Organophosphorus Reinforced Poly(vinyl alcohol) Nanocomposites Doped with Silver-Loaded Zeolite L Nanoparticles as Sustainable Materials for Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oligophosphonate, PFR-4
- FTIR (KBr, cm−1): 3292 (NH), 3065 (C–H aromatic), 1475 (P–Ar), 1210 and 1140 (P=O), 1045 (P–O–C), 925 (P–O–Ar).
- 1H-NMR (400 MHz, DMSO-d6, δ, ppm): 8.52 (d, 2H), 8.18 (m, 4H), 8.02 (d, 4H), 8–7.5 (m, 6H), 7.99 (d, 4H), 7.5–7.05 (m, 18H), 6.52 (m, 8H), 6.1 and 5.7 (m, 2H, N–H), 5.4 and 4.85 (m, 2H, CH–P).
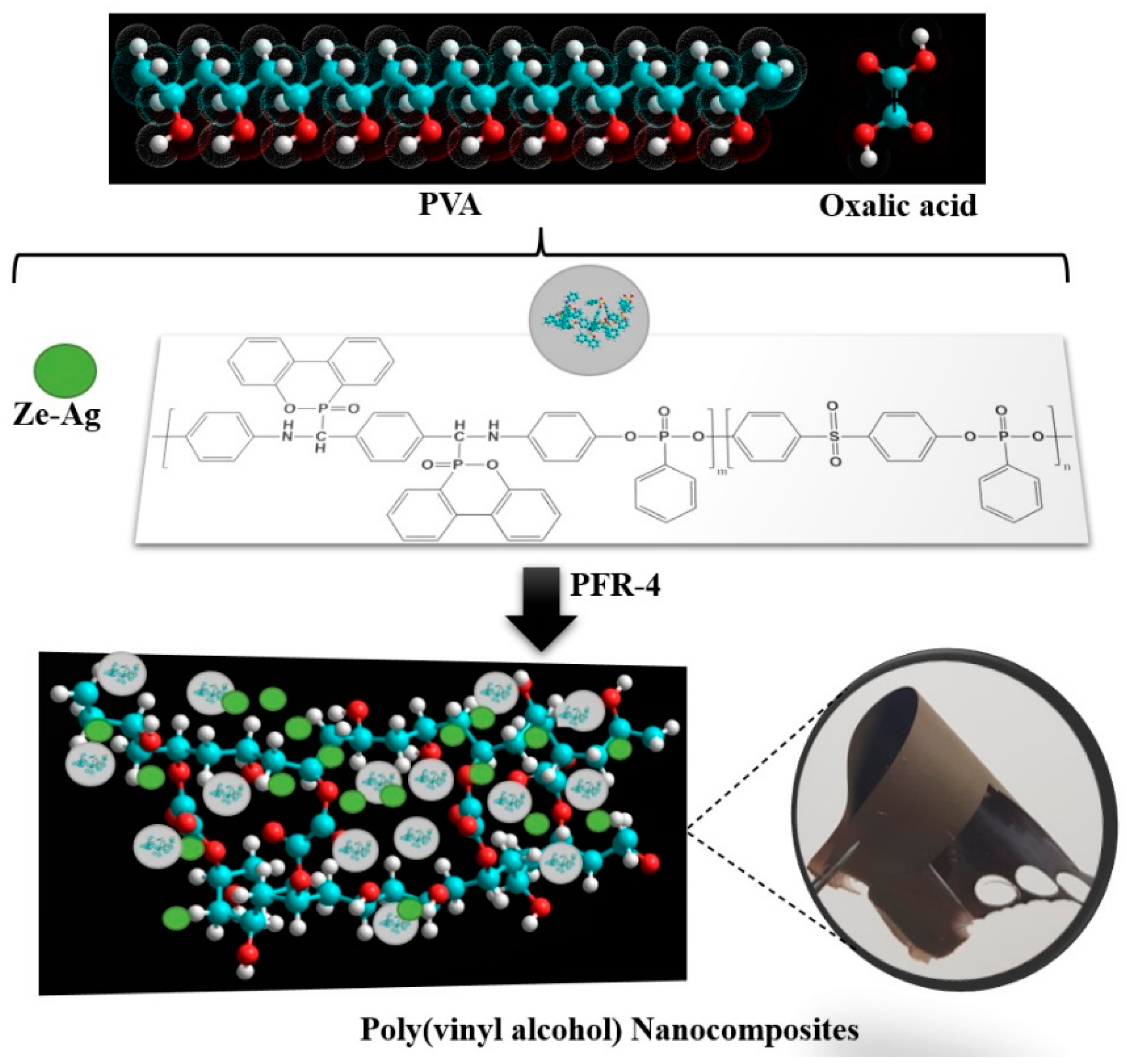
2.3. Preparation of PVA/OA Films
2.4. Methods
2.4.1. FTIR Spectra
2.4.2. Scanning Electron Microscopy
2.4.3. Contact Angle
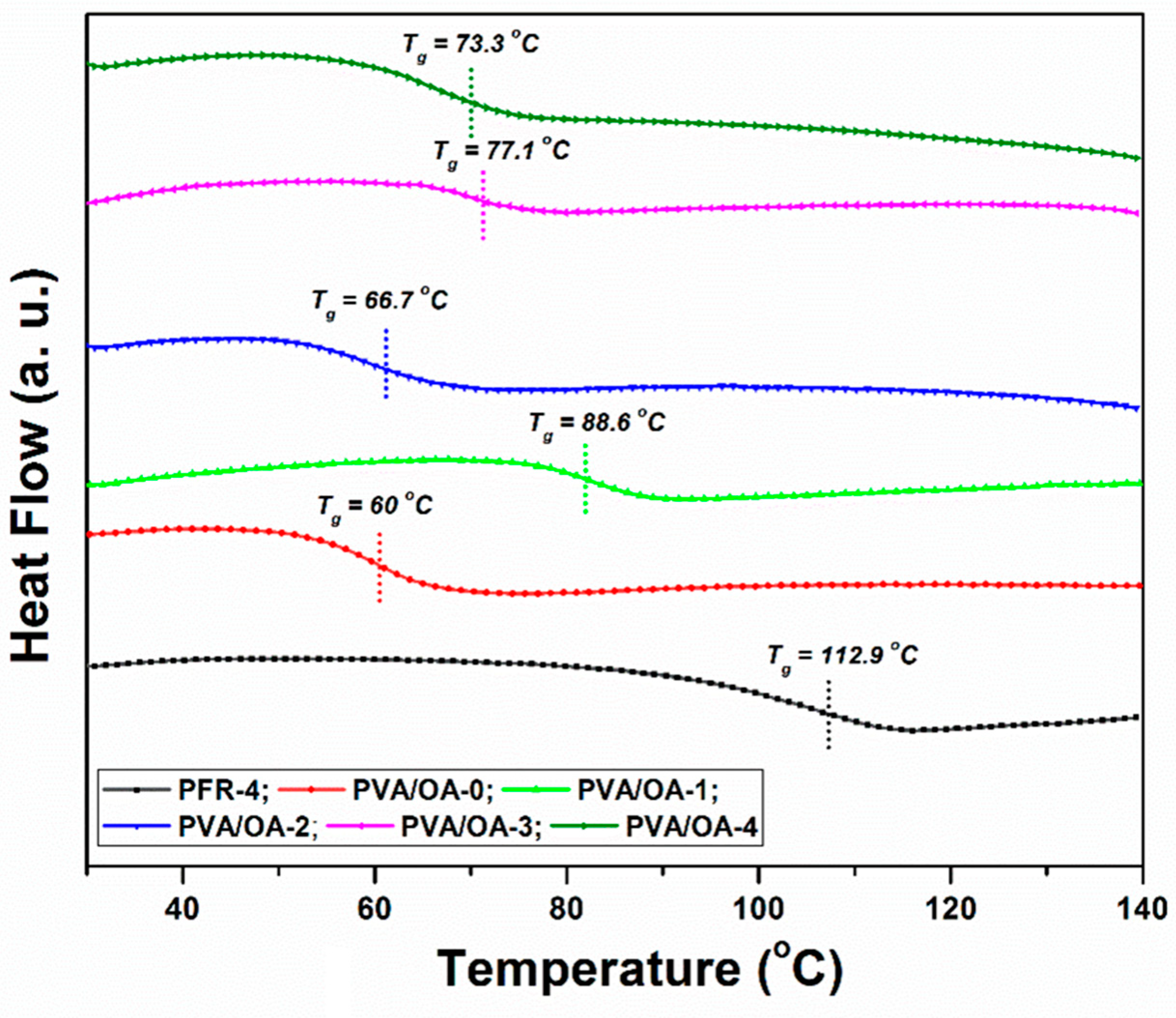
2.4.4. Differential Scanning Calorimetry (DSC) Measurements
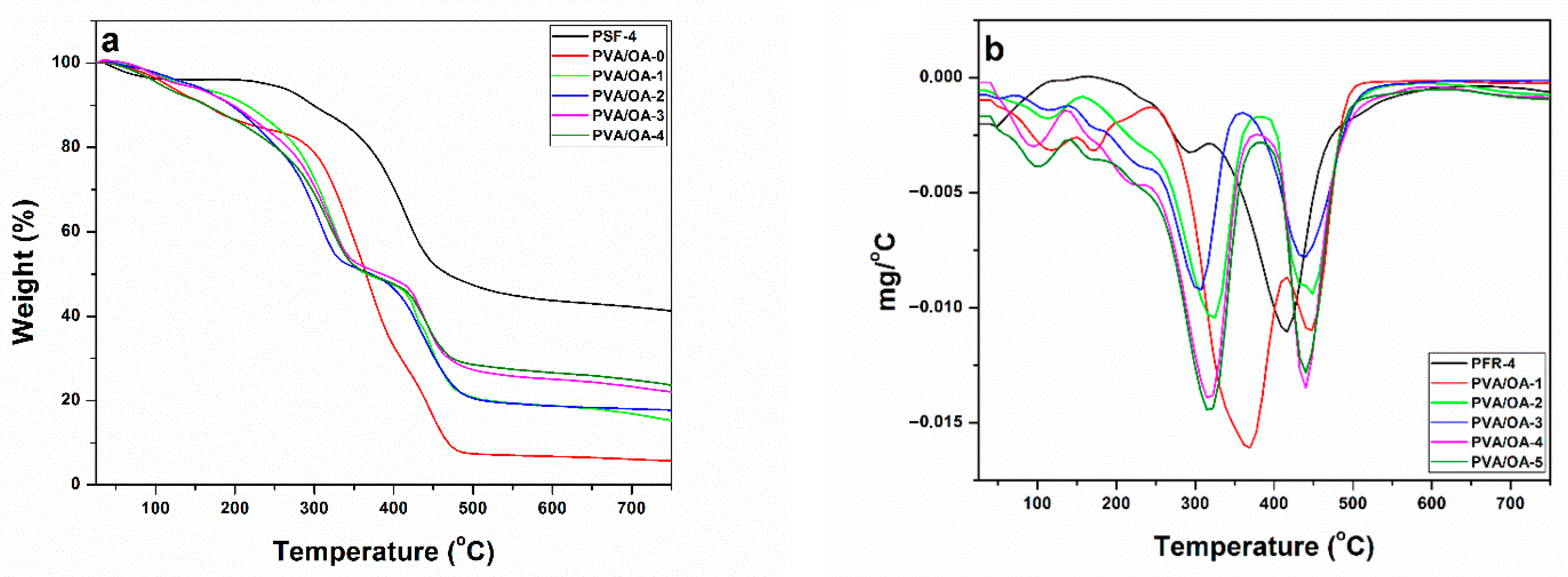
2.4.5. TGA Measurements
2.4.6. Microscale Combustion Calorimetry (MCC)
2.4.7. Mechanical Testing
2.4.8. Antimicrobial Activity
3. Results
3.1. Structural Investigation
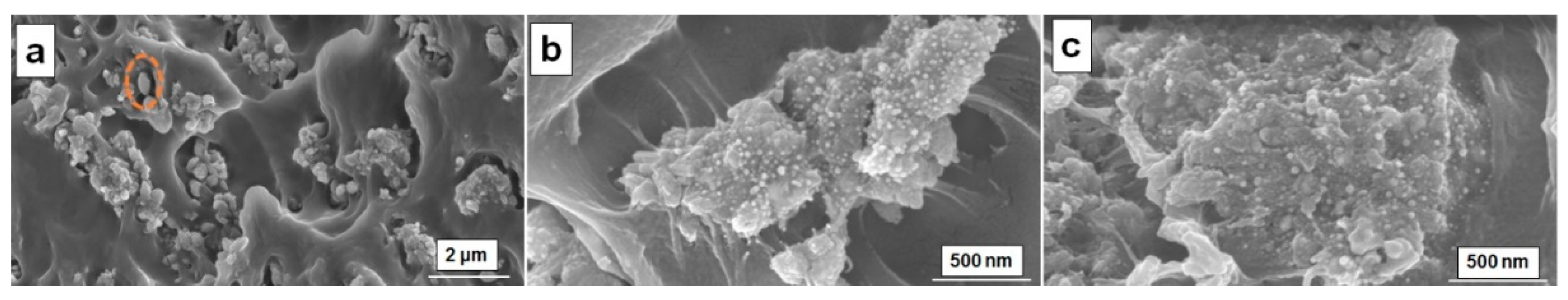
3.2. Morphology Investigation
3.3. Surface Characteristics
