.; Colonna, M.; Marchese, P.; Celli, A. End of Life of Biodegradable Plastics: Composting versus Re/Upcycling. ChemSusChem 2021, 14, 4167–4175. [Google Scholar] [CrossRef]
- Bamps, B.; Guimaraes, R.M.; Duijsters, G.; Hermans, D.; Vanminsel, J.; Vervoort, E.; Buntinx, M.; Peeters, R. Characterizing Mechanical, Heat Seal, and Gas Barrier Performance of Biodegradable Films to Determine Food Packaging Applications. Polymers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Ramesh, M.; Muthukrishnan, M. 25-Biodegradable Polymer Blends and Composites for Food-Packaging Applications. In Woodhead Publishing Series in Composites Science and Engineering; Mavinkere Rangappa, S., Parameswaranpillai, J., Siengchin, S., Ramesh, M., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 693–716. ISBN 978-0-12-823791-5. [Google Scholar]
- Homthawornchoo, W.; Kaewprachu, P.; Pinijsuwan, S.; Romruen, O.; Rawdkuen, S. Enhancing the UV-Light Barrier, Thermal Stability, Tensile Strength, and Antimicrobial Properties of Rice Starch–Gelatin Composite Films through the Incorporation of Zinc Oxide Nanoparticles. Polymers 2022, 14, 2505. [Google Scholar] [CrossRef]
- Karimi Sani, I.; Masoudpour-Behabadi, M.; Alizadeh Sani, M.; Motalebinejad, H.; Juma, A.S.M.; Asdagh, A.; Eghbaljoo, H.; Khodaei, S.M.; Rhim, J.-W.; Mohammadi, F. Value-Added Utilization of Fruit and Vegetable Processing by-Products for the Manufacture of Biodegradable Food Packaging Films. Food Chem. 2023, 405, 134964. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U. Novel Biopolymer-Based Sustainable Composites for Food Packaging Applications: A Narrative Review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental Impact of Bioplastic Use: A Review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef] [PubMed]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of Biodegradable Plastics: New Problem or Solution to Solve the Global Plastic Pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in Applications and Prospects of Bioplastics and Biopolymers: A Review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Global Warming of 1.5 Celcius. Available online: https://www.ipcc.ch/sr15/ (accessed on 11 June 2023).
- Sun, Y.; Bai, Y.; Yang, W.; Bu, K.; Tanveer, S.K.; Hai, J. Global Trends in Natural Biopolymers in the 21st Century: A Scientometric Review. Front. Chem. 2022, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Pathak, A.; Kumar, P.; Chetana, S.; Sharma, N. Commercial Production of Bioplastic from Organic Waste–Derived Biopolymers Viz-a-Viz Waste Treatment: A Minireview. Biomass Convers Biorefinery 2022. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Saha, P.; Adhikari, J.; Deshmukh, K.; Ahamed, M.B.; Cabibihan, J.J. Recent Advances in Mechanical Properties of Biopolymer Composites: A Review. Polym. Compos. 2020, 41, 32–59. [Google Scholar] [CrossRef]
- Ranganathan, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Utilization of Food Waste Streams for the Production of BioPolymers. Heliyon 2020, 6, e04891. [Google Scholar] [CrossRef]
- Behera, A.K.; Manna, S.; Das, N. Effect of Soy Waste/Cellulose on Mechanical, Water Sorption, and Biodegradation Properties of Thermoplastic Starch Composites. Starch-Stärke 2022, 74, 2100123. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Lee, W.Y. Development and Characterization of Biocomposite Films Based on Polysaccharides Derived from Okra Plant Waste for Food Packaging Application. Polymers 2022, 14, 4884. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rojas, A.; Arango Ospina, A.; Rodríguez-Vélez, P.; Arana-Florez, R. What Is the New about Food Packaging Material? A Bibliometric Review during 1996–2016. Trends Food Sci. Technol. 2019, 85, 252–261. [Google Scholar] [CrossRef]
- Palechor-Trochez, J.J.; Ramírez-Gonzales, G.; Villada-Castillo, H.S.; Solanilla-Duque, J.F. A Review of Trends in the Development of Bionanocomposites from Lignocellulosic and Polyacids Biomolecules as Packing Material Making Alternative: A Bibliometric Analysis. Int. J. Biol. Macromol. 2021, 192, 832–868. [Google Scholar] [CrossRef] [PubMed]
- Pluye, P.; Hong, Q.N. Combining the Power of Stories and the Power of Numbers: Mixed Methods Research and Mixed Studies Reviews. Annu. Rev. Public Health 2014, 35, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H. Literature Review as a Research Methodology: An Overview and Guidelines. J. Bus. Res. 2019, 104, 333–339. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. VOSviewer Manual. Available online: http://www.vosviewer.com/documentation/Manual_VOSviewer_1.6.1.pdf (accessed on 29 January 2023).
