Synthesis of Methacryloylated Hydroxyethylcellulose and Development of Mucoadhesive Wafers for Buccal Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
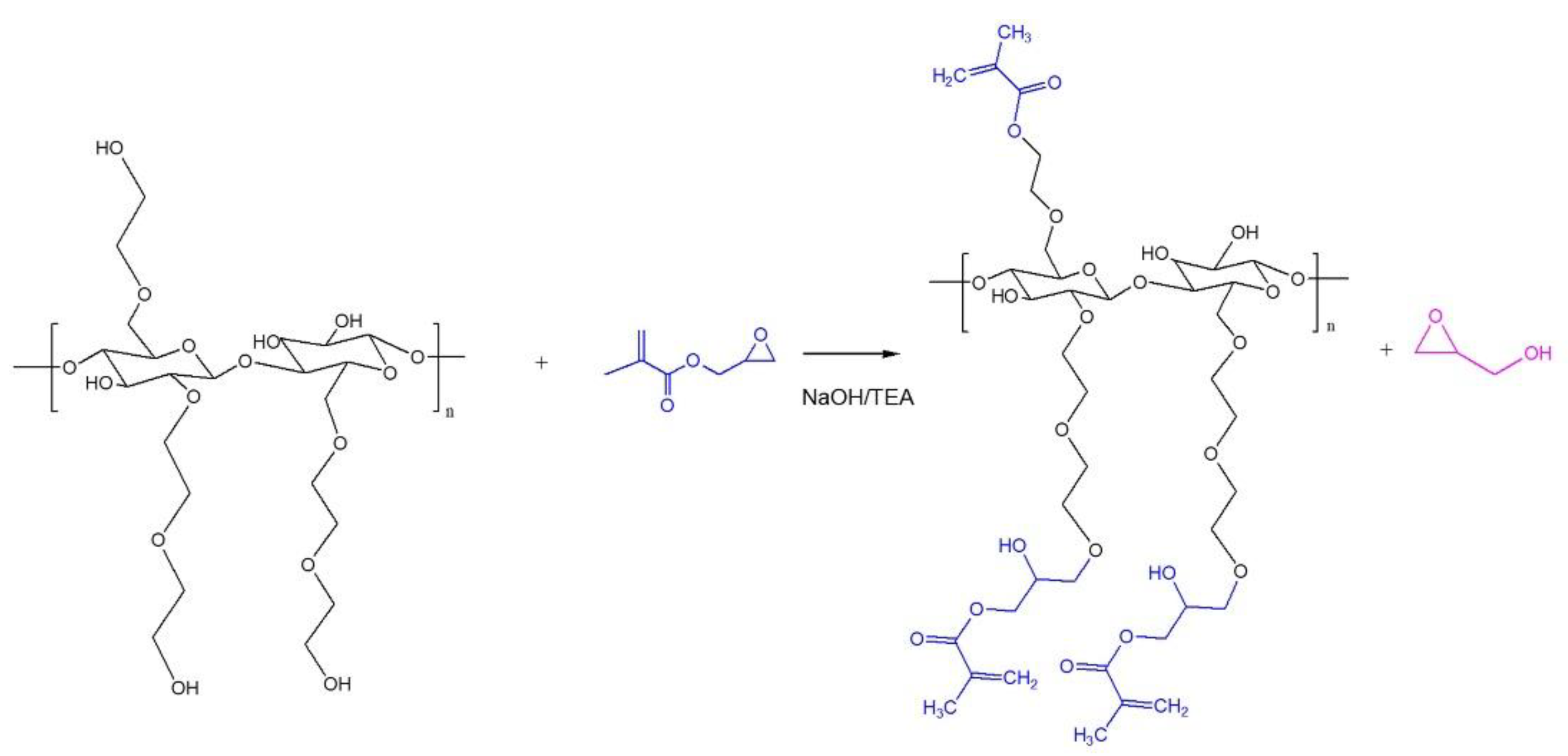
2.2. Modification of HEC
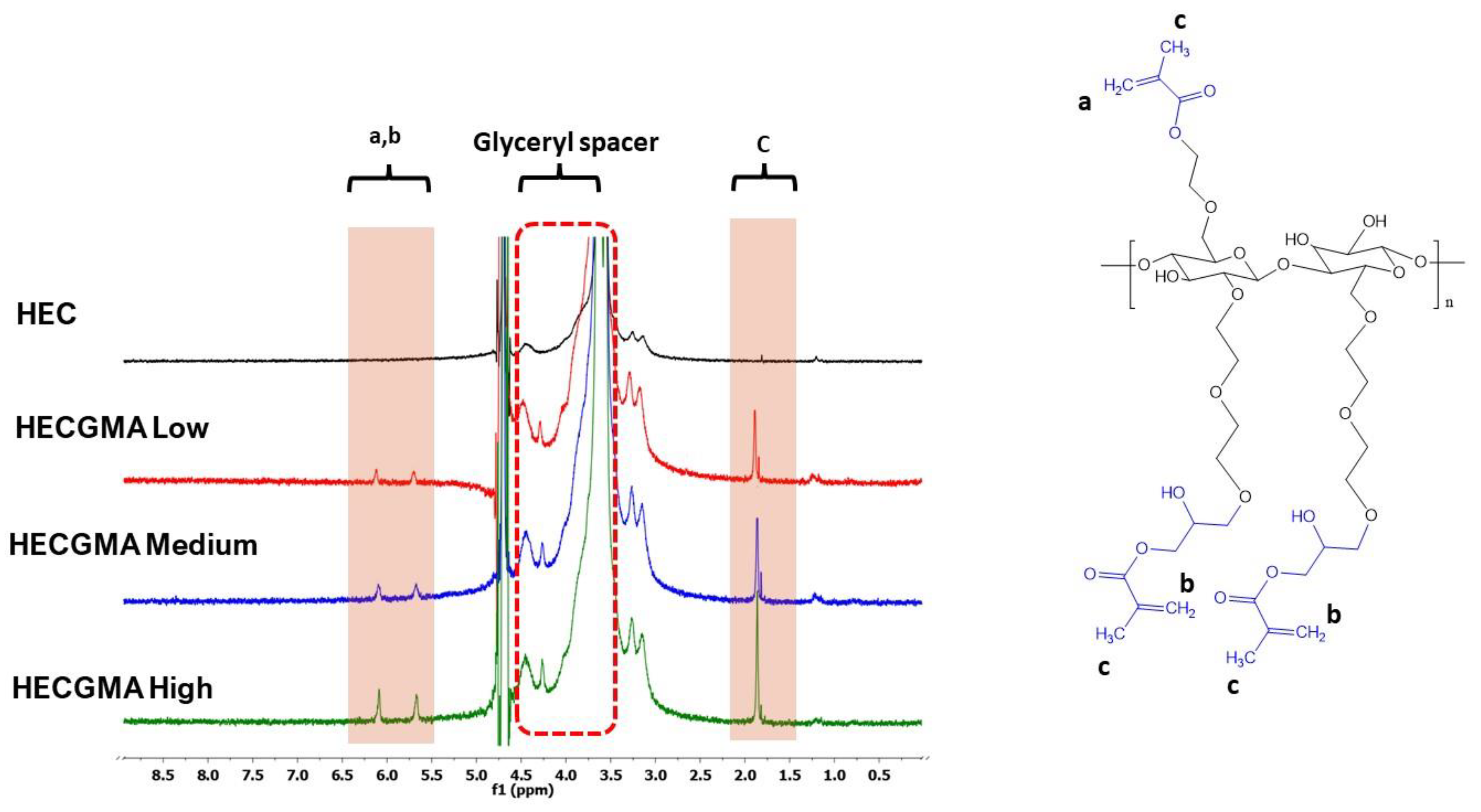
2.3. H Nuclear Magnetic Resonance Spectroscopy (1H NMR)
2.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.5. High-Performance Liquid Chromatography (HPLC)
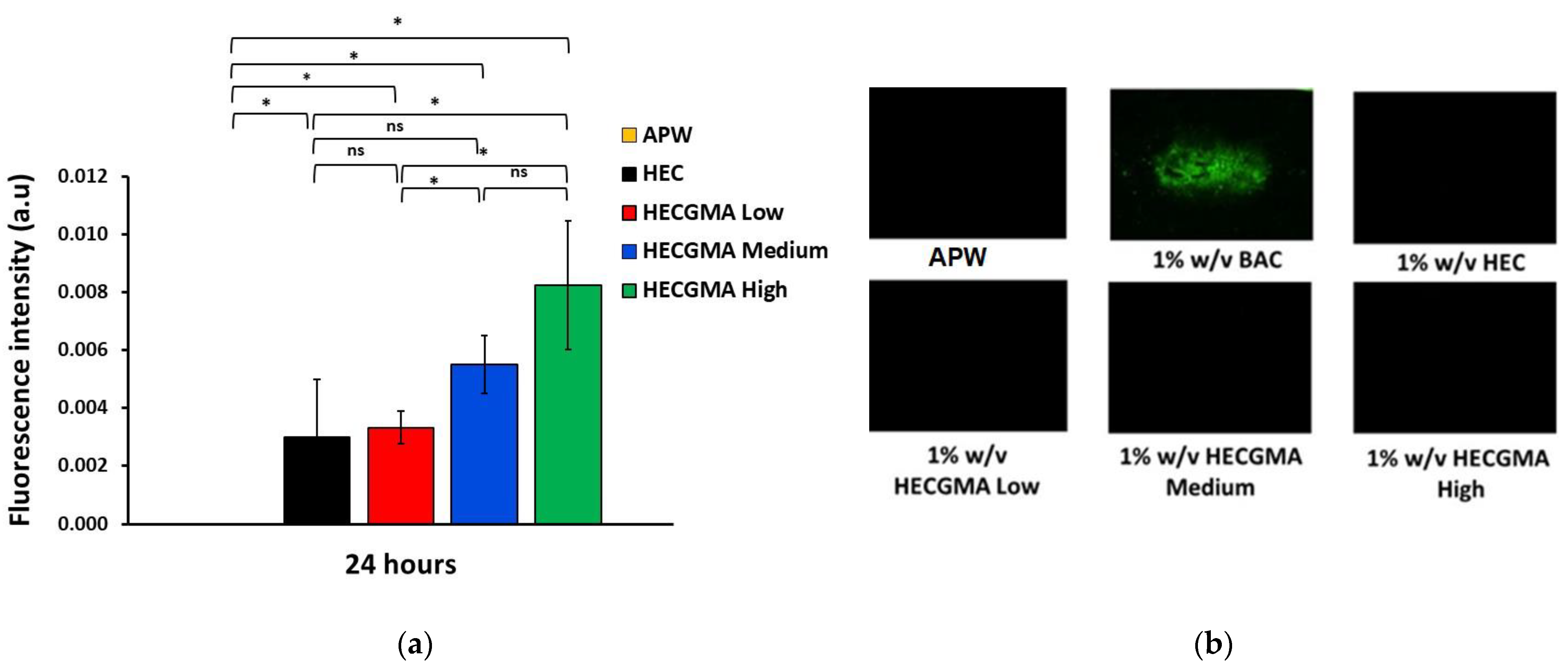
2.6. Planarian Acute Toxicity Assay
2.7. Planarian Toxicity Fluorescent Assay
2.8. In Vitro Cytotoxicity of Polymers
2.9. Preparation of Wafers
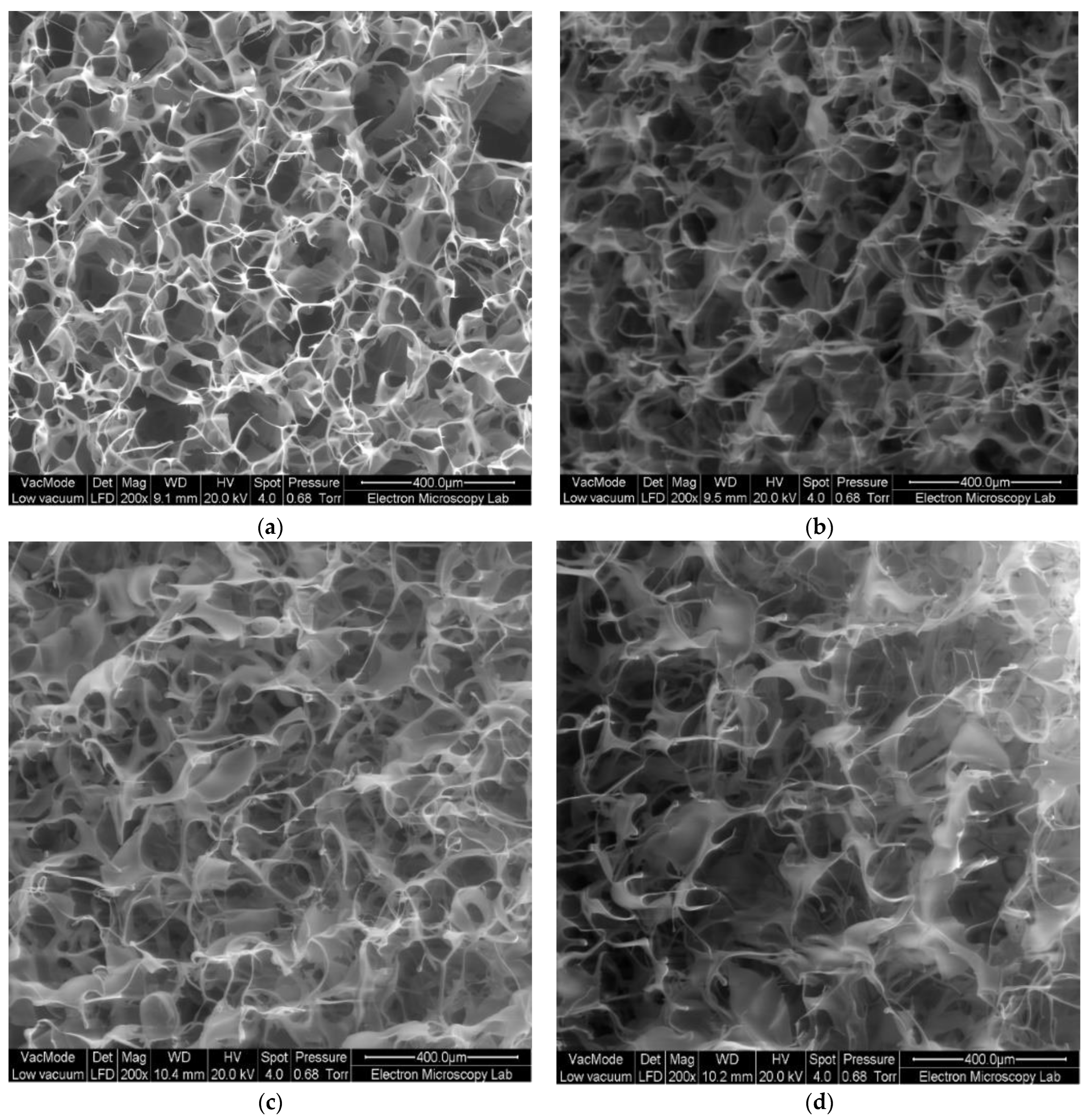
2.10. Physical Characterisation of Wafers
2.11. Ex Vivo Mucoadhesion Study of Wafers
2.12. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Methacryloylated HEC
3.2. Acute Toxicity Assay and Fluorescent Assay in Planaria
3.3. In Vitro Cytotoxicity
3.4. Preparation and Physical Characterisation of Wafers
3.5. Ex Vivo Evaluation of Mucoadhesive Properties of Wafers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioall. Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef]
- Huang, Y.; Leobandung, W.; Foss, A.; Peppas, N.A. Molecular aspects of muco- and bioadhesion:: Tethered structures and site-specific surfaces. J. Control. Release 2000, 65, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Brannigan, R.; Khutoryanskiy, V.V. Progress and Current Trends in the Synthesis of Novel Polymers with Enhanced Mucoadhesive Properties. Macromol. Biosci. 2019, 19, e1900194. [Google Scholar] [CrossRef]