3.4. Thermal Stability
3.5. Microscale Combustion Calorimetry (MCC) Tests
3.6. Mechanical Properties of Films
3.7. Antimicrobial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borg, K.; Lennox, A.; Kaufman, S.; Tull, F.; Prime, R.; Rogers, L.; Dunstan, E. Curbing plastic consumption: A review of single-use plastic behaviour change interventions. J. Clean. Prod. 2022, 344, 131077. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Le, M.T.T.; Do, L.T. Intrinsic motivation for reducing single-use plastics: The compensation effects of basic psychological needs. Resour. Conserv. Recycl. 2022, 185, 106482. [Google Scholar] [CrossRef]
- Patrício Silva, A.L.; Prata, J.C.; Walker, T.R.; Campos, D.; Duarte, A.C.; Soares, A.M.V.M.; Barcelò, D.; Rocha-Santos, T. Rethinking and optimising plastic waste management under COVID-19 pandemic: Policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci. Total Environ. 2020, 742, 140565. [Google Scholar] [CrossRef]
- Shams, M.; Alam, I.; Mahbub, M.S. Plastic pollution during COVID-19: Plastic waste directives and its long-term impact on the environment. Environ. Adv. 2021, 5, 100119. [Google Scholar] [CrossRef] [PubMed]
- Charles, D.; Kimman, L. Plastic Waste Makers Index 2023; Minderoo Foundation: Perth, Australia, 2023. [Google Scholar]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Wandosell, G.; Parra-Meroño, M.C.; Alcayde, A.; Baños, R. Green Packaging from Consumer and Business Perspectives. Sustainability 2021, 13, 1356. [Google Scholar] [CrossRef]
- Orzan, G.; Cruceru, A.F.; Bălăceanu, C.T.; Chivu, R.-G. Consumers’ Behavior Concerning Sustainable Packaging: An Exploratory Study on Romanian Consumers. Sustainability 2018, 10, 1787. [Google Scholar] [CrossRef]
- Granato, G.; Fischer, A.R.H.; van Trijp, H.C.M. The price of sustainability: How consumers trade-off conventional packaging benefits against sustainability. J. Clean. Prod. 2022, 365, 132739. [Google Scholar] [CrossRef]
- Anjoran, R. Advantages of Bioplastics vs. Disadvantages: Memo for Product Designers. Available online: https://qualityinspection.org/advantages-of-bioplastics-vs-disadvantages/ (accessed on 11 April 2023).
