Preparation and Properties of Medium-Density Fiberboards Bonded with Vanillin Crosslinked Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Crosslinked Chitosan Binder (CS-V)
2.3. Preparation of Wood-Based Fiberboard Bonded by V-Crosslinked CS (F-CS-V)
2.4. Physical and Mechanical Properties Test
2.5. Characterization
3. Results and Discussion
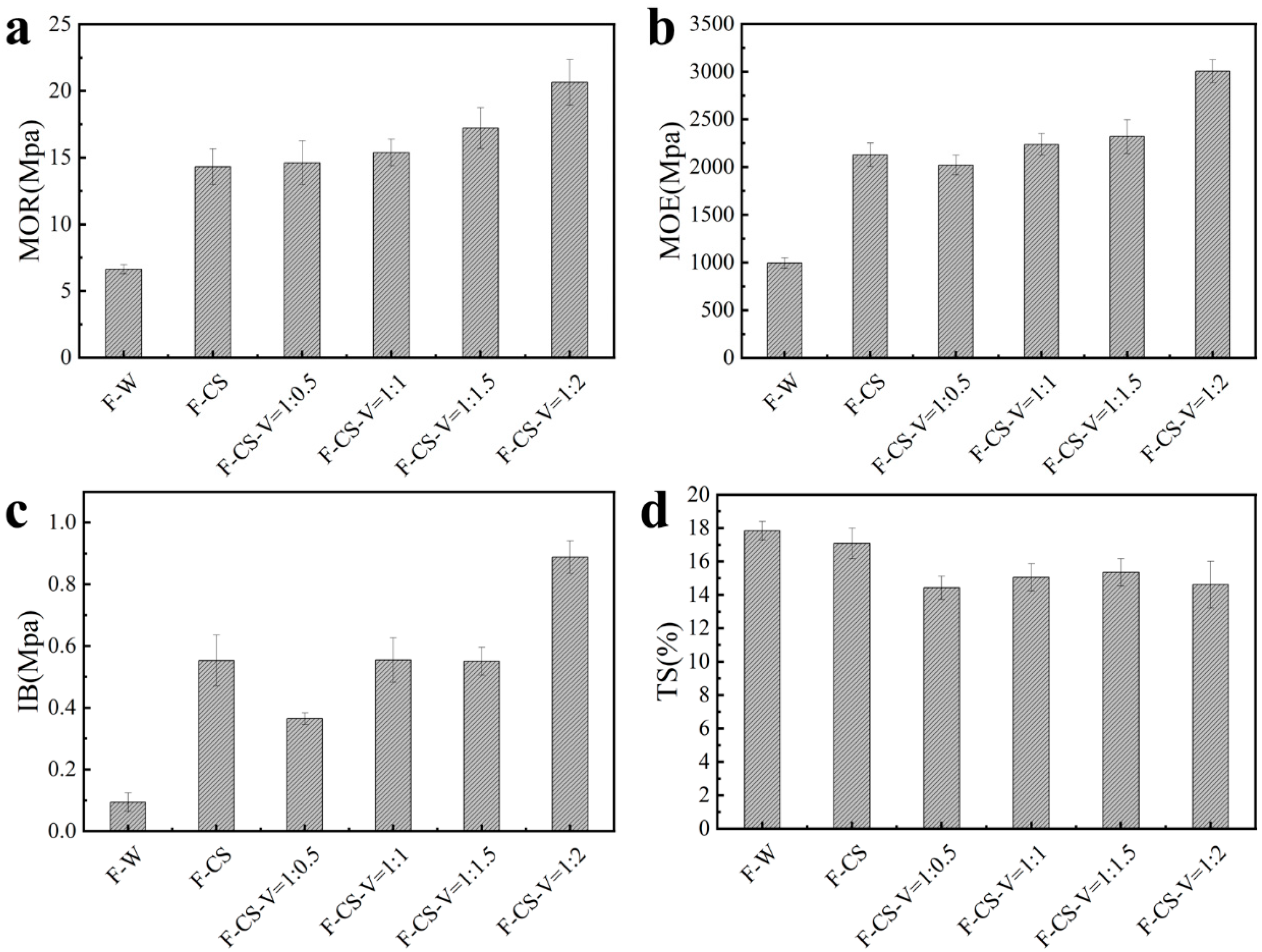
3.1. Mechanical Properties Analysis
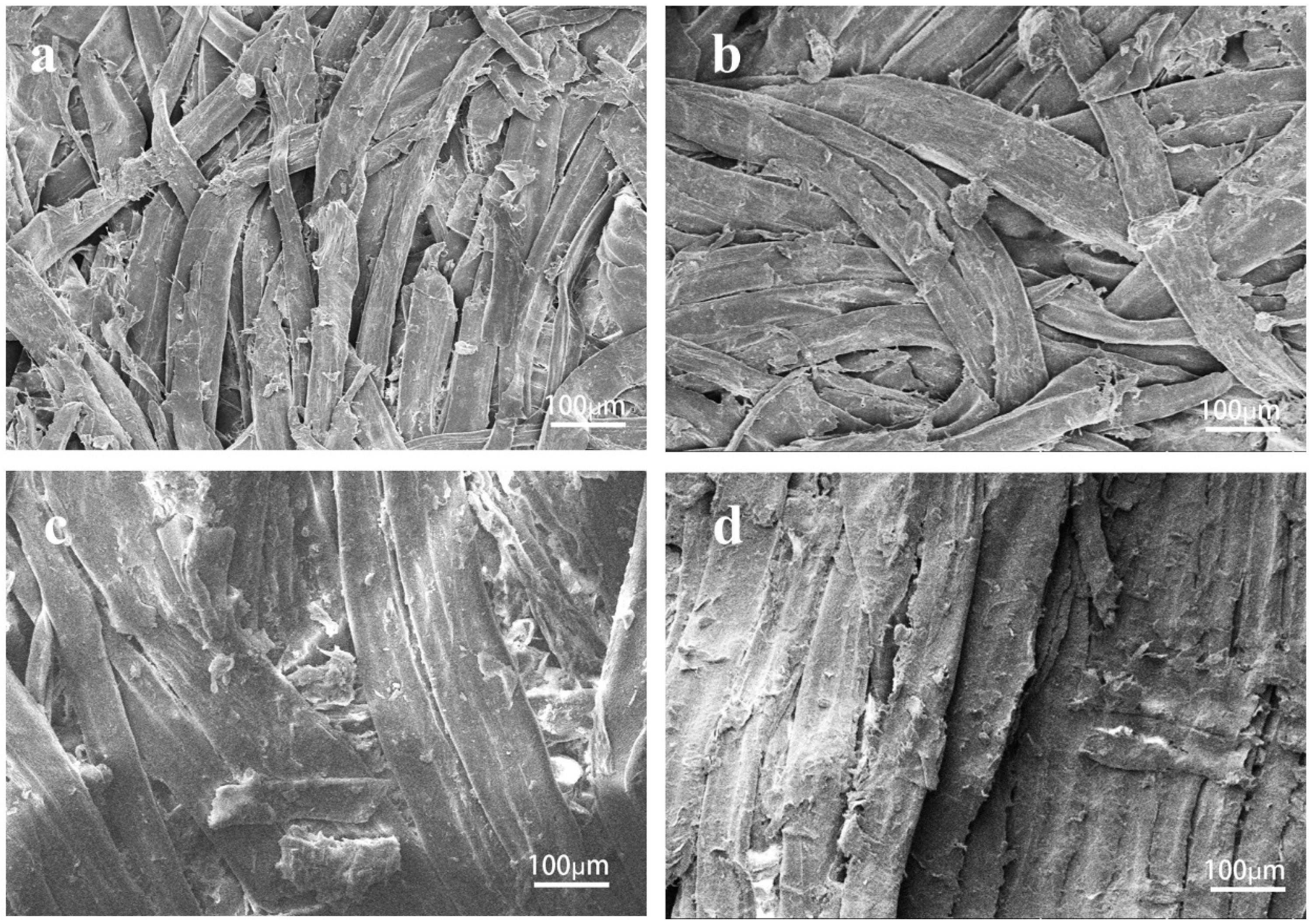
3.2. SEM Analysis
3.3. FT-IR Analysis
3.4. NMR Analysis
3.5. XRD Analysis
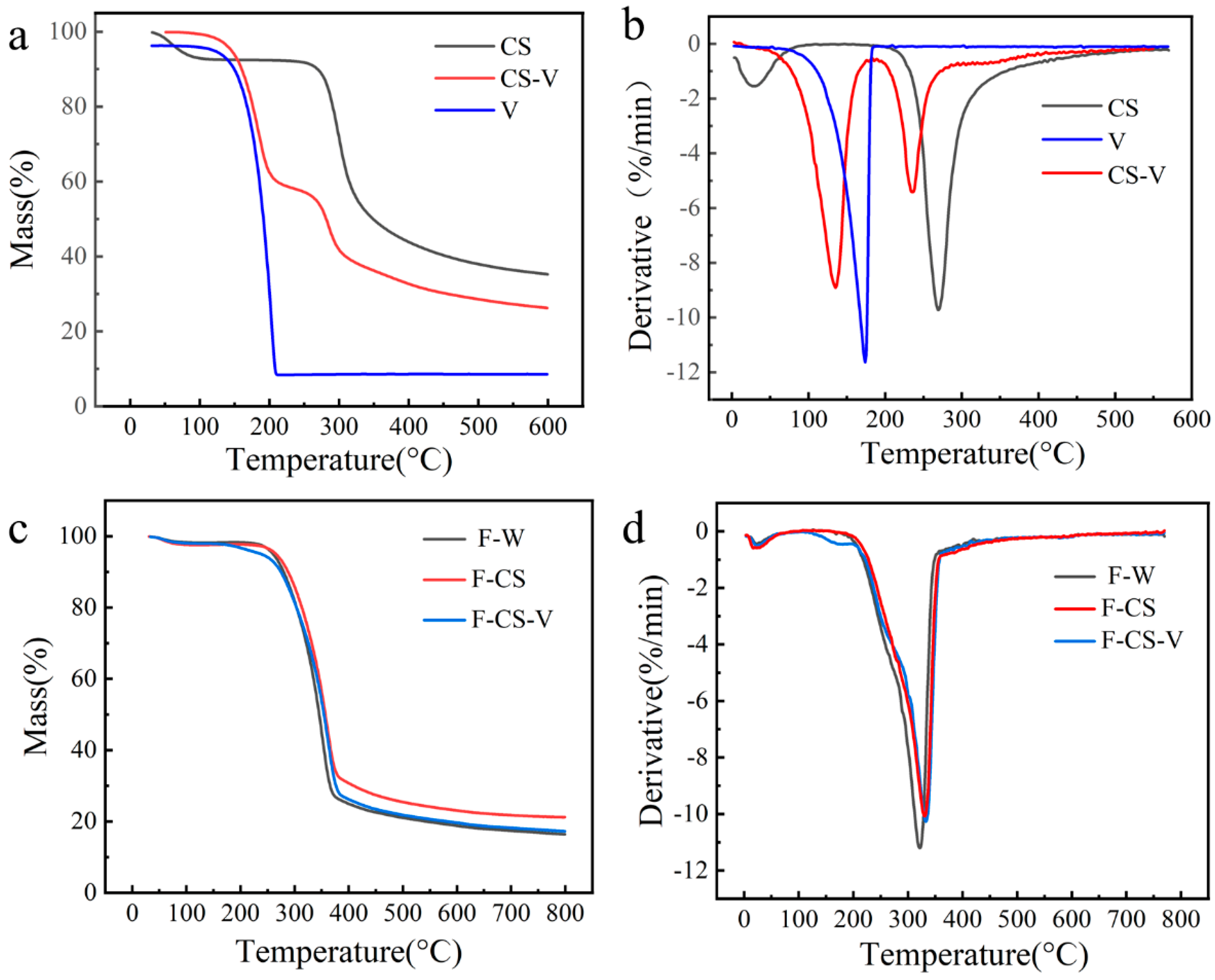
3.6. TG Analysis
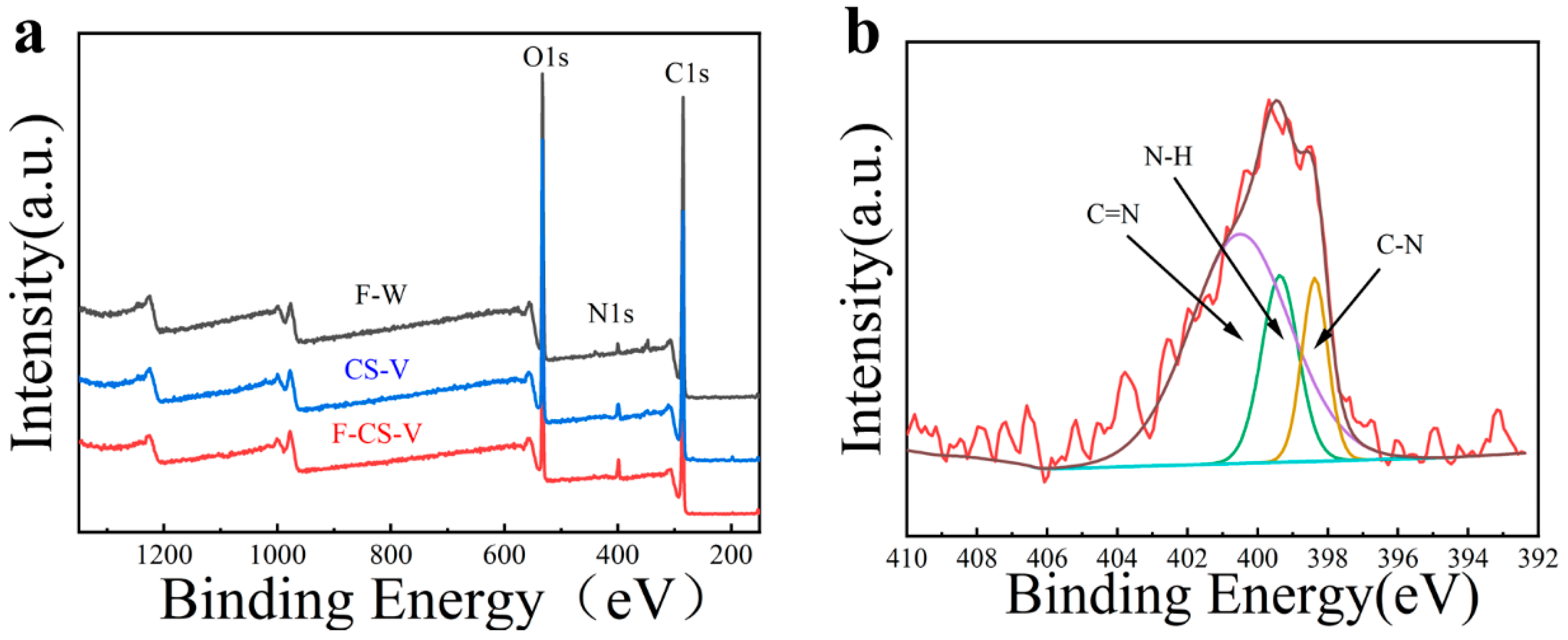
3.7. XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raydan, N.D.; Leroyer, L.; Charrier, B.; Roles, E. Recent advances on the development of protein-based adhesives for wood composite materials—A review. Molecules 2021, 26, 7617. [Google Scholar] [CrossRef]
- Song, Y.H.; Seo, J.H.; Choi, Y.S.; Kim, D.H.; Chio, B.H.; Cha, H.J. Mussel adhesive protein as an environmentally-friendly harmless wood furniture adhesive. Int. J. Adhes. Adhes. 2016, 70, 260–264. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.S.; Zhang, H.; Jiang, Q.X.; Xia, W.S. Preliminary purification and characterization of adhesive proteins from freshwater mussels. J. Adhes. 2014, 90, 607–617. [Google Scholar] [CrossRef]
- Arias, A.; Gonzalez-Rodriguez, S.; Barros, M.V.; Salvador, R.; de Francisco, A.C.; Piekarski, C.M.; Moreira, M.T. Recent developments in bio-based adhesives from renewable natural resources. J. Clean. Prod. 2021, 314, 127892. [Google Scholar] [CrossRef]
