Preparation of Eco-Friendly Chelating Resins and Their Applications for Water Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Purification and Activation of Strongly Basic Anionic Resin
2.3. UV-Vis Determination of TAR and AB 10B
2.4. ICP-MS Analysis of MX+
2.5. Methodology for Evaluation Influence of Contact Time (Liquid-Solid Phases)
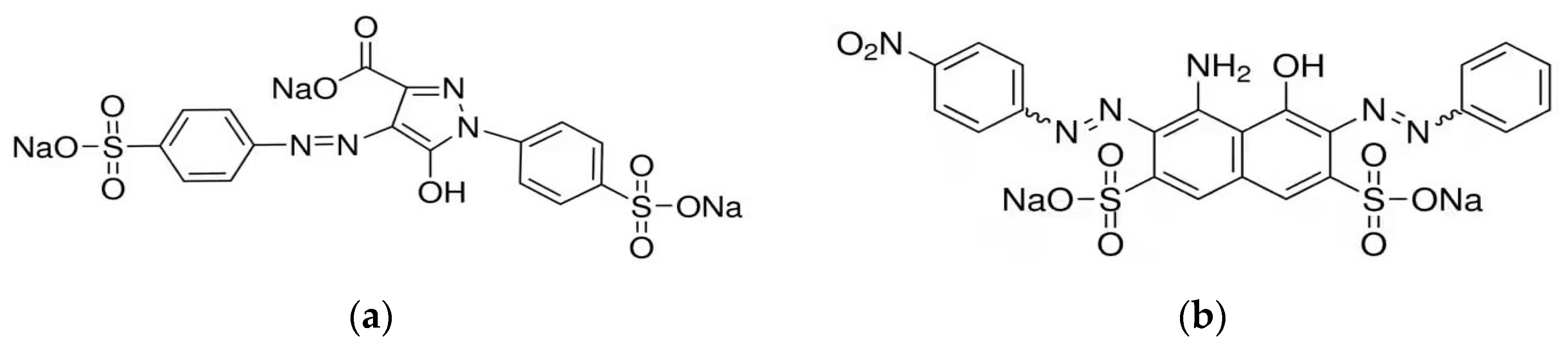
2.6. Procedure for Obtained Chelating Resin IRA 402/TAR
2.7. Procedure for Obtained Chelating Resin IRA402/AB 10B
2.8. Procedure for Evaluation of the Stability of Chelating Resins
2.9. Procedure for Retention of Metal Ions in Chelated Resins
2.10. Procedure for Recycling the Chelated Resins Exhausted with Metal Ions
2.11. Solid Phases Analysis
3. Results and Discussion
3.1. Effect of pH of the Chelating Agent Solution
3.2. Effect of Contact Time
3.3. Adsorption of TAR and AB 10B in Function of Effect Initial Concentration
3.4. Evaluation of Stability for Resins in Form IRA 402/TAR and ITA 402/AB 10B
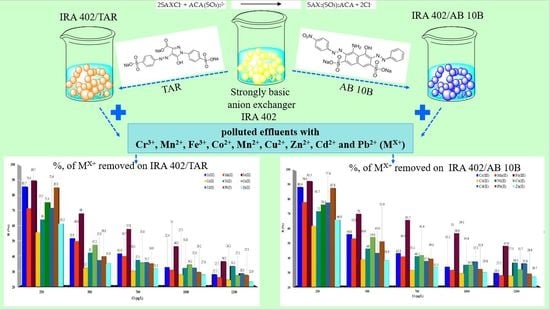
3.5. Application of Chelating Resins
3.5.1. Metal Ion Removal on IRA 402/TAR and IRA 402/AB 10B
3.5.2. Adsorption Capacity of Metal Ions on IRA 402/TAR and IRA 402/AB 10B
3.6. Reusability of Chelating Resins
3.7. Solid Phases Analysis
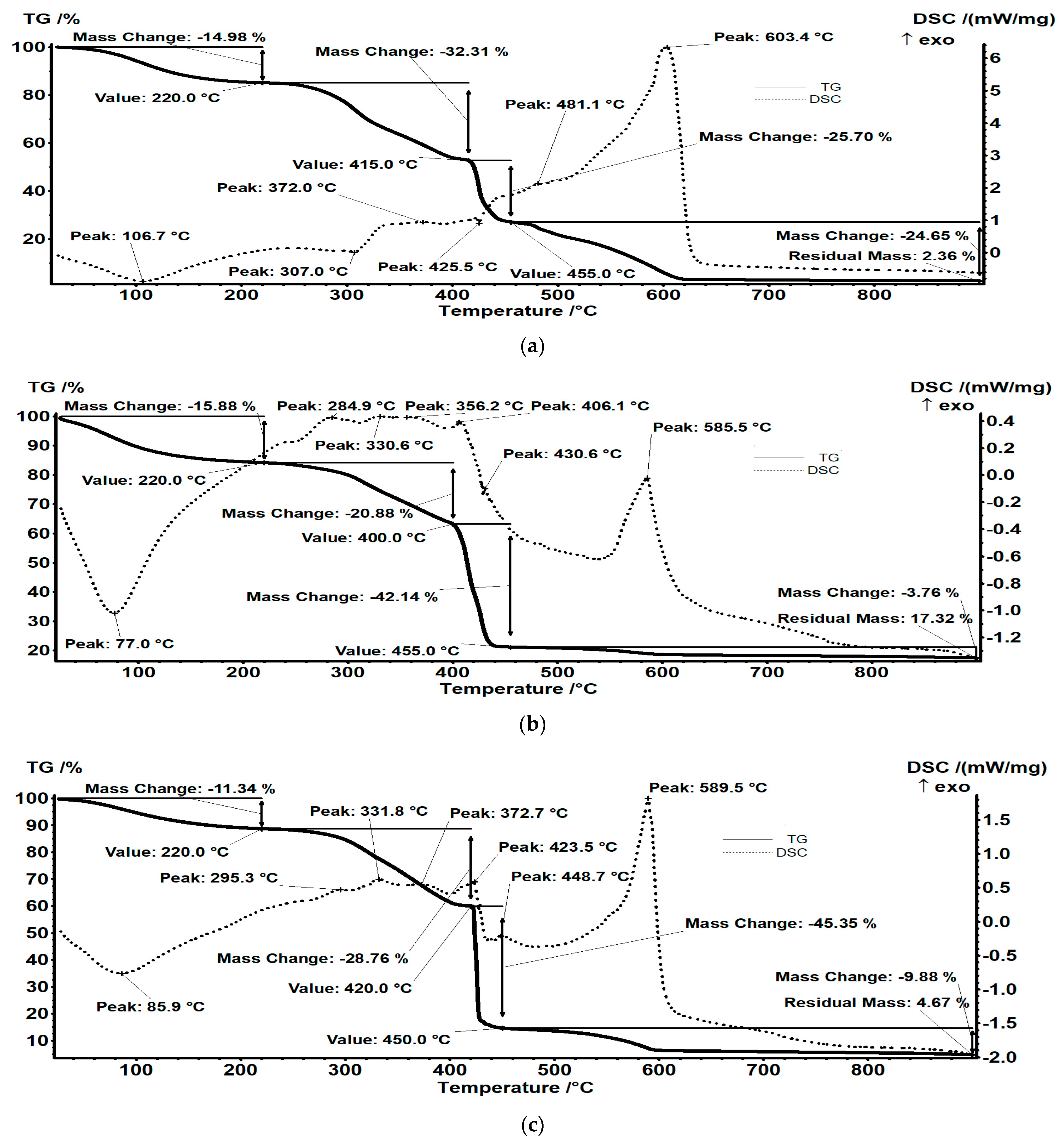
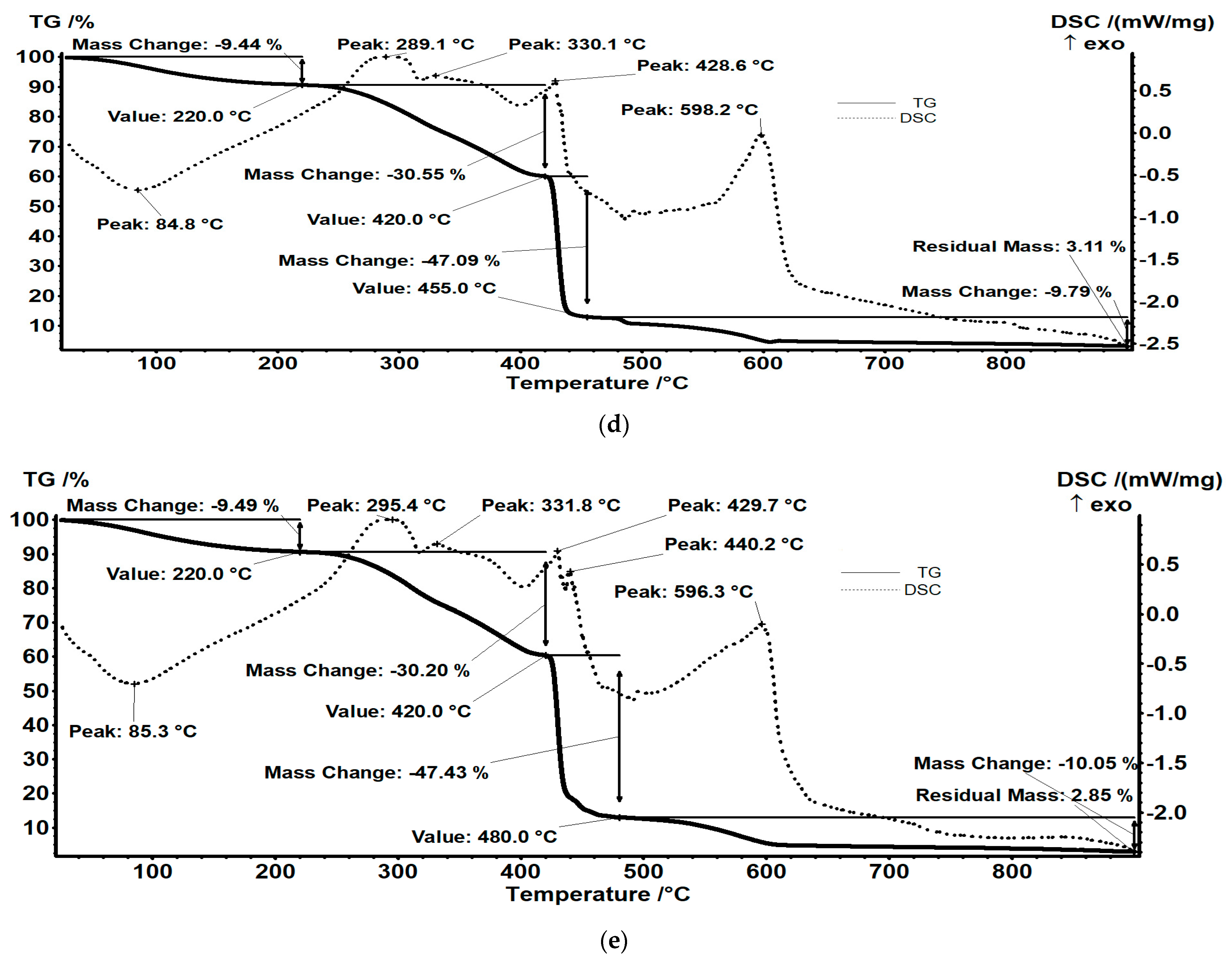
3.7.1. TG Analysis
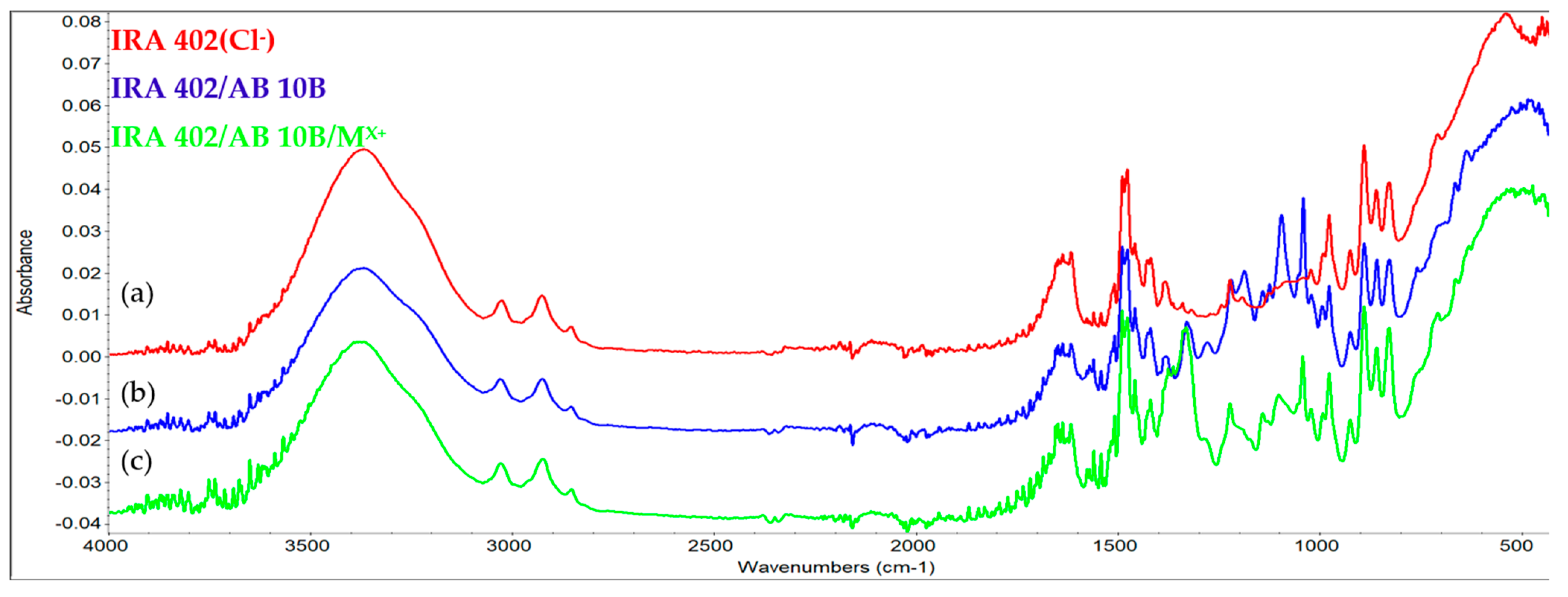
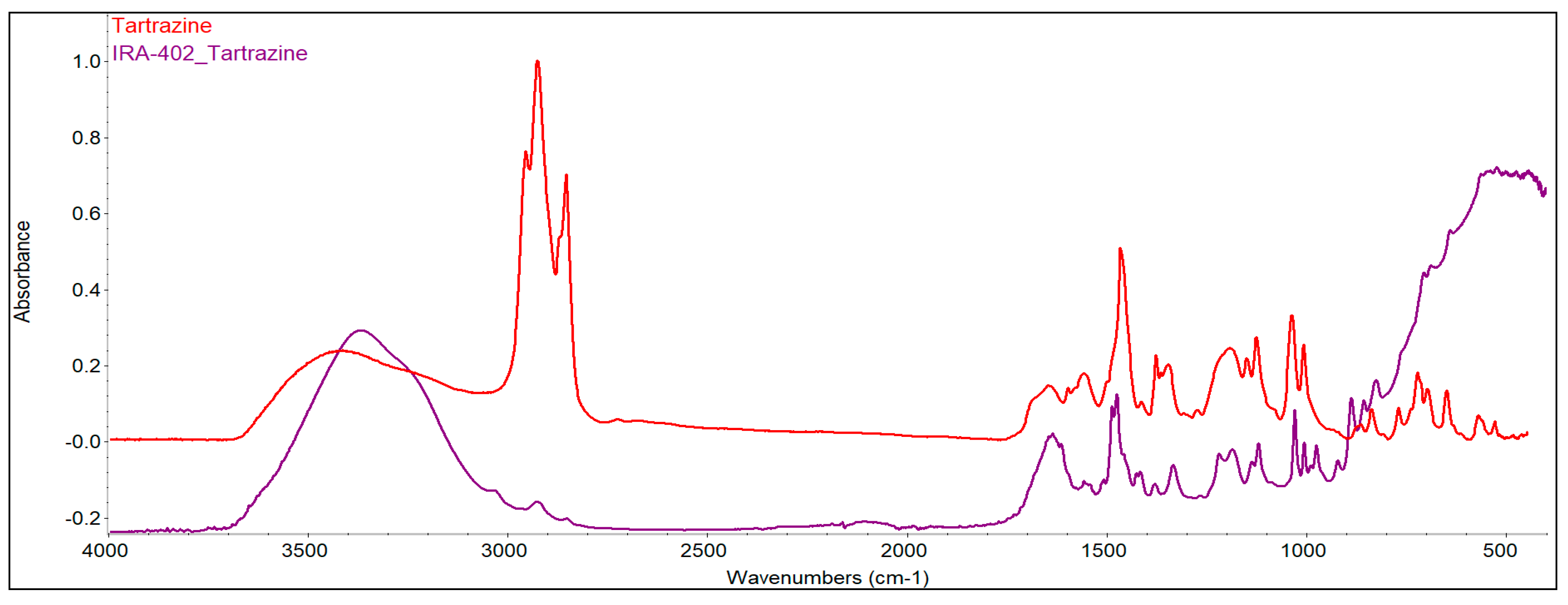
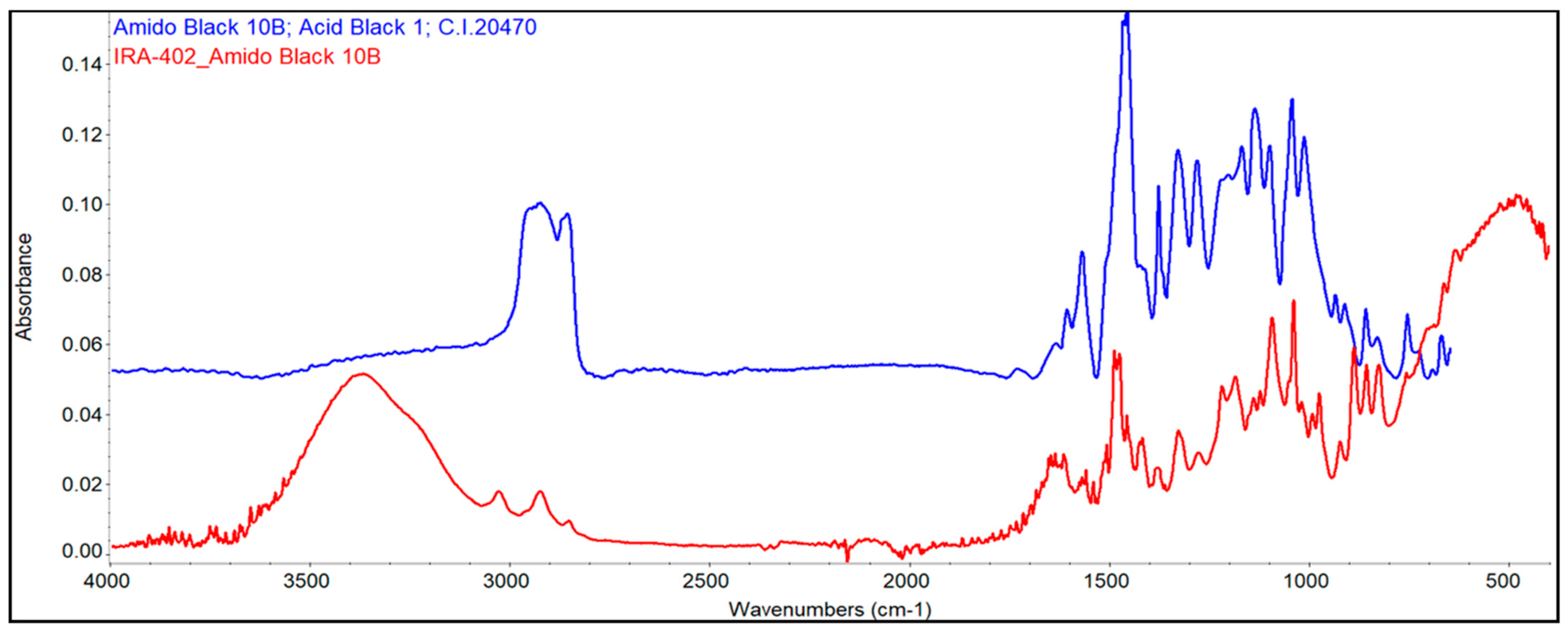
3.7.2. FTIR Analysis
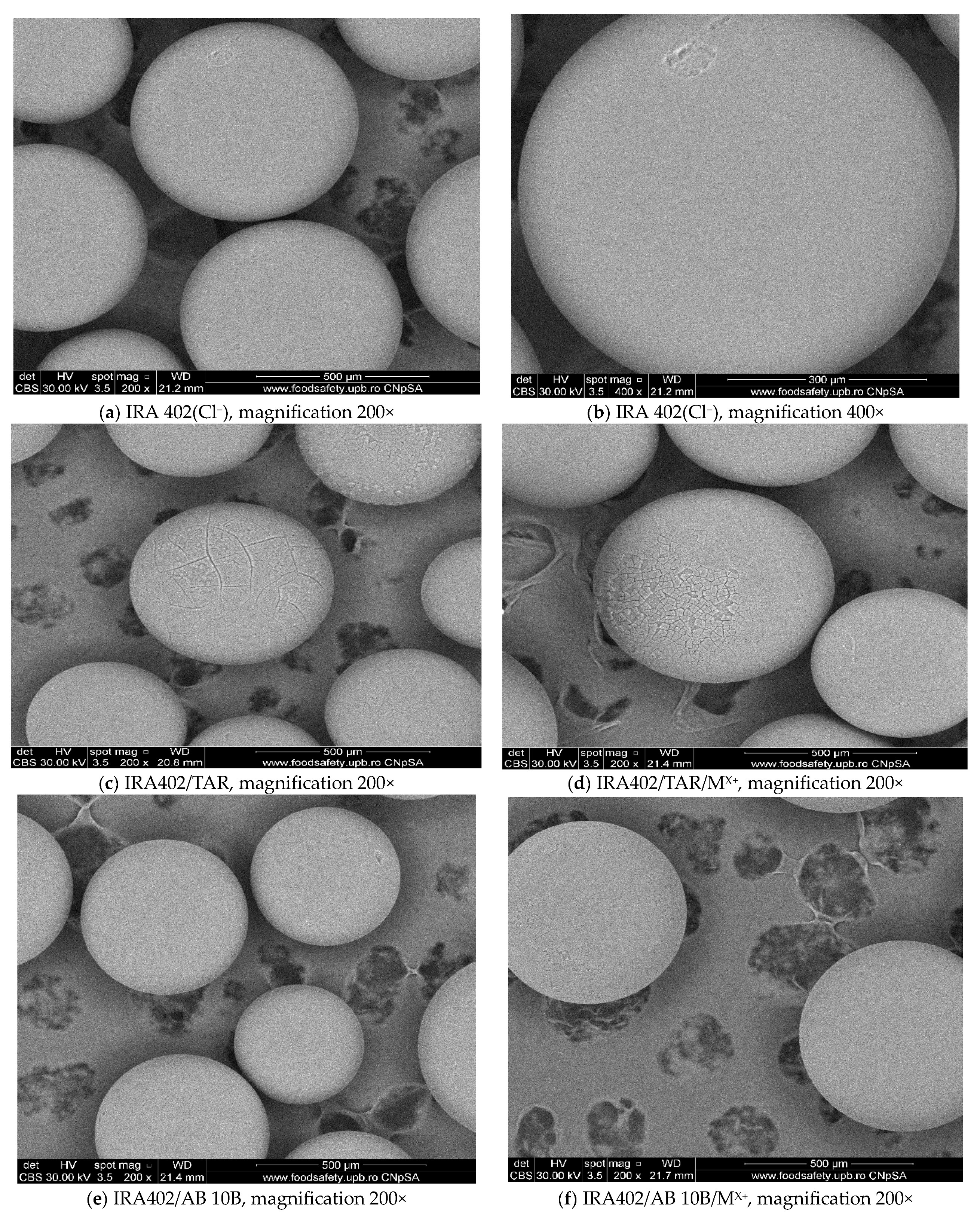
3.7.3. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, L.N.; Nguyen, N.C.T.; Trinh, A.M.H.; Do, N.H.N.; Le, K.A.; Le, P.K. Eco-friendly synthesis of durable aerogel composites from chitosan and pineapple leaf-based cellulose for Cr(VI) removal. Sep. Purif. Technol. 2023, 304, 122415. [Google Scholar] [CrossRef]
