Stimulation of Chondrocyte and Bone Marrow Mesenchymal Stem Cell Chondrogenic Response by Polypyrrole and Polypyrrole/Gold Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Ppy and Ppy/Au NPs
2.2. BMMSCs and Chondrocyte Isolation and Culture
2.3. Cell Proliferation Analysis
2.4. Cell Viability Test
2.5. Chondrogenic Differentiation of Cells
2.6. COMP ELISA
2.7. RNA Extraction from and RT-qPCR
2.8. Histology and Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. AuNPs, Ppy, and Ppy/Au NPs Synthesis
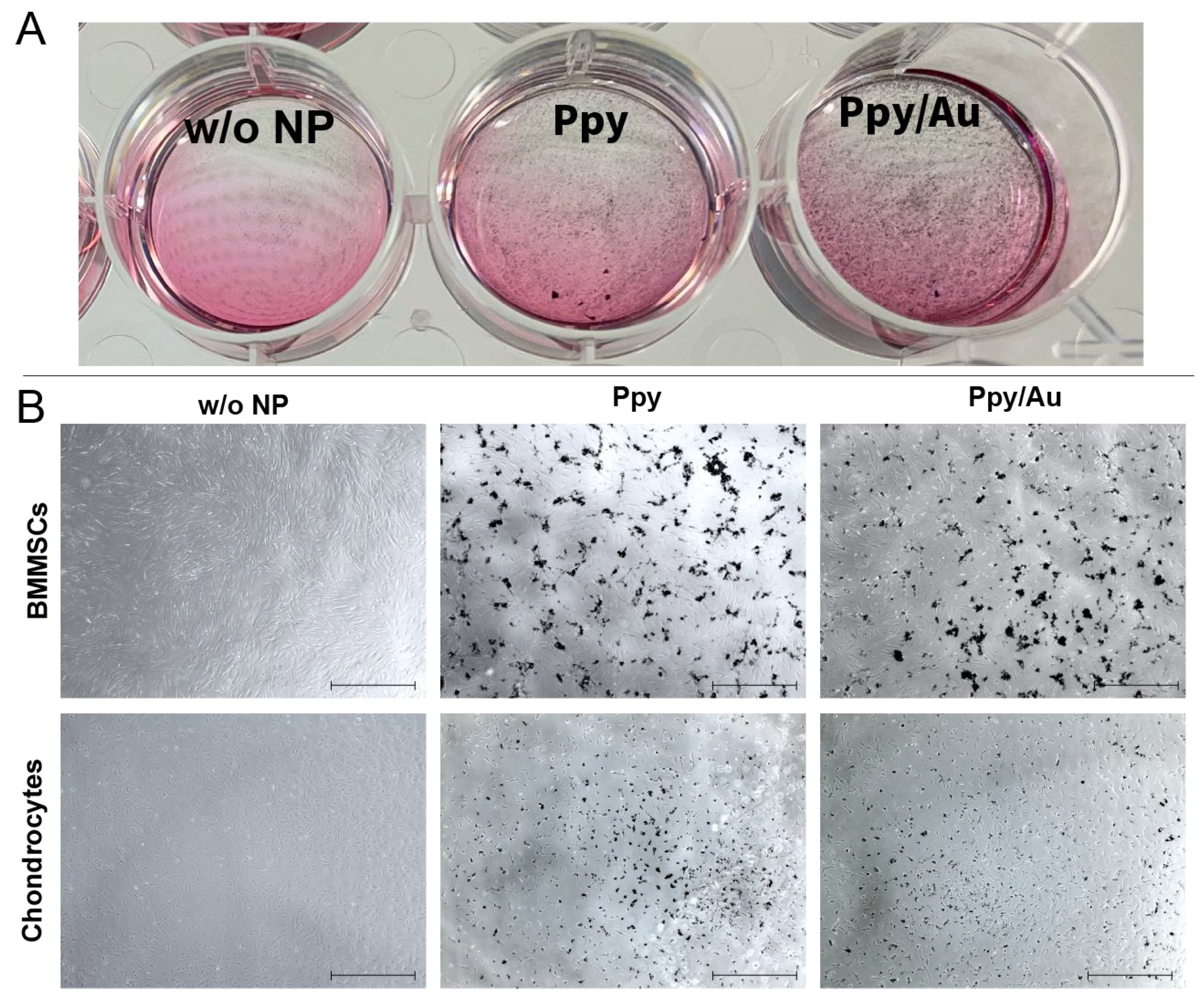
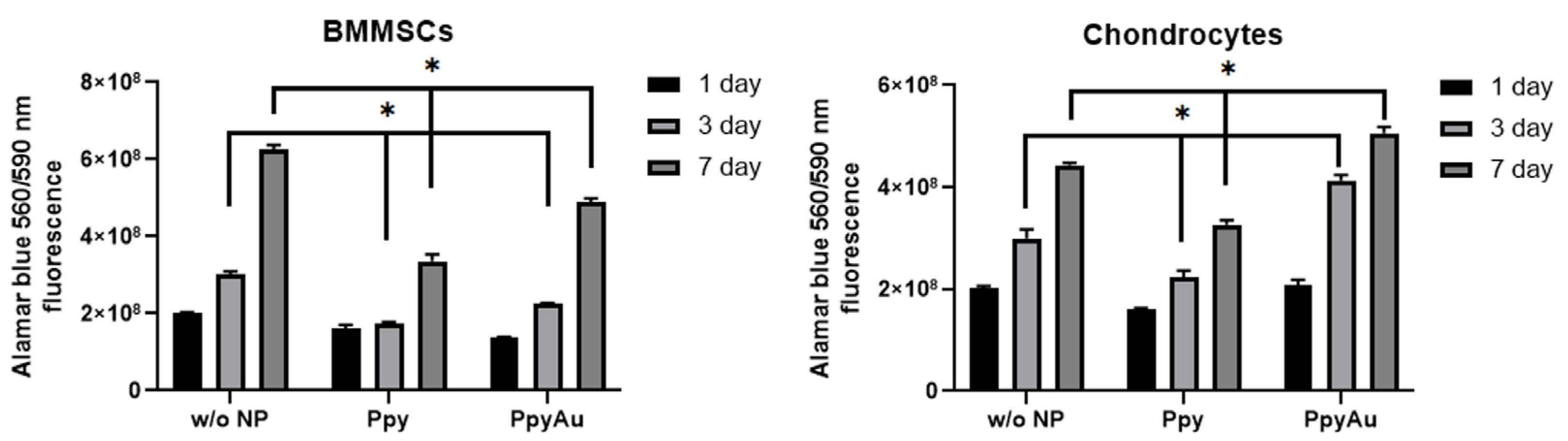
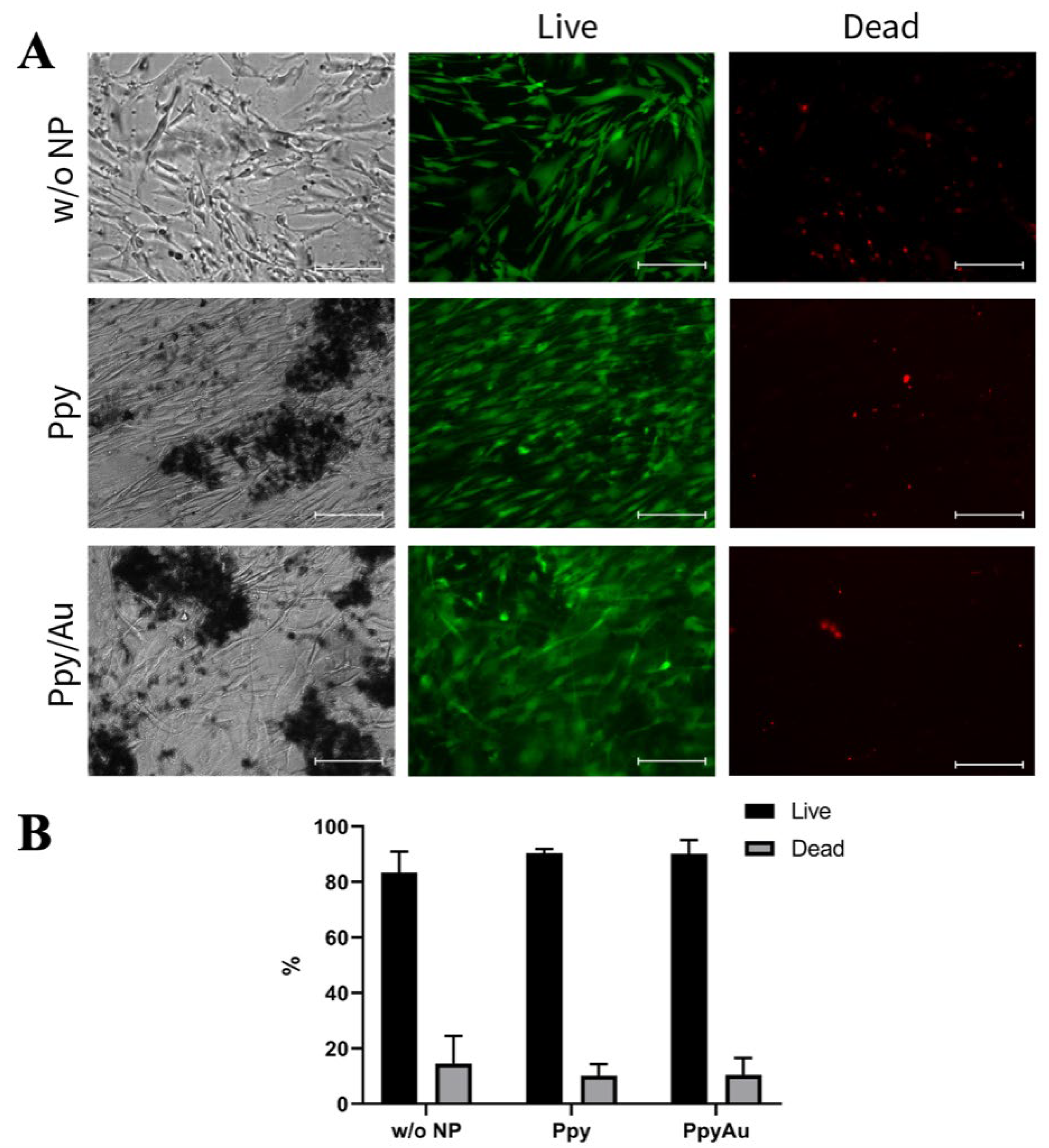
3.2. Cell Morphology, Proliferation, and Viability after Incubation with Ppy and Ppy/Au NPs
3.3. Chondrogenic Differentiation after Incubation with Ppy and Ppy/Au NPs
3.3.1. Cartilage Oligomeric Matrix Protein Level in Cell Supernatants was Higher after Incubation with Ppy and Ppy/Au NPs
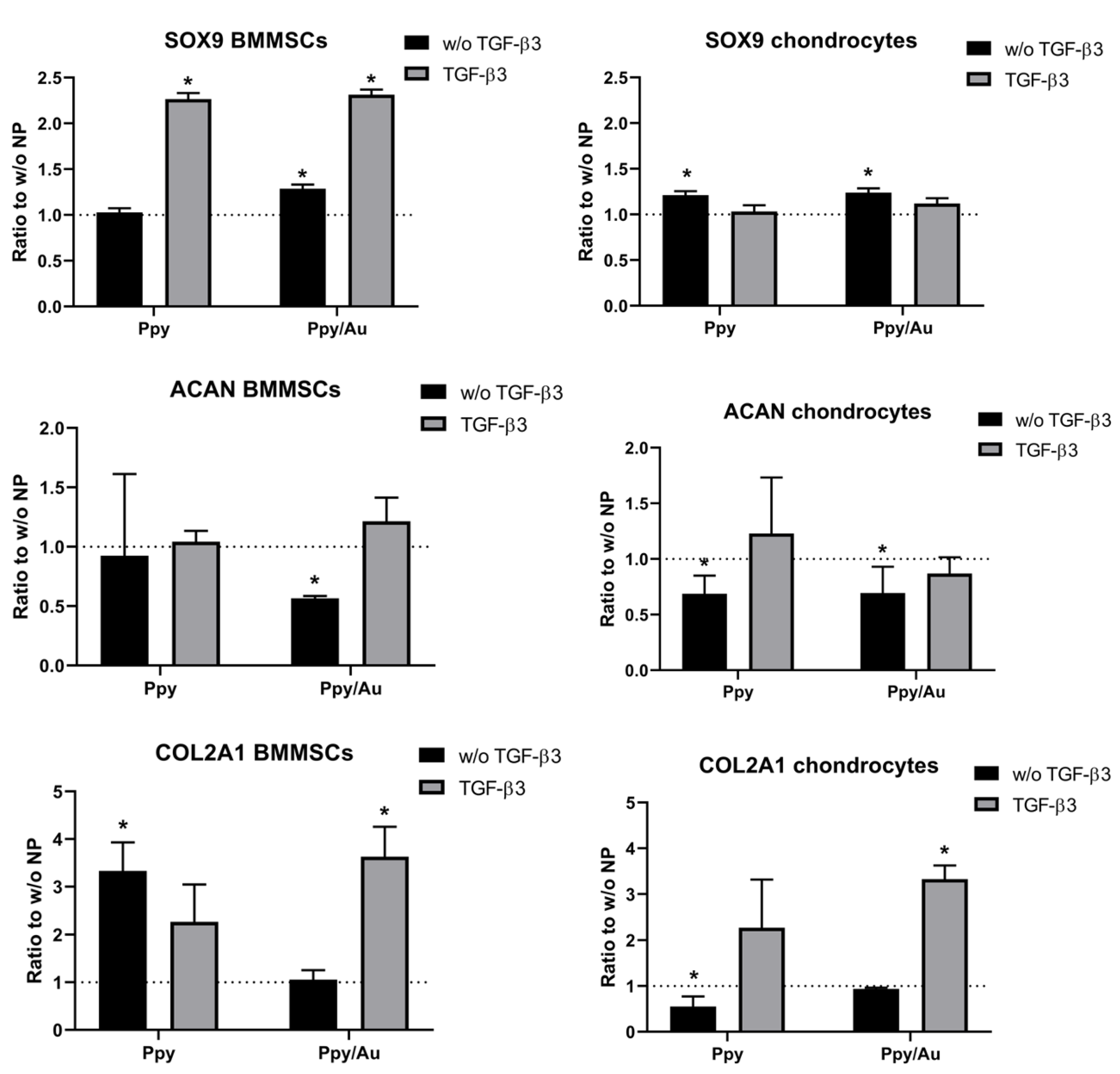
3.3.2. Chondrogenic Gene Expression in Cells was Higher after Incubation with Ppy and Ppy/Au NPs
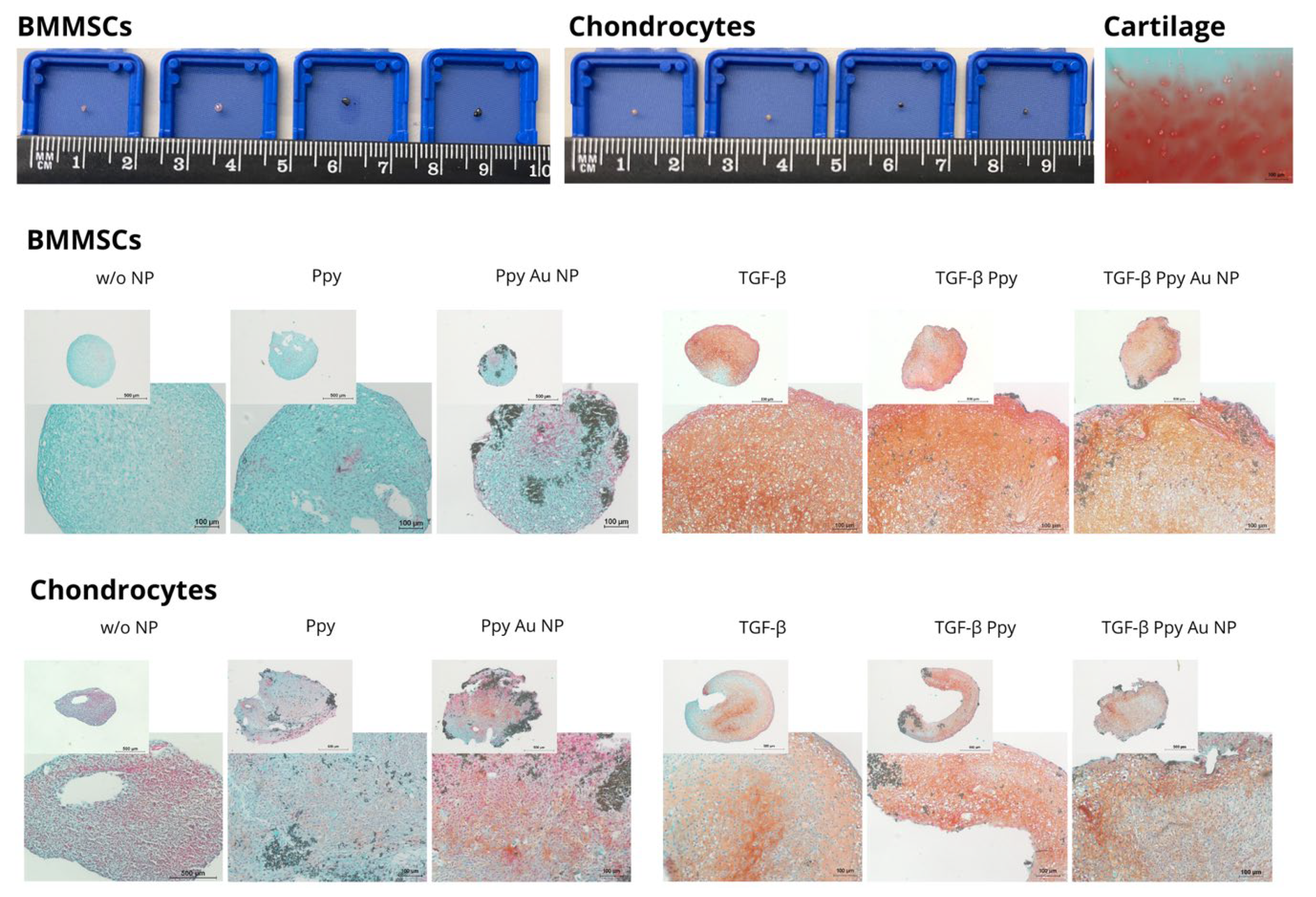
3.3.3. Chondrogenic Differentiation in Pellets after Incubation with Ppy and Ppy/Au NPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACAN | aggrecan gene |
| Au NPs | gold NPs |
| BMMSCs | bone marrow mesenchymal stem cells |
| CCK-8 | cell counting kit 8 |
| COL2A1 | collagen type II gene |
| COMP | cartilage oligomeric matrix protein |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ECM | extracellular matrix |
| ES | electrical stimulation |
| FBS | fetal bovine serum |
| FGF2 | fibroblast growth factor-2 |
| MSCs | mesenchymal stem cells |
| NCDCs | nasal crest-derived chondrocytes |
| NP | nanoparticle |
| OA | osteoarthritis |
| PBS | phosphate-buffered saline |