- Boateng, J.; Okeke, O.; Khan, S. Polysaccharide Based Formulations for Mucosal Drug Delivery: A Review. Curr. Pharm. Des. 2015, 21, 4798–4821. [Google Scholar] [CrossRef]
- Karolewicz, B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm. J. 2016, 24, 525–536. [Google Scholar] [CrossRef]
- Smart, J.D. Drug delivery using buccal-adhesive systems. Adv. Drug Deliv. Rev. 1993, 11, 253–270. [Google Scholar] [CrossRef]
- Gales, R.B.; Ghonaim, H.M.; Gardouh, A.R.; Ghorab, M.M.; Badawy, S.S. Preparation and Characterization of Polymeric Mucoadhesive Film for Buccal Administration. Br. J. Pharm. Res. 2014, 4, 453–476. [Google Scholar] [CrossRef]
- Mazoniene, E.; Joceviciute, S.; Kazlauske, J.; Niemeyer, B.; Liesiene, J. Interaction of cellulose-based cationic polyelectrolytes with mucin. Colloids Surf. B Biointerfaces 2011, 83, 160–164. [Google Scholar] [CrossRef]
- Sarti, F.; Staaf, A.; Sakloetsakun, D.; Bernkop-Schnürch, A. Thiolated hydroxyethylcellulose: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2010, 76, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated chitosan as a polymer with enhanced mucoadhesive properties for transmucosal drug delivery. Int. J. Pharm. 2018, 550, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Agibayeva, L.E.; Kaldybekov, D.B.; Porfiryeva, N.N.; Garipova, V.R.; Mangazbayeva, R.A.; Moustafine, R.I.; Semina, I.I.; Mun, G.A.; Kudaibergenov, S.E.; Khutoryanskiy, V.V. Gellan gum and its methacrylated derivatives as in situ gelling mucoadhesive formulations of pilocarpine: In vitro and in vivo studies. Int. J. Pharm. 2020, 577, 119093. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Aspinall, S.; Kaldybekov, D.B.; Buang, F.; Williams, A.C.; Khutoryanskiy, V.V. Synthesis and Evaluation of Methacrylated Poly(2-ethyl-2-oxazoline) as a Mucoadhesive Polymer for Nasal Drug Delivery. ACS Appl. Polym. Mater. 2021, 3, 5882–5892. [Google Scholar] [CrossRef]
