The Effect of Alkyl Terminal Chain Length of Schiff-Based Cyclotriphosphazene Derivatives towards Epoxy Resins on Flame Retardancy and Mechanical Properties
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Syntheses
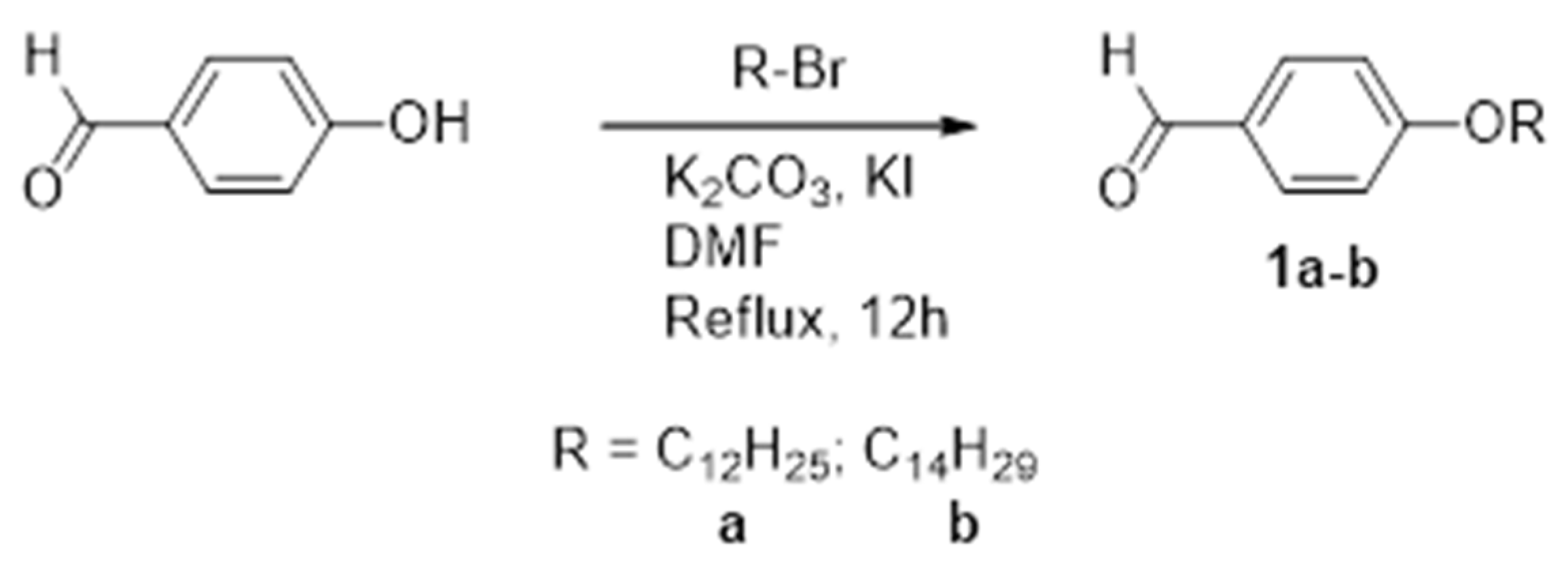
2.2.1. Synthesis of 4-alkoxybenzaldehyde, 1a–b
- 4-dodecyloxybenzaldehyde, 1a:
- 4-tetradecyloxybenzaldehyde, 1b:
2.2.2. Synthesis of 4-(substituted benzylidene)hydrazinium, 2a–b
- 4-Dodecyloxybenzylidene hydrazinium, 2a:
- 4-Tetradecyloxybenzylidene hydrazinium, 2b:
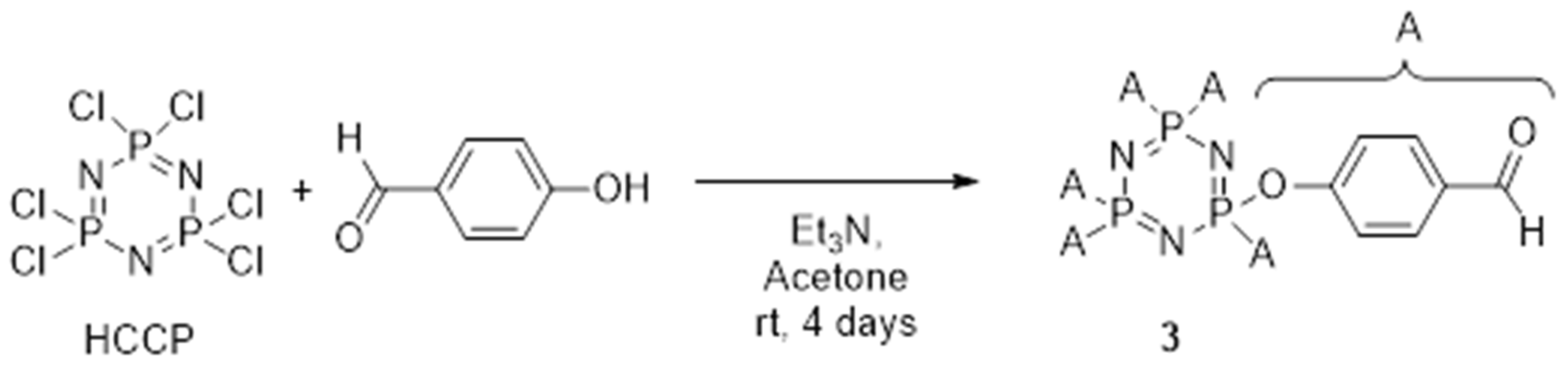
2.2.3. Synthesis of hexakis(4-formlyphenoxy)cyclotriphosphazene, 3
- Hexakis(4-formlyphenoxy)cyclotriphosphazene, 3:
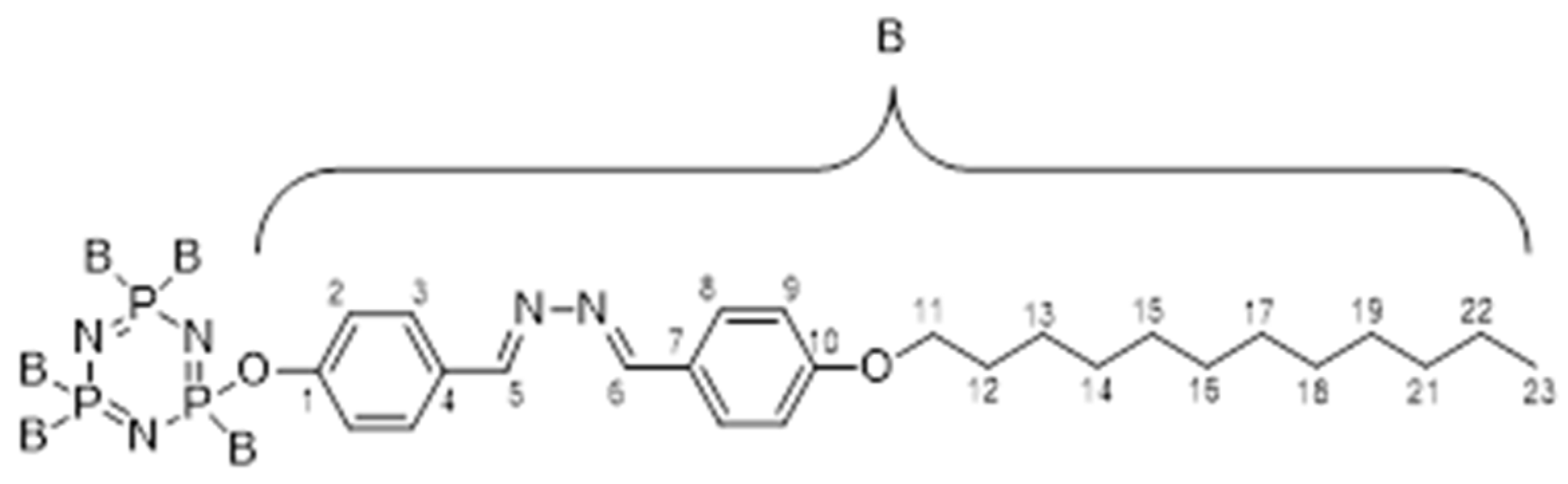
2.2.4. Synthesis of hexakis{4-((E)-((4-((E)-4-substituted-benzylidene)hydrazine-1-ylidene)methyl)phenoxy}triazophosphazene, 4a–b
- Hexakis{4-((E)-((4-((E)-4-dodecyloxy-benzylidene)hydrazine-1 ylidene)methyl)phenoxy} triazophosphazene, 4a:
- Hexakis{4-((E)-((4-((E)-4-tetradecyloxy-benzylidene)hydrazine-1 ylidene)methyl)phenoxy} triazophosphazene, 4b:
2.2.5. Preparation of Molded Epoxy Resin with Compounds 4a and 4b
2.3. Measurement and Characterization
3. Results and Discussion
3.1. Synthesis of the Intermediates and Final Compounds
3.2. Characterization of Chemical Structure
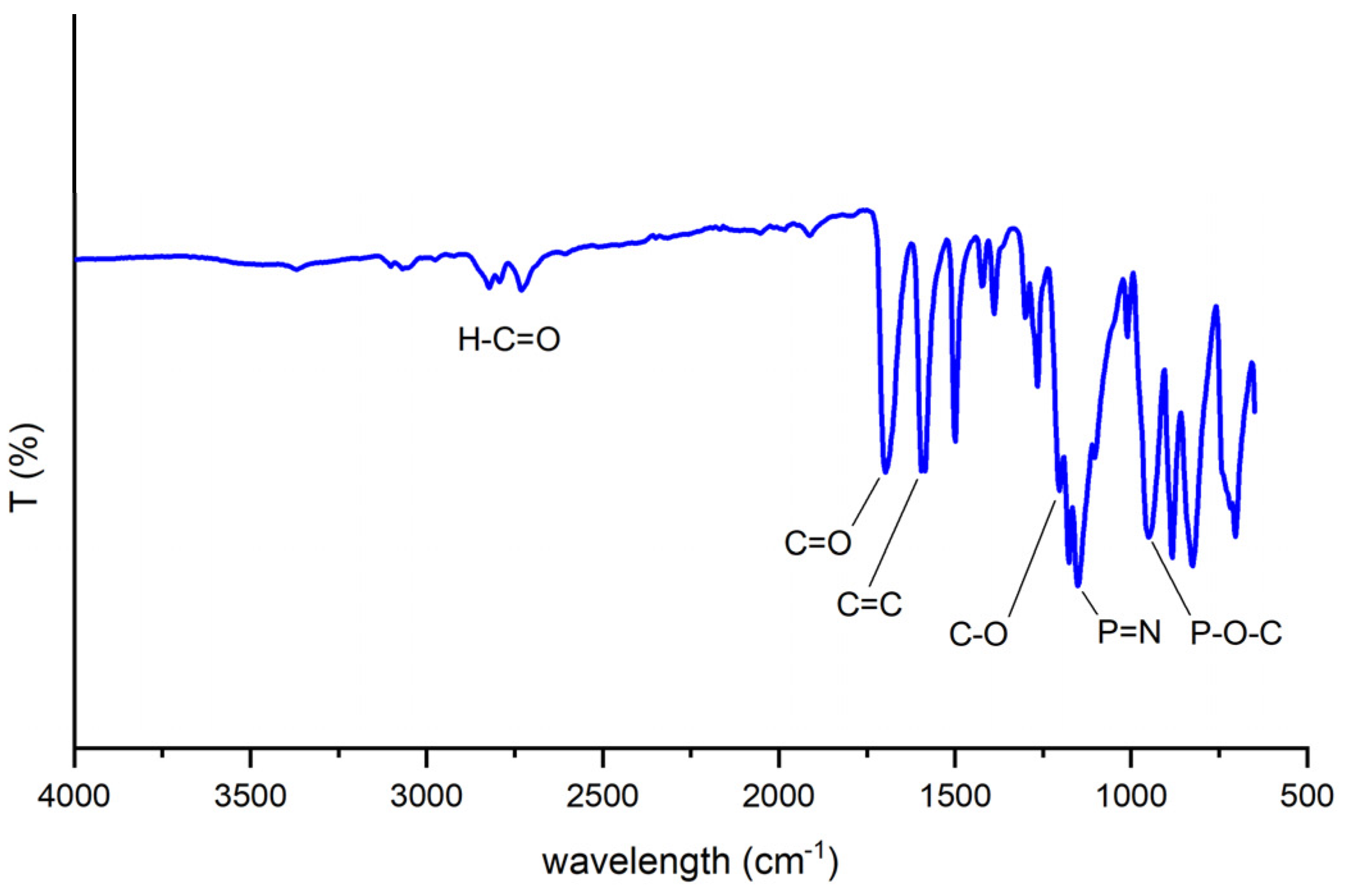
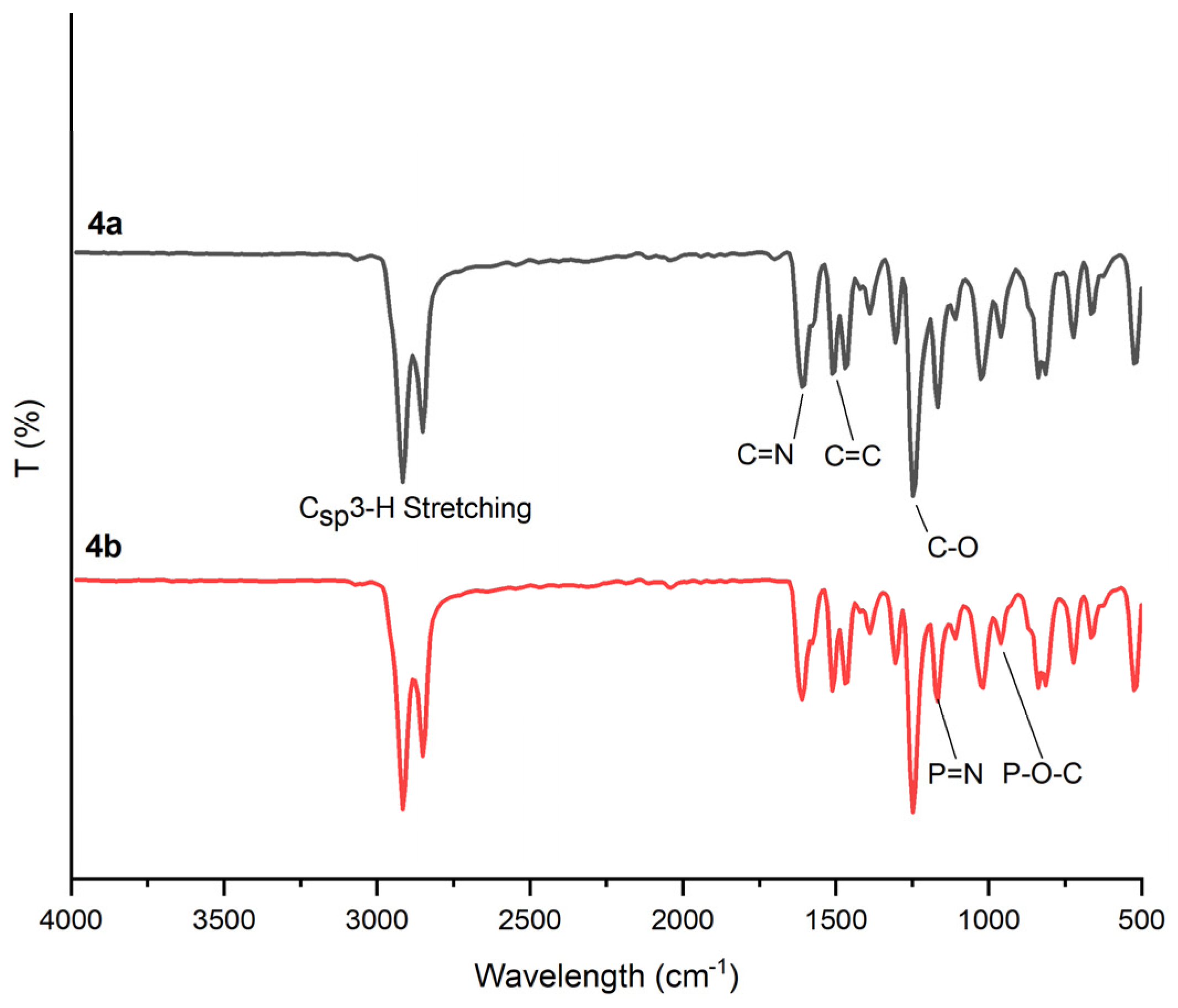
3.2.1. Fourier-Transform Infrared (FT-IR) Spectral Analysis
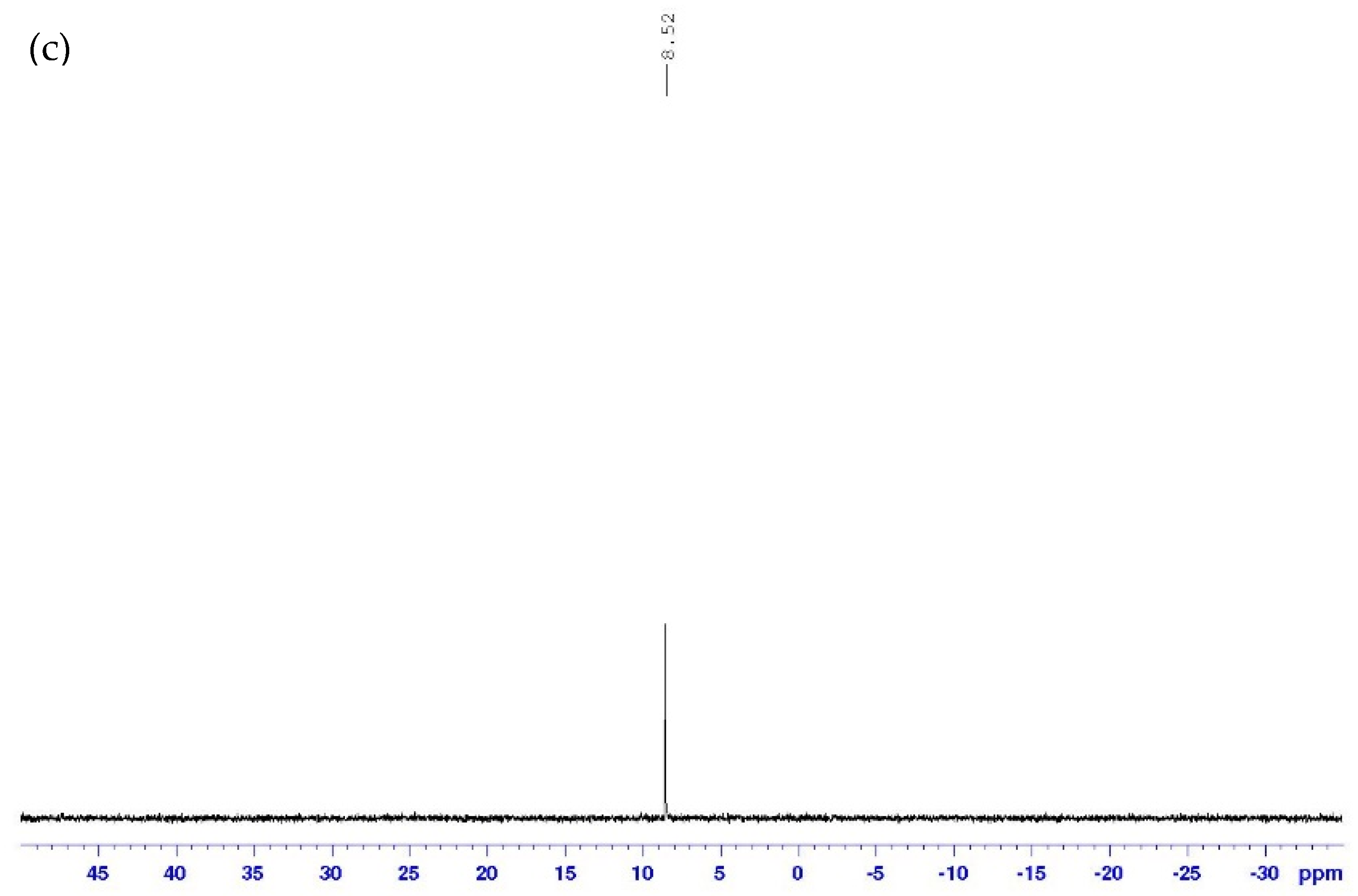
3.2.2. Nuclear Magnetic Resonance (NMR) Characterization of Final Compound
3.2.3. CHN Elemental Analysis
3.3. Effect of Hexasusbstituted Cyclotriphosphazene Derivatives on the Flame Retardancy
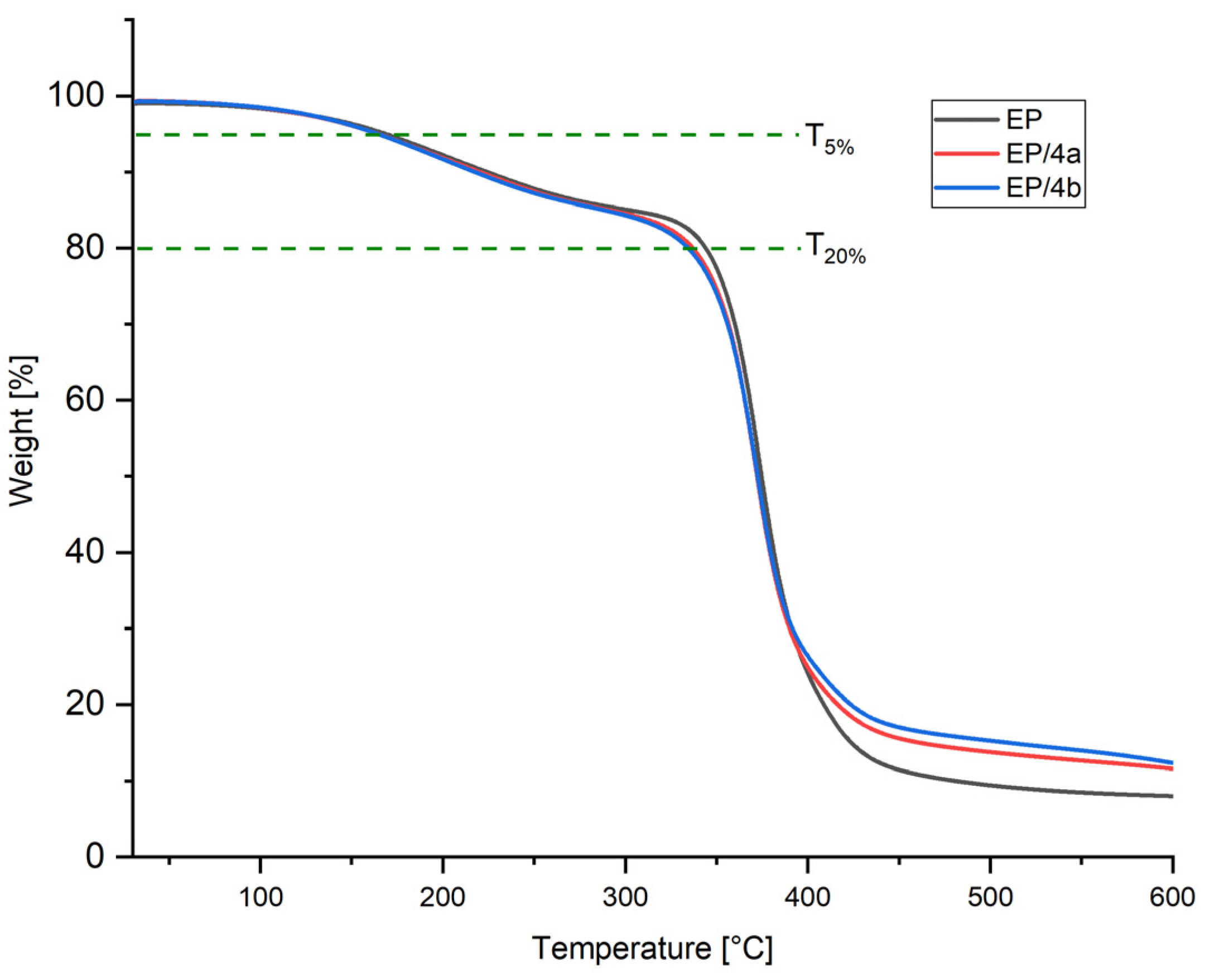
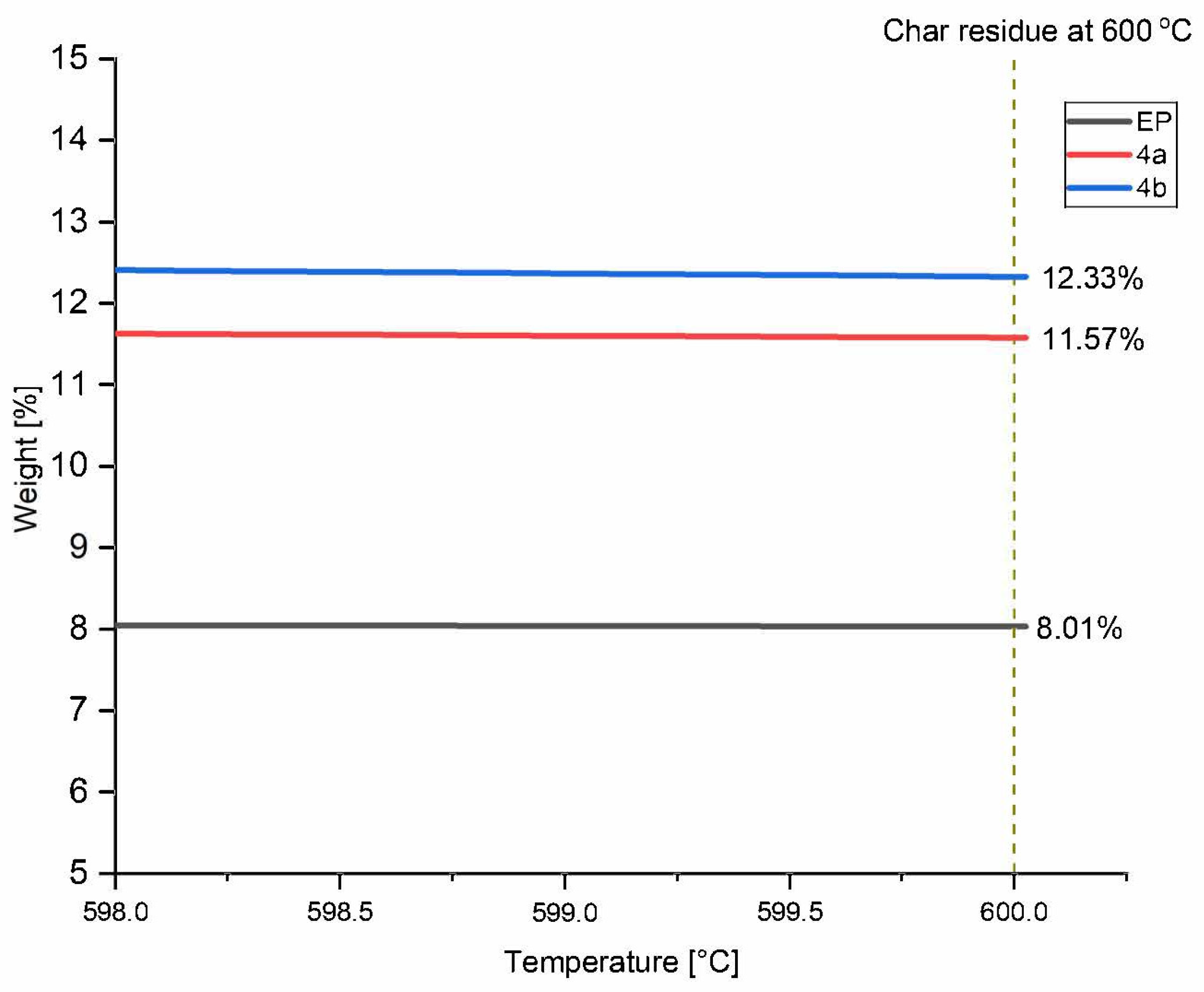
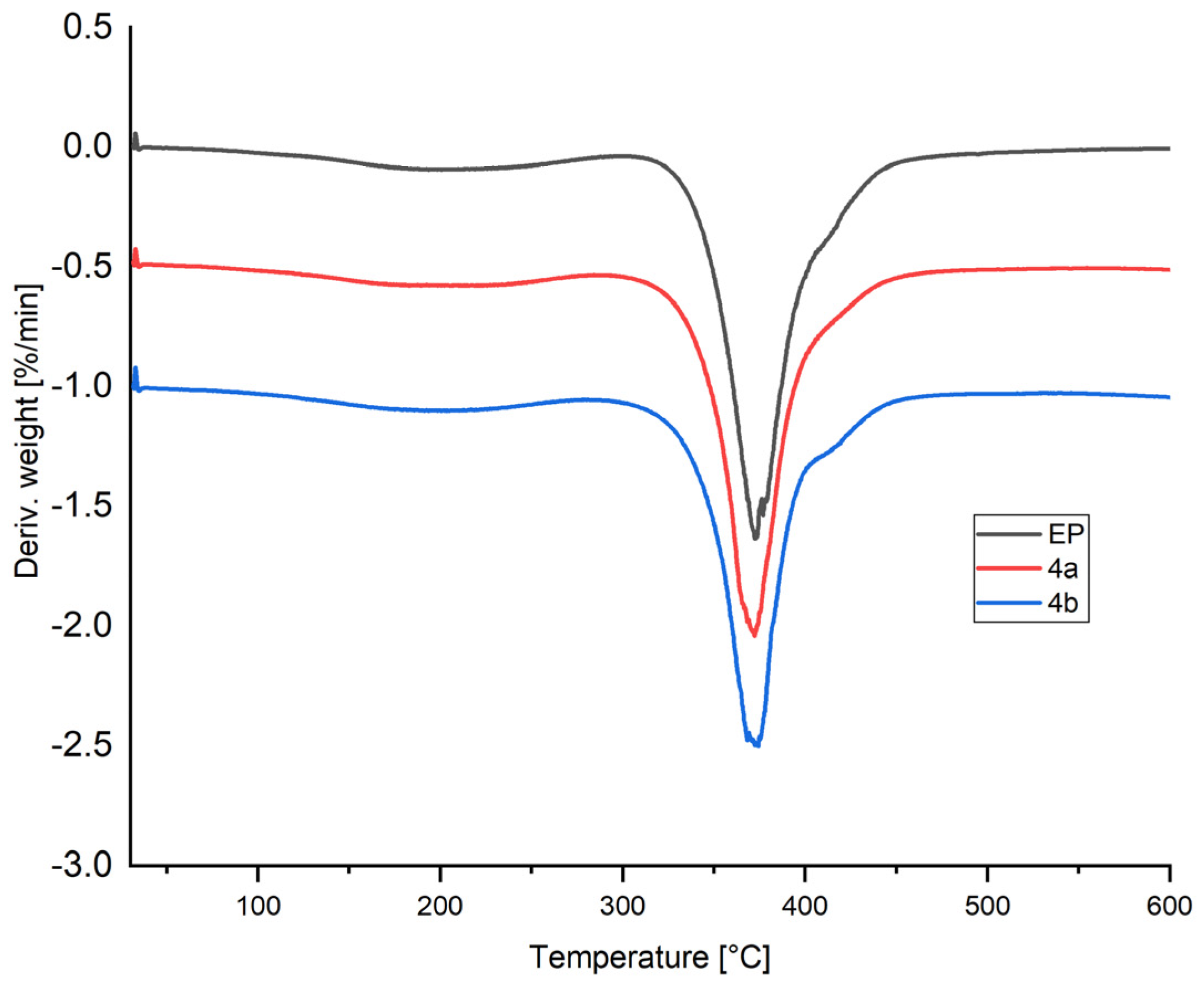
3.3.1. Thermogravimetric Analysis of Final Compounds
3.3.2. Limiting Oxygen Index (LOI) Test
3.3.3. Morphology Study of Char Residue