- Ji, X.D.; Dong, Y.; Nguyen, T.T.; Chen, X.Q.; Guo, M.H. Environment-friendly wood fibre composite with high bonding strength and water resistance. R. Soc. Open Sci. 2018, 5, 172002. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; Delattre, C.; de Baynast, H.; Grediac, M.; Mathias, J.D.; Ursu, A.V.; Desbrieres, J.; Michaud, P. Alkyl-Chitosan-Based Adhesive: Water Resistance Improvement. Molecules 2019, 24, 1987. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; Elchinger, P.H.; de Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Patel, A.K.; Michaud, P.; Petit, E.; de Baynast, H.; Grediac, M.; Mathias, J.D. Development of a chitosan-based adhesive. Application to wood bonding. J. Appl. Polym. Sci. 2013, 127, 5014–5021. [Google Scholar] [CrossRef]
- Patel, A.K. Chitosan: Emergence as potent candidate for green adhesive market. Biochem. Eng. J. 2015, 102, 74–81. [Google Scholar] [CrossRef]
- Hassannejad, H.; Shalbafan, A.; Rahmaninia, M. Reduction of formaldehyde emission from medium density fiberboard by chitosan as scavenger. J. Adhes. 2018, 96, 797–813. [Google Scholar] [CrossRef]
- Todorovic, T.; Norström, E.; Khabbaz, F.; Brucher, J.; Malmstrom, E.; Fogelstrom, L. A fully bio-based wood adhesive valorising hemicellulose-rich sidestreams from the pulp industry. Green Chem. 2021, 23, 3322–3333. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.T.; Shen, X.P.; Qian, T.M.; Wang, J.J.; Sun, Q.F.; Jin, C.D. Lignocellulose-chitosan-multiwalled carbon nanotube composites with improved mechanical strength, dimensional stability and fire retardancy. Polymers 2018, 10, 341. [Google Scholar] [CrossRef]
- Huang, E.Z.; Cao, Y.W.; Duan, X.P.; Yan, Y.T.; Wang, Z.; Jin, C.D. Cross-linked chitosan as an eco-friendly binder for high-performance wood-based fiberboard. Int. J. Polym. Sci. 2021, 2021, 8671384. [Google Scholar] [CrossRef]
- Ji, X.D.; Dong, Y.; Yuan, B.N.; Li, B.; Guo, M.H. Influence of glutaraldehyde on the performance of a lignosulfonate/chitosan-based medium density fiberboard adhesive. J. Appl. Polym. Sci. 2018, 135, 45870. [Google Scholar] [CrossRef]
- Xi, X.D.; Pizzi, A.; Lei, H.; Lei, H.; Zhang, B.G.; Chen, X.Y.; Du, G.B. Environmentally friendly chitosan adhesives for plywood bonding. Int. J. Adhes. Adhes. 2022, 112, 103027. [Google Scholar] [CrossRef]
- Augugliaro, V.; Camera-Roda, G.; Loddo, V.; Palmisano, G.; Palmisano, L.; Parrino, F.; Puma, M.A. Synthesis of vanillin in water by TiO2 photocatalysis. Appl. Catal. B Environ. 2012, 111, 555–561. [Google Scholar] [CrossRef]
- Li, M.Y.; Liu, J.; Hu, Y.M.; Gao, X.P.; Yuan, Q.D.; Zhao, F.G. Investigation of the specularite/chlorite separation using chitosan as a novel depressant by direct flotation. Carbohydr. Polym. 2020, 240, 116334. [Google Scholar] [CrossRef]
- Ghosh, P.; Rameshbabu, A.P.; Dogra, N.; Dhara, S. 2, 5-Dimethoxy 2, 5-dihydrofuran crosslinked chitosan fibers enhance bone regeneration in rabbit femur defects. RSC Adv. 2014, 4, 19516–19524. [Google Scholar] [CrossRef]
