Influence of Epoxy Functional Chain-Extenders on the Thermal and Rheological Properties of Bio-Based Polyamide 10.10
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing
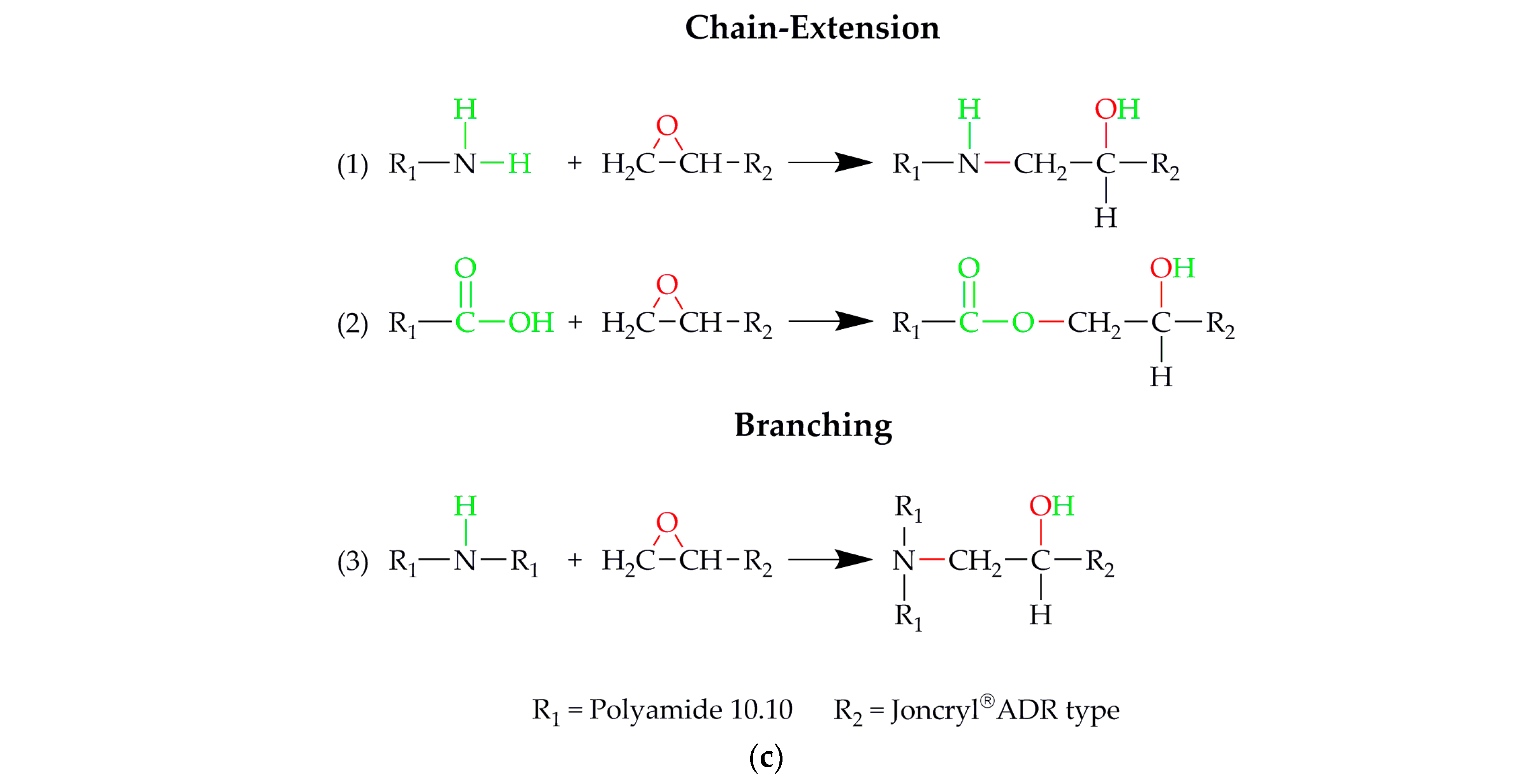
2.2.1. Compounding of Polyamide 10.10 including Different Chain-Extender Types
2.2.2. Heatpressing of Thin Films
2.3. Test Methods and Sample Preparation
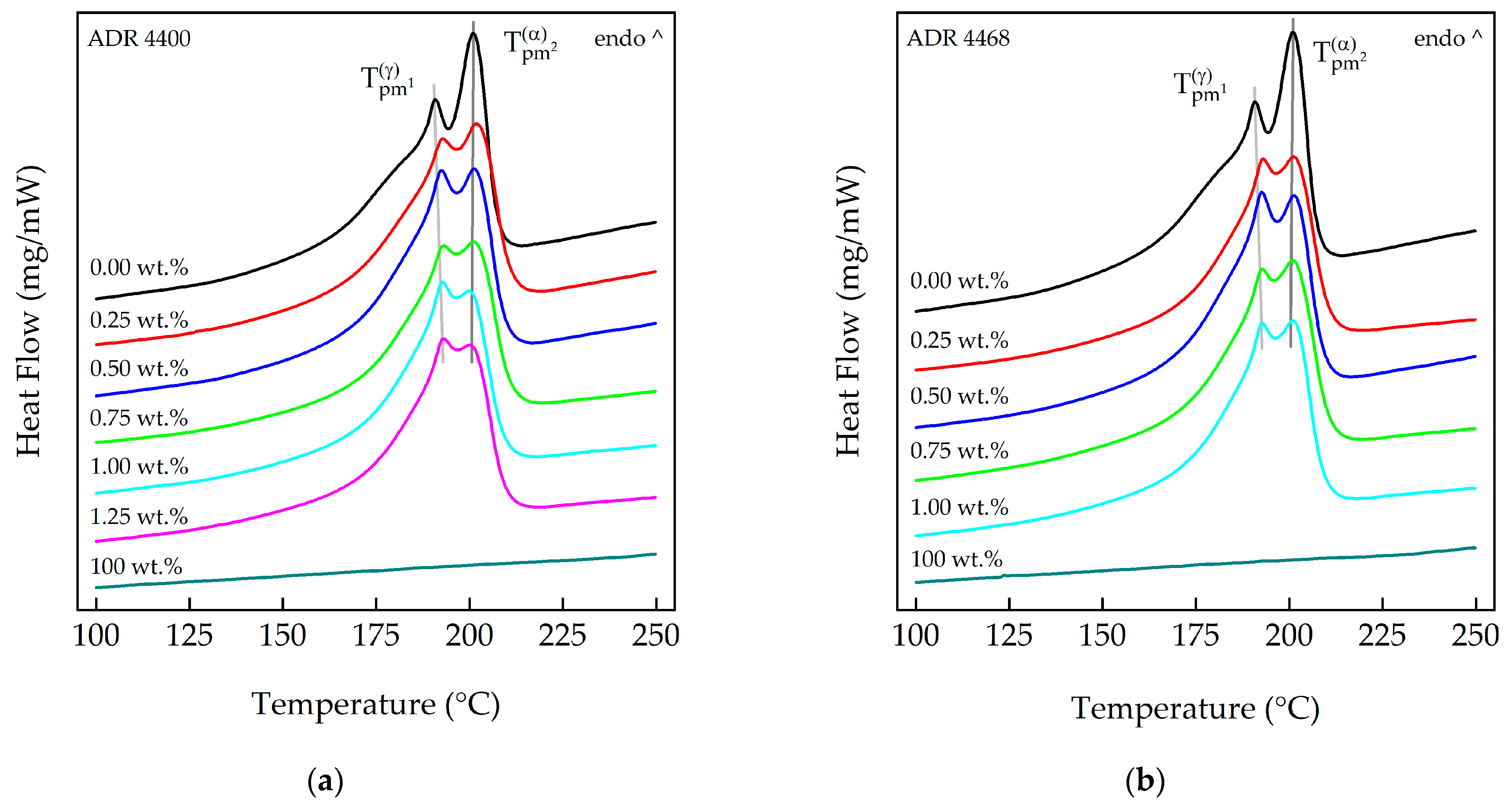
2.3.1. Differential Scanning Calorimetry (DSC)
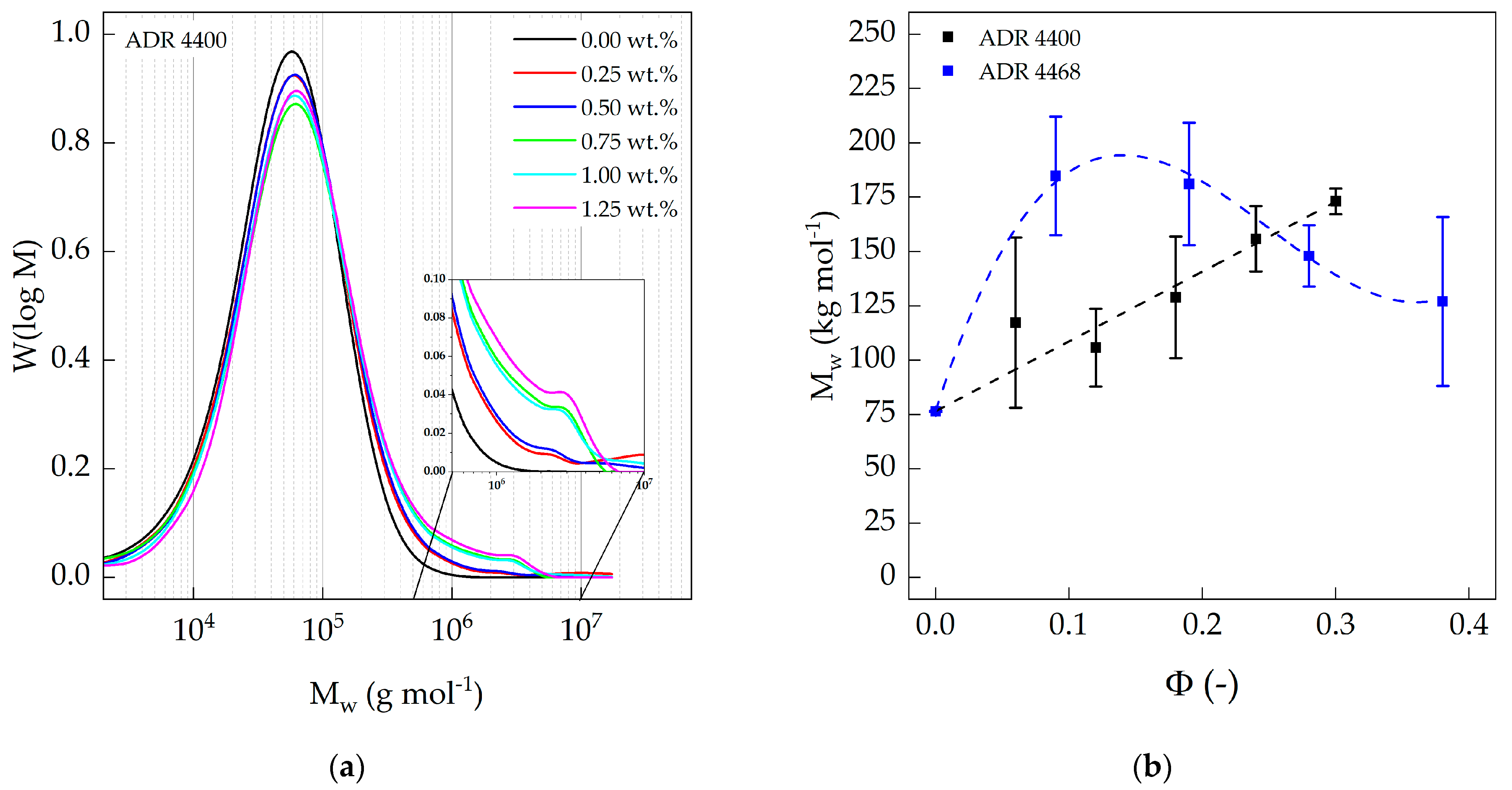
2.3.2. Size Exclusion Chromatography (SEC)
2.3.3. Oscillation Rheology (Plate–Plate)
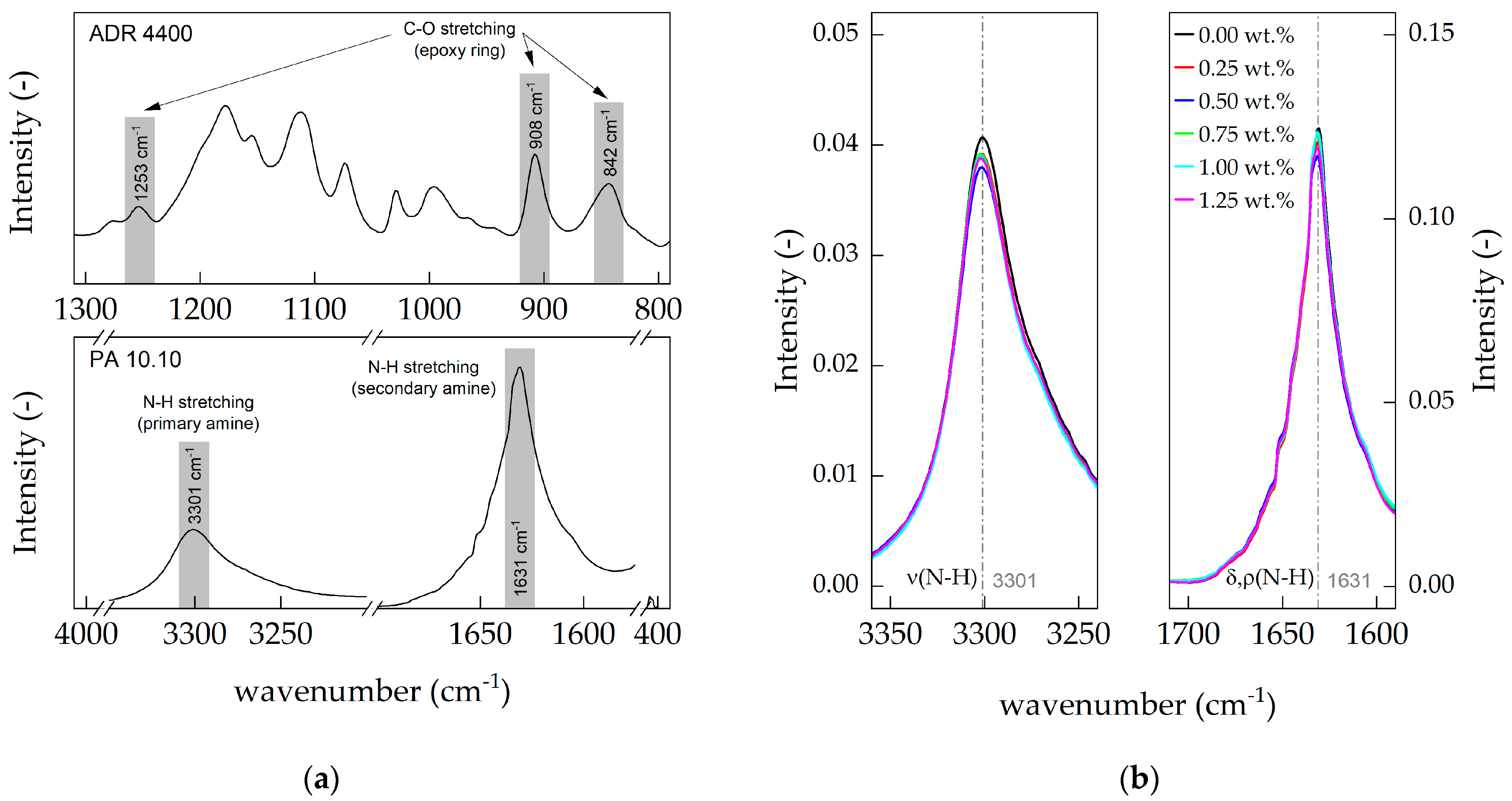
2.3.4. ATR-FTIR Spectroscopy
3. Results and Discussion
3.1. Differential Scanning Calorimetry (DSC)
3.2. Size Exclusion Chromatography (SEC)
| Compound | PA 10.10/ADR Ratio | ||||
|---|---|---|---|---|---|
| [wt.%] | [-] | [kg mol−1] | [kg mol−1] | [-] | |
| PA 10.10 (processed) | 100 | 0.00 | 77 ± 0.3 | 23 ± 0.9 | 3.3 ± 0.1 |
| PA 10.10/ADR 4400 | 100/0.25 | 0.06 | 117 ± 39.2 | 27 ± 0.2 | 4.3 ± 1.4 |
| 100/0.50 | 0.12 | 106 ± 17.8 | 27 ± 2.1 | 4.0 ± 0.4 | |
| 100/0.75 | 0.18 | 129 ± 28.0 | 28 ± 1.0 | 4.6 ± 1.2 | |
| 100/1.00 | 0.24 | 156 ± 15.1 | 29 ± 1.2 | 5.4 ± 0.3 | |
| 100/1.25 | 0.30 | 173 ± 5.9 | 29 ± 4.8 | 6.2 ± 1.2 | |
| PA 10.10/ADR 4468 | 100/0.25 | 0.09 | 185 ± 27.3 | 27 ± 0.2 | 6.8 ± 1.1 |
| 100/0.50 | 0.19 | 181 ± 28.1 | 29 ± 0.1 | 6.2 ± 1.0 | |
| 100/0.75 | 0.28 | 148 ± 14.1 | 28 ± 3.0 | 5.3 ± 0.1 | |
| 100/1.00 | 0.38 | 127 ± 38.8 | 25 ± 2.0 | 5.0 ± 1.2 | |
| 100/1.25 | - | - | - | - |
3.3. Oscillation Rheology (Plate–Plate)
3.4. Attenuated Total Reflectance (ATR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | PA 10.10/ADR Ratio | ||||||
|---|---|---|---|---|---|---|---|
| [wt.%] | [°C] | [°C] | [°C] | [°C] | [J g−1] | [%] | |
| PA 10.10 (processed) | 100 | 179 | 191 | 201 | 207 | 81.36 | 33.3 |
| PA 10.10/ADR 4400 | 100/0.25 | 180 | 193 | 202 | 211 | 80.09 | 32.8 |
| 100/0.50 | 182 | 193 | 202 | 210 | 78.81 | 32.3 | |
| 100/0.75 | 179 | 193 | 202 | 211 | 80.66 | 33.1 | |
| 100/1.00 | 180 | 193 | 200 | 209 | 82.82 | 33.9 | |
