1. Introduction
Bioeconomic approaches are garnering widespread attention in order to develop sustainable sources of raw materials by valorization of existing biomass. In particular, regional concepts are favorable to further reducing emissions by using local material streams. An agricultural industry that exists in various regions of the world is viticulture. Wine has been produced for over 6000 years in human history [
1]. With the integration of industrial machinery to increase efficiency, the processing of grapes into wine has evolved, but the main process steps have remained the same. As illustrated in
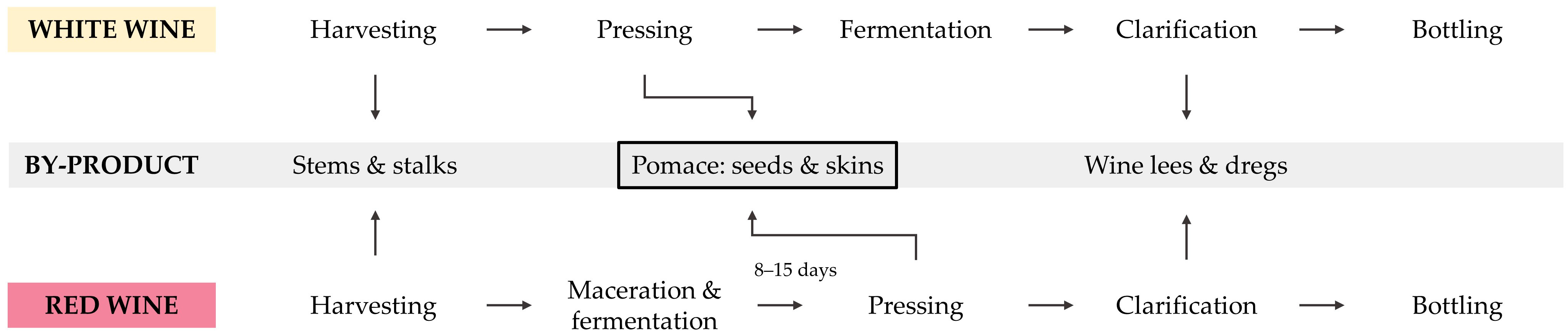
Figure 1, various by-products are generated during winemaking. In total, these by-products represent about one third of the initial grapes [
2,
3]. After harvesting, most of the stems and stalks are separated from the grapes [
4]. While white grapes are subsequently pressed to obtain the grape juice for fermentation, red grapes are macerated and fermented for several days prior to pressing [
5]. Through pressing, the (macerated) grape juice is separated from the wine grape pomace (WP). The by-product, WP, mainly consists of grape seeds and skins, with some remaining stems not fully separated during destemming [
6]. It can be declared the main winemaking by-product, representing about 20–30 wt.-% of the initial grapes [
5]. Accordingly, over 10 Mt of WP are produced worldwide every year [
7]. Only a few percent of these large quantities of biomass are reused in the animal feed industry or to produce distilled spirits [
6,
8]. Despite containing valuable bioactive compounds, most of the by-product is declared agricultural waste, facing challenges in terms of limited disposal options and handling due to high moisture of up to 80% [
9,
10,
11].
Multiple studies have been conducted to determine the composition of WP and its antioxidant activity and reevaluate its potential for valorization [
5,
12,
13,
14]. It is well-known that by-products of the winemaking industry—and especially WP—are rich in functional compounds; e.g., phytochemicals such as polyphenols [
4,
15]. Studies have revealed that about 70% of the grape phenolic compounds remain in WP [
13,
16]. Polyphenolic compounds are classified as flavonoids and non-flavonoids. The flavonoids present in WP are flavonols, such as rutin, kaempferol, and quercetin; flavan-3-ols, such as catechin and its chiral isomer epicatechin; and anthocyanins, such as malvidin and condensed tannins. Non-flavonoids in WP include stilbenes, such as resveratrol, as well as phenolic acids, such as chlorogenic acid and gallic acid [
5,
12,
13,
16,
17,
18]. Although WP generally shows high antioxidant potential, the exact chemical composition and antioxidant activity (e.g., radical scavenging activity) depend on the grape variety, the winemaking process, and the region and weather conditions of cultivation [
8,
19,
20].