| PFA | paraformaldehyde |
| SD | standard deviation |
| TGF-β3 | transforming growth factor β3 |
| Ppy | polypyrrole |
| Ppy NPs | polypyrrole nanoparticles |
| Ppy/Au NPs | polypyrrole/gold nanocomposites |
References
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation of polyaniline and polypyrrole nanocomposites with embedded glucose oxidase and gold nanoparticles. Polymers 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Fadel, M.; Fadeel, D.A.; Ibrahim, M.; Hathout, R.M.; El-Kholy, A.I. One-step synthesis of polypyrrole-coated gold nanoparticles for use as a photothermally active nano-system. Int. J. Nanomed. 2020, 15, 2605–2615. [Google Scholar] [CrossRef] [PubMed]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- Borges, M.H.R.; Nagay, B.E.; Costa, R.C.; Souza, J.G.S.; Mathew, M.T.; Barão, V.A.R. Recent advances of polypyrrole conducting polymer film for biomedical application: Toward a viable platform for cell-microbial interactions. Adv. Colloid Interface Sci. 2023, 314, 102860. [Google Scholar] [CrossRef]
- Liu, L.; Li, P.; Zhou, G.; Wang, M.; Jia, X.; Liu, M.; Niu, X.; Song, W.; Liu, H.; Fan, Y. Increased proliferation and differentiation of pre-osteoblasts MC3T3-E1 cells on nanostructured polypyrrole membrane under combined electrical and mechanical stimulation. J. Biomed. Nanotechnol. 2013, 9, 1532–1539. [Google Scholar] [CrossRef]
- Liang, Y.; Goh, J.C. Polypyrrole-incorporated conducting constructs for tissue engineering applications: A Review. Bioelectricity 2020, 2, 101–119. [Google Scholar] [CrossRef]