- Zou, Q.; Li, J.F.; Li, Y.B. Preparation and characterization of vanillin-crosslinked chitosan therapeutic bioactive microcarriers. Int. J. Biol. Macromol. 2015, 79, 736–747. [Google Scholar] [CrossRef]
- Peng, H.; Xiong, H.; Li, J.H.; Xie, M.Y.; Liu, Y.Z.; Bai, C.Q.; Chen, L.X. Vanillin cross-linked chitosan microspheres for controlled release of resveratrol. Food Chem. 2010, 121, 23–28. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.Z.; Miszuk, J.M.; Zhu, M.; Lansakara, T.I.; Tivanski, A.V.; Banas, J.A.; Sun, H.L. Vanillin-bioglass cross-linked 3D porous chitosan scaffolds with strong osteopromotive and antibacterial abilities for bone tissue engineering. Carbohydr. Polym. 2021, 271, 118440. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Zhan, W.; Tang, X.Z.; Mo, F.; Fu, L.H.; Lin, B.F. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Costa-Júnior, E.S.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Preparation and characterization of chitosan/poly (vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr. Polym. 2009, 76, 472–481. [Google Scholar] [CrossRef]
- Devi, D.A.; Smitha, B.; Sridhar, S.; Aminabhavi, T.M. Pervaporation separation of isopropanol/water mixtures through crosslinked chitosan membranes. J. Membr. 2005, 262, 91–99. [Google Scholar] [CrossRef]