Due to their high potential for use as comparatively cheap and highly sustainable bio-additives and bio-fillers, research on the use of by-products from the winemaking industry in the field of plastics and polymer processing has been conducted in recent years [
21]. In conventional petroleum-based polyolefins, such as polypropylene (PP) and polyethylene (PE), wine by-products and their extracts were able to improve the stability of the matrix polymers to suppress oxidative degradation and (UV-)aging [
11,
22,
23]. As far as the combination of wine by-products and biopolymers is concerned, previous studies have focused either on the use of the by-products as low-cost, non-functional fillers or on the use of elaborately prepared extracts as stabilizers. Gowman et al. [
24] incorporated WP as a filler in poly(lactic acid) (PLA) and developed a model to predict the mechanical properties of the resulting biocomposites. Monari et al. [
4] performed an extraction procedure on WP to obtain functional compounds usable in different applications. With respect to the full valorization of the by-product, the solid extraction residues were used as fillers for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) with only minor effects on the thermal and mechanical properties. The results of this ecofriendly approach were validated by Ferri et al. [
25]. The increase in the elastic modulus after adding wine lees to PHBV has been investigated [
26]. Nanni et al. [
27] further investigated the effects of wine lees and grape seed extracts on the mechanical and thermal properties of poly(3-hydroxybutyrate) (PHB). This study reported a reinforcement of PHB by wine lees and minor stabilization effects from grape seed extracts comparable to conventional antioxidants, such as Irganox 1010. In comparison, Persico et al. [
2] observed increased thermal stabilization effects from WP extracts in PHB.
Another promising sustainable substitute for conventional plastics, besides the mentioned PLA and polyhydroxyalkanoates, is biobased poly(butylene succinate) (PBS). Its high ductility, comparable to PE, enables applications with injection-molded products and extruded films [
28]. As for other polyesters, biobased PBS is susceptible to degradation mechanisms based on mechanical, thermal, and thermo-oxidative stresses [
29,
30,
31]. Hallstein et al. [
30] studied the degradation reactions of biobased PBS during processing. Thermo-oxidative degradation via radical reactions was found to be one of the main degradation mechanisms, especially under industrial conditions. By adding phenolic and phosphite antioxidants, branching reactions through thermo-oxidative reaction modes were prevented. Rizzarelli et al. [
31] reported a significant reduction in the molar mass of PBS with thermo-oxidative degradation, confirming the need for antioxidative stabilizers for these types of polyesters.
Several studies on the combination of biobased PBS and winemaking by-products have been published [
32,
33,
34,
35]. They either focused on the use of the by-products as non-functional fillers or on the use of extensively prepared extracts in PBS, as mentioned above. Gowman et al. [
32] successfully incorporated WP in biobased PBS and observed an improvement in the mechanical properties, such as tensile strength and modulus, after adding a compatibilizer. Nanni et al. [
33] reported an increase in the stiffness of PBS after adding dried and ground grape stalks as a reinforcing filler. Further, biocomposites based on PBS and wine lees showed improved mechanical properties and creep resistance with mostly unaltered thermal properties compared to neat PBS [
34]. Regarding the stabilization of PBS by wine by-products, the effects of lab-made grape pomace extracts and commercial grape seed tannin extract were compared to the conventional stabilizer Irganox 1010 [
35]. While the lab-made extract did not act as a stabilizer, the commercial extract prevented thermo-oxidative degradation comparably to the conventional stabilizer. These findings suggest that these by-products demonstrate high potential for use as antioxidant agents in biopolymers. Simultaneously, these results highlight the significant influence of the preparation methods on the efficiency of the bio-fillers and demonstrate the importance of appropriate processing parameters and material handling.