3.4. Effect of Hexasusbstituted Cyclotriphosphazene Derivatives on the Mechanical Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singewald, T.D.; Bruckner, T.M.; Gruber, R.; Schimo-Aichhorn, G.; Hader-Kregl, L.; Andronescu, S.; Klotz, M.; Müller, M.; Kern, C.; Rosner, M.; et al. Systematic Variation of Inorganic Additives and Their Impact on Interfacial Delamination Processes of Automotive Coating Systems. Prog. Org. Coat. 2022, 173, 107172. [Google Scholar] [CrossRef]
- Miturska-Barańska, I.; Rudawska, A.; Doluk, E. Influence of Physical Modification of the Adhesive Composition on the Strength Properties of Aerospace Aluminum Alloy Sheet Adhesive Joints. Materials 2022, 15, 7799. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, X.; Wu, D. Novel Cyclolinear Cyclotriphosphazene-Linked Epoxy Resin for Halogen-Free Fire Resistance: Synthesis, Characterization, and Flammability Characteristics. Ind. Eng. Chem. Res. 2012, 51, 15064–15074. [Google Scholar] [CrossRef]
- Younis, A.A. Flammability Properties of Polypropylene Containing Montmorillonite and Some of Silicon Compounds. Egypt. J. Pet. 2017, 26, 1–7. [Google Scholar] [CrossRef]
- Venier, M.; Salamova, A.; Hites, R.A. Halogenated Flame Retardants in the Great Lakes Environment. Acc. Chem. Res. 2015, 48, 1853–1861. [Google Scholar] [CrossRef]
- Shaw, S.D.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.P.; Dobraca, D.; Hanson, S.; Birnbaum, L.S.; et al. Halogenated Flame Retardants: Do the Fire Safety Benefits Justify the Risks? Rev. Environ. Health 2010, 5, 261–305. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Wang, C.; Huang, W.; Tian, Q.; Fu, Q.; Yan, W. Flame-Retardant Performance of Epoxy Resin Composites with SiO2 Nanoparticles and Phenethyl-Bridged DOPO Derivative. ACS Omega 2021, 6, 666–674. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wang, D.Y. Renewable Cardanol-Based Surfactant Modified Layered Double Hydroxide as a Flame Retardant for Epoxy Resin. ACS Sustain. Chem. Eng. 2015, 3, 3281–3290. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Nurtazina, A.S.; Kadykova, Y.A.; Bekeshev, A.Z. Highly Efficient Plasticizers-Antipirenes for Epoxy Polymers. Inorg. Mater. Appl. Res. 2019, 10, 1135–1139. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Kadykova, Y.; Akhmetova, M.; Tastanova, L.; Lopukhova, M. Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites. Polymers 2021, 13, 2421. [Google Scholar] [CrossRef]