- Pelto, J.; Björninen, M.; Pälli, A.; Talvitie, E.; Hyttinen, J.; Mannerström, B.; Suuronen Seppanen, R.; Kellomäki, M.; Miettinen, S.; Haimi, S. Novel polypyrrole-coated polylactide scaffolds enhance adipose stem cell proliferation and early osteogenic differentiation. Tissue Eng. Part A 2013, 19, 882–892. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Kang, E.T.; Neoh, K.G. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016, 32, 46–56. [Google Scholar] [CrossRef]
- Björninen, M.; Siljander, A.; Pelto, J.; Hyttinen, J.; Kellomäki, M.; Miettinen, S.; Seppänen, R.; Haimi, S. Comparison of chondroitin sulfate and hyaluronic acid doped conductive polypyrrole films for adipose stem cells. Ann. Biomed. Eng. 2014, 42, 1889–1900. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Kalamegam, G.; Memic, A.; Budd, E.; Abbas, M.; Mobasheri, A. A comprehensive review of stem cells for cartilage regeneration in osteoarthritis. Adv. Exp. Med. Biol. 2018, 1089, 23–36. [Google Scholar]
- Jang, S.; Lee, K.; Ju, J.H. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Farooqi, A.R.; Bader, R.; Van Rienen, U. Numerical study on electromechanics in cartilage tissue with respect to its electrical properties. Tissue Eng. Part B Rev. 2019, 25, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Vaiciuleviciute, R.; Uzieliene, I.; Bernotas, P.; Novickij, V.; Alaburda, A.; Bernotiene, E. Electrical stimulation in cartilage tissue engineering. Bioengineering 2023, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Matta, C.; Uzielienè, I.; Budd, E.; Martín-Vasallo, P.; Bernotiene, E. The chondrocyte channelome: A narrative review. Jt. Bone Spine 2019, 86, 29–35. [Google Scholar] [CrossRef]
- Parate, D.; Franco-Obregón, A.; Fröhlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci. Rep. 2017, 7, 9421. [Google Scholar] [CrossRef] [PubMed]
- Mikoliunaite, L.; Kubiliute, R.; Popov, A.; Voronovič, J.; Šakirzanovas, S.; Ramanavičiene, A.; Ramanavičius, A. Development of gold nanoparticle-polypyrrole nanocomposites. Chemija 2014, 25, 63–69. [Google Scholar]
