Boron Adsorption Using NMDG-Modified Polypropylene Melt-Blown Fibers Induced by Ultraviolet Grafting
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Preparation of Adsorbent
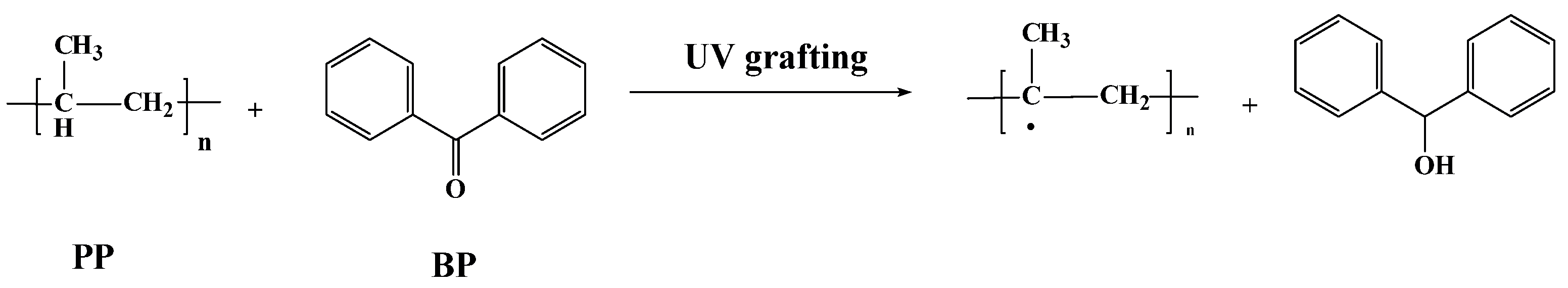
2.2.1. PP Melt-Blown Fiber Pretreatment
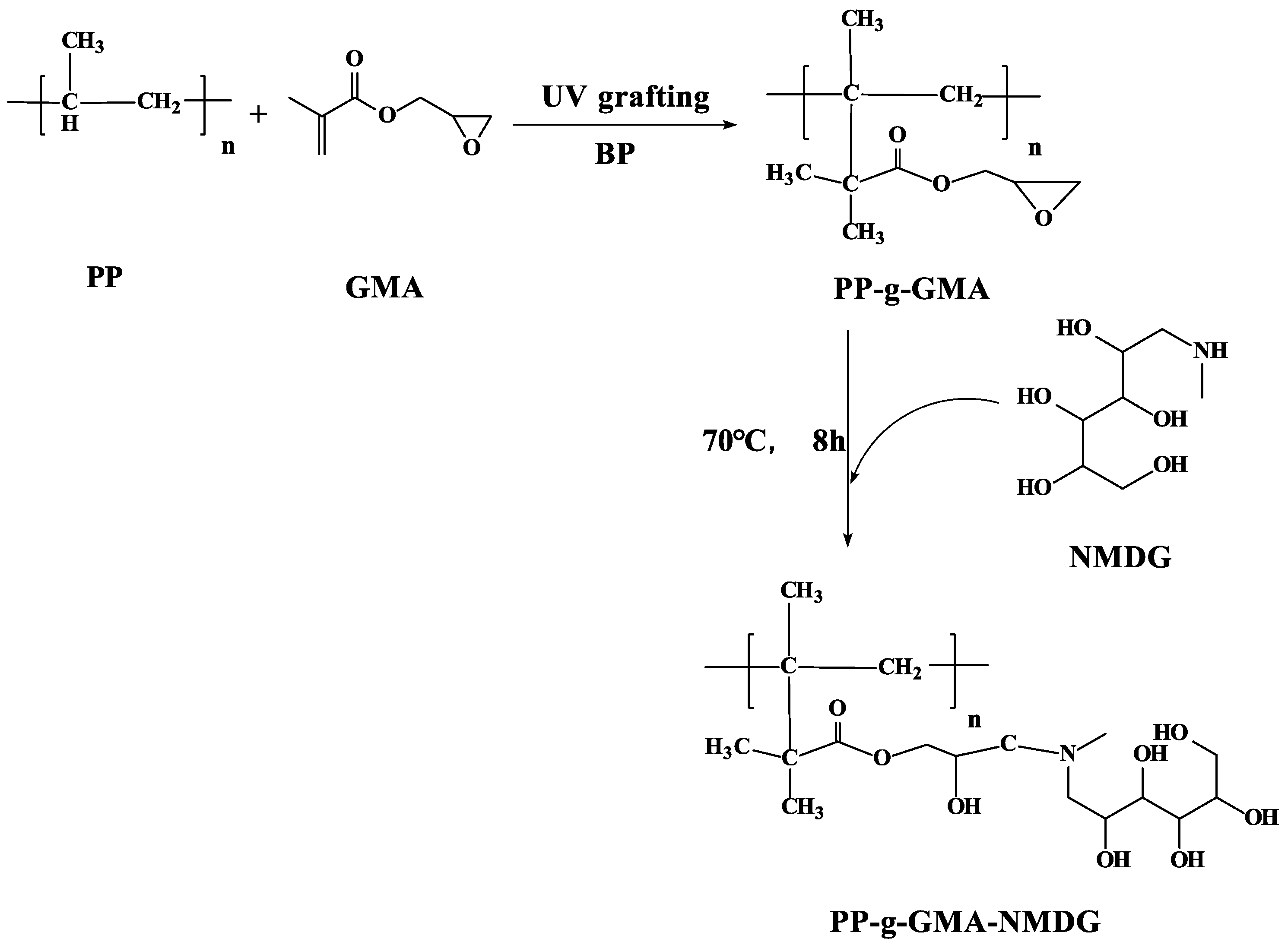
2.2.2. PP-g-GMA Preparation
2.2.3. PP-g-GMA-NMDG Preparation
2.3. Characterization
2.4. Adsorption Experiments
2.4.1. pH
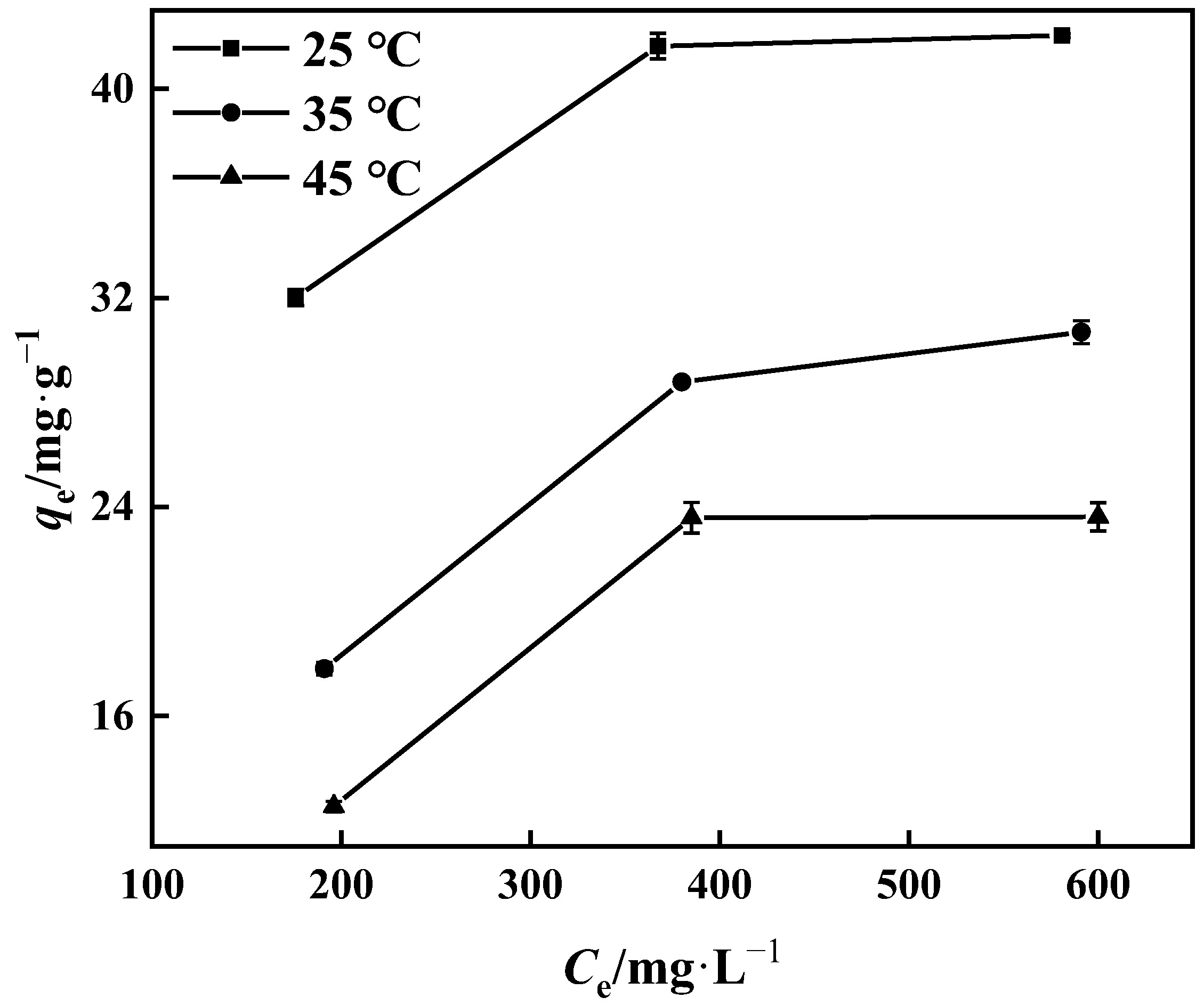
2.4.2. Adsorption Isotherm
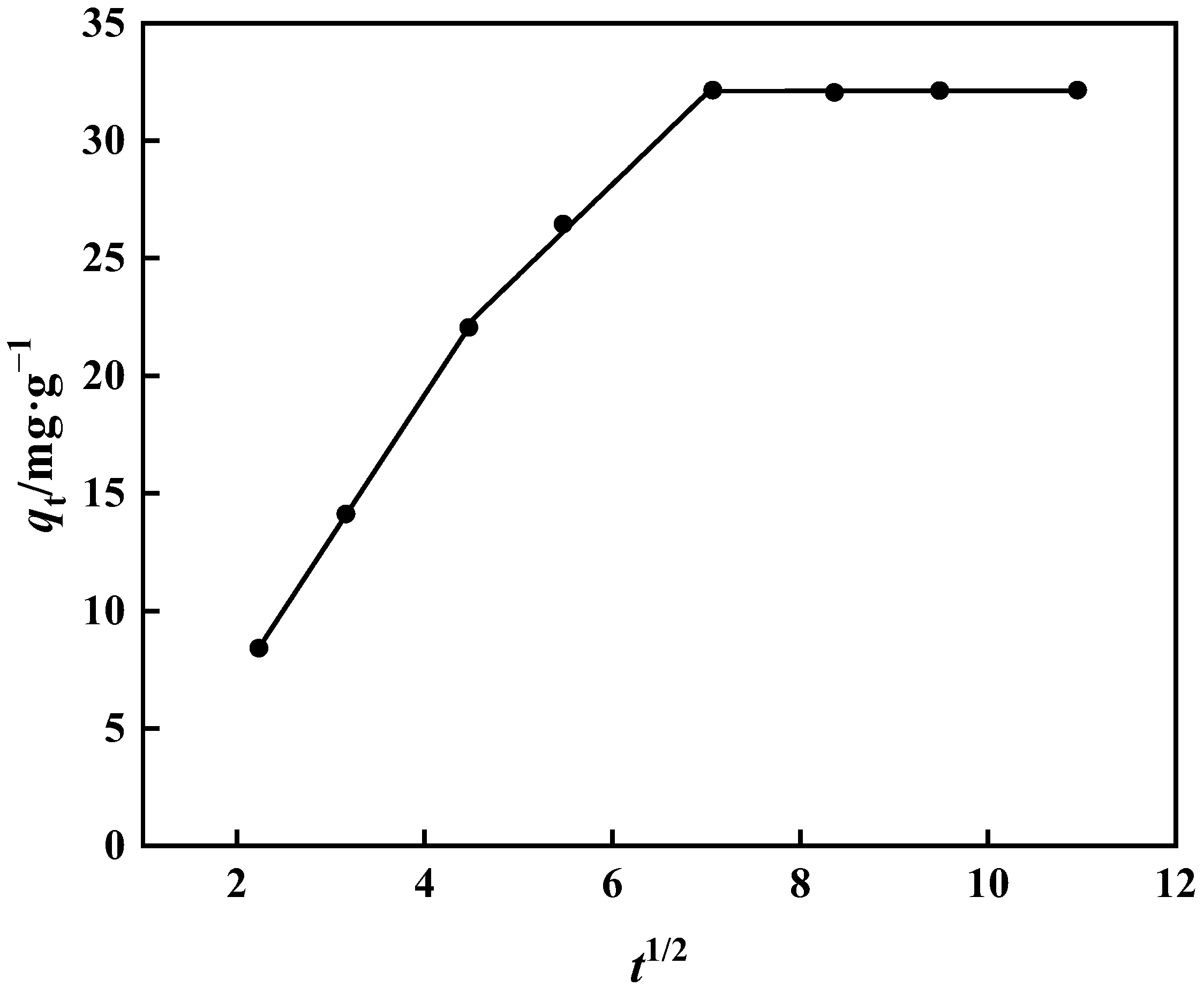
2.4.3. Adsorption Kinetics and Internal Diffusion Model
2.4.4. Thermodynamics of Adsorption
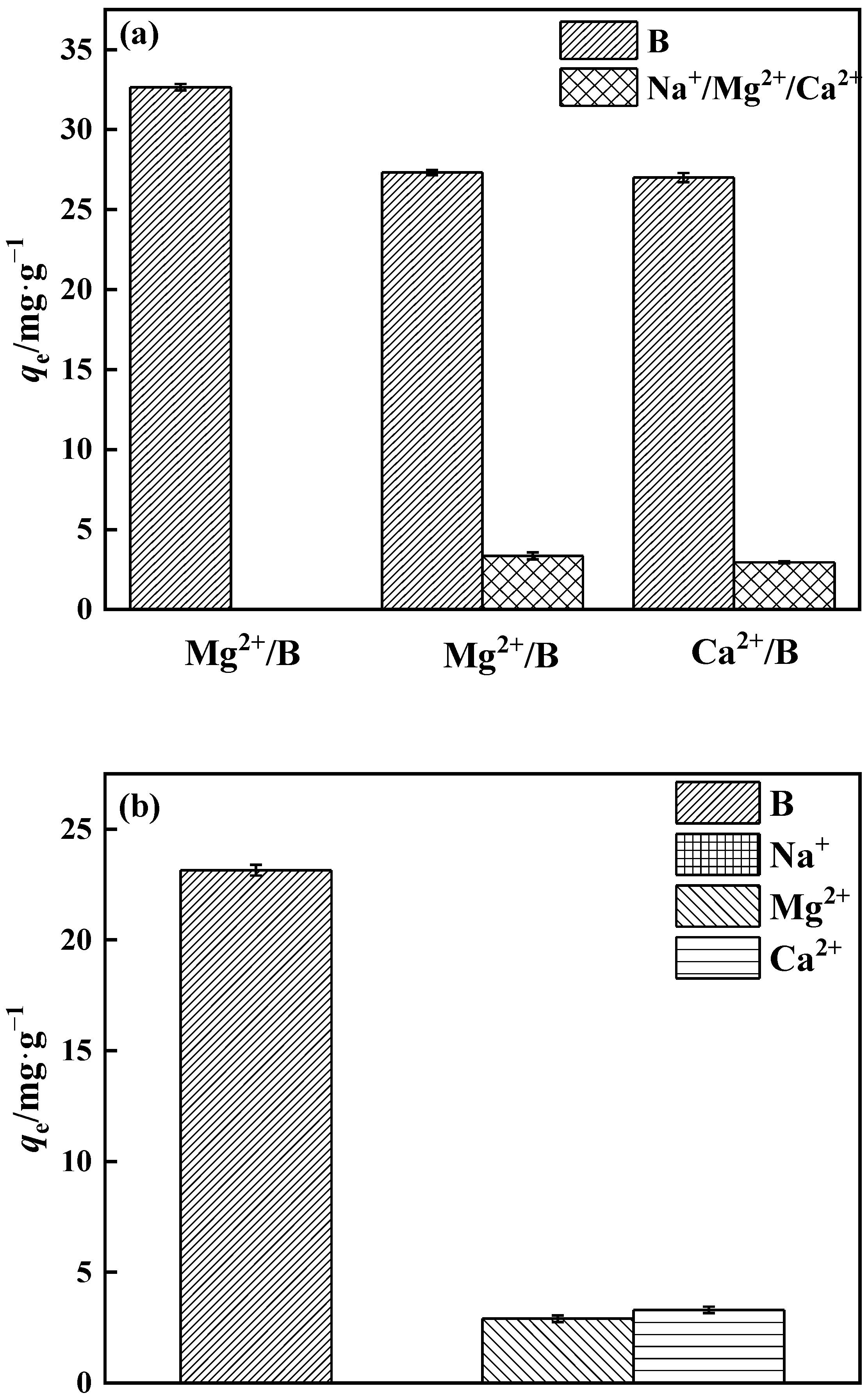
2.4.5. Selectivity in Adsorption
2.4.6. Stability
3. Results and Discussion
3.1. Modification of UV Grafting Conditions
3.1.1. Concentration of GMA
3.1.2. Dosage of BP
3.1.3. Time Required for Grafting
3.2. Characterization
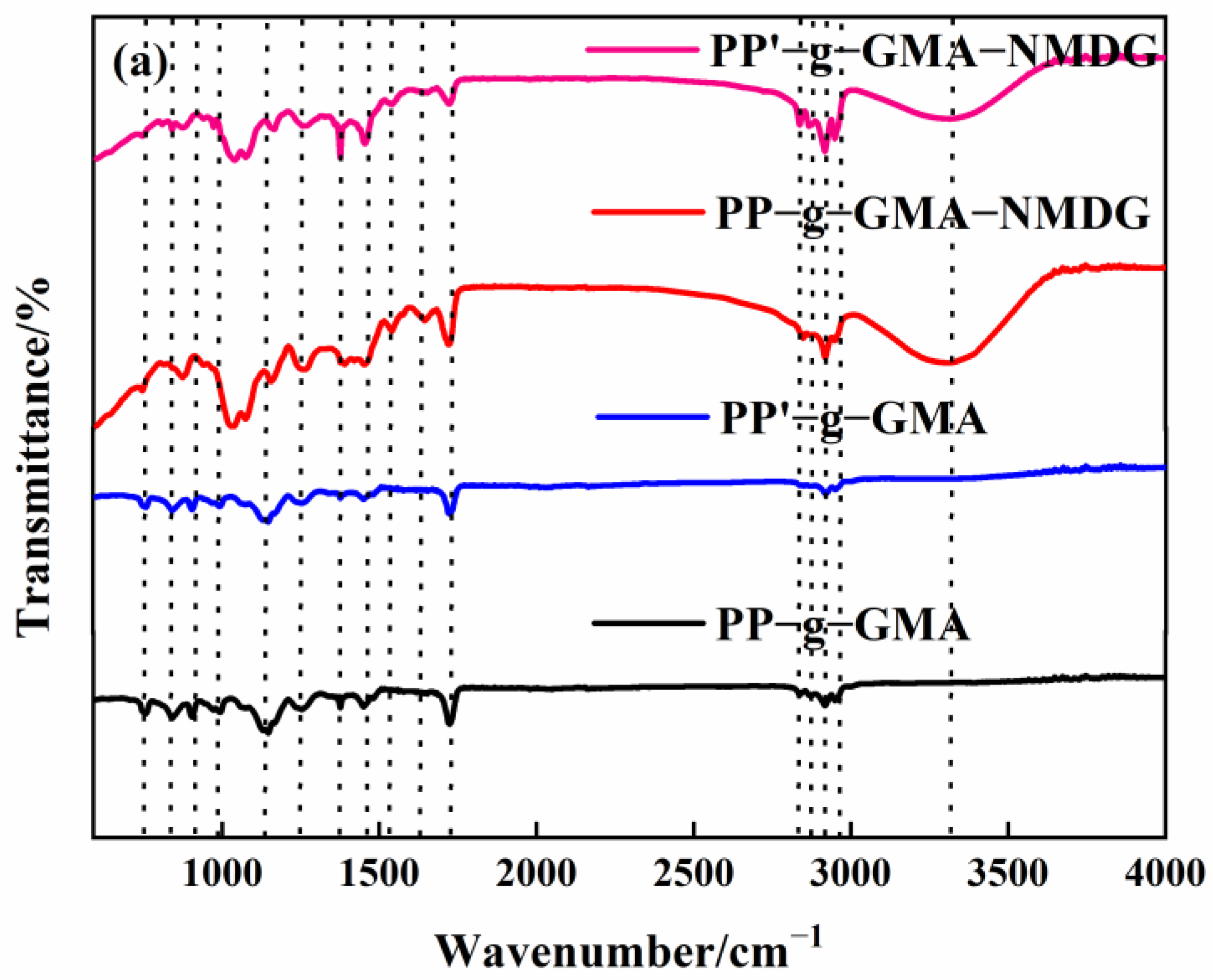
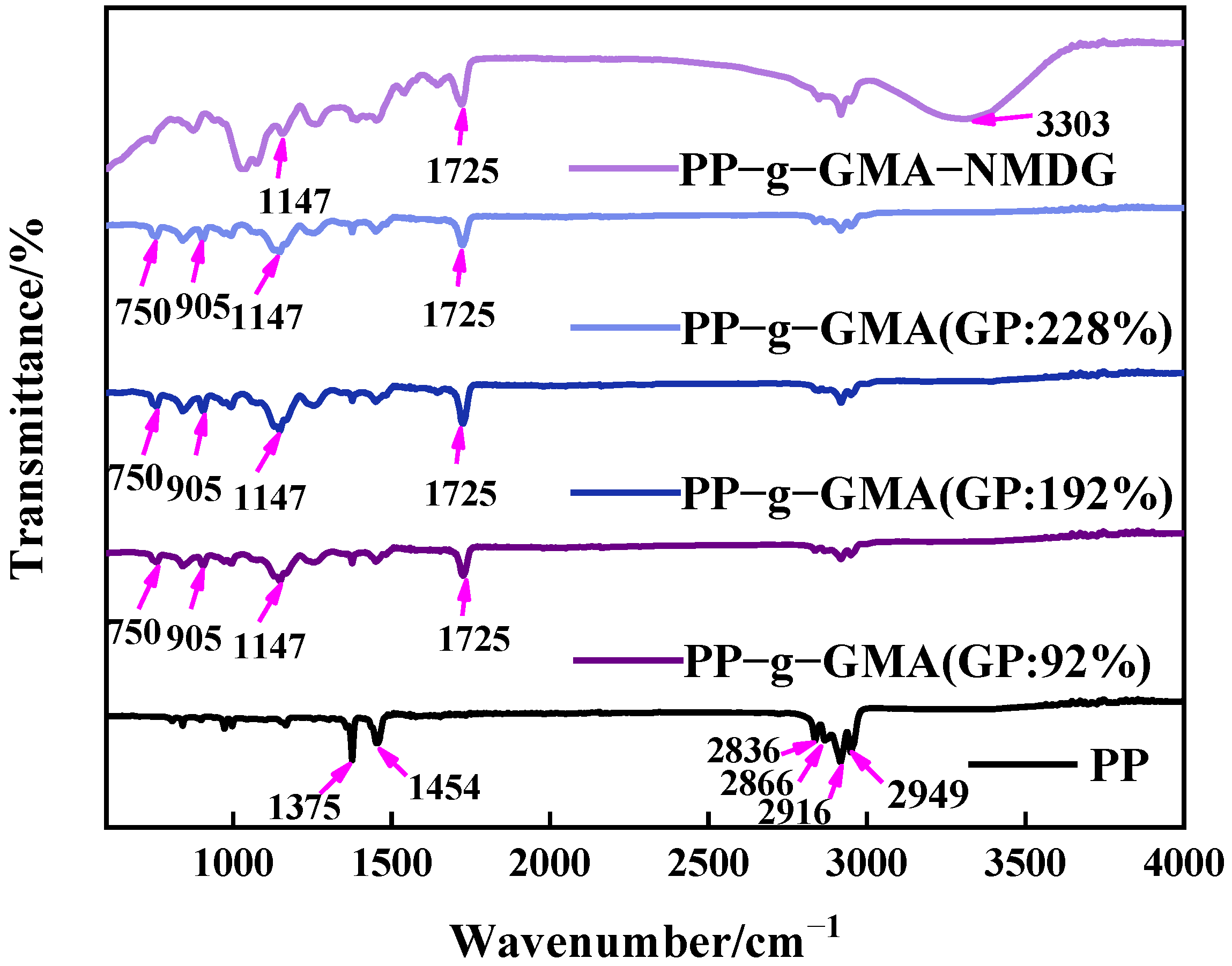
3.2.1. FTIR
3.2.2. XRD
3.2.3. TGA
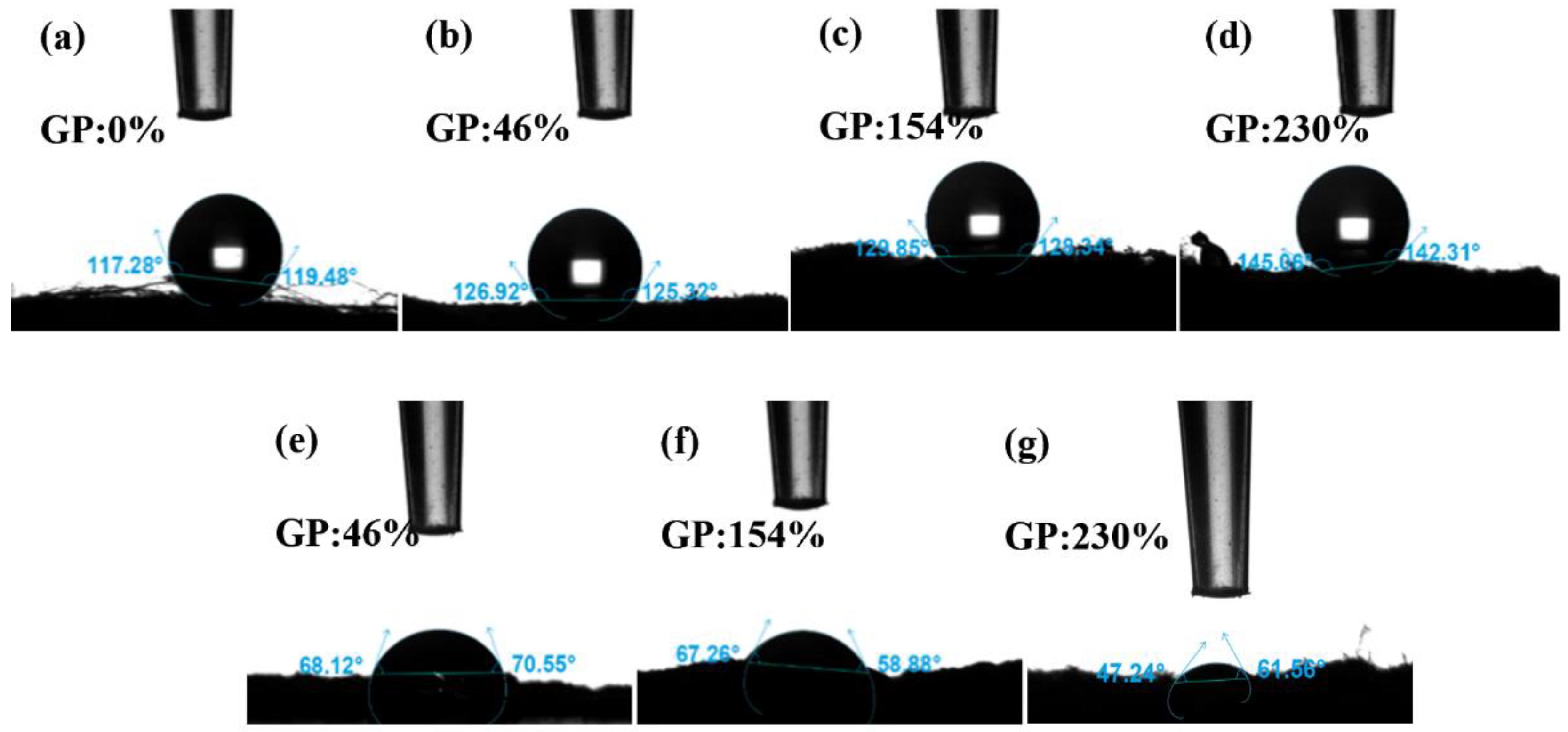
3.2.4. Contact Angle
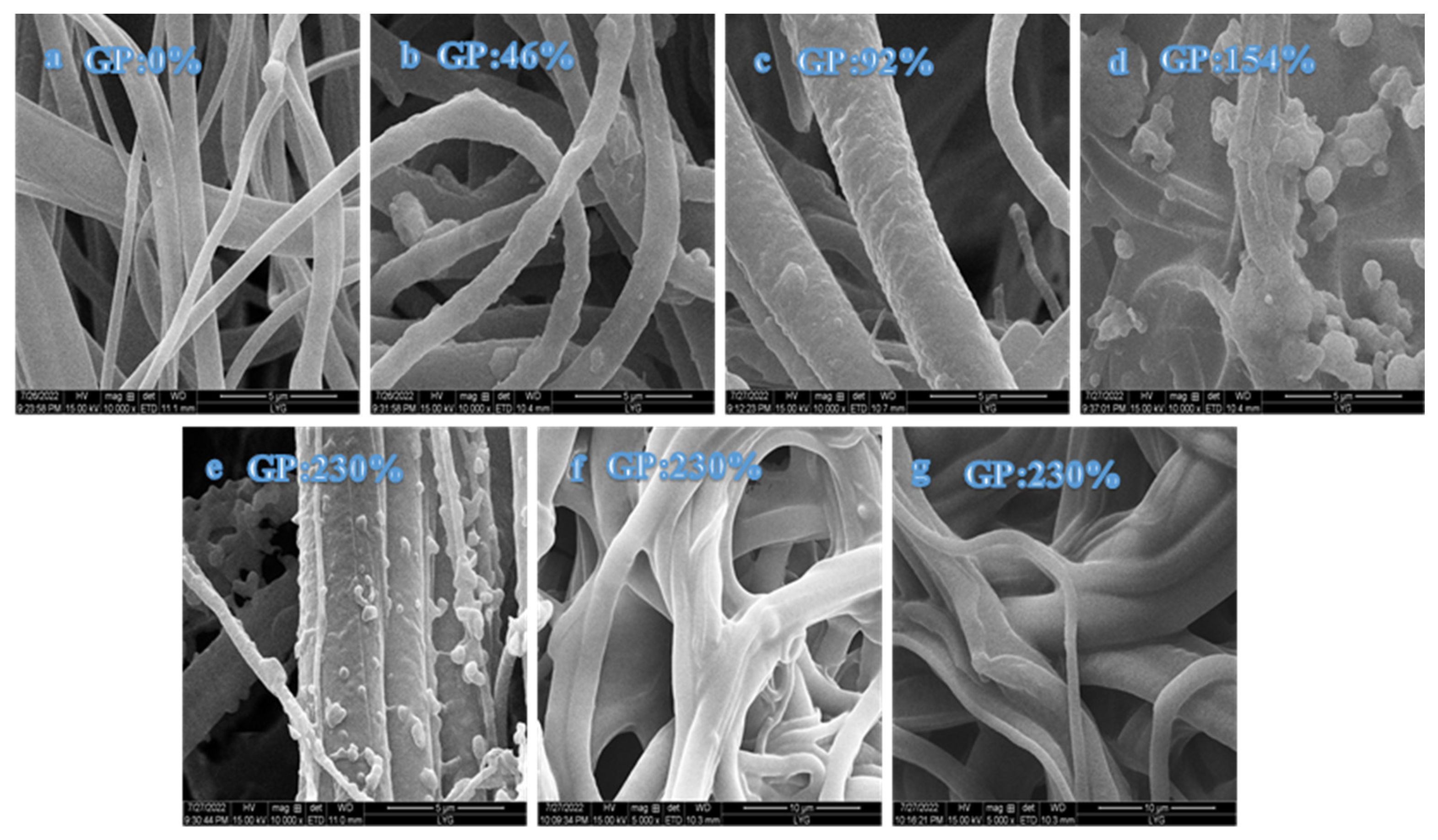
3.2.5. SEM
3.3. Adsorption Mechanism of PP-g-GMA-NMDG
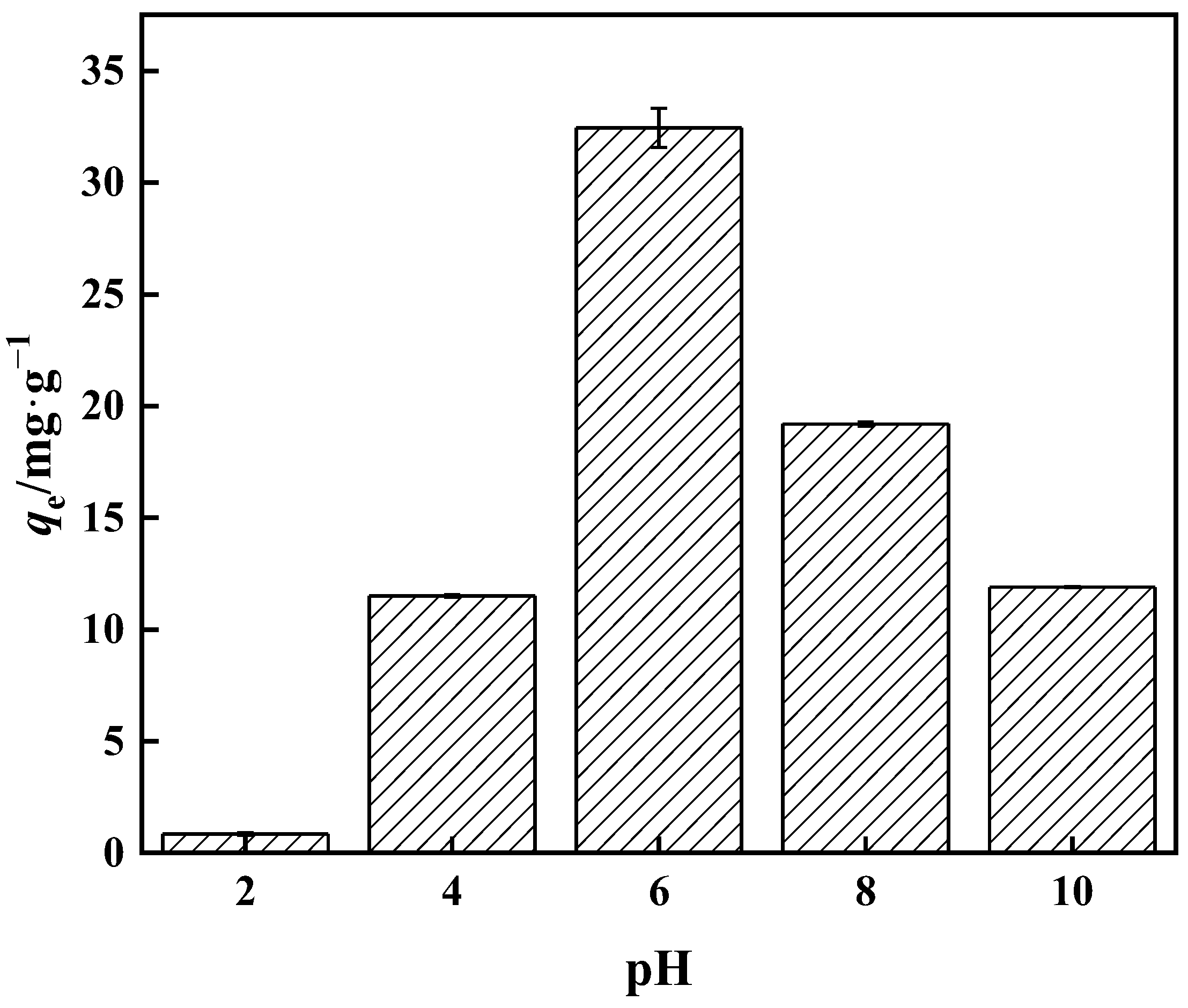
3.3.1. Relationship between pH and Adsorption Capacity
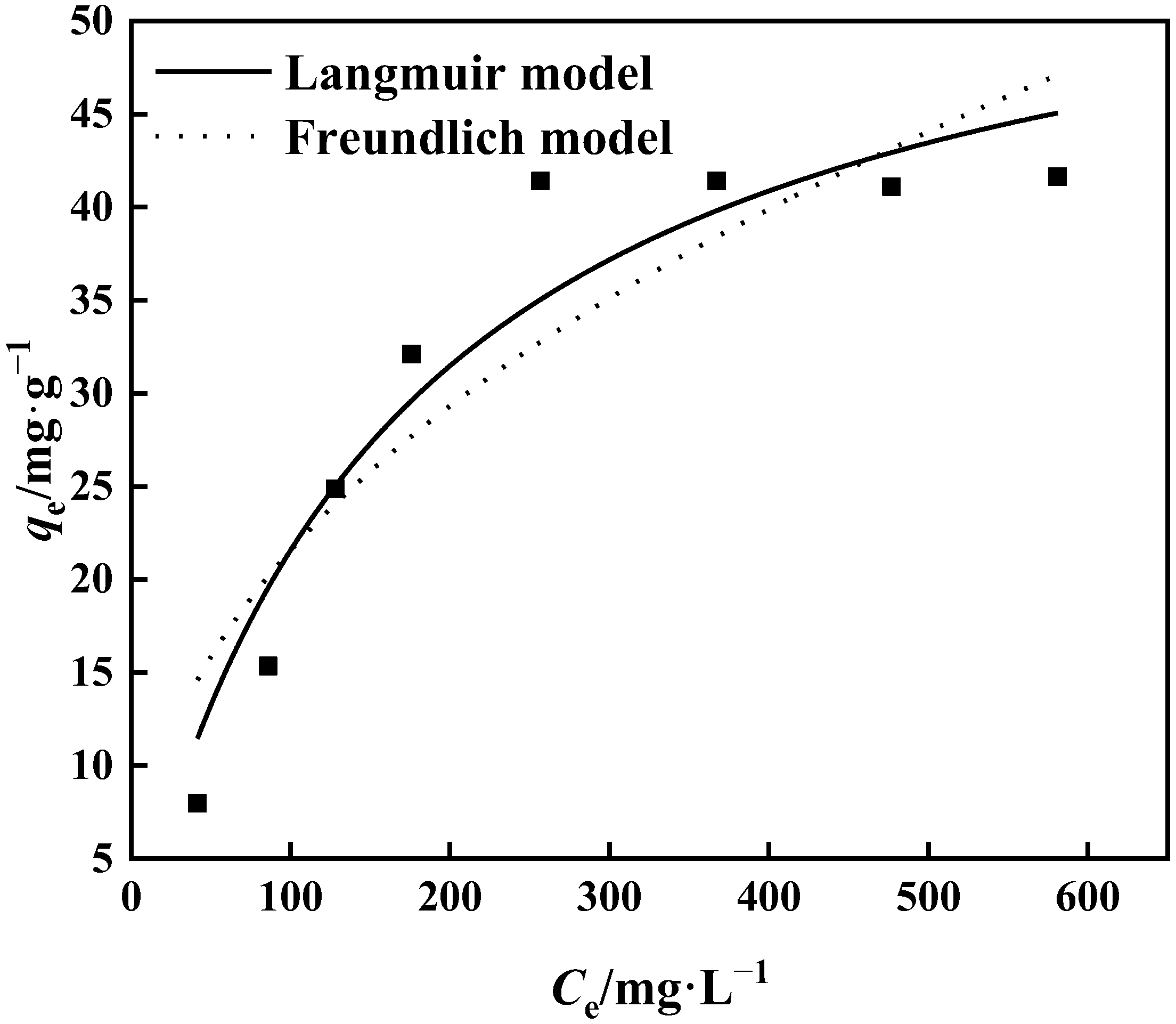
3.3.2. Adsorption Isotherm
3.3.3. Kinetics of Adsorption and Internal Diffusion Model
3.3.4. Adsorption Thermodynamics
3.3.5. Adsorption Selectivity
3.3.6. Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Reguera, M.; Abreu, I.; Sentis, C.; Bonilla, I.; Bolaos, L. Altered plant organogenesis under boron deficiency is associated with changes in high-mannose N-glycan profile that also occur in animals. J. Plant Physiol. 2019, 243, 153058. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Abd, E.M.E.; Swelum, A.A.; Perillo, A.; Losacco, C. The vital roles of boron in animal health and production: A comprehensive review. J. Trace Elem. Med. Biol. 2018, 50, 50296–50304. [Google Scholar] [CrossRef] [PubMed]