| 100/1.25 | 181 | 194 | 200 | 209 | 78.48 | 32.2 | |
| Joncryl® ADR 4400 | 100 | - | - | - | - | - | - |
| PA 10.10/ADR 4468 | 100/0.25 | 183 | 193 | 202 | 211 | 73.73 | 30.2 |
| 100/0.50 | 183 | 193 | 201 | 209 | 77.01 | 31.6 | |
| 100/0.75 | 181 | 193 | 201 | 211 | 81.19 | 33.3 | |
| 100/1.00 | 181 | 193 | 201 | 209 | 80.16 | 32.9 | |
| 100/1.25 | - | - | - | - | - | - | |
| Joncryl® ADR 4468 | 100 | - | - | - | - | - | - |
| Compound | PA 10.10/ADR Ratio | ||
|---|---|---|---|
| [wt.%] | [kJ mol−1] | [kJ mol−1] | |
| PA 10.10 (processed) | 100 | 39.0 ± 7.4 | 33.2 ± 7.4 |
| PA 10.10/ADR 4400 | 100/0.25 | 67.2 ± 9.6 | 61.6 ± 9.6 |
| 100/0.50 | 73.6 ± 6.6 | 68.0 ± 6.7 | |
| 100/0.75 | 68.9 ± 3.2 | 63.3 ± 3.3 | |
| 100/1.00 | 62.1 ± 7.9 | 56.6 ± 7.9 | |
| 100/1.25 | 57.9 ± 5.0 | 52.3 ± 5.0 | |
| PA 10.10/ADR 4468 | 100/0.25 | 60.1 ± 7.8 | 54.5 ± 7.9 |
| 100/0.50 | 70.1 ± 7.7 | 64.6 ± 7.7 | |
| 100/0.75 | 66.7 ± 6.6 | 61.1 ± 6.7 | |
| 100/1.00 | 61.6 ± 10.1 | 56.0 ± 10.1 | |
| 100/1.25 | - | - |
| Compound | PA 10.10/ADR Ratio | ||
|---|---|---|---|
| [wt.%] | [Nm] | [bar] | |
| PA 10.10 (processed) | 100 | 34.3 | 11 |
| PA 10.10/ADR 4400 | 100/0.25 | 35.0 | 14 |
| 100/0.50 | 35.0 | 17 | |
| 100/0.75 | 35.7 | 22 | |
| 100/1.00 | 36.4 | 28 | |
| 100/1.25 | 37.8 | 30 | |
| PA 10.10/ADR 4468 | 100/0.25 | 37.8 | 14 |
| 100/0.50 | 39.2 | 16 | |
| 100/0.75 | 37.1 | 11 | |
| 100/1.00 | 37.8 | 14 | |
| 100/1.25 | - | - |
References
- Kind, S.; Wittmann, C. Bio-based production of the platform chemical 1,5-diaminopentane. Appl. Microbiol. Biotechnol. 2011, 91, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, B. Polyamides from Biomass Derived Monomers. In Bio-Based Plastics; Kabasci, S., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 275–293. ISBN 9781118676646. [Google Scholar]
- Pagacz, J.; Raftopoulos, K.N.; Leszczyńska, A.; Pielichowski, K. Bio-polyamides based on renewable raw materials. J. Therm. Anal. Calorim. 2016, 123, 1225–1237. [Google Scholar] [CrossRef]
- Wang, X.; Gao, S.; Wang, J.; Xu, S.; Li, H.; Chen, K.; Ouyang, P. The production of biobased diamines from renewable carbon sources: Current advances and perspectives. Chin. J. Chem. Eng. 2021, 30, 4–13. [Google Scholar] [CrossRef]