- Bao, X.; Wu, F.; Wang, J. Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes. Polymers 2021, 13, 3332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tao, R.; Dai, P.; Luo, Z.; He, M. Two-Step Fabrication of Lignin-Based Flame Retardant for Enhancing the Thermal and Fire Retardancy Properties of Epoxy Resin Composites. Polym. Compos. 2020, 41, 2025–2035. [Google Scholar] [CrossRef]
- Orlov, A.; Konstantinova, A.; Korotkov, R.; Yudaev, P.; Mezhuev, Y.; Terekhov, I.; Gurevich, L.; Chistyakov, E. Epoxy Compositions with Reduced Flammability Based on DER-354 Resin and a Curing Agent Containing Aminophosphazenes Synthesized in Bulk Isophoronediamine. Polymers 2022, 14, 3592. [Google Scholar] [CrossRef] [PubMed]
- Jeevananthan, V.; Shanmugan, S. Halogen-Free Layered Double Hydroxide-Cyclotriphosphazene Carboxylate Flame Retardants: Effects of Cyclotriphosphazene Di, Tetra and Hexacarboxylate Intercalation on Layered Double Hydroxides against the Combustible Epoxy Resin Coated on Wood Substrates. RSC Adv. 2022, 12, 23322–23336. [Google Scholar] [CrossRef]
- Zarybnicka, L.; Machotova, J.; Kopecka, R.; Sevcik, R.; Hudakova, M.; Pokorny, J.; Sal, J. Effect of Cyclotriphosphazene-Based Curing Agents on the Flame Resistance of Epoxy Resins. Polymers 2021, 13, 8. [Google Scholar] [CrossRef]
- Palabıyık, D.; Mutlu Balcı, C.; Tümay, S.O.; Sengul, I.F.; Beşli, S. New Design of Cyclotriphosphazene Derivatives Bearing Carbazole Units: The Syntheses, Characterization, and Photophysical Properties. Inorg. Chim. Acta 2022, 539, 121022. [Google Scholar] [CrossRef]
- Fontenot, K.R.; Nguyen, M.M.; Al-Abdul-Wahid, M.S.; Easson, M.W.; Chang, S.; Lorigan, G.A.; Condon, B.D. The Thermal Degradation Pathway Studies of a Phosphazene Derivative on Cotton Fabric. Polym. Degrad. Stab. 2015, 120, 32–41. [Google Scholar] [CrossRef]