- Marin, N.M. Maize Stalk Obtained after Acid Treatment and Its Use for Simultaneous Removal of Cu2+, Pb2+, Ni2+, Cd2+, Cr3+, and Fe3+. Polymers 2022, 14, 3141. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Singh, N.; Rai, S.N.; Kumar, A.; Singh, A.K.; Singh, M.P.; Sahoo, A.; Shekhar, S.; Vamanu, E.; Mishra, V. Heavy Metal Contamination in the Aquatic Ecosystem: Toxicity and Its Remediation Using Eco-Friendly Approaches. Toxics 2023, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Goci, M.C.; Leudjo Taka, A.; Martin, L.; Klink, M.J. Chitosan-Based Polymer Nanocomposites for Environmental Remediation of Mercury Pollution. Polymers 2023, 15, 482. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; González-Delgado, Á. Adsorption Study of Continuous Heavy Metal Ions (Pb2+, Cd2+, Ni2+) Removal Using Cocoa (Theobroma cacao L.) Pod Husks. Materials 2022, 15, 6937. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Soad, A.M.; Lazzara, G.; Abd El-Magied, M.O.; Cavallaro, G.; Al-Otaibi, J.S.; Sayyed, M.I.; Kovaleva, E.G. Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media. Int. J. Mol. Sci. 2022, 23, 2396. [Google Scholar] [CrossRef]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Achour, S.; Menasra, H. Removal of Heavy Metals from Industrial Wastewater by Chemical Precipitation: Mechanisms and Sludge Characterization. Arab. J. Sci. Eng. 2022, 47, 5587–5599. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Li, B.; Huang, H.; Shi, W.; Liu, Z. Removal and recovery of vanadium from waste by chemical precipitation, adsorption, solvent extraction, remediation, photo-catalyst reduction and membrane filtration. A review. Environ. Chem. Lett. 2022, 20, 1763–1776. [Google Scholar] [CrossRef]