- Incarnato, L.; Scarfato, P.; Di Maio, L.; Acierno, D. Structure and rheology of recycled PET modified by reactive extrusion. Polymer 2000, 41, 6825–6831. [Google Scholar] [CrossRef]
- Forsythe, J.S.; Cheah, K.; Nisbet, D.R.; Gupta, R.K.; Lau, A.; Donovan, A.R.; O’Shea, M.S.; Moad, G. Rheological properties of high melt strength poly(ethylene terephthalate) formed by reactive extrusion. J. Appl. Polym. Sci. 2006, 100, 3646–3652. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Duchet, J.; Reignier, J.; Maazouz, A. Melt strengthening of poly (lactic acid) through reactive extrusion with epoxy-functionalized chains. Rheol. Acta 2011, 50, 613–629. [Google Scholar] [CrossRef]
- Tuna, B.; Benkreira, H. Reactive Extrusion of Polyamide 6 Using a Novel Chain extender. Polym. Eng. Sci. 2019, 59, E25–E31. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Jeziórska, R. Studies on reactive compatibilisation of polyamide 6/poly(butylene terephthalate) blends by low molecular weight bis-oxazoline. Polym. Degrad. Stab. 2005, 90, 224–233. [Google Scholar] [CrossRef]
- Lu, C.; Chen, T.; Zhao, X.; Ren, X.; Cai, X. Chemical modification of polyamide-6 by chain extension with 2,2′-bis(2-oxazoline). J. Polym. Sci. B Polym. Phys. 2007, 45, 1976–1982. [Google Scholar] [CrossRef]
- Lu, C.; Chen, L.; Ye, R.; Cai, X. Chain extension of Polyamide 6 Using Bisoxazoline Coupling Agents. J. Polym. Sci. B Polym. Phys. 2008, 47, 986–999. [Google Scholar] [CrossRef]
- Néry, L.; Lefebvre, H.; Fradet, A. Chain extension of Carboxy-Terminated Aliphatic Polyamides and Polyesters by Arylene and Pyridylene Bisoxazolines. Macromol. Chem. Phys. 2004, 205, 448–455. [Google Scholar] [CrossRef]
- Xu, M.; Yan, H.; He, Q.; Wan, C.; Liu, T.; Zhao, L.; Park, C.B. Chain extension of polyamide 6 using multifunctional chain extenders and reactive extrusion for melt foaming. Eur. Polym. J. 2017, 96, 210–220. [Google Scholar] [CrossRef]