- Jamain, Z.; Khairuddean, M.; Saidin, S.A. Synthesis and Characterization of 1,4-Phenylenediamine Derivatives Containing Hydroxyl and Cyclotriphosphazene as Terminal Group. J. Mol. Struct. 2019, 1186, 293–302. [Google Scholar] [CrossRef]
- Mohd Taip, N.A.; Jamain, Z.; Palle, I. Fire-Retardant Property of Hexasubstituted Cyclotriphosphazene Derivatives with Schiff Base Linking Unit Applied as an Additives in Polyurethane Coating for Wood Fabrication. Polymers 2022, 14, 3768. [Google Scholar] [CrossRef]
- Shi, L.; Ge, H.M.; Tan, S.H.; Li, H.Q.; Song, Y.C.; Zhu, H.L.; Tan, R.X. Synthesis and Antimicrobial Activities of Schiff Bases Derived from 5-Chloro-Salicylaldehyde. Eur. J. Med. Chem. 2007, 42, 558–564. [Google Scholar] [CrossRef]
- Xiong, G.; Gao, S.; Zhang, Q.; Ren, B.; You, L.; Ding, F.; He, Y.; Sun, Y. High Porosity Cyclotriphosphazene-Based Hyper-Crosslinked Polymers as Efficient Cationic Dye MB Adsorbents. Polymer 2022, 247, 124787. [Google Scholar] [CrossRef]
- İbişoğlu, H.; Erdemir, E.; Atilla, D.; Zorlu, Y.; Şenkuytu, E. Synthesis, Characterization and Photophysical Properties of Cyclotriphosphazenes Including Heterocyclic Rings. Inorg. Chim. Acta 2019, 498, 119120. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, M.; Qu, L.; Sun, G. Effect of Phosphorus-Containing Flame Retardants on Flame Retardancy and Thermal Stability of Tetrafunctional Epoxy Resin. Polym. Adv. Technol. 2015, 26, 1531–1536. [Google Scholar] [CrossRef]
- Qu, L.; Zhang, C.; Li, P.; Dai, X.; Xu, T.; Sui, Y.; Gu, J.; Dou, Y. Improved Thermal Properties of Epoxy Resin Modified with Polymethyl Methacrylate-Microencapsulated Phosphorus-Nitrogen-Containing Flame Retardant. RSC Adv. 2018, 8, 29816–29829. [Google Scholar] [CrossRef]
- Tarasov, I.v.; Oboishchikova, A.v.; Borisov, R.S.; Kireev, V.v.; Sirotin, I.S. Phosphazene-Containing Epoxy Resins Based on Bisphenol F with Enhanced Heat Resistance and Mechanical Properties: Synthesis and Properties. Polymers 2022, 14, 4547. [Google Scholar] [CrossRef]