- Bourakadi, K.E.; Merghoub, N.; Fardioui, M.; Mekhzoum, M.E.M.; Kadmiri, I.M.; Essassi, E.M.; Qaiss, A.e.K.; Bouhfid, R. Chitosan/polyvinyl alcohol/thiabendazoluim-montmorillonite bio-nanocomposite films: Mechanical, morphological and antimicrobial properties. Compos. Part B Eng. 2019, 172, 103–110. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Saha, P.; Adhikari, J.; Deshmukh, K.; Ahamed, M.B.; Cabibihan, J.-J. Recent advances in mechanical properties of biopolymer composites: A review. Polym. Compos. 2020, 41, 32–59. [Google Scholar] [CrossRef]
- Dogra, V.; Verma, D.; Fortunati, E. Chapter 15-Biopolymers and nanomaterials in food packaging and applications. In Nanotechnology-Based Sustainable Alternatives for the Management of Plant Diseases; Balestra, G.M., Fortunati, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 355–374. [Google Scholar]
- Pokharel, A.; Falua, K.J.; Babaei-Ghazvini, A.; Acharya, B. Biobased Polymer Composites: A Review. J. Compos. Sci. 2022, 6, 255. [Google Scholar] [CrossRef]
- Gutierrez Cisneros, C.; Bloemen, V.; Mignon, A. Synthetic, Natural, and Semisynthetic Polymer Carriers for Controlled Nitric Oxide Release in Dermal Applications: A Review. Polymers 2021, 13, 760. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, B.C.; Ivanov, D. Natural and Synthetic Polymers for Designing Composite Materials. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–54. [Google Scholar]
- Liu, B.; Zhang, J.; Guo, H. Research Progress of Polyvinyl Alcohol Water-Resistant Film Materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Gaume, J.; Wong-Wah-Chung, P.; Rivaton, A.; Thérias, S.; Gardette, J.-L. Photochemical behavior of PVA as an oxygen-barrier polymer for solar cell encapsulation. RSC Adv. 2011, 1, 1471–1481. [Google Scholar] [CrossRef]
- Sapalidis, A.A. Porous Polyvinyl Alcohol Membranes: Preparation Methods and Applications. Symmetry 2020, 12, 960. [Google Scholar] [CrossRef]
- Abd El-aziz, A.M.; El-Maghraby, A.; Taha, N.A. Comparison between polyvinyl alcohol (PVA) nanofiber and polyvinyl alcohol (PVA) nanofiber/hydroxyapatite (HA) for removal of Zn2+ ions from wastewater. Arab. J. Chem. 2017, 10, 1052–1060. [Google Scholar] [CrossRef]
- Rezaei, I.; Sadeghi, A. Investigating the Mechanical Properties of Polyvinyl Alcohol Nanofibers Based on Aligned and Random Orientations. Arab. J. Sci. Eng. 2021, 46, 12479–12486. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Ma, S.; Li, Z.; Xin, Z.; Zhang, Z.; Zhou, B.; Shen, J.; Qin, L.; Du, A. Ultrablack Poly(vinyl alcohol)-Graphite Composite Xerogel with Vertically Arranged Pores for Highly Efficient Solar Steam Generation and Desalination. Adv. Energy Sustain. Res. 2022, 3, 2100188. [Google Scholar] [CrossRef]