- Kumar, P.P.P.; Lim, D.K. Gold-polymer nanocomposites for future therapeutic and tissue engineering applications. Pharmaceutics 2022, 14, 70. [Google Scholar] [CrossRef]
- Aoki, T.; Tanino, M.; Sanui, K.; Ogata, N.; Kumakura, K. Secretory function of adrenal chromaffin cells cultured on polypyrrole films. Biomaterials 1996, 17, 1971–1974. [Google Scholar] [CrossRef]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. Part A 2004, 68, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ramanaviciene, A.; Voronovic, J.; Popov, A.; Drevinskas, R.; Kausaite-Minkstimiene, A.; Ramanavicius, A. Investigation of biocatalytic enlargement of gold nanoparticles using dynamic light scattering and atomic force microscopy. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 510, 183–189. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization model for hydrogen peroxide initiated synthesis of polypyrrole nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef] [PubMed]
- Uzieliene, I.; Bironaite, D.; Pachaleva, J.; Bagdonas, E.; Sobolev, A.; Tsai, W.B.; Kvedaras, G.; Bernotiene, E. Chondroitin sulfate-tyramine-based hydrogels for cartilage tissue repair. Int. J. Mol. Sci. 2023, 24, 3451. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bagdonas, E.; Hoshi, K.; Sakamoto, T.; Hikita, A.; Tachtamisevaite, Z.; Rakauskiene, G.; Kvederas, G.; Mobasheri, A.; Bernotiene, E. Different phenotypes and chondrogenic responses of human menstrual blood and bone marrow mesenchymal stem cells to activin A and TGF-β3. Stem Cell Res. Ther. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Haleem-smith, H.; Calderon, R.; Song, Y.; Tuan, R.S.; Faye, H. Cartilage oligomeric matrix protein enhances. J. Cell. Biochem. 2013, 113, 1245–1252. [Google Scholar] [CrossRef]