- Al-Shukri, S.M.; Mahmood, A.T.; al Hanbali, O.A. Spiro-Cyclotriphosphazene with Three Functional End Groups: Synthesis and Structural Characterization of New Polycyclotriphosphazenes with Schiff-Base Groups. Polimery/Polymers 2021, 66, 341–349. [Google Scholar] [CrossRef]
- Xu, M.J.; Xu, G.R.; Leng, Y.; Li, B. Synthesis of a Novel Flame Retardant Based on Cyclotriphosphazene and DOPO Groups and Its Application in Epoxy Resins. Polym. Degrad. Stab. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Wang, H.; Du, X.; Wang, S.; Du, Z.; Wang, H.; Cheng, X. Improving the Flame Retardancy of Waterborne Polyurethanes Based on the Synergistic Effect of P-N Flame Retardants and a Schiff Base. RSC Adv. 2020, 10, 12078–12088. [Google Scholar] [CrossRef]
- İbişoğlu, H.; Ün, Ş.Ş.; Erdemir, E.; Tümay, S.O. Synthesis, Characterization, and Photophysical Properties of Cyclotriphosphazenes Containing Quinoline-4-Aldehyde-p-Oxyanil Moieties. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 760–768. [Google Scholar] [CrossRef]
- Jamain, Z.; Khairuddean, M.; Guan-Seng, T.; Rahman, A.B.A. Synthesis, Characterisation and Mesophase Transition of Hexasubstituted Cyclotriphosphazene Molecules with Schiff Base and Azo Linking Units and Determination of Their Fire Retardant Properties. Macromol. Res. 2021, 29, 331–341. [Google Scholar] [CrossRef]
- Fan, S.; Peng, B.; Yuan, R.; Wu, D.; Wang, X.; Yu, J.; Li, F. A Novel Schiff Base-Containing Branched Polysiloxane as a Self-Crosslinking Flame Retardant for PA6 with Low Heat Release and Excellent Anti-Dripping Performance. Compos. B Eng. 2020, 183, 107684. [Google Scholar] [CrossRef]
- Wu, J.N.; Chen, L.; Fu, T.; Zhao, H.B.; Guo, D.M.; Wang, X.L.; Wang, Y.Z. New Application for Aromatic Schiff Base: High Efficient Flame-Retardant and Anti-Dripping Action for Polyesters. Chem. Eng. J. 2018, 336, 622–632. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, Y.; Li, Y.; Tan, X.; Li, Q.; Cheng, J.; Zhang, J. High Mechanical Properties of Epoxy Networks with Dangling Chains and Tunable Microphase Separation Structure. RSC Adv. 2017, 7, 49074–49082. [Google Scholar] [CrossRef]