- Gungor, A.; Akbay, I.K.; Ozdemir, T. EPDM Rubber with hexagonal Boron Nitride: A Thermal Neutron Shielding Composite. Radiat. Phys. Chem. 2019, 165, 108391. [Google Scholar] [CrossRef]
- Ozmen, O.T.; Karaman, M.; Sedani, S.H.; Sagban, H.M.; Turan, R. Solid phase epitaxial thickening of boron and phosphorus doped polycrystalline silicon thin films formed by aluminium induced crystallization technique on glass substrate. Solid Films 2019, 689, 45–53. [Google Scholar]
- Guo, G.; Lu, Y.; Yang, D.; Li, X.; Gong, M. Purification of thorium by precipitation. J. Radioanal. Nucl. Chem. 2021, 327, 667–671. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, Y.; Song, J.; Xing, L.; Kong, D.; Li, X.; He, T. Extraction of boron from salt lake brine using 2-ethylhexanol. Hydrometallurgy 2016, 160, 129–136. [Google Scholar] [CrossRef]
- Hu, G.Z.; Zhang, W.; Chen, Y.T.; Xu, C.; Liu, R.; Zhen, H. Removal of boron from water by GO/ZIF-67 hybrid material adsorption. Environ. Sci. Pollut. Res. 2020, 27, 28396–28407. [Google Scholar] [CrossRef]
- Demetriou, A.; Pashalidis, I.; Nicolaides, A.V.; Kumke, M.U. Surface mechanism of the boron adsorption on alumina in aqueous solutions. Desalination Water Treat. Sci. Eng. 2013, 51, 6130–6136. [Google Scholar] [CrossRef]
- Farhat, A.; Ahmad, F.; Hilal, N.; Arafat, H.A. Boron removal in new generation reverse osmosis (RO) membranes using two-pass RO without pH adjustment. Desalination 2013, 310, 50–59. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, M.Y.; Wang, X. A novel chitosan based adsorbent for boron separation. Sep. Purif. Technol. 2019, 211, 162–169. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Al-Ghouti, M.A.; Kasak, P.; Krupa, I. An updated review on boron removal from water through adsorption processes. Emergent Mater. 2021, 4, 1167–1186. [Google Scholar] [CrossRef]
- Wang, B.Y.; Lin, H.; Guo, X.H.; Peng, B. Boron removal using chelating resins with pyrocatechol functional groups. Desalination 2014, 347, 138–143. [Google Scholar] [CrossRef]