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 1–9. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Imran, R.; Arif, Z.U.; Akram, N.; Arshad, H.; Al Rashid, A.; García Márquez, F.P. Developments in Chemical Treatments, Manufacturing Techniques and Potential Applications of Natural-Fibers-Based Biodegradable Composites. Coatings 2021, 11, 293. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current Status of Biobased and Biodegradable Food Packaging Materials: Impact on Food Quality and Effect of Innovative Processing Technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Ochoa, T.A.; Almendárez, B.E.G.; Reyes, A.A.; Pastrana, D.M.R.; López, G.F.G.; Belloso, O.M.; González, C.R. Design and Characterization of Corn Starch Edible Films Including Beeswax and Natural Antimicrobials. Food Bioprocess Technol. 2017, 10, 103–114. [Google Scholar] [CrossRef]
- Chaichi, M.; Badii, F.; Mohammadi, A.; Hashemi, M. Novel Bioactive Composite Films Based on Pectin-Nanocellulose-Synergistic Triple Essential Oils: Development and Characterization. Food Bioprocess Technol. 2023. [Google Scholar] [CrossRef]
- Bizymis, A.-P.; Giannou, V.; Tzia, C. Contribution of Hydroxypropyl Methylcellulose to the Composite Edible Films and Coatings Properties. Food Bioprocess Technol. 2023, 16, 1488–1501. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable Compatibilized Polymer Blends for Packaging Applications: A Literature Review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Chakraborty, P.; Nath, D.; Hoque, M.; Sarkar, P.; Hati, S.; Mishra, B.K. Biopolymer-Based Antimicrobial Coatings for Aquatic Food Products: A Review. J. Food Process Preserv. 2022, 46, e16465. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, Citral and α-Terpineol Essential Oil Incorporated Biodegradable Films for Functional Active Packaging of Pacific White Shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef]
- de Souza, A.G.; Barbosa, R.F.D.S.; Quispe, Y.M.; Rosa, D.D.S. Essential Oil Microencapsulation with Biodegradable Polymer for Food Packaging Application. J. Polym. Environ. 2022, 30, 3307–3315. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and Plasticization Effects of Carvacrol in Biodegradable Poly(Lactic acid) and Poly(Butylene Adipate Terephthalate) Blend Films for Bakery Packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Global Data. Market Size of Biodegradable Plastics in Brazil (2017–2021). Available online: https://www.globaldata.com/data-insights/packaging/market-size-of-biodegradable-plastics-in-brazil-2017-2021/ (accessed on 28 January 2023).
- Grand View Research. Biodegradable Plastic Market Size, Share & Trends Analysis Report By Product (Starch Based, PLA, PHA, PBAT, PBS), By Application (Packaging, Consumer Goods, Agriculture), And Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/biodegradable-plastics-market# (accessed on 28 January 2023).
- United Nations Iran. Together, for Less Usage of Single-Use #Plastic. Available online: https://iran.un.org/en/191423-together-less-usage-single-use-plastic (accessed on 20 January 2023).