- Saw, S.K.; Purwar, R.; Nandy, S.; Ghose, J.; Sarkhel, G. Fabrication, Characterization, and Evaluation of Luffa Cylindrica Fiber Reinforced Epoxy Composites. BioResources 2013, 8, 4805–4826. [Google Scholar] [CrossRef]
- Jamain, Z.; Khairuddean, M.; Guan-Seng, T. Liquid-Crystal and Fire-Retardant Properties of New Hexasubstituted Cyclotriphosphazene Compounds with Two Schiff Base Linking Units. Molecules 2020, 25, 2122. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.J.; Park, J.H.; Lee, C.; Chang, J.Y. Discotic Liquid Crystalline Hydrazone Compounds: Synthesis and Mesomorphic Properties. Org. Lett. 2006, 8, 2221–2224. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Hu, W.; Xu, L.; Song, Y.; Li, J. Synthesis, Mechanical Properties and Fire Behaviors of Rigid Polyurethane Foam with a Reactive Flame Retardant Containing Phosphazene and Phosphate. Polym. Degrad. Stab. 2015, 122, 102–109. [Google Scholar] [CrossRef]
- Kandioller, W.; Theiner, J.; Keppler, B.K.; Kowol, C.R. Elemental Analysis: An Important Purity Control but Prone to Manipulations. Inorg. Chem. Front. 2022, 9, 412–416. [Google Scholar] [CrossRef]
- Manfredi, L.B.; Rodríguez, E.S.; Wladyka-Przybylak, M.; Vázquez, A. Thermal Degradation and Fire Resistance of Unsaturated Polyester, Modified Acrylic Resins and Their Composites with Natural Fibres. Polym. Degrad. Stab. 2006, 91, 255–261. [Google Scholar] [CrossRef]
- Kandola, B.K.; Biswas, B.; Price, D.; Horrocks, A.R. Studies on the Effect of Different Levels of Toughener and Flame Retardants on Thermal Stability of Epoxy Resin. Polym. Degrad. Stab. 2010, 95, 144–152. [Google Scholar] [CrossRef]
- Rhili, K.; Chergui, S.; Eldouhaibi, A.S.; Siaj, M. Hexachlorocyclotriphosphazene Functionalized Graphene Oxide as a Highly Efficient Flame Retardant. ACS Omega 2021, 6, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, P.; Fan, H.; Sun, Z.; Wen, J. Covalently Functionalized Graphene towards Molecular-Level Dispersed Waterborne Polyurethane Nanocomposite with Balanced Comprehensive Performance. Appl. Surf. Sci. 2019, 471, 595–606. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Jiang, Y.; He, H.; Liu, H.; Huang, H.; Wan, C. Facile Synthesis of a Novel Transparent Hyperbranched Phosphorous/Nitrogen-Containing Flame Retardant and Its Application in Reducing the Fire Hazard of Epoxy Resin. J. Hazard. Mater. 2019, 379, 120793. [Google Scholar] [CrossRef] [PubMed]