- Peyravi, M.; Rezaei, H. Chapter 17—Metals Removal by Membrane Filtration. In Metals in Water; Shukla, S.K., Kumar, S., Madhav, S., Mishra, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 331–351. [Google Scholar] [CrossRef]
- Yan, L.; Yang, X.; Zeng, H.; Zhao, Y.; Li, Y.; He, X.; Ma, J.; Shao, L. Nanocomposite hydrogel engineered hierarchical membranes for efficient oil/water separation and heavy metal removal. J. Membr. Sci. 2023, 668, 121243. [Google Scholar] [CrossRef]
- Pérez-Silva, I.; Páez-Hernández, M.; Ibarra, I.S.; Camacho-Mendoza, R.L. Evaluation of the Hybrid Membrane of ZnO Particles Supported in Cellulose Acetate for the Removal of Lead. Membranes 2023, 13, 123. [Google Scholar] [CrossRef]
- Pan, S.; Shen, J.; Deng, Z.; Zhang, X.; Pan, B. Metastable nano-zirconium phosphate inside gel-type ion exchanger for enhanced removal of heavy metals. J. Hazard. Mater. 2022, 423, 127158. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.K.; Tamang, N.; Chatterjee, A.; Bhattarai, A.; Saha, B. Ion Exchange Resins for Selective Separation of Toxic Metals. Ion Exch. Resins Biomed. Environ. Sci. 2023, 137, 55–74. [Google Scholar]
- Montaño-Medina, C.U.; Lopéz-Martínez, L.M.; Ochoa-Terán, A.; López-Maldonado, E.A.; Salazar-Gastelum, M.I.; Trujillo-Navarrete, B.; Pérez-Sicairos, S.; Cornejo-Bravo, J.M. New pyridyl and aniline-functionalized carbamoylcarboxylic acids for removal of metal ions from water by coagulation-flocculation process. Chem. Eng. J. 2023, 451, 138396. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Zhang, W.; Fan, Y. Study on Flocculation Behavior of Cr (VI) Using a Novel Chitosan Functionalized with Thiol Groups. Polymers 2023, 15, 1117. [Google Scholar] [CrossRef] [PubMed]