Thermo-oxidative stabilization of PBS is not only crucial for processing but also for technical and long-term applications. Moreover, previous studies partly contradict the approach using a full valorization of the by-products. For these reasons, this research investigated the potential of WP as a whole for the improvement of the thermo-oxidative stability of biobased PBS. Additionally, in all previous studies, the wine by-products were dried and ground separately using non-industrial methods. The preparation methods could have a significant influence on the functional compounds, such as polyphenols, in the biobased fillers. Improper drying conditions and increased mechanical and thermal stresses during grinding can degrade the natural antioxidants [
10]. Guaita et al. [
7] identified drying at high temperatures for a short duration as the best strategy to prevent extensive degradation of phenolic compounds in WP. Nanni et al. [
35] confirmed the need for appropriate by-product preparation to avoid degradation during handling and maintain the stabilization effects. In the present research, an industrial-scale method for WP preparation was used. WP was simultaneously dried and micronized with a TurboRotor mill-dryer, which is well-established in the food industry; e.g., for milling spices [
36]. This method is characterized by high throughputs enabling upscaling [
37] and its suitability for processing temperature-sensitive materials, such as phenolic compounds, due to its very short residence times and reduced thermal stress compared to conventional methods [
38].
To the best of the knowledge of the authors, this is the first study to investigate the influence of industrial TurboRotor mill-dried WP on the thermo-oxidative stability of a biopolyester. Furthermore, this is the first study to characterize and use German WP as a functional filler, following a global approach with the principle transferable to different regions worldwide.
2. Materials and Methods
2.1. Materials and Chemicals
Commercially available biobased PBS (BioPBS FZ71PM) was purchased from PTT MCC Biochem Co., Ltd. (Bangkok, Thailand). The semi-crystalline polyester polymerized with biobased succinic acid and 1,4-butanediol has a density of 1.26 g/cm3, an MFR of 22 g/10 min (measured at 190 °C and 2.16 kg), and a melting temperature of 115 °C. The mechanical properties include a yield stress of 40 MPa, a stress at break of 30 MPa, and a strain at break of 170%. These data were taken from the material data sheet provided by the material supplier.
White WP from Vitis vinifera subsp. vinifera of the variety Silvaner (WWP-Silv), as well as red WP of the variety Domina (RWP-Dom), was kindly provided by the winery Richard Dahms GmbH (Schweinfurt, Germany) during harvesting in October 2021. White grapes were pressed immediately after harvest, while the red grapes had been previously macerated for 12 days. Both varieties were pressed on the same day. The fresh WP designated for processing was packed in mash barrels for transport and kept in cool storage until mill-drying. Native samples of WP without further preparation were stored in sealed bags at −18 °C until analysis.
Bioethanol (99.9%) was supplied by Höfer Chemie GmbH (Kleinbittersdorf, Germany). All chemicals purchased for chemical analysis were analytical grade. Anhydrous sodium carbonate and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were purchased from Applichem GmbH (Darmstadt, Germany). Folin–Ciocâlteu (FC) reagent was supplied by Merck KGaA (Darmstadt, Germany). Potassium persulfate, (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), gallic acid, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany).
2.2. Preparation of Functional Bio-Fillers
Industrial mill-drying of the fresh WP using a TurboRotor mill was performed by Mahltechnik Görgens GmbH (Dormagen, Germany). The processing of the fresh WP to obtain dry and fine powders occurred in two stages. In the first stage, both WPs were processed with a rotor speed of 94 m/s at an air flow of 2000 kg/h and an elevated air intake temperature of 220 °C for simultaneous drying. The second stage for fine micronization was performed at a rotor speed of 113 m/s and an air flow of 1800 kg/h. In this stage, the air was not pre-heated to increase the intake temperature. These parameters were set by Mahltechnik Görgens based on their experience in processing biogenic materials. After milling, the micronized particles were separated from the air stream with a cyclone and manually bagged. The resulting dry bio-filler powders were stored in sealed bags in the absence of light to avoid degradation until processing and analysis.