- Kumar, A.M.; Suresh, B.; Das, S.; Obot, I.B.; Adesina, A.Y.; Ramakrishna, S. Promising bio-composites of polypyrrole and chitosan: Surface protective and in vitro biocompatibility performance on 316L SS implants. Carbohydr. Polym. 2017, 173, 121–130. [Google Scholar] [CrossRef]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.-A.; Maiti, T.K.; et al. Electroconductive multi-functional polypyrrole composites for biomedical applications. Appl. Mater. Today 2021, 24, 101117. [Google Scholar] [CrossRef]
- Golabi, M.; Turner, A.P.F.; Jager, E.W.H. Tuning the surface properties of polypyrrole films for modulating bacterial adhesion. Macromol. Chem. Phys. 2016, 217, 1128–1135. [Google Scholar] [CrossRef]
- Gelmi, A.; Cieslar-Pobuda, A.; de Muinck, E.; Los, M.; Rafat, M.; Jager, E.W.H. Direct mechanical stimulation of stem cells: A beating electromechanically active scaffold for cardiac tissue engineering. Adv. Healthc. Mater. 2016, 5, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H.; et al. Electrically conductive and 3D-printable oxidized alginate-gelatin polypyrrole: PSS Hydrogels for Tissue Engineering. Adv. Healthc. Mater. 2021, 10, e2001876. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Hwang, T.I.; Kim, J.I.; Lee, J.H.; Chun, S.; Kim, B.S.; Park, C.H.; Kim, C.S. Synthesis of polypyrrole nanorods via sacrificial removal of aluminum oxide nanopore template: A study on cell viability, electrical stimulation and neuronal differentiation of PC12 cells. Mater. Sci. Eng. C 2020, 107, 110325. [Google Scholar] [CrossRef]