- Bicak, N.; Senkal, B.F. Sorbitol-modified poly (N-glycidyl styrene sulfonamide) for removal of boron. J. Appl. Polym. Sci. 1998, 68, 2113–2119. [Google Scholar] [CrossRef]
- Li, D.J.; Chen, Y.; Liu, Z. Boronate affinity materials for separation and molecular recognition: Structure, properties and applications. Chem. Soc. Rev. 2015, 44, 8097–8123. [Google Scholar] [CrossRef]
- Zhang, J.L.; Cai, Y.N.; Liu, K.X. Extremely effective boron removal from water by stable metal organic framework ZIF-67. Ind. Eng. Chem. Res. 2019, 58, 4199–4207. [Google Scholar] [CrossRef]
- Kamcev, J.; Taylor, M.K.; Shin, D.M.; Jarenwattananon, N.N.; Colwell, K.A.; Long, J.R. Functionalized porous aromatic frameworks as high-performance adsorbents for the rapid removal of boric acid from water. Adv. Mater. 2019, 31, 1808027. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, A.; Cimen, A.; Kursunlu, A.N.; Karapinar, H.S.; Guler, E. Synthesis, characterization, and application of functionalized pillar[5]arene silica gel (si-aptms-pillar[5]arene) adsorbent for selectivity and effective removal of Cu(II) ion. J. Mater. Res. 2022, 37, 3587–3598. [Google Scholar] [CrossRef]
- Adeiga, O.I.; Velempini, T.; Pillay, K. Polyaniline-decorated macadamia nutshell composite: An adsorbent for the removal of highly toxic Cr(VI) and efficient catalytic activity of the spent adsorbent for reuse. Polym. Bull. 2023, 80, 1951–1973. [Google Scholar] [CrossRef]
- Smart, G.; Kandola, B.K.; Horrocks, A.R.; Nazare, S.; Marney, D. Polypropylene fibers containing dispersed clays having improved fire performance. Part II: Characterization of fibers and fabrics from PP–nanoclay blends. Polym. Adv. Technol. 2008, 19, 658–670. [Google Scholar] [CrossRef]
- Yoon, C.S.; Ji, D.S. Modification and properties of polypropylene fibers using aluminosiloxane. Fibers Polym. 2003, 4, 210–214. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Jeong, S.H.; Lee, H.J. Preparation of nanocomposite fibers for permanent antibacterial effect. J. Mater. Sci. 2003, 38, 2143–2147. [Google Scholar] [CrossRef]