2.3. Characterization of Native By-Products and Functional Bio-Fillers
2.3.1. Characterization of Native Wine Grape Pomace
For both varieties, representative amounts of a minimum of 25 g WP were manually separated into their constituents (grape skins, seeds, and stalks) and weighed to obtain the initial mass on a fresh-weight basis (fwb). The separated constituents were dried in a dry-air T 5050 E laboratory oven (Heraeus Noblelight GmbH, Hanau, Germany) at a temperature of 105 °C for 48 h to ensure that all moisture was removed. After cooling in a desiccator to prevent moisture absorption from the atmosphere, the mass on a dry-weight basis (dwb) was determined. The relative moisture (
) of the native by-products and their constituents was calculated using Equation (1):
where
and
represent the sample mass before and after the drying procedure, respectively. The shares on both fresh- and dry-weight bases were calculated as the ratio of the mass of the constituent and the total mass of the WP sample considering all constituents. The results are average values of two measurements.
2.3.2. Characterization of Bio-Filler Powders
The particle size distribution of the prepared bio-filler powders was determined with a Cilas particle size analyzer 1090 (Cilas S.A., Orleans, France) in the range of 0.02–500.00 µm. A mixture of bio-filler powder, water, and 5 vol.-% tenside was prepared. Measurements were performed at an obscuration of 15% in triplicate. Results are expressed as the top cut , mean and median particle size and , density, and cumulative distribution.
The RM of the prepared WP powders was determined in duplicate with a KERN moisture analyzer DBS (Kern and Sohn GmbH, Balingen-Frommern, Germany). At least 5 g of powders was used. Measurements were performed in AUTO mode at a temperature of 105 °C using a preset weight loss ΔM of 0.05% as the shutoff criterion. Drying was completed when the weight loss remained constant (below the preset ΔM) for 30 s.
Thermal properties of the bio-filler powders were characterized using thermogravimetric analysis (TGA) with a Netzsch TG 209 F3 Tarsus (NETZSCH-Gerätebau GmbH, Selb, Germany). For each bio-filler, 9.5 ± 0.5 mg of powder was measured in a temperature range from 40 °C to 600 °C at a constant heating rate of 10 °C/min. The gas flows for purge and protective gas were set at a constant rate of 20 mL/min of nitrogen. Decomposition of the material was determined using the temperature at a mass loss of 5% (), as well as the tangential onset temperature () at the maximum degradation rate obtained from the peak () of the first derivative of the TG signal (DTG).
2.3.3. Chemical Analysis by Assays
Different spectrophotometric assays were carried out for chemical analysis of the by-products regarding their potential for use as stabilizing functional fillers. Prior to the spectrophotometric assays, liquid extracts of WP powders were produced via ultrasound-assisted extraction (UAE). Sample masses of 1 g were dissolved in 10 mL of extraction solvent (50/50 v/v ethanol/water) in a sealed container and well-agitated. The container was then sonicated for 1 h at 50 °C, a frequency of 40 kHz, and a power of 600 W in a Palssonic Eco UD30 ultrasound bath (Allpax GmbH and Co. KG, Papenburg, Germany). Sonication was briefly paused after 30 min to re-mix the samples. The liquid and solid phases of the samples were separated via centrifugation at 3500 rpm for 15 min in a Labofuge 200 (Heraeus Noblelight GmbH, Hanau, Germany). For each of the following assays, absorbance measurements were performed on a DR6000 UV-VIS spectrophotometer (Hach Lange GmbH, Düsseldorf, Germany). All assay measurements were performed in duplicate, and the results were calculated while considering the RM of the samples and averaged.