- Matheus, J.R.V.; de Farias, P.M.; Satoriva, J.M.; de Andrade, C.J.; Fai, A.E.C. Cassava Starch Films for Food Packaging: Trends over the Last Decade and Future Research. Int. J. Biol. Macromol. 2023, 225, 658–672. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of Chitosan-Based Biodegradable Active Films Using Bio-Waste Enriched with Polyphenol Propolis Extract Envisaging Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; da Rosa, C.G.; Agostinetto, L.; de Lima Veeck, A.P.; Bretanha, L.C.; Micke, G.A.; Dalla Costa, M.; Bertoldi, F.C.; et al. Chitosan Packaging Functionalized with Cinnamodendron Dinisii Essential Oil Loaded Zein: A Proposal for Meat Conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Sobral, P.J.A. Disintegrability under Composting Conditions of Films Based on Gelatin, Chitosan and/or Sodium Caseinate Containing Boldo-of-Chile Leafs Extract. Int. J. Biol. Macromol. 2020, 151, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; do Amaral Sobral, P.J. Production and Characterization of Films Based on Blends of Chitosan from Blue Crab (Callinectes sapidus) Waste and Pectin from Orange (Citrus sinensis Osbeck) Peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; Bittante, A.M.Q.B.; Lourenço, R.V.; do Amaral Sobral, P.J. Properties of Gelatin-Based Films Incorporated with Chitosan-Coated Microparticles Charged with Rutin. Int. J. Biol. Macromol. 2017, 101, 643–652. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; da Rosa, C.G.; da Silva, A.P.G.; Ferrareze, J.P.; Azevedo, M.S.; Forster-Carneiro, T.; Nunes, M.R.; de Lima Veeck, A.P. Application in Situ of Biodegradable Films Produced with Starch, Citric Pectin and Functionalized with Feijoa (Acca Sellowiana (Berg) Burret) Extracts: An Effective Proposal for Food Conservation. Int. J. Biol. Macromol. 2021, 189, 544–553. [Google Scholar] [CrossRef]
- de Lima Barizão, C.; Crepaldi, M.I.; Oscar de Oliveira, S.; de Oliveira, A.C.; Martins, A.F.; Garcia, P.S.; Bonafé, E.G. Biodegradable Films Based on Commercial κ-Carrageenan and Cassava Starch to Achieve Low Production Costs. Int. J. Biol. Macromol. 2020, 165, 582–590. [Google Scholar] [CrossRef]
- Tibolla, H.; Czaikoski, A.; Pelissari, F.M.; Menegalli, F.C.; Cunha, R.L. Starch-Based Nanocomposites with Cellulose Nanofibers Obtained from Chemical and Mechanical Treatments. Int. J. Biol. Macromol. 2020, 161, 132–146. [Google Scholar] [CrossRef]
- Silva, O.A.; Pellá, M.G.; Pellá, M.G.; Caetano, J.; Simões, M.R.; Bittencourt, P.R.S.; Dragunski, D.C. Synthesis and Characterization of a Low Solubility Edible Film Based on Native Cassava Starch. Int. J. Biol. Macromol. 2019, 128, 290–296. [Google Scholar] [CrossRef]
- Wang, R.-L.; Hsu, T.-F.; Hu, C.-Z. A Bibliometric Study of Research Topics and Sustainability of Packaging in the Greater China Region. Sustainability 2021, 13, 5384. [Google Scholar] [CrossRef]
- Lam, W.S.; Lee, P.F.; Lam, W.H. Cellulose Nanofiber for Sustainable Production: A Bibliometric Analysis. Mater. Today Proc. 2022, 62, 6460–6467. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In Vitro Antibacterial and Antioxidant Properties of Chitosan Edible Films Incorporated with Thymus Moroderi or Thymus Piperella Essential Oils. Food Control. 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Bashir, A.; Jabeen, S.; Gull, N.; Islam, A.; Sultan, M.; Ghaffar, A.; Khan, S.M.; Iqbal, S.S.; Jamil, T. Co-Concentration Effect of Silane with Natural Extract on Biodegradable Polymeric Films for Food Packaging. Int. J. Biol. Macromol. 2018, 106, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Natural Polymers in Bio-Degradable/Edible Film: A Review on Environmental Concerns, Cold Plasma Technology and Nanotechnology Application on Food Packaging- A Recent Trends. Food Chem. Adv. 2022, 1, 100135. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Barone, A.S.; Matheus, J.R.V.; de Souza, T.S.P.; Moreira, R.F.A.; Fai, A.E.C. Green-Based Active Packaging: Opportunities beyond COVID-19, Food Applications, and Perspectives in Circular Economy—A Brief Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4881–4905. [Google Scholar] [CrossRef]