- Lu, C.; Ye, R.; Yang, Y.; Ren, X.; Cai, X. Chemical Modification of Polyamide 6 by Chain Extension with Terephthaloyl-biscaprolactam. J. Macromol. Sci. B Phys. 2010, 50, 350–362. [Google Scholar] [CrossRef]
- Buccella, M.; Dorigato, A.; Pasqualini, E.; Caldara, M.; Fambri, L. Chain extension behavior and thermo-mechanical properties of polyamide 6 chemically modified with 1,1′-carbonyl-bis-caprolactam. Polym. Eng. Sci. 2014, 54, 158–165. [Google Scholar] [CrossRef]
- Ozmen, S.C.; Ozkoc, G.; Serhatli, I.E. Effect of Reactive Extrusion Process Parameters on Thermal, Mechanical, and Physical Properties of Recycled Polyamide-6: Comparison of Two Novel Chain Extenders. J. Macromol. Sci. B Phys. 2021, 60, 350–367. [Google Scholar] [CrossRef]
- Ozmen, S.C.; Ozkoc, G.; Serhatli, E. Thermal, mechanical and physical properties of chain extended recycled polyamide 6 via reactive extrusion: Effect of chain extender types. Polym. Degrad. Stab. 2019, 162, 76–84. [Google Scholar] [CrossRef]
- Xu, M.; Lu, J.; Zhao, J.; Wei, L.; Liu, T.; Zhao, L.; Park, C.B. Rheological and foaming behaviors of long-chain branched polyamide 6 with controlled branch length. Polymer 2021, 224, 123730. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, X.; Xu, J.; Guo, B. Chain extension of PA1010 by reactive extrusion by diepoxide 711 and diepoxide TDE85 as chain extenders. J. Appl. Polym. Sci. 2004, 94, 2347–2355. [Google Scholar] [CrossRef]
- Jorda, M.; Montava-Jorda, S.; Balart, R.; Lascano, D.; Montanes, N.; Quiles-Carrillo, L. Functionalization of Partially Bio-Based Poly(Ethylene Terephthalate) by Blending with Fully Bio-Based Poly(Amide) 10,10 and a Glycidyl Methacrylate-Based Compatibilizer. Polymers 2019, 11, 1331. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Fenollar, O.; Balart, R.; Torres-Giner, S.; Rallini, M.; Dominici, F.; Torre, L. A comparative study on the reactive compatibilization of melt-processed polyamide 1010/polylactide blends by multi-functionalized additives derived from linseed oil and petroleum. Express Polym. Lett. 2020, 14, 583–604. [Google Scholar] [CrossRef]