The FC assay, used to evaluate the total phenolic content (TPC), followed a procedure derived from Singleton et al. [
39]. The previously obtained liquid extracts were diluted by a factor of 10 such that the absorbance was within a range covered by the gallic acid standard. A volume of 0.1 mL of the diluted liquid extract was mixed with 7.9 mL of deionized water and 0.5 mL of FC reagent. After an incubation time of 8 min, 1.5 mL of a 20%
w/v aqueous sodium carbonate solution was added. The solutions were well-agitated and left to react in the dark for 30 min at 40 °C. Sample absorbance values were measured at 765 nm. A blank solution was studied using deionized water instead of liquid extract and subtracted from the absorbance values for all measurements. A calibration line using a gallic acid standard (y = 0.9568x; R
2 = 0.9982) was generated. Results for each sample were compared to the gallic acid standard to determine the TPC of the sample in units of mg gallic acid equivalents (GAE) per g of WP dwb.
The DPPH assay was performed using the procedure described by Brand-Williams et al. [
40]. Six aliquots of the liquid sample extract were produced through dilution by a factor of 1–500, depending on the by-product, to obtain data points from 10 to 90% inhibition. A volume of 3.9 mL of a 60 µM DPPH radical cation solution in ethanol was mixed with 0.1 mL of the extract aliquot. The reaction proceeded in the dark for 30 min at room temperature (RT) before measurement. At 515 nm, the absorbance of each sample was measured. A blank solution (only ethanol) and a control solution (3.9 mL of DPPH solution and 0.1 mL ethanol) were also measured. The blank was subtracted from the absorbance value for each aliquot. The
of each aliquot was calculated using Equation (2):
with
representing the absorbance of the sample and
representing the absorbance of the control (where no DPPH was inhibited). From this, a curve representing the %
inhibition against the concentration of WP (dwb) in the extract was obtained. The value for inhibition of 50% of the DPPH (
) was determined for each sample using linear regression analysis. The
of the sample was compared to that derived from a Trolox standard curve (y = 497.5701x; R
2 = 0.9985) to obtain the radical scavenging activity in units of mmol Trolox equivalents (TE) per 100 g dwb.
The ABTS assay method was adapted from Re et al. [
41] with minor modifications. An ABTS radical solution was prepared by mixing a 7 mM aqueous ABTS solution with a 140 mM aqueous potassium persulfate solution to obtain a final concentration of 2.45 mM. The solution was left to activate at RT in the dark for 16 h before use. Subsequently, the solution was diluted with ethanol until it adopted an absorbance of 0.700 ± 0.005 at a wavelength of 734 nm. For each sample studied, six aliquots were prepared by diluting the liquid extract by a factor of 1 to 500 depending on the by-product studied. A volume of 3.4 mL of the ABTS solution was mixed with 0.1 mL of the aliquot. The reaction was allowed to proceed for 6 min at RT before its absorbance at 734 nm was measured. A blank sample consisting of 100% ethanol and a control sample using 3.4 mL ABTS working solution and 0.1 mL ethanol were also measured. The blank was subtracted from the sample absorbances. Using the control sample, a
versus sample concentration curve was generated using Equation (2). The
value for the curve was determined through linear regression analysis and compared to that obtained from a Trolox standard curve (y = 456.3230x; R
2 = 0.9995). Values for the antioxidant capacity are expressed in units of mmol TE per 100 g dwb sample.
2.4. Preparation of Biocomposites Based on PBS and Bio-Fillers
Biocomposites based on PBS and WP bio-fillers were compounded with a Labtech type LTE20-44 laboratory co-rotating twin-screw extruder with an L/D ratio of 44 and screw diameters of 20 mm (Labtech Engineering Co., Ltd., Samutprakarn, Thailand). Prior to processing, the matrix material was pre-dried according to the manufacturer recommendations for at least 5 h at 80 °C in a BIN S 15 in combination with a LUXOR 50 dry air generator (Motan Holding GmbH, Konstanz, Germany). Filler concentrations of 0, 5, 10, 15, and 20 wt.-% were compounded. The fillers were incorporated using a Labtech type LSF20-10 side feeder controlled by a gravimetric dosing system (Scholz Dosiertechnik GmbH, Großostheim, Germany). The temperature profile for compounding is reported in
Table 1. The screw speed was set at 260 rpm for all recipes at throughputs of 6.0–6.5 kg/h. The melt-mixed materials were cooled in a water bath and granulated with a Labtech type LZ-120/hp granulator for subsequent processing and analysis. The compounds were stored in sealed bags in the absence of light until subsequent processing and/or analysis.