- Byrne, D.; Boeije, G.; Croft, I.; Hüttmann, G.; Luijkx, G.; Meier, F.; Parulekar, Y.; Stijntjes, G. Biodegradability of Polyvinyl Alcohol Based Film Used for Liquid Detergent Capsules. Biol. Abbaubarkeit Der Für Flüssigwaschmittelkapseln Verwendeten Folie Auf Polyvinylalkoholbasis 2021, 58, 88–96. [Google Scholar] [CrossRef]
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Shen, Z.; Rajabi-Abhari, A.; Oh, K.; Yang, G.; Youn, H.J.; Lee, H.L. Improving the Barrier Properties of Packaging Paper by Polyvinyl Alcohol Based Polymer Coating-Effect of the Base Paper and Nanoclay. Polymers 2021, 13, 1334. [Google Scholar] [CrossRef] [PubMed]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Chandio, A.D.; Yousuf, S.; Makhdoom, M.A.; Jumah, M.N. Sustainable and Eco-Friendly Packaging Films Based on Poly (Vinyl Alcohol) and Glass Flakes. Membranes 2022, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Suganthi, S.; Vignesh, S.; Kalyana Sundar, J.; Raj, V. Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl. Water Sci. 2020, 10, 100. [Google Scholar] [CrossRef]
- Mustafa, P.; Niazi, M.B.K.; Jahan, Z.; Rafiq, S.; Ahmad, T.; Sikander, U.; Javaid, F. Improving functional properties of PVA/starch-based films as active and intelligent food packaging by incorporating propolis and anthocyanin. Polym. Polym. Compos. 2020, 29, 1472–1484. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, Q.; Huang, J.; Zhao, Y.; Li, Q.; Wang, S. Improved Hydrophobic, UV Barrier and Antibacterial Properties of Multifunctional PVA Nanocomposite Films Reinforced with Modified Lignin Contained Cellulose Nanofibers. Polymers 2022, 14, 1705. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Chen, P.; Duan, M.; Lin, X.; Zhang, Y. Biodegradation analysis of polyvinyl alcohol during the compost burial course. J. Basic Microbiol. 2019, 59, 368–374. [Google Scholar] [CrossRef]
- Mititelu-Mija, A.; Cascavala, C.N.; Navard, P. Advanced ordered material based on liquid crystalline azomethine diepoxide. J. Optoelectron. Adv. Mater. 2007, 9, 3628–3632. [Google Scholar]
- Hamciuc, C.; Vlad-Bubulac, T.; Serbezeanu, D.; Carja, I.-D.; Hamciuc, E.; Lisa, G.; Pérez, V.F. Environmentally friendly fire-resistant epoxy resins based on a new oligophosphonate with high flame retardant efficiency. RSC Adv. 2016, 6, 22764–22776. [Google Scholar] [CrossRef]
- Hamciuc, C.; Vlad-Bubulac, T.; Bercea, M.; Suflet, D.M.; Doroftei, F.; Rîmbu, C.M.; Enache, A.A.; Kalvachev, Y.; Todorova, T.; Butnaru, M.; et al. Electrospun Copoly(ether imide) Nanofibers Doped with Silver-Loaded Zeolite as Materials for Biomedical Applications. ACS Appl. Polym. Mater. 2022, 4, 6080–6091. [Google Scholar] [CrossRef]
- Fischer, P.J.A.R. Surface Chemistry of Solid and Liquid Interfaces. Appl. Rheol. 2007, 17, 129. [Google Scholar] [CrossRef]
- Islam, M.S.; Karim, M.R.J.C.; Physicochemical, S.A.; Aspects, E. Fabrication and characterization of poly (vinyl alcohol)/alginate blend nanofibers by electrospinning method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 135–140. [Google Scholar] [CrossRef]