- Leal Filho, W.; Salvia, A.L.; Minhas, A.; Paço, A.; Dias-Ferreira, C. The COVID-19 Pandemic and Single-Use Plastic Waste in Households: A Preliminary Study. Sci. Total Environ. 2021, 793, 148571. [Google Scholar] [CrossRef]
- Castillo, L.A.; Farenzena, S.; Pintos, E.; Rodríguez, M.S.; Villar, M.A.; García, M.A.; López, O. V Active Films Based on Thermoplastic Corn Starch and Chitosan Oligomer for Food Packaging Applications. Food Packag. Shelf Life 2017, 14, 128–136. [Google Scholar] [CrossRef]
- Wen, L.; Liang, Y.; Lin, Z.; Xie, D.; Zheng, Z.; Xu, C.; Lin, B. Design of Multifunctional Food Packaging Films Based on Carboxymethyl Chitosan/Polyvinyl Alcohol Crosslinked Network by Using Citric Acid as Crosslinker. Polymer 2021, 230, 124048. [Google Scholar] [CrossRef]
- Joanne Kam, W.-Y.; Mirhosseini, H.; Abas, F.; Hussain, N.; Hedayatnia, S.; Florence Chong, H.-L. Antioxidant Activity Enhancement of Biodegradable Film as Active Packaging Utilizing Crude Extract from Durian Leaf Waste. Food Control. 2018, 90, 66–72. [Google Scholar] [CrossRef]
- Dewi, E.N.; Tassakka, A.C.M.A.R.; Yuwono, M.; Suyono, E.A.; Purnamayati, L.; Alam, J.F. Effect of Chlorophyll in Alginate-Based Edible Film in Inhibiting Spoilage of Fish Snacks. Canrea J. Food Technol. Nutr. Culin. J. 2022, 5, 57–68. [Google Scholar] [CrossRef]
- Xu, D.; Chen, T.; Liu, Y. The Physical Properties, Antioxidant and Antimicrobial Activity of Chitosan–Gelatin Edible Films Incorporated with the Extract from Hop Plant. Polym. Bull. 2021, 78, 3607–3624. [Google Scholar] [CrossRef]
- Verdi, A.G.; de Souza, A.G.; Rocha, D.B.; de Oliveira, S.A.; Alves, R.M.V.; dos Santos Rosa, D. Biodegradable Films Functionalized with Moringa Oleifera Applied in Food Packaging. Iran. Polym. J. 2021, 30, 235–246. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active Biodegradable Films Based on the Whole Potato Peel Incorporated with Bacterial Cellulose and Curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Go, E.-J.; Song, K. Bin Antioxidant Properties of Rye Starch Films Containing Rosehip Extract and Their Application in Packaging of Chicken Breast. Starch-Stärke 2019, 71, 1900116. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chawla, S.P. Shelf Life Extension of Chicken Packed in Active Film Developed with Mango Peel Extract. J. Food Saf. 2018, 38, e12385. [Google Scholar] [CrossRef]
- Apicella, A.; Adiletta, G.; Albanese, D.; Di Matteo, M.; Incarnato, L. Biodegradable Films Based on Poly(Lactic acid) Coatings and Natural Olive-Wastewater Extracts for Active Food Packaging. Chem. Eng. Trans. 2021, 87, 85–90. [Google Scholar] [CrossRef]
- Sisilia Yolanda, D.; Dirpan, A.; Nur Faidah Rahman, A.; Djalal, M.; Hatul Hidayat, S. The Potential Combination of Smart and Active Packaging in One Packaging System in Improving and Maintaining the Quality of Fish. Canrea J. Food Technol. Nutr. Culin. J. 2020, 3, 74–86. [Google Scholar] [CrossRef]
- Kamkar, A.; Molaee-aghaee, E.; Khanjari, A.; Akhondzadeh-basti, A.; Noudoost, B.; Shariatifar, N.; Alizadeh Sani, M.; Soleimani, M. Nanocomposite Active Packaging Based on Chitosan Biopolymer Loaded with Nano-Liposomal Essential Oil: Its Characterizations and Effects on Microbial, and Chemical Properties of Refrigerated Chicken Breast Fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef]