Tensile test specimens (type four according to the technical standard DIN EN ISO 8256) were injection-molded using a BOY XS injection-molding machine (BOY Machines, Inc., Exton, PA, USA). Before injection molding, the compounds were pre-dried for 5 h at 80 °C, similar to the pre-drying for compounding. Injection molding was performed at temperatures of 155 °C, 160 °C, and 170 °C from hopper to nozzle and with a mold temperature of 25 °C. The injection pressure of 800 bar maximum, the injection speed of 3 cm3/s, the holding pressure of 600 bar maximum, the holding time of 2 s, and the cooling time of 20 s were kept constant throughout the injection molding of all test specimens.
2.5. Characterization of Biocomposites
2.5.1. Thermal Properties
Thermal properties of the composites were determined using differential scanning calorimetry (DSC) on a Netzsch DSC Polyma 214 system (NETZSCH-Gerätebau GmbH, Selb, Germany). A sample mass of 15 ± 1 mg and gas flows of 40 mL/min (purge) and 60 mL/min (protective) of nitrogen were used. The program consisted of a first heating cycle from −60 °C to 180 °C, followed by a cooling cycle from 180 °C to −60 °C and a second heating cycle from −60 °C to 180 °C. Each cycle was performed at a constant heating rate of 20 °C/min and separated from the others by isothermal segments of 3 min. The melting peak temperature (
) and the melting enthalpy (
) were determined from the second heating cycle, whereas the crystallization peak temperature (
) and the crystallization enthalpy (
) were obtained from the cooling cycle. Thereby, the degree of crystallinity (
) was calculated considering the weight fractions (
) of the filler and the theoretical melting enthalpy of 100% crystalline PBS (
) of 200 J/g [
32,
42], following Equation (3):
As for the measurements of the bio-filler powders, a Netzsch TG 209 F3 Tarsus (NETZSCH-Gerätebau GmbH, Selb, Germany) was used for TGA. A sample mass of 9.5 ± 0.5 mg was heated from 40 °C to 600 °C at a constant heating rate of 10 °C/min. During heating, the chamber was purged with gas flows of 20 mL/min of nitrogen for both purge and protective gas. Initial decomposition was obtained from the temperature () at a mass loss of 10%, while the onset temperature (Ton) was determined using a tangential procedure at the maximum degradation rate obtained from the DTG curve. Both the DSC and the TGA measurements were performed in duplicate, and the results are expressed as mean values.