- Pedroza-Toscano, M.A.; López-Cuenca, S.; Rabelero-Velasco, M.; Moreno-Medrano, E.D.; Mendizabal-Ruiz, A.P.; Salazar-Peña, R. Silver Nanoparticles Obtained by Semicontinuous Chemical Reduction Using Carboxymethyl Cellulose as a Stabilizing Agent and Its Antibacterial Capacity. J. Nanomater. 2017, 2017, 1390180. [Google Scholar] [CrossRef]
- Yan, L.; Tang, X.; Xie, X.; Xu, Z. Fire Resistance, Thermal and Anti-Ageing Properties of Transparent Fire-Retardant Coatings Modified with Different Molecular Weights of Polyethylene Glycol Borate. Polymers 2021, 13, 4206. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Vlad-Bubulac, T.; Onofrei, M.D.; Doroftei, F.; Hamciuc, C.; Ipate, A.-M.; Anisiei, A.; Lisa, G.; Anghel, I.; Şofran, I.-E.; et al. Phosphorylated Poly(vinyl alcohol) Electrospun Mats for Protective Equipment Applications. Nanomaterials 2022, 12, 2685. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Vlad-bubulac, T.; Hamciuc, E.; Hamciuc, C.; Lisa, G.; Anghel, I.; Sofran, I.-E.; Preda, D.-M. Study on Thermal and Flame Retardant Properties of Phosphorus-containing Polyimides. Rev. Chim. 2021, 72, 13–21. [Google Scholar] [CrossRef]
- Homocianu, M.; Serbezeanu, D.; Lisa, G.; Brebu, M.; Vlad-Bubulac, T. Optical and Flame-Retardant Properties of a Series of Polyimides Containing Side Chained Bulky Phosphaphenanthrene Units. Int. J. Mol. Sci. 2022, 23, 13174. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Cheng, L. Synthesis of a Phosphorus/Nitrogen-Containing Additive with Multifunctional Groups and Its Flame-Retardant Effect in Epoxy Resin. Ind. Eng. Chem. Res. 2015, 54, 7777–7786. [Google Scholar] [CrossRef]
- You, G.; Cheng, Z.; Tang, Y.; He, H. Functional Group Effect on Char Formation, Flame Retardancy and Mechanical Properties of Phosphonate–Triazine-based Compound as Flame Retardant in Epoxy Resin. Ind. Eng. Chem. Res. 2015, 54, 7309–7319. [Google Scholar] [CrossRef]
- Zhao, P.; Rao, W.; Luo, H.; Wang, L.; Liu, Y.; Yu, C. Novel organophosphorus compound with amine groups towards self-extinguishing epoxy resins at low loading. Mater. Des. 2020, 193, 108838. [Google Scholar] [CrossRef]
- Gu, J.; Liang, C.; Dang, J.; Dong, W.; Zhang, Q.J.R.a. Ideal dielectric thermally conductive bismaleimide nanocomposites filled with polyhedral oligomeric silsesquioxane functionalized nanosized boron nitride. RSC. Adv. 2016, 6, 35809–35814. [Google Scholar] [CrossRef]
- Saengmee-Anupharb, S.; Srikhirin, T.; Thaweboon, B.; Thaweboon, S.; Amornsakchai, T.; Dechkunakorn, S.; Suddhasthira, T. Antimicrobial effects of silver zeolite, silver zirconium phosphate silicate and silver zirconium phosphate against oral microorganisms. Asian Pac. J. Trop. Biomed. 2013, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tsaplin, D.E.; Ostroumova, V.A.; Kulikov, L.A.; Zolotukhina, A.V.; Sadovnikov, A.A.; Kryuchkov, M.D.; Egazaryants, S.V.; Maksimov, A.L.; Wang, K.; Luo, Z.; et al. Synthesis and Investigation of Zeolite TiO2/Al-ZSM-12 Structure and Properties. Catalysts 2023, 13, 216. [Google Scholar] [CrossRef]