- Carrasco, F.; Santana Pérez, O.; Maspoch, M.L. Kinetics of the Thermal Degradation of Poly(lactic acid) and Polyamide Bioblends. Polymers 2021, 13, 3996. [Google Scholar] [CrossRef] [PubMed]
- García-Masabet, V.; Santana Pérez, O.; Cailloux, J.; Abt, T.; Sánchez-Soto, M.; Carrasco, F.; Maspoch, M.L. PLA/PA Bio-Blends: Induced Morphology by Extrusion. Polymers 2019, 12, 10. [Google Scholar] [CrossRef]
- Feldmann, M.; Verheyen, F. Impact Behavior of Continuous Biaxial Reinforced Composites Based on Bio-Polyamides and Man-Made Cellulose Fibres. Int. Polym. Process. 2016, 31, 198–206. [Google Scholar] [CrossRef]
- Feldmann, M.; Heim, H.-P.; Zarges, J.-C. Influence of the process parameters on the mechanical properties of engineering biocomposites using a twin-screw extruder. Compos. Part A Appl. Sci. Manuf. 2016, 83, 113–119. [Google Scholar] [CrossRef]
- Rinberg, R.; Hartmann, T.; Nikiforov, A.; Doynikov, A.; Volfson, S.; Kroll, L. Investigation of bio-based polyamide with short fibers for lightweight structures. Technol. Lightweight Struct. 2018, 1, 147–156. [Google Scholar] [CrossRef]
- Nikiforov, A.A.; Volfson, S.I.; Rinberg, R.; Okhotina, N.A.; Fayzullin, I.Z. Effect of Lubricants on Fiber Length Distribution and Properties of Glass Fiber Reinforced Composites Based on Polyamide 1010. Key Eng. Mater. 2019, 816, 202–207. [Google Scholar] [CrossRef]
- Bazan, P.; Nosal, P.; Wierzbicka-Miernik, A.; Kuciel, S. A novel hybrid composites based on biopolyamide 10.10 with basalt/aramid fibers: Mechanical and thermal investigation. Compos. B Eng. 2021, 223, 109125. [Google Scholar] [CrossRef]
- Kuciel, S.; Kuznia, P.; Jakubowska, P. Properties of composites based on polyamide 10.10 reinforced with carbon fibers. Polimery 2016, 61, 106–112. [Google Scholar] [CrossRef]
- BASF Corporation. Joncryl® ADR 4400: Technical Information; BASF Corporation: Ludwigshafen, Germany, 2014. [Google Scholar]
- BASF Corporation. Joncryl® ADR 4468: Technical Information; BASF Corporation: Ludwigshafen, Germany, 2014. [Google Scholar]
- Shanghai H&B New Material Technology Co., Ltd. Available online: http://www.lithlon.com/en/ProductDetail/2887400.html (accessed on 17 May 2023).