2.5.2. Thermo-Oxidative Properties
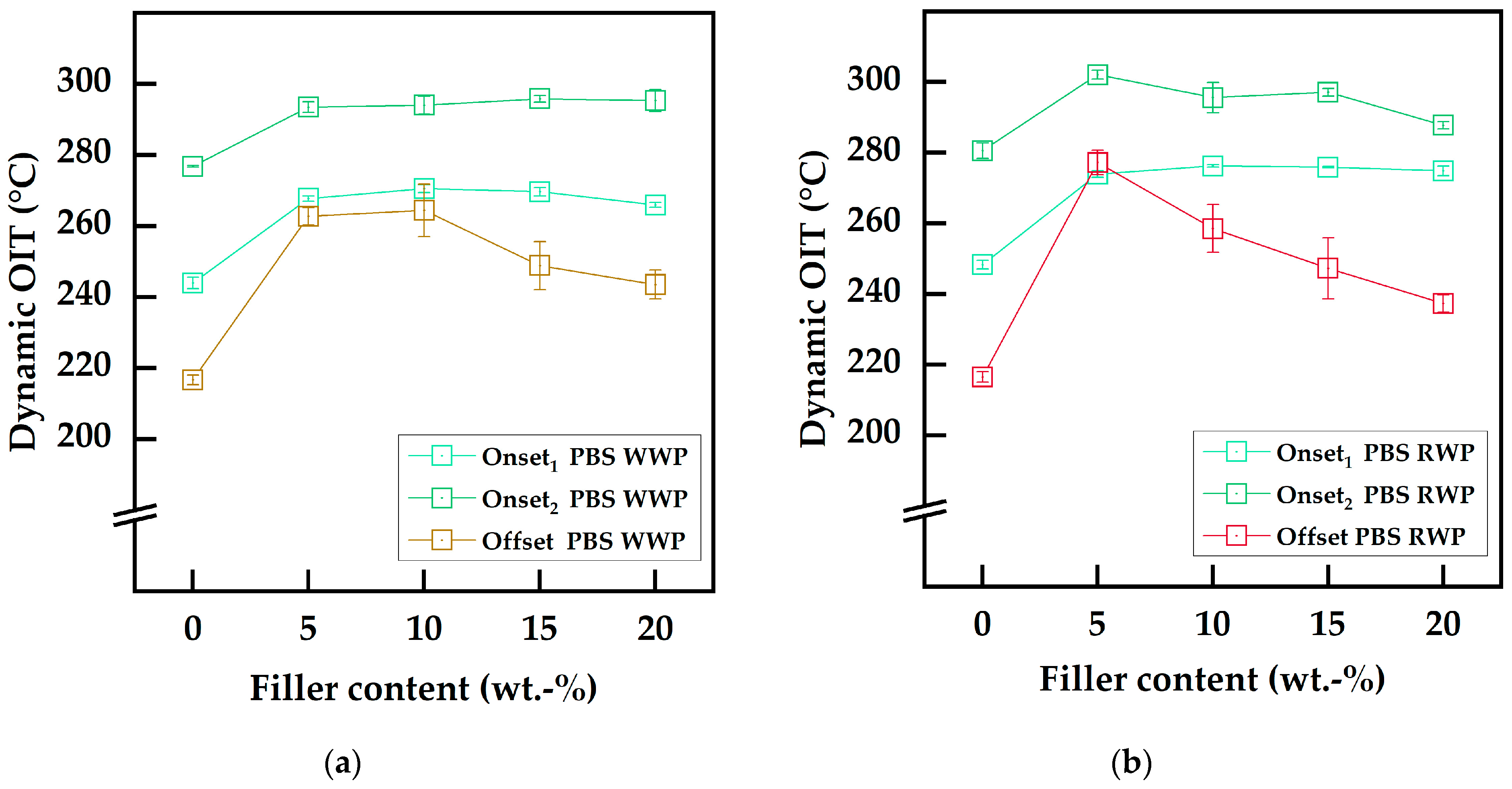
To determine the thermo-oxidative stability of the compounds, dynamic oxidation induction temperature (OIT) measurements were conducted on a Netzsch DSC Polyma 214 (NETZSCH-Gerätebau GmbH, Selb, Germany). An open aluminum crucible with a sample mass of 5.0 ± 0.5 mg was used. The measurement cell was purged with oxygen for 5 min at 25 °C at a flow rate of 50 mL/min (protective: 60 mL/min nitrogen). Subsequently, the oven was heated from 25 °C to 330 °C at a constant heating rate of 20 °C/min. Degradation temperatures were determined using the offset method with a Δ of 0.05 W/g () and the tangential onset procedure at the maxima of the first derivative of the curve according to the technical standard DIN EN ISO 11357-6. Since PBS shows two-step oxidative degradation, both maxima of the first derivative of the curve were considered for onset temperature determination (, ). All OIT measurements were performed in triplicate.