- Paleologos, E.K.; Kontominas, M.G. Determination of acrylamide and methacrylamide by normal phase high performance liquid chromatography and UV detection. J. Chromatogr. A 2005, 1077, 128–135. [Google Scholar] [CrossRef]
- Shah, S.I.; Williams, A.C.; Lau, W.M.; Khutoryanskiy, V.V. Planarian toxicity fluorescent assay: A rapid and cheap pre-screening tool for potential skin irritants. Toxicol. In Vitro 2020, 69, 105004. [Google Scholar] [CrossRef]
- Pagán, O.R.; Rowlands, A.L.; Urban, K.R. Toxicity and behavioral effects of dimethylsulfoxide in planaria. Neurosci. Lett. 2006, 407, 274–278. [Google Scholar] [CrossRef]
- Cosmet Ingredient Rev Expert Panel Staff (USA). Final report on the safety assessment of benzalkonium chloride. J. Am. Coll. Toxicol. 1989, 8, 589–625. [Google Scholar] [CrossRef]
- Boateng, J.S. Composite Sodium Alginate and Chitosan Based Wafers for Buccal Delivery of Macromolecules. Austin J. Anal. Pharm. Chem. 2014, 1, 1022. [Google Scholar]
- Boateng, J.; Okeke, O. Evaluation of Clay-Functionalized Wafers and Films for Nicotine Replacement Therapy via Buccal Mucosa. Pharmaceutics 2019, 11, 104. [Google Scholar] [CrossRef]
- Laffleur, F.; Netsomboon, K.; Bernkop-Schnürch, A.; Westmeier, D.; Stauber, R.H.; Docter, D. Comprehensive mucoadhesive study of anionic polymers and their derivate. Eur. Polym. J. 2017, 93, 314–322. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Reis, A.V.; Guilherme, M.R.; Schuquel, I.T.A.; Vidotti, G.J.; Rubira, A.F.; Muniz, E.C. Clearing the mechanism of Reaction of Glycidyl Methacrylate at the Hydroxyl and Carboxylic Groups of Poly(vinyl alcohol) and Poly(acrylic acid). Polymer 2009, 47, 2023–2029. [Google Scholar]
- Reis, A.V.; Guilherme, M.R.; Cavalcanti, O.A.; Rubira, A.; Muniz, E. Synthesis and characterization of pH-responsive hydrogels based on chemically modified Arabic gum polysaccharide. Polymer 2006, 47, 2023–2029. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Reis, A.V.; Takahashi, S.H.; Rubira, A.; Feitosa, J.P.; Muniz, E.C. Synthesis of a novel superabsorbent hydrogel by copolymerization of acrylamide and cashew gum modified with glycidyl methacrylate. Carbohydr. Polym. 2005, 61, 464–471. [Google Scholar] [CrossRef]
- Crispim, E.G.; Piai, J.F.; Schuquel, I.T.A.; Rubira, A.F.; Muniz, E.C. Functionalization of poly(vinyl alcohol) by addition of methacryloyl groups: Characterization by FTIR and NMR and optimization of reaction conditions by RSM. e-Polymers 2006, 6, 793–810. [Google Scholar] [CrossRef]
- Reis, A.V.; Guilherme, M.R.; Moia, T.A.; Mattoso, L.H.C.; Muniz, E.C.; Tambourgi, E.B. Synthesis and characterization of a starch-modified hydrogel as potential carrier for drug delivery system. J. Polym. Sci. A Polym. Chem. 2008, 46, 2567–2574. [Google Scholar] [CrossRef]
- Reis, A.V.; Fajardo, A.R.; Schuquel, I.T.A.; Guilherme, M.R.; Vidotti, G.J.; Rubira, A.F.; Muniz, E.C. Reaction of Glycidyl Methacrylate at the Hydroxyl and Carboxylic Groups of Poly(vinyl alcohol) and Poly(acrylic acid): Is This Reaction Mechanism Still Unclear? J. Org. Chem. 2009, 74, 3750–3757. [Google Scholar] [CrossRef]