- Rokhade, A.P.; Patil, S.A.; Aminabhavi, T.M. Synthesis and characterization of semi-interpenetrating polymer network microspheres of acrylamide grafted dextran and chitosan for controlled release of acyclovir. Carbohydr. Polym. 2007, 67, 605–613. [Google Scholar] [CrossRef]
- Mo, F.; Lin, B.F.; Lai, F.J.; Xu, C.H.; Zou, H.Z. A green modified microsphere of chitosan encapsulating dimethyl fumarate and cross-linked by vanillin and its application for litchi preservation. Ind. Eng. Chem. Res. 2016, 55, 4490–4498. [Google Scholar] [CrossRef]
- Kayaci, F.; Uyar, T. Solid inclusion complexes of vanillin with cyclodextrins: Their formation, characterization, and high-temperature stability. J. Agric. Food Chem. 2011, 59, 11772–11778. [Google Scholar] [CrossRef]
- Walke, S.; Srivastava, G.; Nikalje, M.; Doshi, J.; Kumar, R.; Ravetkar, S.; Doshi, P. Fabrication of chitosan microspheres using vanillin/TPP dual crosslinkers for protein antigens encapsulation. Carbohydr. Polym. 2015, 128, 188–198. [Google Scholar] [CrossRef]
- Demetgül, C.; Serin, S. Synthesis and characterization of a new vic-dioxime derivative of chitosan and its transition metal complexes. Carbohydr. Polym. 2008, 72, 506–512. [Google Scholar] [CrossRef]
- Ahmadi, M.; Moezzipour, B.; Moezzipour, A. Thermal Stability of Wood Fibers Produced from Recycled Medium Density Fiberboards. Drv. Ind. 2019, 70, 149–155. [Google Scholar] [CrossRef]
- Wu, L.Z.; Chen, Y.Z.; Li, Y.; Meng, Q.; Duan, T. Functionally integrated g-C3N4@ wood-derived carbon with an orderly interconnected porous structure. Appl. Surf. Sci. 2021, 540, 148440. [Google Scholar] [CrossRef]