- Jamain, Z.; Khairuddean, M.; Guan-Seng, T. Synthesis of Novel Liquid Crystalline and Fire Retardant Molecules Based on Six-Armed Cyclotriphosphazene Core Containing Schiff Base and Amide Linking Units. RSC Adv. 2020, 10, 28918–28934. [Google Scholar] [CrossRef]
- Li, N.; Ming, J.; Yuan, R.; Fan, S.; Liu, L.; Li, F.; Wang, X.; Yu, J.; Wu, D. Novel Eco-Friendly Flame Retardants Based on Nitrogen-Silicone Schiff Base and Application in Cellulose. ACS Sustain. Chem. Eng. 2020, 8, 290–301. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent Developments in the Fire Retardancy of Polymeric Materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Jimenez, M.; Duquesne, S.; Bourbigot, S. Intumescent Fire Protective Coating: Toward a Better Understanding of Their Mechanism of Action. Thermochim. Acta 2006, 449, 16–26. [Google Scholar] [CrossRef]
- Djafari Petroudy, S.R. Physical and Mechanical Properties of Natural Fibers. In Advanced High Strength Natural Fibre Composites in Construction; Woodhead Publishing: Sawston, UK, 2017; pp. 59–83. [Google Scholar] [CrossRef]
- Leng, B.; Yang, J.; Zhu, C.; Wang, Z.; Shi, C.; Liu, Y.; Zhang, H.; Xu, W.; Liu, B. Synthesis of a Cyclophosphazene Derivative Containing Multiple Cyano Groups for Electron-Beam Irradiated Flame-Retardant Materials. Polymers 2021, 13, 3460. [Google Scholar] [CrossRef]






| Properties of Epoxy Resin [34] | |
| Appearance | Clear liquid |
| Specific gravity at 25 °C | 1.12 gm/cc |
| Viscosity at 25 °C | 550 centipoises |
| Tensile strength | 6.9 MPa |
| Properties of Phosphonitrilic Chloride Trimer, HCCP (SDS, Sigma-Aldrich) | |
| Appearance | Crystalline/light gray powder |
| Formula | Cl6N3P3 |
| Molecular weight | 347.66 g/mol |
| Melting point | 112–115 °C |
| Initial boiling point and boiling range | 127 °C at 17 hPa |