- Kraljević Pavelić, S.; Simović Medica, J.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef]
- Król, M.; Syguła-Cholewińska, J.; Sawoszczuk, T. Zeolite-Supported Aggregate as Potential Antimicrobial Agents in Gypsum Composites. Materials 2022, 15, 3305. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Env. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Sacarescu, L.; Pascariu, P.; Ghilan, A.; Doroftei, F.; Ursu, E.L.; Rimbu, C.M.; Horhogea, C.E.; et al. Phyto-Functionalized Silver Nanoparticles Derived from Conifer Bark Extracts and Evaluation of Their Antimicrobial and Cytogenotoxic Effects. Molecules 2021, 27, 217. [Google Scholar] [CrossRef]
- Jiraroj, D.; Tungasmita, S.; Tungasmita, D.N. Silver ions and silver nanoparticles in zeolite A composites for antibacterial activity. Powder Technol. 2014, 264, 418–422. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Wang, B. Zeolite-supported silver as antimicrobial agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar] [CrossRef]







| Sample | PVA (g) | Oxalic Acid (g) | PFR-4 (g) | Ze-Ag (g) |
|---|---|---|---|---|
| PVA/OA-0 | 1.70 | 0.30 | - | - |
| PVA/OA-1 | 1.53 | 0.27 | 0.20 | - |
| PVA/OA-2 | 1.36 | 0.24 | 0.40 | - |
| PVA/OA-3 | 1.42 | 0.25 | 0.20 | 0.13 |
| PVA/OA-4 | 1.31 | 0.23 | 0.20 | 0.26 |
| Sample | Contact Angles (°) | ||||
|---|---|---|---|---|---|
| PVA/OA-0 | PVA/OA-1 | PVA/OA-2 | PVA/OA-3 | PVA/OA-4 | |
| Water | 131.49 ± 0.28 | 99.41 ± 1.02 | 114.63 ± 1.95 | 113.04 ± 0.92 | 124.66 ± 0.87 |
| EG | 94.38 ± 0.96 | 73.69 ± 1.12 | 62.66 ± 0.47 | 70.57 ± 0.56 | 63.85 ± 0.49 |
| Sample | W | γSVp | γSVd | γSV | γSL |
|---|---|---|---|---|---|
| PVA/OA-0 | 24.6/44.3 | 4.25 | 33.5 | 37.7 | 27.04 |
| PVA/OA-1 | 60.9/61.5 | 1.27 | 22.9 | 24.3 | 36.2 |
| PVA/OA-2 | 42.5/70.1 | 7.38 | 75.75 | 83.1 | 35.8 |
| PVA/OA-3 | 44.3/63.9 | 2.75 | 53.03 | 55.8 | 36.9 |
| PVA/OA-4 | 31.4/69.1 | 18.04 | 97.2 | 115.2 | 35.3 |
| Samples | T5% a (°C) | T30% b (°C) | THRI c (°C) | Tmax d (°C) | Residue at 750 °C |
|---|---|---|---|---|---|
| PFR-4 | 244 | 401 | 165.72 | 294; 415 | 42 |
| PVA/OA-0 | 119 | 323 | 118.29 | 371; 446 | 6 |
| PVA/OA-1 | 131 | 308 | 116.23 | 324; 448 | 15 |
| PVA/OA-2 | 140 | 290 | 112.7 | 305; 435 | 18 |
| PVA/OA-3 | 127 | 307 | 115.15 | 220; 319; 436 | 22 |
| PVA/OA-4 | 103 | 299 | 108.09 | 319; 436 | 24 |
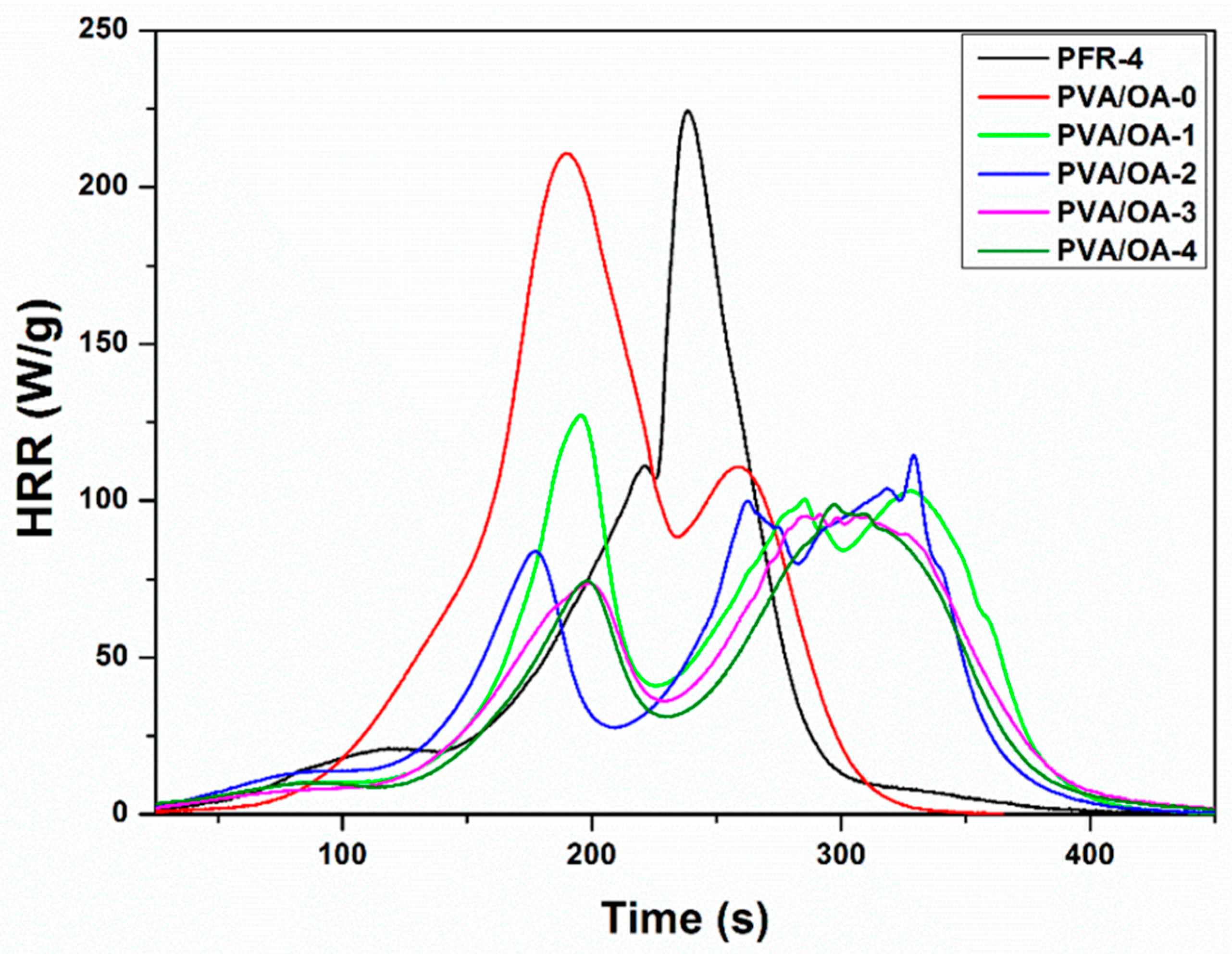
| Samples | Weight (mg) | Char Yield (mg) | Char Yield (wt%) | Decomposition Rate (%) | HRC (J/(g*K)) | THR (kJ/g) | PHRR (W/g) | TPHRR (°C) | Time (s) |
|---|---|---|---|---|---|---|---|---|---|