- Dirpan, A.; Djalal, M.; Kamaruddin, I. Application of an Intelligent Sensor and Active Packaging System Based on the Bacterial Cellulose of Acetobacter Xylinum to Meat Products. Sensors 2022, 22, 544. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Lee, J.-H.; Yang, H.-J.; Song, K. Bin Production and Characterisation of Skate Skin Gelatin Films Incorporated with Thyme Essential Oil and Their Application in Chicken Tenderloin Packaging. Int. J. Food Sci. Technol. 2016, 51, 1465–1472. [Google Scholar] [CrossRef]
- Sayadi, M.; Mojaddar Langroodi, A.; Amiri, S.; Radi, M. Effect of Nanocomposite Alginate-Based Film Incorporated with Cumin Essential Oil and TiO2 Nanoparticles on Chemical, Microbial, and Sensory Properties of Fresh Meat/Beef. Food Sci. Nutr. 2022, 10, 1401–1413. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Silva, J.B.A.D.A.; Silva, J.A.D.A.; Camilloto, G.P.; Souza, C.O.D.E.; Druzian, J.I.; Guimaraes, A.G. Development and Characterization of Antioxidant and Antimicrobial Poly (Butylene Adipate-Co-Terephtalate) (PBAT) Film Incorporated with Oregano Essential Oil and Applied in Sliced Mozzarella Cheese. An. Acad. Bras. Cienc. 2022, 94, 1–16. [Google Scholar] [CrossRef]
- Aytac, Z.; Huang, R.; Vaze, N.; Xu, T.; Eitzer, B.D.; Krol, W.; MacQueen, L.A.; Chang, H.; Bousfield, D.W.; Chan-Park, M.B.; et al. Development of Biodegradable and Antimicrobial Electrospun Zein Fibers for Food Packaging. ACS Sustain. Chem. Eng. 2020, 8, 15354–15365. [Google Scholar] [CrossRef]
- Júnior, A.V.; Fronza, N.; Foralosso, F.B.; Dezen, D.; Huber, E.; dos Santos, J.H.Z.; Machado, R.A.F.; Quadri, M.G.N. Biodegradable Duo-Functional Active Film: Antioxidant and Antimicrobial Actions for the Conservation of Beef. Food Bioprocess Technol. 2015, 8, 75–87. [Google Scholar] [CrossRef]
- Yildiz, E.; Emir, A.A.; Sumnu, G.; Kahyaoglu, L.N. Citric Acid Cross-Linked Curcumin/Chitosan/Chickpea Flour Film: An Active Packaging for Chicken Breast Storage. Food Biosci. 2022, 50, 102121. [Google Scholar] [CrossRef]
- Souza, V.G.; Rodrigues, C.; Valente, S.; Pimenta, C.; Pires, J.R.; Alves, M.M.; Santos, C.F.; Coelhoso, I.M.; Fernando, A.L. Eco-Friendly ZnO/Chitosan Bionanocomposites Films for Packaging of Fresh Poultry Meat. Coatings 2020, 10, 110. [Google Scholar] [CrossRef]
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal Packaging of Sorbate and Benzoate Incorporated Biodegradable Films for Fresh Noodles. Food Control. 2021, 123, 107763. [Google Scholar] [CrossRef]
- Martins da Costa, J.C.; Lima Miki, K.S.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of Biodegradable Films Based on Purple Yam Starch/Chitosan for Food Application. Heliyon 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Romero, V.; Borneo, R.; Passalacqua, N.; Aguirre, A. Biodegradable Films Obtained from Triticale (x Triticosecale wittmack) Flour Activated with Natamycin for Cheese Packaging. Food Packag. Shelf Life 2016, 10, 54–59. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Yang, H.-J.; Song, K. Bin Application of a Puffer Fish Skin Gelatin Film Containing Moringa Oleifera Lam. Leaf Extract to the Packaging of Gouda Cheese. J. Food Sci. Technol. 2016, 53, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Li, Y.; Luo, C.; Yang, C.; Shi, W.; Li, L. Covalent Immobilization of Polypeptides on Polylactic Acid Films and Their Application to Fresh Beef Preservation. J. Agric. Food Chem. 2020, 68, 10532–10541. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.M.; Soares Júnior, M.S.; Yamashita, F. Active Biodegradable Films Produced with Blends of Rice Flour and Poly(Butylene Adipate Co-Terephthalate): Effect of Potassium Sorbate on Film Characteristics. Mater. Sci. Eng. C 2013, 33, 3153–3159. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Bian, L.; Wang, K.; Chia, W.Y.; Khoo, K.S.; Zhang, C.; Chew, K.W. Preparation and Characterization of Curdlan/Nanocellulose Blended Film and Its Application to Chilled Meat Preservation. Chemosphere 2021, 266, 128948. [Google Scholar] [CrossRef]