- Lu, J.H.; Jiang, P.; Chen, Z.L.; Li, L.M.; Huang, Y.X. Flame retardancy, thermal stability, and hygroscopicity of wood materials modified with melamine and amino trimethylene phosphonic acid. Constr. Build. Mater. 2021, 267, 121042. [Google Scholar] [CrossRef]




| Mass Ratio a | Chitosan (g) | Acetic Acid (g) | Vanillin (g) | Deionized Water (mL) |
|---|---|---|---|---|
| 0.5:1 | 3.24 | 3.5 | 1.62 | 270 |
| 1:1 | 3.24 | 3.5 | 3.24 | 270 |
| 1.5:1 | 3.24 | 3.5 | 4.86 | 270 |
| 2:1 | 3.24 | 3.5 | 6.48 | 270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Qin, C.; Zhao, Z.; Wang, Z.; Jin, C. Preparation and Properties of Medium-Density Fiberboards Bonded with Vanillin Crosslinked Chitosan. Polymers 2023, 15, 2509. https://doi.org/10.3390/polym15112509
Cao Y, Qin C, Zhao Z, Wang Z, Jin C. Preparation and Properties of Medium-Density Fiberboards Bonded with Vanillin Crosslinked Chitosan. Polymers. 2023; 15(11):2509. https://doi.org/10.3390/polym15112509
Chicago/Turabian StyleCao, Yanwei, Chen Qin, Zhengbo Zhao, Zhe Wang, and Chunde Jin. 2023. "Preparation and Properties of Medium-Density Fiberboards Bonded with Vanillin Crosslinked Chitosan" Polymers 15, no. 11: 2509. https://doi.org/10.3390/polym15112509
APA StyleCao, Y., Qin, C., Zhao, Z., Wang, Z., & Jin, C. (2023). Preparation and Properties of Medium-Density Fiberboards Bonded with Vanillin Crosslinked Chitosan. Polymers, 15(11), 2509. https://doi.org/10.3390/polym15112509