- Strecka, Z.; Ujhelyiova, A.; Bolhova, E.; Alexy, P.; Borsig, E. Polypropylene fibers modified by polyvinyl alcohol and montmorillonite. J. Text. Inst. 2010, 101, 315–323. [Google Scholar] [CrossRef]
- Kristofic, M.; Vassova, I.; Ujhelyiova, A.; Ryba, J. Functionalisation of polypropylene. Part I. Mechanical, electric and sorptive properties of PP fibres modified with concentrates consisting of copolyamides and nanoclay. Fibres Text. East. Eur. 2011, 19, 14–18. [Google Scholar]
- Li, C.S.; Liang, T.X.; Lu, W.Z.; Tang, C.H.; Hu, X.Q.; Cao, M.S.; Liang, J. Improving the antistatic ability of polypropylene fibers by inner antistatic agent filled with carbon nanotubes. Compos. Sci. Technol. 2004, 64, 2089–2096. [Google Scholar] [CrossRef]
- Nia, N.D.; Lee, S.W.; Bae, S.; Kim, T.H.; Hwang, Y. Surface modification of polypropylene non-woven filter by O2plasma/acrylic acid enhancing Prussian blue immobilization for aqueous cesium adsorption. Appl. Surf. Sci. 2022, 590, 153101. [Google Scholar]
- Dai, J.D.; Liang, M.; Ren, P.F.; Fu, Y.F.; Wang, F.M.; Ge, X.; Zhang, T.Z. Surface modification of polypropylene with porous polyacrylamide coating. Mater. Lett. 2020, 266, 127487. [Google Scholar] [CrossRef]
- Luo, Z.W.; Guo, M.L.; Jiang, H.; Geng, W.H.; Wei, W.J.; Lian, Z.Y. Plasma polymerization mediated construction of surface ion-imprinted polypropylene fibers for the selective adsorption of Cr(VI). React. Funct. Polym. 2020, 150, 104552. [Google Scholar] [CrossRef]
- Haji, A.; Mehrizi, M.K.; Sarani, M. Surface modification of Polypropylene nonwoven by plasma and β-Cyclodextrin: Optimization and cationic dye removal studies. Surf. Interfaces 2021, 25, 101278. [Google Scholar] [CrossRef]
- Chan, M.A.; Obendorf, S.K. Surface modification of microporous polypropylene membrane by UV-initiated grafting with poly (ethylene glycol) diacrylate. Fibers Polym. 2014, 15, 2032–2039. [Google Scholar] [CrossRef]