- Yang, H.-J.; Lee, J.-H.; Lee, K.-Y.; Song, K. Bin Antimicrobial Effect of an Undaria Pinnatifida Composite Film Containing Vanillin against Escherichia Coli and Its Application in the Packaging of Smoked Chicken Breast. Int. J. Food Sci. Technol. 2017, 52, 398–403. [Google Scholar] [CrossRef]
- Xiao, L.; Kang, S.; Lapu, M.; Jiang, P.; Wang, X.; Liu, D.; Li, J.; Liu, M. Preparation and Characterization of Chitosan/Pullulan Film Loading Carvacrol for Targeted Antibacterial Packaging of Chilled Meat. Int. J. Biol. Macromol. 2022, 211, 140–149. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Wang, Q.; Lin, G.; Yang, H.; Yu, D.; Cui, S.W.; Xia, W. Antimicrobial and Antioxidant Films Formed by Bacterial Cellulose, Chitosan and Tea Polyphenol–Shelf Life Extension of Grass Carp. Food Packag. Shelf Life 2022, 33, 100866. [Google Scholar] [CrossRef]
- Zehra, A.; Wani, S.M.; Jan, N.; Bhat, T.A.; Rather, S.A.; Malik, A.R.; Hussain, S.Z. Development of Chitosan-Based Biodegradable Films Enriched with Thyme Essential Oil and Additives for Potential Applications in Packaging of Fresh Collard Greens. Sci. Rep. 2022, 12, 16923. [Google Scholar] [CrossRef]
- Mohan, S.; Unnikrishnan, T.G.; Dubey, U.; Ramesh, M.; Panneerselvam, K. Development and Characterization of Mustard Oil Incorporated Biodegradable Chitosan Films for Active Food Packaging Applications. J. Polym. Environ. 2022, 31, 2190–2203. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in Situ Enhanced PVA/Chitosan Biodegradable Films for Food Packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Indumathi, M.P.; Rajarajeswari, G.R. Mahua Oil-Based Polyurethane/Chitosan/Nano ZnO Composite Films for Biodegradable Food Packaging Applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar] [CrossRef]
- Su, C.; Li, D.; Wang, L.; Wang, Y. Biodegradation Behavior and Digestive Properties of Starch-Based Film for Food Packaging—A Review. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent Progress in Biodegradable Polymers and Nanocomposite-Based Packaging Materials for Sustainable Environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Di Piazza, S.; Houbraken, J.; Meijer, M.; Cecchi, G.; Kraak, B.; Rosa, E.; Zotti, M. Thermotolerant and Thermophilic Mycobiota in Different Steps of Compost Maturation. Microorganisms 2020, 8, 880. [Google Scholar] [CrossRef]
- Mohammed, A.; Gaduan, A.; Chaitram, P.; Pooran, A.; Lee, K.-Y.; Ward, K. Sargassum Inspired, Optimized Calcium Alginate Bioplastic Composites for Food Packaging. Food Hydrocoll. 2023, 135, 108192. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and Smart Biodegradable Packaging Based on Starch and Natural Extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef]
- Wongphan, P.; Panrong, T.; Harnkarnsujarit, N. Effect of Different Modified Starches on Physical, Morphological, Thermomechanical, Barrier and Biodegradation Properties of Cassava Starch and Polybutylene Adipate Terephthalate Blend Film. Food Packag. Shelf Life 2022, 32, 100844. [Google Scholar] [CrossRef]
- Abdillah, A.A.; Charles, A.L. Characterization of a Natural Biodegradable Edible Film Obtained from Arrowroot Starch and Iota-Carrageenan and Application in Food Packaging. Int. J. Biol. Macromol. 2021, 191, 618–626. [Google Scholar] [CrossRef]
- Ruggero, F.; Carretti, E.; Gori, R.; Lotti, T.; Lubello, C. Monitoring of Degradation of Starch-Based Biopolymer Film under Different Composting Conditions, Using TGA, FTIR and SEM Analysis. Chemosphere 2020, 246, 125770. [Google Scholar] [CrossRef]