- Parvin, F.; Niloy, N.M.; Haque, M.M.; Tareq, S.M. Activated Carbon as Potential Material for Heavy Metals Removal from Wastewater. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Elsevier: Amsterdam, The Netherlands, 2023; pp. 117–130. [Google Scholar] [CrossRef]
- Temesgen Abeto, A.; Beyan, S.M.; Abreham Bekele, B.; Worku Firomsa, K. Optimization and Modeling of Cr (VI) Removal from Tannery Wastewater onto Activated Carbon Prepared from Coffee Husk and Sulfuric Acid (H2SO4) as Activating Agent by Using Central Composite Design (CCD). J. Environ. Public Health. 2023, 5663261. [Google Scholar] [CrossRef]
- Culita, D.C.; Simonescu, C.M.; Patescu, R.-E.; Dragne, M.; Stanica, N.; Oprea, O. o-Vanillin functionalized mesoporous silica–coated magnetite nanoparticles for efficient removal of Pb (II) from water. J. Solid State Chem. 2016, 238, 311–320. [Google Scholar] [CrossRef]
- Enache, D.F.; Vasile, E.; Simonescu, C.M.; Culita, D.; Vasile, E.; Oprea, O.; Pandele, A.M.; Razvan, A.; Dumitru, F.; Nechifor, G. Schiff base-functionalized mesoporous silicas (MCM-41, HMS) as Pb (II) adsorbents. RSC Adv. 2018, 8, 176–189. [Google Scholar] [CrossRef]
- Culita, D.C.; Simonescu, C.M.; Dragne, M.; Stanica, N.; Munteanu, C.; Preda, S.; Oprea, O. Effect of surfactant concentration on textural, morphological and magnetic properties of CoFe2O4 nanoparticles and evaluation of their adsorptive capacity for Pb (II) ions. Ceram. Int. 2015, 41, 13553–13560. [Google Scholar] [CrossRef]
- Duan, G.; Li, X.; Ma, X.; Zhong, W.; Wang, S. High-efficiency adsorption removal for Cu(II) and Ni(II) using a novel acylamino dihydroxamic acid chelating resin. Sci. Total Environ. 2023, 864, 160984. [Google Scholar] [CrossRef]