- Sadeghi, K.; Seo, J. Ultraviolet-cured p-phenylenediamine functionalized polypropylene film as a non-migratory antioxidant. Food Packag. Shelf Life 2022, 33, 100907. [Google Scholar] [CrossRef]
- He, D.; Susanto, H.; Ulbricht, M. Photo-irradiation for preparation, modification and stimulation of polymeric membranes. Prog. Polym. Sci. 2009, 34, 62–98. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Wang, J.X. Optimizing interfacial polymerization with UV-introduced photo-fries rearrangement for enhancing RO membrane performance. Chem. Eng. J. 2022, 437, 135380. [Google Scholar] [CrossRef]
- Wang, W.F.; Sun, S.B.; Zhao, X.X.; Cui, S.P.; Wang, J.C.; Shi, Y.; Liu, H. The surface modification of extruded polystyrene foams through UV curing and its stable adhesion to mortar. Constr. Build. Mater. 2022, 359, 129507. [Google Scholar] [CrossRef]
- Yu, L.H.; Zhang, S.; Liu, W.; Zhu, X.J.; Chen, X.P.; Chen, X.P.; Chen, X.S. Improving the flame retardancy of PET fabric by photo-induced grafting. Polym. Degrad. Stab. 2010, 95, 1934–1942. [Google Scholar] [CrossRef]
- Yang, Y.F.; Xie, Y.L.; Pang, L.C.; Li, M.; Song, X.H.; Wen, J.G.; Zhao, H.Y. Preparation of reduced graphene oxide/poly(acrylamide) nanocomposite and its adsorption of Pb(II) and methylene blue. Langmuir 2013, 29, 10727–10736. [Google Scholar] [CrossRef] [PubMed]
- Shek, T.; Ma, A.; Lee, V.K.C.; Mckay, G. Kinetics of zinc ions removal from effluents using ion exchange resin. Chem. Eng. J. 2009, 146, 63–70. [Google Scholar] [CrossRef]
- Kubota, H.; Ogiwara, Y. Effect of water in vapor-phase photografting of vinyl monomers on polymer-films. J. Appl. Polym. Sci. 1991, 43, 1001–1005. [Google Scholar] [CrossRef]
- Yu, H.J.; Cao, Y.M.; Kang, G.D.; Liu, J.H.; Li, M.; Yuan, Q. Enhancing antifouling property of polysulfone ultrafiltration membrane by grafting zwitterionic copolymer via UV-initiated polymerization. J. Membr. Sci. 2009, 342, 6–13. [Google Scholar] [CrossRef]
- Yusof, A.; Ulbricht, M. Polypropylene-based membrane adsorbers via photo-initiated graft copolymerization: Optimizing separation performance by preparation conditions. J. Membr. Sci. 2008, 311, 294–305. [Google Scholar] [CrossRef]