- Di Filippo, M.F.; Dolci, L.S.; Liccardo, L.; Bigi, A.; Bonvicini, F.; Gentilomi, G.A.; Passerini, N.; Panzavolta, S.; Albertini, B. Cellulose Derivatives-Snail Slime Films: New Disposable Eco-Friendly Materials for Food Packaging. Food Hydrocoll. 2021, 111, 106247. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Chandio, A.D.; Yousuf, S.; Makhdoom, M.A.; Jumah, M.N. Sustainable and Eco-Friendly Packaging Films Based on Poly (Vinyl alcohol) and Glass Flakes. Membranes 2022, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Bharimalla, A.K.; Mahapatra, A.; Dhakane-Lad, J.; Arputharaj, A.; Kumar, M.; Raja, A.S.M.; Kambli, N. Effect of Polymer Blending on Mechanical and Barrier Properties of Starch-Polyvinyl Alcohol Based Biodegradable Composite Films. Food Biosci. 2021, 44, 101352. [Google Scholar] [CrossRef]
- Sutay Kocabaş, D.; Erkoç Akçelik, M.; Bahçegül, E.; Özbek, H.N. Bulgur Bran as a Biopolymer Source: Production and Characterization of Nanocellulose-Reinforced Hemicellulose-Based Biodegradable Films with Decreased Water Solubility. Ind. Crops Prod. 2021, 171, 113847. [Google Scholar] [CrossRef]
- Lal, S.; Kumar, V.; Arora, S. Eco-Friendly Synthesis of Biodegradable and High Strength Ternary Blend Films of PVA/Starch/Pectin: Mechanical, Thermal and Biodegradation Studies. Polym. Polym. Compos. 2020, 29, 1505–1514. [Google Scholar] [CrossRef]
- Hasan, M.; Zarlaida, F.; Susilawati, D.; Zulfadli; Nasir, M.; Hanum, L. Robust Biodegradable Chitosan Film Reinforced Cellulose Isolated from Straw Waste. Rasayan J. Chem. 2021, 14, 2147–2153. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.-R.; Zhang, Y.-C.; Han, X.-D.; Chen, C.; Chen, A. In Situ Generated Silica Reinforced Polyvinyl Alcohol/Liquefied Chitin Biodegradable Films for Food Packaging. Carbohydr. Polym. 2020, 238, 116182. [Google Scholar] [CrossRef]
- Nogueira, D.; Martins, V.G. Use of Different Proteins to Produce Biodegradable Films and Blends. J. Polym. Environ. 2019, 27, 2027–2039. [Google Scholar] [CrossRef]
- Pérez-Arauz, A.O.; Aguilar-Rabiela, A.E.; Vargas-Torres, A.; Rodríguez-Hernández, A.-I.; Chavarría-Hernández, N.; Vergara-Porras, B.; López-Cuellar, M.R. Production and Characterization of Biodegradable Films of a Novel Polyhydroxyalkanoate (PHA) Synthesized from Peanut Oil. Food Packag. Shelf Life 2019, 20, 100297. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Xu, Y.; Li, X.; Li, D.; Mu, C. Development of Antimicrobial and Controlled Biodegradable Gelatin-Based Edible Films Containing Nisin and Amino-Functionalized Montmorillonite. Food Bioprocess Technol. 2017, 10, 1727–1736. [Google Scholar] [CrossRef]
- De Campo, C.; Pagno, C.H.; Costa, T.M.H.; De Oliveira Rios, A.; Flôres, S.H. Gelatin Capsule Waste: New Source of Protein to Develop a Biodegradable Film. Polimeros 2017, 27, 100–107. [Google Scholar] [CrossRef]
- Vargas, C.G.; Costa, T.M.H.; de Oliveira Rios, A.; Flôres, S.H. Comparative Study on the Properties of Films Based on Red Rice (Oryza glaberrima) Flour and Starch. Food Hydrocoll. 2017, 65, 96–106. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Reddy, G.S.M.; Varaprasad, K.; Sadiku, E.R.; Sinha Ray, S.; Varada Rajulu, A. Preparation and Properties of Biodegradable Films from Sterculia Urens Short Fiber/Cellulose Green Composites. Carbohydr. Polym. 2013, 93, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Gasti, T.; Dixit, S.; Sataraddi, S.P.; Hiremani, V.D.; Masti, S.P.; Chougale, R.B.; Malabadi, R.B. Physicochemical and Biological Evaluation of Different Extracts of Edible Solanum Nigrum L. Leaves Incorporated Chitosan/Poly (Vinyl alcohol) Composite Films. J. Polym. Environ. 2020, 28, 2918–2930. [Google Scholar] [CrossRef]
- Chen, L.; Qiang, T.; Chen, X.; Ren, W.; Zhang, H.J. Gelatin from Leather Waste to Tough Biodegradable Packaging Film: One Valuable Recycling Solution for Waste Gelatin from Leather Industry. Waste Manag. 2022, 145, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Bioplastic Packaging Market Size, Share & Trends Analysis Report By Material (Biodegradable, Non-Biodegradable), By Type (Flexible, Rigid), By Application, By Region, And Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/bioplastic-packaging-market (accessed on 28 January 2023).