- Tan, L.; Liu, T.; Zhang, Y.; Xue, N.; Hu, P. Enhance selective separation of vanadium and iron from acid-leaching solution with sulfamic acid modified resin. J. Chem. Eng. 2023, 457, 141286. [Google Scholar] [CrossRef]
- Long, W.; Yang, C.; Wang, G.; Hu, J. Effective adsorption of Hg(II) ions by new ethylene imine polymer/β-cyclodextrin crosslinked functionalized magnetic composite. Arab. J. Chem. 2023, 16, 104439. [Google Scholar] [CrossRef]
- Marin, N.M.; Ficai, A.; Constantin, L.A.; Motelica, L.; Trusca, R. New Chelate Resins Prepared with Direct Red 23 for Cd2+, Ni2+, Cu2+ and Pb2+ Removal. Polymers 2022, 14, 5523. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Feng, X.; Liu, C.; Wu, H.; Liu, X. Advances in Chelating Resins for Adsorption of Heavy Metal Ions. Ind. Eng. Chem. 2022, 61, 11309–11328. [Google Scholar] [CrossRef]
- Marin, N.M. Natural and Synthetic Polymers Modified with Acid Blue 113 for Removal of Cr3+, Zn2+ and Mn2+. Polymers 2022, 14, 2139. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.M.; Tiron, O.; Pascu, L.F.; Costache, M.; Nita-Lazar, M.; Badea, I.A. Synergistic methodology based on ion exchange and biodegradation mechanisms applied for metal complex dye removal from waste waters. Rev. Chim. 2018, 69, 38–44. [Google Scholar] [CrossRef]
- Marin, N.M.; Pascu, L.F.; Demba, A.; Nita-Lazar, M.; Badea, I.A.; Aboul-Enein, H. Removal of the Acid Orange 10 by ion exchange and microbiological methods. Int. J. Environ. Sci. Technol. 2019, 16, 6357–6366. [Google Scholar] [CrossRef]
- Marin, N.M.; Stanculescu, I. Application of amberlite IRA 402 resin adsorption and laccase treatment for acid blue 113 removal from aqueous media. Polymers 2021, 13, 3991. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, V.; Mittal, S.; Sharma, R.K. Design, Synthesis and Application of Chemically Modified Amberlite XAD-2 for Separation and Preconcentration of Pb (II). Chem.Select 2023, 8, e202202966. [Google Scholar] [CrossRef]