- Zheng, X.C.; Wu, C.L.; Xiong, J.; Lei, H. UV Photoinitiated Temperature-sensitive modification of polypropylene grafted with poly(N-isopropylacrylamide). Polym. Sci. Ser. B 2022, 64, 644–650. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.L.; Liu, X.H.; Huo, T.G.; Qin, Y.W. Preparation of durable flame retardant PAN fabrics based on amidoximation and phosphorylation. Appl. Surf. Sci. 2018, 428, 395–403. [Google Scholar] [CrossRef]
- Sokker, H.H.; Badawy, S.M.; Zayed, E.M.; Eldien, E.M.; Farag, A.M. Radiation-induced grafting of glycidyl methacrylate onto cotton fabric waste and its modification for anchoring hazardous wastes from their solutions. J. Hazard. Mater. 2009, 168, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Su, D.F.; Su, Z.Q.; Chen, X. Fabrication of multiwalled carbon nanotube/polypropylene conductive fibrous membranes by melt electrospinning. Ind. Eng. Chem. Res. 2014, 53, 2308–2317. [Google Scholar] [CrossRef]
- Kolyaganova, O.V.; Duridivko, M.O.; Klimov, V.V.; Le, M.D.; Kharlamov, V.O.; Bryuzgin, E.V.; Navrotsky, A.V.; Novakov, I.A. Highly hydrophobic and superhydrophobic coatings based on linseed oil and copolymers of glycidyl methacrylate and (fluoro)alkyl methacrylates for wood surfaces. Colloid J. 2022, 84, 416–426. [Google Scholar] [CrossRef]
- Suresh, K.; Islam, M.A.; Rastgar, M.; Mohammadnezhad, A.; Fleck, B.A.; Sadrzadeh, M. Poly (methyl methacrylate) grafted wheat straw for economical and eco-friendly treatment of oily wastewater. Cellulose 2022, 29, 3351–3374. [Google Scholar] [CrossRef]
- Ingri, N.; Dahlen, J.; Buchardt, O.; Kvande, P.C.; Meisingseth, E. Equilibrium studies of polyanions 10. On the first equilibrium steps in the acidification of B(OH), an application of the self-medium method. Acta Chem. Scand. 1963, 17, 573–580. [Google Scholar] [CrossRef]