- Standau, T.; Nofar, M.; Dörr, D.; Ruckdäschel, H.; Altstädt, V. A Review on Multifunctional Epoxy-Based Joncryl® ADR Chain Extended Thermoplastics. Polym. Rev. 2022, 62, 296–350. [Google Scholar] [CrossRef]
- Moynihan, C.T.; Easteal, A.J.; Wilder, J.; Tucker, J. Dependence of the glass transition temperature on heating and cooling rate. J. Phys. Chem. 1974, 78, 2673–2677. [Google Scholar] [CrossRef]
- Levinta, N.; Corobea, M.C.; Vuluga, Z.; Nicolae, C.-A.; Gabor, A.R.; Raditoiu, V.; Osiac, M.; Teodorescu, G.-M.; Teodorescu, M. Bio-Based Polyamide 1010 with a Halogen-Free Flame Retardant Based on Melamine-Gallic Acid Complex. Polymers 2020, 12, 1482. [Google Scholar] [CrossRef] [PubMed]
- Ishisue, T.; Okamoto, M.; Tashiro, K. Real-time investigation of crystallization in nylon 6-clay nano-composite probed by infrared spectroscopy. Polymer 2010, 51, 5585–5591. [Google Scholar] [CrossRef]
- Karkhanis, S.S.; Matuana, L.M. Extrusion blown films of poly(lactic acid) chain-extended with food grade multifunctional epoxies. Polym. Eng. Sci. 2019, 59, 2211–2219. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Taylor, S.; Misra, M.; Mohanty, A.K. Thermo-mechanical characterization of bioblends from polylactide and poly(butylene adipate-co-terephthalate) and lignin. Macromol. Mater. Eng. 2015, 300, 299–311. [Google Scholar] [CrossRef]
- Paredes, N.; Rodrguez-Galn, A.; Puiggal, J. Synthesis and characterization of a family of biodegradable poly(ester amide)s derived from glycine. J. Polym. Sci. A Polym. Chem. 1998, 36, 1271–1282. [Google Scholar] [CrossRef]
- Galimberti, D.; Quarti, C.; Milani, A.; Brambilla, L.; Civalleri, B.; Castiglioni, C. IR spectroscopy of crystalline polymers from ab initio calculations: Nylon 6,6. Vib. Spectrosc. 2013, 66, 83–92. [Google Scholar] [CrossRef]
- Jasinska-Walc, L.; Dudenko, D.; Rozanski, A.; Thiyagarajan, S.; Sowinski, P.; van Es, D.; Shu, J.; Hansen, M.R.; Koning, C.E. Structure and Molecular Dynamics in Renewable Polyamides from Dideoxy–Diamino Isohexide. Macromolecules 2012, 45, 5653–5666. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, X.; Berglund, L.A. FT-IR spectroscopic study of hydrogen bonding in PA6/clay nanocomposites. Polymer 2002, 43, 2445–2449. [Google Scholar] [CrossRef]
- Vasanthan, N.; Salem, D.R. FTIR spectroscopic characterization of structural changes in polyamide-6 fibers during annealing and drawing. J. Polym. Sci. B Polym. Phys. 2001, 39, 536–547. [Google Scholar] [CrossRef]
- Rotter, G.; Ishida, H. FTIR separation of nylon-6 chain conformations: Clarification of the mesomorphous and γ-crystalline phases. J. Polym. Sci. B Polym. Phys. 1992, 30, 489–495. [Google Scholar] [CrossRef]
- Nair, S.S.; Ramesh, C. Studies on the Crystallization Behavior of Nylon-6 in the Presence of Layered Silicates Using Variable Temperature WAXS and FTIR. Macromolecules 2005, 38, 454–462. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, W.; Liu, H.; Sun, A.; Yoo, Y.; He, S.; Zhu, C.; Yang, M. The Thermal Behavior of γ-PA1010: Evolution of Structure and Morphology in the Simultaneous Thermal Stretched Films. Materials 2020, 13, 1722. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, M.; Awojulu, A.; Greeley, T.; Turco, G.; Deeter, G. Oligomeric chain extenders for economic reprocessing and recycling of condensation plastics. Energy 2006, 31, 3227–3234. [Google Scholar] [CrossRef]
- Yu, T.; Chen, J.S.; Wu, F.M.; Rocks, J. Crosslinking of Polyamide 6 by Reactive Processing. Mater. Sci. Forum 2015, 815, 576–582. [Google Scholar] [CrossRef]
- Wu, W.-J.; Sun, X.-L.; Chen, Q.; Qian, Q. Recycled Poly(Ethylene Terephthalate) from Waste Textiles with Improved Thermal and Rheological Properties by Chain Extension. Polymers 2022, 14, 510. [Google Scholar] [CrossRef]
- Härth, M.; Dörnhöfer, A.; Kaschta, J.; Münstedt, H.; Schubert, D.W. Molecular structure and rheological properties of a poly(ethylene terephthalate) modified by two different chain extenders. J. Appl. Polym. Sci. 2021, 138, 50110. [Google Scholar] [CrossRef]
- Härth, M.; Kaschta, J.; Schubert, D.W. Shear and Elongational Flow Properties of Long-Chain Branched Poly(ethylene terephthalates) and Correlations to Their Molecular Structure. Macromolecules 2014, 47, 4471–4478. [Google Scholar] [CrossRef]
- Jaszkiewicz, A.; Bledzki, A.K.; Duda, A.; Galeski, A.; Franciszczak, P. Investigation of Processability of Chain-Extended Polylactides During Melt Processing—Compounding Conditions and Polymer Molecular Structure. Macromol. Mater. Eng. 2014, 299, 307–318. [Google Scholar] [CrossRef]