- Gelmi, A.; Ljunggren, M.K.; Rafat, M.; Jager, E.W.H. Influence of conductive polymer doping on the viability of cardiac progenitor cells. J. Mater. Chem. B 2014, 2, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, L.; Jing, J.; Zhan, J.; Xu, C.; Xie, W.; Ye, S.; Zhao, Y.; Zhang, C.; Huang, F. Conductive collagen-based hydrogel combined with electrical stimulation to promote neural stem cell proliferation and differentiation. Front. Bioeng. Biotechnol. 2022, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Castano, H.; O’Rear, E.A.; McFetridge, P.S.; Sikavitsas, V.I. Polypyrrole thin films formed by admicellar polymerization support the osteogenic differentiation of mesenchymal stem cells. Macromol. Biosci. 2004, 4, 785–794. [Google Scholar] [CrossRef]
- Maharjan, B.; Kaliannagounder, V.K.; Jang, S.R.; Awasthi, G.P.; Bhattarai, D.P.; Choukrani, G.; Park, C.H.; Kim, C.S. In-situ polymerized polypyrrole nanoparticles immobilized poly(ε-caprolactone) electrospun conductive scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 114, 111056. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in-vivo study in mice. J. Pharm. Pharmacol. 2010, 59, 311–315. [Google Scholar] [CrossRef]
- Kim, M.; Ericksona, I.E.; Choudhurya, M.; Pleshkoc, N.; Maucka, R.L. Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels Minwook. J. Mech. Behav. Biomed. Mater. 2012, 11, 92–101. [Google Scholar] [CrossRef]
- Maumus, M.; Fonteneau, G.; Ruiz, M.; Assou, S.; Boukhaddaoui, H.; Pastoureau, P.; De Ceuninck, F.; Jorgensen, C.; Noel, D. Neuromedin B promotes chondrocyte differentiation of mesenchymal stromal cells via calcineurin and calcium signaling. Cell Biosci. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Matta, C.; Zakany, R. Calcium signalling in chondrogenesis: Implications for cartilage repair. Front. Bioeng. Biotechnol. 