- Yin, G.C.; Tao, L.; Chen, X.L.; Bolan, N.S.; Sarkar, B.; Lin, Q.T. Quantitative analysis on the mechanism of Cd2+ removal by MgCl2 modified biochar in aqueous solutions. J. Hazard. Mater. 2021, 420, 126487. [Google Scholar] [CrossRef]
- Fu, H.B.; Wang, B.Y.; Li, D.T.; Xue, L.H.; Hua, Y.; Feng, Y.F.; Xie, H.F. Anaerobic fermentation treatment improved Cd2+ adsorption of different feedstocks based hydrochars. Chemosphere 2021, 263, 127981. [Google Scholar] [CrossRef]
- Maslova, M.V.; Ivanenko, V.I.; Gerasimova, L.G. Effect of temperature on the kinetics of the sorption of strontium cations by a sorbent based on titanium phosphate. Russ. J. Phys. Chem. A 2019, 93, 1245–1251. [Google Scholar] [CrossRef]
- Abou Taleb, M.F.; Albalwi, H.; Abou El Fadl, F.I. Removal of mercury (II) from aqueous solution using silver nanocomposite: Synthesis and adsorption mechanism. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1825–1835. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, X.; Yu, S.; Bai, P.; Guo, X.H.; Lyu, J.F. Unveiling the nature of boric acid adsorption by metal-organic frameworks with hexanuclear clusters. Chem. Eng. J. 2022, 433, 133543. [Google Scholar] [CrossRef]
- Kluczka, J.; Gnus, M.; Kazek-Kesik, A.; Dudek, G. Zirconium-chitosan hydrogel beads for removal of boron from aqueous solutions. Polymer 2018, 150, 109–118. [Google Scholar] [CrossRef]
- Abbasi, A.; Yahya, W.Z.N.; Nasef, M.M.; Moniruzzaman, M.; Ghumman, A.S.M.; Afolabi, H.K. Boron removal by glucamine-functionalized inverse vulcanized sulfur polymer. React. Funct. Polym. 2017, 177, 105311. [Google Scholar] [CrossRef]
- Luo, Q.L.; Zeng, M.T.; Wang, X.Y.; Huang, H.; Wang, X.F.; Liu, N.; Huang, X.L. Glycidol-functionalized macroporous polymer for boron removal from aqueous solution. React. Funct. Polym. 2020, 150, 104543. [Google Scholar] [CrossRef]









| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| kL | qm (mg·g−1) | R2 | kF | 1/n | R2 |
| 0.006 | 58.26 | 0.924 | 2.782 | 0.444 | 0.836 |
| qe (mg·g−1) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qm (mg·g−1) | k1 | R2 | qm (mg·g−1) | k2 | R2 | |