- Amcor. Flexible Packaging. Available online: https://www.amcor.com/products/search?type=Flexible+Packaging&category=Food&category=Beverages (accessed on 30 December 2022).
- Novamont. Packaging Attributes. Available online: https://www.novamont.com/eng/mater-bi (accessed on 29 December 2022).
- Innovia Films. Cellulose Film Attributes. Available online: https://innoviafilms.com/products//?cat=3 (accessed on 29 December 2022).
- Allied Market Research. Polylactic Acid Market by End Use Industry (Packaging, Textile, Agricultural, Electronics, Bio-Medical and Others): Global Opportunity Analysis and Industry Forecast, 2021–2030. Available online: https://www.alliedmarketresearch.com/polylactic-acid-market (accessed on 28 January 2023).
- Bio4Pack. Food Packaging That Makes Your Mouth Water. Available online: https://www.bio4pack.com/products/food/ (accessed on 30 December 2022).
- Plantic. Sustainability Packaging. Available online: https://plantic.com.au/product/plantic-flexible (accessed on 29 December 2022).
- Taghleef Industries. Food Packaging Films. Available online: https://www.ti-films.com/en/food-packaging-films/products (accessed on 29 December 2022).
- Sidaplax. Packaging Attributes. Available online: https://earthfirstfilms.com/products?page=0&substrates%5B%5D=polyflex-films&units=gauge&applications%5B%5D=all (accessed on 30 December 2022).
- Clondalkin Group. Packaging Products. Available online: https://clondalkingroup.com/wentus/ (accessed on 30 December 2022).
- United Nations. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 29 January 2023).
- World Health Organization. Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 6 February 2023).
- Condor Ferries Company. Shocking Ocean Plastic Statistics: The Threat to Marine Life, The Ocean & Humanity. Available online: https://www.condorferries.co.uk/plastic-in-the-ocean-statistics (accessed on 22 February 2023).





| Rank | Country | Number of Documents | Number of Citations | Total Link Strength |
|---|---|---|---|---|
| 1 | Brazil | 82 | 1422 | 19 |
| 2 | China | 50 | 1378 | 19 |
| 3 | Iran | 44 | 2234 | 18 |
| 4 | India | 42 | 908 | 15 |
| 5 | Malaysia | 30 | 1217 | 14 |
| 6 | Spain | 26 | 2044 | 21 |
| 7 | United States | 19 | 884 | 22 |
| 8 | Italy | 18 | 177 | 6 |
| 9 | Mexico | 18 | 1081 | 5 |
| 10 | South Korea | 18 | 448 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dirpan, A.; Ainani, A.F.; Djalal, M. A Review on Biopolymer-Based Biodegradable Film for Food Packaging: Trends over the Last Decade and Future Research. Polymers 2023, 15, 2781. https://doi.org/10.3390/polym15132781
Dirpan A, Ainani AF, Djalal M. A Review on Biopolymer-Based Biodegradable Film for Food Packaging: Trends over the Last Decade and Future Research. Polymers. 2023; 15(13):2781. https://doi.org/10.3390/polym15132781
Chicago/Turabian StyleDirpan, Andi, Andi Fadiah Ainani, and Muspirah Djalal. 2023. "A Review on Biopolymer-Based Biodegradable Film for Food Packaging: Trends over the Last Decade and Future Research" Polymers 15, no. 13: 2781. https://doi.org/10.3390/polym15132781
APA StyleDirpan, A., Ainani, A. F., & Djalal, M. (2023). A Review on Biopolymer-Based Biodegradable Film for Food Packaging: Trends over the Last Decade and Future Research. Polymers, 15(13), 2781. https://doi.org/10.3390/polym15132781