As a second method to evaluate the thermo-oxidative properties of the materials, TGA was performed in an oxidative atmosphere. Due to the design of the Netzsch TG 209 F3 Tarsus (NETZSCH-Gerätebau GmbH, Selb, Germany), a maximum oxygen concentration of 50% was achievable at a total gas flow of 40 mL/min (purge: 20 mL/min oxygen, protective: 20 mL/min nitrogen). The values ox. , ox. , and ox. (obtained from the DTG) were determined in duplicate in the same manner as the TGA measurements performed in a nitrogen atmosphere.
2.5.3. Mechanical Properties
Mechanical properties of neat and filled PBS were characterized via tensile testing of the injection-molded specimen. A Zwick RetroLine Z 2.5 (ZwickRoell GmbH and Co. KG, Ulm, Germany) equipped with a 2.5 kN Xforce P load cell was used. Testing was performed at ambient conditions with a clamp length of 30 mm and 1 mm/min clamp separation speed for determination of the tensile modulus () and 50 mm/min for the tensile strength () and the elongation at break (), respectively. All mechanical data are expressed as the average for at least five specimens, which were previously stored in a desiccator for at least 48 h.
4. Conclusions
With an increasing demand for sustainable materials and access to existing material streams, especially substantially unused biomass, it is favorable to incorporate biogenic by-products as fillers in biopolyesters, such as biobased PBS. In this way, green biocomposites from renewable resources can contribute to reducing emissions and supporting the shift to sustainable materials. In this study, WP, as the main by-product from the winemaking industry, was investigated not only for use as a low-cost filler in PBS but also as a functional filler to act as a stabilizer while combining economic, sustainable, and technical benefits. Analysis of the composition confirmed the international transferability of the approach, as the constitution of two different varieties of German WP was comparable to WP from other regions in Europe and North America. Furthermore, industrial mill-drying was identified as a viable method for bio-filler preparation. The resulting WP powders were small in particle size with a narrow distribution and showed high total phenolic content. Chemical analysis using spectrophotometric assays revealed high antioxidant and radical scavenging activities for the WP, strengthening the potential for its application as stabilizing functional filler in polymers. Both WP bio-fillers were suitable for PBS-based biocomposites since their thermal stability, investigated using TGA, was sufficient for compounding. Different filler contents of up to 20 wt.-% for both WPs were realized by co-rotating twin-screw extrusion. The resulting biocomposites showed almost unchanged thermal properties and the degree of crystallinity remained constant. TGA measurements showed that WP does not act as a pure thermal stabilizer to suppress β-hydrogen-transfer bond scission. Regarding the main degradation mechanism of PBS, the incorporation of WP led to an outstanding improvement in thermo-oxidative stability. Dynamic OIT analysis revealed that RWP-Dom outperformed WWP-Silv, with optimum stabilization at 5 wt.-% filler content compared to 10 wt.-%. This was consistent with the findings from the chemical analysis using assays. The effect of WP in acting as a thermo-oxidative stabilizer in PBS was verified using TGA measurements in an oxidative atmosphere. Mechanical properties of the biocomposites were altered within expected ranges, as demonstrated by tensile testing. The incorporation of the bio-filler led to an increase in the tensile modulus while reducing the tensile strength and decreasing elongation at break, as is well-known for composite materials. Overall, this study proves that WP, a biogenic by-product generated globally in high amounts, can be valorized for use as a low-cost functional filler in PBS. The stability against thermo-oxidative degradation was increased while maintaining the characteristic thermal properties and altering the mechanical properties within expected ranges. According to the findings from this study, WP filler contents of up to 10 wt.-% are favorable for obtaining biocomposites with balanced thermal, thermo-oxidative, and mechanical properties and additional bio-economic benefits through the valorization of existing biogenic by-products from winemaking. Future research in this area should investigate WP from different years to qualify the potential for sustained use of the materials in industries such as food packaging.