| 31.15 | 46.81 | 0.084 | 0.992 | 36.87 | 0.002 | 0.993 |
| kd1 (mg·g−1·min−1/2) | R2 | kd2 (mg·g−1·min−1/2) | R2 | kd3 (mg·g−1·min−1/2) | R2 |
|---|---|---|---|---|---|
| 6.095 | 0.999 | 3.858 | 0.997 | 0.006 | 0.041 |
| T/°C | Thermodynamic Parameter | R2 | ||
|---|---|---|---|---|
| ∆H (kJ·mol−1) | ∆S (J·mol−1·K−1) | ∆G (kJ·mol−1) | ||
| 25 °C | 62.063 | 245.796 | 135.347 | 0.909 |
| 35 °C | 137.805 | |||
| 45 °C | 140.263 | |||
| Adsorbents | Substrate Material | qe (mg·g−1) | References |
|---|---|---|---|
| Zr-NU-1008 | MOFs | 23.28 | [51] |
| Hf-NU-1008 | 24.56 | ||
| Zr-NU-903 | 19.66 | ||
| Hf-NU-903 | 28.02 | ||
| Zr-NU-1008 * | 9.83 | ||
| Hf-NU-1008 * | 12.12 | ||
| CTS-MG | Chitosan | 20.36 | [10] |
| Zr-CTS | Chitosan | 22.2–24.5 | [52] |
| S-VBC-NMDG | Sulfur-based polymers prepared from sulfur and 4-vinylbenzyl chloride (VBC) revulcanization | 7.186 | [53] |
| P(GMA-co-TRIM)-EN-PG | Glycide-functionalized polymeric nanomaterials | 29.22 | [54] |
| P(GMA-CO-TRIM)-TETA-PG | 23.25 | ||
| PP-g-GMA-NMDG | PP melt-blow fiber | 41.65 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, N.; Jiang, H.; Luo, Z.; Geng, W.; Zhu, J. Boron Adsorption Using NMDG-Modified Polypropylene Melt-Blown Fibers Induced by Ultraviolet Grafting. Polymers 2023, 15, 2252. https://doi.org/10.3390/polym15102252
Yu N, Jiang H, Luo Z, Geng W, Zhu J. Boron Adsorption Using NMDG-Modified Polypropylene Melt-Blown Fibers Induced by Ultraviolet Grafting. Polymers. 2023; 15(10):2252. https://doi.org/10.3390/polym15102252
Chicago/Turabian StyleYu, Ning, Hui Jiang, Zhengwei Luo, Wenhua Geng, and Jianliang Zhu. 2023. "Boron Adsorption Using NMDG-Modified Polypropylene Melt-Blown Fibers Induced by Ultraviolet Grafting" Polymers 15, no. 10: 2252. https://doi.org/10.3390/polym15102252
APA StyleYu, N., Jiang, H., Luo, Z., Geng, W., & Zhu, J. (2023). Boron Adsorption Using NMDG-Modified Polypropylene Melt-Blown Fibers Induced by Ultraviolet Grafting. Polymers, 15(10), 2252. https://doi.org/10.3390/polym15102252