- Ray, J.; Jana, S.; Bhanja, S.K.; Tripathy, T. Efficient removal of Co(II), Ni(II), and Zn(II) metal ions from binary and ternary solutions using a pH responsive bifunctional graft copolymer. Colloid Polym. Sci. 2018, 296, 1275–1291. [Google Scholar] [CrossRef]
- Hagstrom, D.; Cochet-Escartin, O.; Collins, E.S. Planarian brain regeneration as a model system for developmental neurotoxicology. Regeneration 2016, 3, 65–77. [Google Scholar] [CrossRef]
- Delongeas, J.-L.; de Conchard, G.V.; Beamonte, A.; Bertheux, H.; Spire, C.; Maisonneuve, C.; Becourt-Lhote, N.; Goldfain-Blanc, F.; Claude, N. Assessment of Labrasol®/Labrafil®/Transcutol® (4/4/2, v/v/v) as a non-clinical vehicle for poorly water-soluble compounds after 4-week oral toxicity study in Wistar rats. Regul. Toxicol. Pharmacol. 2010, 57, 284–290. [Google Scholar] [CrossRef]
- Lopez, F.L.; Bowles, A.; Gul, M.O.; Clapham, D.; Ernest, T.B.; Tuleu, C. Effect of formulation variables on oral grittiness and preferences of multiparticulate formulations in adult volunteers. Eur. J. Pharm. Sci. 2016, 92, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ashri, L.Y.; Amal El Sayeh, F.; Ibrahim, M.A.; Alshora, D.H.; Naguib, M.J. Optimization and evaluation of chitosan buccal films containing tenoxicam for treating chronic periodontitis: In vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 57, 101720. [Google Scholar] [CrossRef]