2013, 2013, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hsu, Y.T.; Hu, W.W. The regulation of osteogenesis using electroactive polypyrrole films. Polymers 2016, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, K.J.; Kita, M.; Han, Y.; Gelmi, A.; Higgins, M.J.; Moulton, S.E.; Clark, G.M.; Kapsa, R.; Wallace, G.G. Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. Biomaterials 2009, 30, 5292–5304. [Google Scholar] [CrossRef]
- Björninen, M.; Gilmore, K.; Pelto, J.; Seppänen-Kaijansinkko, R.; Kellomäki, M.; Miettinen, S.; Wallace, G.; Grijpma, D.; Haimi, S. Electrically stimulated adipose stem cells on polypyrrole-coated scaffolds for smooth muscle tissue engineering. Ann. Biomed. Eng. 2017, 45, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Miar, S.; Ong, J.L.; Bizios, R.; Guda, T. Electrically stimulated tunable drug delivery from polypyrrole-coated polyvinylidene fluoride. Front. Chem. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Ulasevich, S.; Ryzhkov, N.V.; Andreeva, D.V.; Özden, D.S.; Piskin, E.; Skorb, E.V. Light-to-Heat photothermal dynamic properties of polypyrrole-based coating for regenerative therapy and lab-on-a-chip applications. Adv. Mater. Interfaces 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Xie, C.; Li, P.; Han, L.; Wang, Z.; Zhou, T.; Deng, W.; Wang, K.; Lu, X. Electroresponsive and cell-affinitive polydopamine/polypyrrole composite microcapsules with a dual-function of on-demand drug delivery and cell stimulation for electrical therapy. NPG Asia Mater. 2017, 9, e358. [Google Scholar] [CrossRef]
- Leprince, L.; Dogimont, A.; Magnin, D.; Demoustier-Champagne, S. Dexamethasone electrically controlled release from polypyrrole-coated nanostructured electrodes. J. Mater. Sci. Mater. Med. 2010, 21, 925–930. [Google Scholar] [CrossRef]