- Cailloux, J.; Santana, O.O.; Franco-Urquiza, E.; Bou, J.J.; Carrasco, F.; Gamez-Perez, J.; Maspoch, M.L. Sheets of branched poly(lactic acid) obtained by one step reactive extrusion calendering process: Melt rheology analysis. Express Polym. Lett. 2013, 7, 304–318. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Maazouz, A.; Reignier, J.; Duchet, J. Influence of the chain extension on the crystallization behavior of polylactide. Polym. Eng. Sci. 2014, 54, 616–625. [Google Scholar] [CrossRef]
- Yang, Z.; Xin, C.; Mughal, W.; Li, X.; He, Y. High-melt-elasticity poly(ethylene terephthalate) produced by reactive extrusion with a multi-functional epoxide for foaming. J. Appl. Polym. Sci. 2018, 135, 45805. [Google Scholar] [CrossRef]
- Costa, A.R.d.M.; Henrique, M.A.; Luna, C.B.B.; de Carvalho, L.H.; Almeida, Y.M.B.d. Influence of a Multifunctional Epoxy Additive on the Performance of Polyamide 6 and PET Post-Consumed Blends during Processing. Sustainability 2022, 14, 16658. [Google Scholar] [CrossRef]
- Ng, H.Y.; Lu, X.; Lau, S.K. Thermal conductivity of boron nitride-filled thermoplastics: Effect of filler characteristics and composite processing conditions. Polym. Compos. 2005, 26, 778–790. [Google Scholar] [CrossRef]
- Standau, T.; Hädelt, B.; Schreier, P.; Altstädt, V. Development of a Bead Foam from an Engineering Polymer with Addition of Chain Extender: Expanded Polybutylene Terephthalate. Ind. Eng. Chem. Res. 2018, 57, 17170–17176. [Google Scholar] [CrossRef]
- McKee, M.G.; Unal, S.; Wilkes, G.L.; Long, T.E. Branched polyesters: Recent advances in synthesis and performance. Prog. Polym. Sci. 2005, 30, 507–539. [Google Scholar] [CrossRef]
- Nishitani, Y.; Hasumi, M.; Kitano, T. Influence of silane coupling agents on the rheological behavior of hemp fiber filled polyamide 1010 biomass composites in molten state. In Proceedings of the PPS-30: The 30th International Conference of the Polymer Processing Society—Conference Papers, Cleveland, OH, USA, 6–12 June 2014; AIP Publishing LLC: Melville, NY, USA, 2015; p. 60007. [Google Scholar]
- Parrini, P.; Romanini, D.; Righi, G.P. Melt rheology of some aliphatic polyamides. Polymer 1976, 17, 377–381. [Google Scholar] [CrossRef]
- Wood-Adams, P.; Costeux, S. Thermorheological Behavior of Polyethylene: Effects of Microstructure and Long Chain Branching. Macromolecules 2001, 34, 6281–6290. [Google Scholar] [CrossRef]
- Hatzikiriakos, S.G. Long chain branching and polydispersity effects on the rheological properties of polyethylenes. Polym. Eng. Sci. 2000, 40, 2279–2287. [Google Scholar] [CrossRef]
- Munari, A.; Pilati, F.; Pezzin, G. Linear and branched poly(butyleneterephthalate): Activation energy for melt flow. Rheol. Acta 1985, 24, 534–536. [Google Scholar] [CrossRef]
- Munari, A.; Pezzin, G.; Pilati, F.; Manaresi, P. Rheological characterization of highly branched poly(ethyleneterephthalate). Rheol. Acta 1989, 28, 25–29. [Google Scholar] [CrossRef]
- Munari, A.; Pezzin, G.; Pilati, F. Linear and branched poly(butyleneisophthalate): Activation energy for melt flow. Rheol. Acta 1990, 29, 469–474. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Reactive extrusion of PLA, PBAT with a multi-functional epoxide: Physico-chemical and rheological properties. Eur. Polym. J. 2014, 58, 90–102. [Google Scholar] [CrossRef]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Schmid, K.; Archodoulaki, V.-M. Influence of the Molar Mass on Long-Chain Branching of Polypropylene. Polymers 2017, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kang, H.-J. Enhancement of the Processability and Properties of Nylon 6 by Blending with Polyketone. Polymers 2021, 13, 3403. [Google Scholar] [CrossRef] [PubMed]





| Chain-Extender | 1 | 2 | Non Vol by GC | ||||
|---|---|---|---|---|---|---|---|
| [g mol−1] | [g cm−3] | [g mol−1] | [-] | [wt.%] | [°C] | [°C] | |
| Joncryl® ADR 4400 | 7100 | 1.16 | 485 | 5 | >99 | 65 | >100 |
| Joncryl® ADR 4468 | 7250 | 1.16 | 310 | 9 | >99 | 59 | - |
| Zone | Die | 9 | 8 | 7 | 6 | 5 | 4 | 3 | 2 | 1 | Feeder |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 265 | 265 | 260 | 260 | 260 | 260 | 260 | 250 | 240 | 230 | 220 |
| Screw design (-) | Conveying | Mixing | Conveying | Mixing and dispersion | Kneading | Conveying | |||||
| Reference Band | PA 10.10 | Band Assignment | Joncryl® ADR 4400 and 4468 Type | Band Assignment |