- Eouani, C.; Piccerelle, P.; Prinderre, P.; Bourret, E.; Joachim, J. In-vitro comparative study of buccal mucoadhesive performance of different polymeric films. Eur. J. Pharm. Biopharm. 2001, 52, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wattanakorn, N.; Asavapichayont, P.; Nunthanid, J.; Limmatvapirat, S.; Sungthongjeen, S.; Chantasart, D.; Sriamornsak, P. Pectin-Based Bioadhesive Delivery of Carbenoxolone Sodium for Aphthous Ulcers in Oral Cavity. AAPS PharmSciTech 2010, 11, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef]




| ID | Molar Ratio [HEC]:[GMA] | GMA (μL) | TEA (μL) |
|---|---|---|---|
| HECGMA Low | [1]:[1] | 225 | 240 |
| HECGMA Medium | [1]:[3] | 675 | 240 |
| HECGMA High | [1]:[6] | 1350 | 240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buang, F.; Chatzifragkou, A.; Amin, M.C.I.M.; Khutoryanskiy, V.V. Synthesis of Methacryloylated Hydroxyethylcellulose and Development of Mucoadhesive Wafers for Buccal Drug Delivery. Polymers 2023, 15, 93. https://doi.org/10.3390/polym15010093
Buang F, Chatzifragkou A, Amin MCIM, Khutoryanskiy VV. Synthesis of Methacryloylated Hydroxyethylcellulose and Development of Mucoadhesive Wafers for Buccal Drug Delivery. Polymers. 2023; 15(1):93. https://doi.org/10.3390/polym15010093
Chicago/Turabian StyleBuang, Fhataheya, Afroditi Chatzifragkou, Mohd Cairul Iqbal Mohd Amin, and Vitaliy V. Khutoryanskiy. 2023. "Synthesis of Methacryloylated Hydroxyethylcellulose and Development of Mucoadhesive Wafers for Buccal Drug Delivery" Polymers 15, no. 1: 93. https://doi.org/10.3390/polym15010093
APA StyleBuang, F., Chatzifragkou, A., Amin, M. C. I. M., & Khutoryanskiy, V. V. (2023). Synthesis of Methacryloylated Hydroxyethylcellulose and Development of Mucoadhesive Wafers for Buccal Drug Delivery. Polymers, 15(1), 93. https://doi.org/10.3390/polym15010093