| PFR-4 | 10.61 | 4.30 | 61.87 | 38.13 | 280.18 | 15.18 | 224.37 | 425.75 | 238.50 |
| PVA/OA-0 | 20.01 | 0.49 | 2.45 | 97.55 | 305.41 | 20.77 | 210.80 | 389.17 | 189.50 |
| PVA/OA-1 | 20.02 | 2.03 | 10.14 | 89.86 | 239.84 | 18.66 | 127.14 | 339.42 | 196.00 |
| PVA/OA-2 | 20.01 | 2.97 | 14.84 | 85.16 | 228.33 | 16.66 | 114.69 | 471.47 | 329.00 |
| PVA/OA-3 | 19.96 | 3.39 | 16.98 | 83.02 | 165.66 | 16.02 | 95.70 | 428.64 | 291.50 |
| PVA/OA-4 | 19.95 | 4.43 | 22.21 | 77.79 | 164.57 | 14.63 | 98.91 | 442.76 | 297.50 |
| Sample | Young’s Modulus, MPa | Maximum Tensile Stress (MPa) | Elongation at Break (mm/mm) |
|---|---|---|---|
| PVA/OA-0 | 70.10 ± 2.36 | 27.46 ± 0.89 | 1.36 ± 0.15 |
| PVA/OA-1 | 286.28 ± 25 | 45.34 ± 1.34 | 1.61 ± 0.23 |
| PVA/OA-2 | 324.73 ± 10 | 33.71 ± 0.5 | 0.87 ± 0.01 |
| PVA/OA-3 | 368.53 ± 15 | 54.08 ± 4.6 | 1.33 ± 0.31 |
| PVA/OA-4 | 565.64 ± 65 | 52.06 ± 2.5 | 0.47 ± 0.02 |
| Samples Ø 10 mm | Inhibition Zone (mm) | ||
|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | Methicillin-Resistant Staphylococcus aureus (MRSA) ATCC 33591 | Escherichia coli ATCC 25922 | |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| PVA/OA-0 | 0 | 0 | 0 |
| PVA/OA-1 | 0 | 0 | 0 |
| PVA/OA-2 | 0 | 0 | 0 |
| PVA/OA-3 | 4.66 ± 0.333 | 1 | 0 |
| PVA/OA-4 | 5.66 ± 0.333 | 2.66 ± 0.333 | 1.33 ± 0.333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlad-Bubulac, T.; Hamciuc, C.; Serbezeanu, D.; Suflet, D.M.; Rusu, D.; Lisa, G.; Anghel, I.; Preda, D.-M.; Todorova, T.; Rîmbu, C.M. Organophosphorus Reinforced Poly(vinyl alcohol) Nanocomposites Doped with Silver-Loaded Zeolite L Nanoparticles as Sustainable Materials for Packaging Applications. Polymers 2023, 15, 2573. https://doi.org/10.3390/polym15112573
Vlad-Bubulac T, Hamciuc C, Serbezeanu D, Suflet DM, Rusu D, Lisa G, Anghel I, Preda D-M, Todorova T, Rîmbu CM. Organophosphorus Reinforced Poly(vinyl alcohol) Nanocomposites Doped with Silver-Loaded Zeolite L Nanoparticles as Sustainable Materials for Packaging Applications. Polymers. 2023; 15(11):2573. https://doi.org/10.3390/polym15112573
Chicago/Turabian StyleVlad-Bubulac, Tăchiță, Corneliu Hamciuc, Diana Serbezeanu, Dana Mihaela Suflet, Daniela Rusu, Gabriela Lisa, Ion Anghel, Dana-Maria Preda, Totka Todorova, and Cristina Mihaela Rîmbu. 2023. "Organophosphorus Reinforced Poly(vinyl alcohol) Nanocomposites Doped with Silver-Loaded Zeolite L Nanoparticles as Sustainable Materials for Packaging Applications" Polymers 15, no. 11: 2573. https://doi.org/10.3390/polym15112573
APA StyleVlad-Bubulac, T., Hamciuc, C., Serbezeanu, D., Suflet, D. M., Rusu, D., Lisa, G., Anghel, I., Preda, D.-M., Todorova, T., & Rîmbu, C. M. (2023). Organophosphorus Reinforced Poly(vinyl alcohol) Nanocomposites Doped with Silver-Loaded Zeolite L Nanoparticles as Sustainable Materials for Packaging Applications. Polymers, 15(11), 2573. https://doi.org/10.3390/polym15112573