- Justin, G.A.; Zhu, S.; Nicholson, T.R.; Maskrod, J.; Mbugua, J.; Chase, M.; Jung, J.H.; Mercado, R.M.L. On-demand controlled release of anti-inflammatory and analgesic drugs from conducting polymer films to aid in wound healing. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2012, 1206–1209. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzieliene, I.; Popov, A.; Lisyte, V.; Kugaudaite, G.; Bialaglovyte, P.; Vaiciuleviciute, R.; Kvederas, G.; Bernotiene, E.; Ramanaviciene, A. Stimulation of Chondrocyte and Bone Marrow Mesenchymal Stem Cell Chondrogenic Response by Polypyrrole and Polypyrrole/Gold Nanoparticles. Polymers 2023, 15, 2571. https://doi.org/10.3390/polym15112571
Uzieliene I, Popov A, Lisyte V, Kugaudaite G, Bialaglovyte P, Vaiciuleviciute R, Kvederas G, Bernotiene E, Ramanaviciene A. Stimulation of Chondrocyte and Bone Marrow Mesenchymal Stem Cell Chondrogenic Response by Polypyrrole and Polypyrrole/Gold Nanoparticles. Polymers. 2023; 15(11):2571. https://doi.org/10.3390/polym15112571
Chicago/Turabian StyleUzieliene, Ilona, Anton Popov, Viktorija Lisyte, Gabija Kugaudaite, Paulina Bialaglovyte, Raminta Vaiciuleviciute, Giedrius Kvederas, Eiva Bernotiene, and Almira Ramanaviciene. 2023. "Stimulation of Chondrocyte and Bone Marrow Mesenchymal Stem Cell Chondrogenic Response by Polypyrrole and Polypyrrole/Gold Nanoparticles" Polymers 15, no. 11: 2571. https://doi.org/10.3390/polym15112571
APA StyleUzieliene, I., Popov, A., Lisyte, V., Kugaudaite, G., Bialaglovyte, P., Vaiciuleviciute, R., Kvederas, G., Bernotiene, E., & Ramanaviciene, A. (2023). Stimulation of Chondrocyte and Bone Marrow Mesenchymal Stem Cell Chondrogenic Response by Polypyrrole and Polypyrrole/Gold Nanoparticles. Polymers, 15(11), 2571. https://doi.org/10.3390/polym15112571