|---|---|---|---|---|
| [cm−1] | [cm−1] | [-] | [cm−1] | [-] |
| General | ||||
| 3300 [37] | 3303 | N-H stretch hydrogen bonded | 1597 [38] | C-C stretching in phenyl |
| 3070 [37] | 3072 | N-H stretch and amide II overtone | 1489 [38] | C-C stretching in phenyl |
| 2935 [37] | 2920 | CH2 asymmetric stretching | 1447 [38] | CH3 scissoring vibration |
| 2860 [37] | 2851 | CH2 symmetric stretching | 1250 [38,39] | Stretching in epoxy C-O |
| 1741 [40] | 1741 | C=O stretch (ester) | 905 [38] | Stretching in epoxy C-O |
| 1660 [37] | 1637 | Amide I: C=O stretch | 844 [38] | Stretching in epoxy C-O |
| 1530 [37] | 1535 | Amide II: N-H in plane bending coupled with C-N and C-O stretch | ||
| 1170 [41] | 1165 | CO-NH skeletal, crystalline | ||
| 1123 [41] | 1122 | Amorphous | ||
| -structure | ||||
| 1466 [42] | 1466 | CH2 scissoring not adjacent to the amide group | ||
| 1416 [43] | 1419 (mi) | CH2 scissoring | ||
| 1373 [43] | 1372 | Amide III and CH2 wagging | ||
| 1262 [37] | - | Amide III | ||
| 1200 [44] | 1191 | CH2 twist-wagging | ||
| 959 [44] | 959 (vw) | CO-NH in plane (shoulder) | ||
| 936 [41] | 936 | Vibration of the N-vicinal CH2 group coupled amide III “crystal band” | ||
| -structure | ||||
| 1439 [45] | 1437 | CH2 scissor vibration | ||
| 1369 [43] | 1360 | CH2 twist-wagging | ||
| 1329 [41] | - | C-H deformation | ||
| 1255 [42] | - | Skeletal C-C stretch | ||
| 1236 [46] | 1237 | CH2 twist-wagging | ||
| 976 [44] | - | CO-NH in-plane | ||
| - and -structure | ||||
| 730 [37] | 721 | Rocking mode of CH2 | ||
| Compound | PA 10.10/ADR Ratio | |||||
|---|---|---|---|---|---|---|
| [wt.%] | [°C] | [J g−1] | [°C] | [J g−1] | [%] | |
| PA 10.10 (processed) | 100 | - | - | 206 | 71.47 | 29.29 |
| PA 10.10/ADR 4400 | 100/0.25 | 172 | −1.01 | 207 | 64.20 | 25.96 |
| 100/0.50 | 168 | −0.70 | 207 | 67.58 | 27.69 | |
| 100/0.75 | 179 | −0.73 | 206 | 57.01 | 23.24 | |
| 100/1.00 | 181 | −0.56 | 205 | 52.63 | 21.55 | |
| 100/1.25 | 172 | −0.10 | 208 | 52.73 | 21.86 | |
| Joncryl® ADR 4400 | 100 | - | - | 152 | 3.63 | - |
| PA 10.10/ADR 4468 | 100/0.25 | 177 | −1.20 | 209 | 59.90 | 24.12 |
| 100/0.50 | 173 | −1.07 | 207 | 58.56 | 23.68 | |
| 100/0.75 | 176 | −0.94 | 208 | 61.29 | 24.92 | |
| 100/1.00 | 175 | −0.87 | 208 | 59.20 | 24.13 | |
| 100/1.25 | - | - | - | - | - | |
| Joncryl® ADR 4468 | 100 | - | - | 135 | 2.34 | - |
| Compound | PA 10.10/ADR Ratio | MFR1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| [wt.%] | [Pa s] | [-] | [-] | [-] | [kJ mol−1] | [Pa] | [g 10 min−1] | [Pa s] | |
| PA 10.10 (processed) | 100 | 1857 | 0.092 | 0.528 | 0.572 | 64.7 | - | 9.8 ± 0.2 | 974 |
| PA 10.10/ADR 4400 | 100/0.25 | 5738 | 0.259 | 0.445 | 0.420 | 72.3 | 127,508 | 3.1 ± 0.2 | 2896 |
| 100/0.50 | 5717 | 0.336 | 0.471 | 0.449 | 117.1 | 145,438 | 2.0 ± 0.1 | 4611 | |
| 100/0.75 | 11,799 | 1.010 | 0.452 | 0.420 | 57.4 | 103,780 | 1.3 ± 0.1 | 7297 | |
| 100/1.00 | 14,420 | 1.546 | 0.461 | 0.407 | 64.3 | 90,019 | 0.6 ± 0.1 | 15,509 | |
| 100/1.25 | 47,062 | 4.761 | 0.414 | 0.355 | 71.0 | 52,177 | 0.5 ± 0.0 | 19,653 | |
| PA 10.10/ADR 4468 | 100/0.25 | 7520 | 0.470 | 0.461 | 0.415 | 63.9 | 141,612 | 2.0 ± 0.4 | 4302 |
| 100/0.50 | 9505 | 0.661 | 0.456 | 0.417 | 89.8 | 123,839 | 1.4 ± 0.1 | 6459 | |
| 100/0.75 | 10,683 | 0.510 | 0.413 | 0.362 | 67.6 | 109,398 | 1.4 ± 0.1 | 6646 | |
| 100/1.00 | 13,245 | 1.121 | 0.451 | 0.423 | 71.4 | 95,137 | 1.1 ± 0.1 | 8220 | |
| 100/1.25 | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdmann, R.; Rennert, M.; Meins, T. Influence of Epoxy Functional Chain-Extenders on the Thermal and Rheological Properties of Bio-Based Polyamide 10.10. Polymers 2023, 15, 3571. https://doi.org/10.3390/polym15173571
Erdmann R, Rennert M, Meins T. Influence of Epoxy Functional Chain-Extenders on the Thermal and Rheological Properties of Bio-Based Polyamide 10.10. Polymers. 2023; 15(17):3571. https://doi.org/10.3390/polym15173571
Chicago/Turabian StyleErdmann, Rafael, Mirko Rennert, and Thomas Meins. 2023. "Influence of Epoxy Functional Chain-Extenders on the Thermal and Rheological Properties of Bio-Based Polyamide 10.10" Polymers 15, no. 17: 3571. https://doi.org/10.3390/polym15173571
APA StyleErdmann, R., Rennert, M., & Meins, T. (2023). Influence of Epoxy Functional Chain-Extenders on the Thermal and Rheological Properties of Bio-Based Polyamide 10.10. Polymers, 15(17), 3571. https://doi.org/10.3390/polym15173571