- Bahsaine, K.; Mekhzoum, M.E.M.; Benzeid, H.; Qaiss, A.E.k.; Bouhfid, R. Chromium (III) adsorption from the phosphoric acid medium using DETA grafted Merrifield resin. Environ. Sci. Pollut. 2023. [Google Scholar] [CrossRef]
- Leonard, J.; Sivalingam, S.; Srinadh, R.V.; Mishra, S. Efficient removal of hexavalent chromium ions from simulated wastewater by functionalized anion exchange resin: Process optimization, isotherm and kinetic studies. J. Environ. Chem. Ecotoxicol. 2023, 5, 98–107. [Google Scholar] [CrossRef]
- Bringas, A.; Bringas, E.; Ibañez, R.; San-Román, M.F. Fixed-bed columns mathematical modeling for selective nickel and copper recovery from industrial spent acids by chelating resins. Sep. Purif. Technol. 2023, 313, 123457. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; Abdel-Salam, A.H.; Khalil, T.E. The impact of an Amberlite XAD-16-based chelating resin for the removal of aqueous Cd(II) and Pb(II)ions. Microchem. J. 2021, 165, 106097. [Google Scholar] [CrossRef]
- Amberlite® IRA402 Chloride Form, Specification Sheet. Available online: https://www.sigmaaldrich.com/RO/en/product/sial/06466 (accessed on 1 February 2023).
- How to Determine the LOD Using the Calibration Curve? Available online: https://mpl.loesungsfabrik.de/en/english-blog/method-validation/calibration-line-procedure (accessed on 1 February 2023).
- Xu, Y.; Qu, Y.; Yang, Y.; Qu, B.; Shan, R.; Yuan, H.; Sun, Y. Study on Efficient Adsorption Mechanism of Pb2+ by Magnetic Coconut Biochar. Int. J. Mol. Sci. 2022, 23, 14053. [Google Scholar] [CrossRef] [PubMed]
- Wawrzkiewicz, M.; Hubicki, Z. Equilibrium and kinetic studies on the adsorption of acidic dye by the gel anion exchanger. J. Hazard. Mater. 2009, 172, 868–874. [Google Scholar] [CrossRef]