| Relative density at 25 °C | 1.98 g/mL |
| Compound | Calculated (%) | Found (%) | ||||
|---|---|---|---|---|---|---|
| C | H | N | C | H | N | |
| C21H34O2, 1b | 79.19 | 10.76 | - | 78.84 | 10.67 | - |
| C19H32N2O, 2a | 74.95 | 10.59 | 9.20 | 74.83 | 10.47 | 9.11 |
| C21H36N2O, 2b | 75.85 | 10.92 | 8.42 | 75.79 | 10.86 | 8.38 |
| C42H30N3O12P3, 3 | 58.55 | 3.51 | 4.88 | 58.30 | 3.45 | 4.80 |
| C156H210N15O12P3, 4a | 72.61 | 8.20 | 8.14 | 72.55 | 8.16 | 8.09 |
| C168H234N15O12P3, 4b | 73.41 | 8.58 | 7.64 | 73.38 | 8.54 | 7.61 |
| Sample | T5% (°C) | T20% (°C) | Tmax (°C) | Char Yield at 600 °C (wt.%) |
|---|---|---|---|---|
| Pure EP | 168.7 | 344.6 | 372.6 | 8.0 |
| EP/4a | 164.3 | 337.9 | 372.3 | 11.57 |
| EP/4b | 164.4 | 334.5 | 374.0 | 12.33 |
| Sample | LOI Value (%) | Sample | LOI Value (%) |
|---|---|---|---|
| Pure EP | 22.75 (±0.00) | EP/3 | 24.90 (±0.00) |
| EP/2a | 23.42 (±0.00) | EP/4a | 26.55 (±0.00) |
| EP/2b | 23.53 (±0.00) | EP/4b | 26.71 (±0.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldin, N.A.; Jamain, Z. The Effect of Alkyl Terminal Chain Length of Schiff-Based Cyclotriphosphazene Derivatives towards Epoxy Resins on Flame Retardancy and Mechanical Properties. Polymers 2023, 15, 1431. https://doi.org/10.3390/polym15061431
Waldin NA, Jamain Z. The Effect of Alkyl Terminal Chain Length of Schiff-Based Cyclotriphosphazene Derivatives towards Epoxy Resins on Flame Retardancy and Mechanical Properties. Polymers. 2023; 15(6):1431. https://doi.org/10.3390/polym15061431
Chicago/Turabian StyleWaldin, Nur Atika, and Zuhair Jamain. 2023. "The Effect of Alkyl Terminal Chain Length of Schiff-Based Cyclotriphosphazene Derivatives towards Epoxy Resins on Flame Retardancy and Mechanical Properties" Polymers 15, no. 6: 1431. https://doi.org/10.3390/polym15061431
APA StyleWaldin, N. A., & Jamain, Z. (2023). The Effect of Alkyl Terminal Chain Length of Schiff-Based Cyclotriphosphazene Derivatives towards Epoxy Resins on Flame Retardancy and Mechanical Properties. Polymers, 15(6), 1431. https://doi.org/10.3390/polym15061431