- Ion Exchange Capacity. Available online: http://dardel.info/IX/capacity.html (accessed on 1 February 2023).
- Wawrzkiewicz, M.; Hubicki, Z. Removal of tartrazine from aqueous solutions by strongly basic polystyrene anion exchange resins. J. Hazard. Mater. 2009, 164, 502–509. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, H. Adsorptive removal of Amido black 10B from aqueous solution using stem carbon of Ricinus communis as adsorbent. Asian J. Chem. 2019, 31, 1071–1076. [Google Scholar] [CrossRef]
- Ma, J.; Shen, J.; Wang, C.; Wei, Y. Preparation of dual-function chelating resin with high capacity and adjustable adsorption selectivity to variety of heavy metal ions. J. Taiwan Inst. Chem. Eng. 2018, 91, 532–538. [Google Scholar] [CrossRef]
- Lv, Y.; Li, T.; Jiang, Y.; Wang, L.; Liu, Z.; Liu, F.; Li, A. Highly selective separation of Pb (II) with a novel aminophosphonic acid chelating resin from strong-acidic hexa-solute media. Sep. Purif. Technol. 2022, 300, 121818. [Google Scholar] [CrossRef]
- Suppapruek, M.; Threepopnatkul, P.; Sittattrakul, A.; Lerdwijitjarud, W. Effect of Chelating Agents on Removal of Heavy Metal Cations of Cellulose-based Ion Exchange Resins from Water Hyacinth. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 302, p. 02020. [Google Scholar]
- Li, W.-T.; Hu, Z.-J.; Meng, J.; Zhang, X.; Gao, W.; Chen, M.-L.; Wang, J.-H. Zn-based metal organic framework-covalent organic framework composites for trace lead extraction and fluorescence detection of TNP. J. Hazard. Mater. 2021, 411, 125021. [Google Scholar] [CrossRef]
- Periodic Table of the Elements Characterization. Available online: https://chemglobe.org/ptoe/_/29.php (accessed on 1 December 2022).
- Kocaoba, S. Determination of some heavy metals from aqueous solutions using modified Amberlite XAD-4 resin by selective solid-phase extraction. J. Anal. Sci. Technol. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Marin, N.M.; Stanculescu, I. Removal of procainamide and lidocaine on Amberlite XAD7HP resin and of As (V), Pb (II) and Cd (II) on the impregnated resin for water treatment. Mater. Chem. Phys. 2022, 277, 125582. [Google Scholar] [CrossRef]
- THE 17 GOALS, Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 1 January 2023).
- Kumar, M.; Rathore, D.P.S.; Singh, A.K. Amberlite XAD-2 functionalized with o-aminophenol: Synthesis and applications as extractant for copper(II), cobalt(II), cadmium(II), nickel(II), zinc(II) and lead(II). Talanta 2000, 51, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xue, N.; Zhang, Y.; Hu, P. Enhanced vanadium adsorption performance on aminophosphonic chelating resin with oxalic acid regeneration. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130220. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Lavric, V.; Musina, A.; Antonescu, O.M.; Culita, D.C.; Marinescu, V.; Tardei, C.; Oprea, O.; Pandele, A.M. Experimental and modeling of cadmium ions removal by chelating resins. J. Mol. Liq. 2020, 307, 112973. [Google Scholar] [CrossRef]






| Type | Strongly Basic Anion Exchanger |
|---|---|
| Matrix | Styrene-divinylbenzene copolymer |
| Ionic form as shipped | Chloride (Cl−) |
| Physical form | Yellow Translucent beads |
| Total ion exchange capacity | ≥1.2 eq/dm3 |
| pH range | 0–14 |
| Moisture holding capacity | 49–60% Chloride form |
| Harmonic mean size | 0.6 mm–0.75 mm; (20–25 mesh) |
| Maximum operating temperature | 77 °C |
| Parameter | TAR | AB 10B |
|---|---|---|
| LOD (mg/L) | 0.07 | 0.10 |
| LOQ (mg/L) | 0.21 | 0.31 |
| Parameter | Operating Condition |
|---|---|
| RF power | 1150 W |
| Sample Depth | 8.0 mm |
| Carrier gas | 1.00 L/min |
| Nebulizer pump | 0.10 rps |
| Spray-Chamber Temperature | 2 °C |
| Cell gas flow (He) | 7 mL/min |
| Dwell time | 10 ms (Cr, Fe) and 12 ms (Mn, Co, Ni, Cu, Zn, Cd, Pb) |
| Sampling cone | Ni-tipped with Cu base |
| Skimmer cone | Ni |
| Sample | Water Content (Mass Loss RT-220 °C) | Mass Loss 220–415 (420) °C, % | Mass Loss 415–455 °C, % | Mass Loss 455–900 °C, % | Residual, % |
|---|---|---|---|---|---|
| IRA 402(Cl−) | 14.98 | 32.31 | 25.70 | 24.65 | 2.36 |
| IRA402/TAR | 15.88 | 20.88 | 42.14 | 3.76 | 17.32 |
| IRA402/AB10B | 11.34 | 28.76 | 45.35 | 9.88 | 4.67 |
| IRA402/Tar/MX+ | 9.44 | 30.55 | 47.09 | 9.79 | 3.11 |
| IRA402/AB10B/MX+ | 9.49 | 30.20 | 47.43 | 10.05 | 2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, N.M.; Dolete, G.; Motelica, L.; Trusca, R.; Oprea, O.C.; Ficai, A. Preparation of Eco-Friendly Chelating Resins and Their Applications for Water Treatment. Polymers 2023, 15, 2251. https://doi.org/10.3390/polym15102251
Marin NM, Dolete G, Motelica L, Trusca R, Oprea OC, Ficai A. Preparation of Eco-Friendly Chelating Resins and Their Applications for Water Treatment. Polymers. 2023; 15(10):2251. https://doi.org/10.3390/polym15102251
Chicago/Turabian StyleMarin, Nicoleta Mirela, Georgiana Dolete, Ludmila Motelica, Roxana Trusca, Ovidiu Cristian Oprea, and Anton Ficai. 2023. "Preparation of Eco-Friendly Chelating Resins and Their Applications for Water Treatment" Polymers 15, no. 10: 2251. https://doi.org/10.3390/polym15102251
APA StyleMarin, N. M., Dolete, G., Motelica, L., Trusca, R., Oprea, O. C., & Ficai, A. (2023). Preparation of Eco-Friendly Chelating Resins and Their Applications for Water Treatment. Polymers, 15(10), 2251. https://doi.org/10.3390/polym15102251