Fabrication of Europium-Doped Barium Titanate/Polystyrene Polymer Nanocomposites Using Ultrasonication-Assisted Method: Structural and Optical Properties
Abstract
1. Introduction
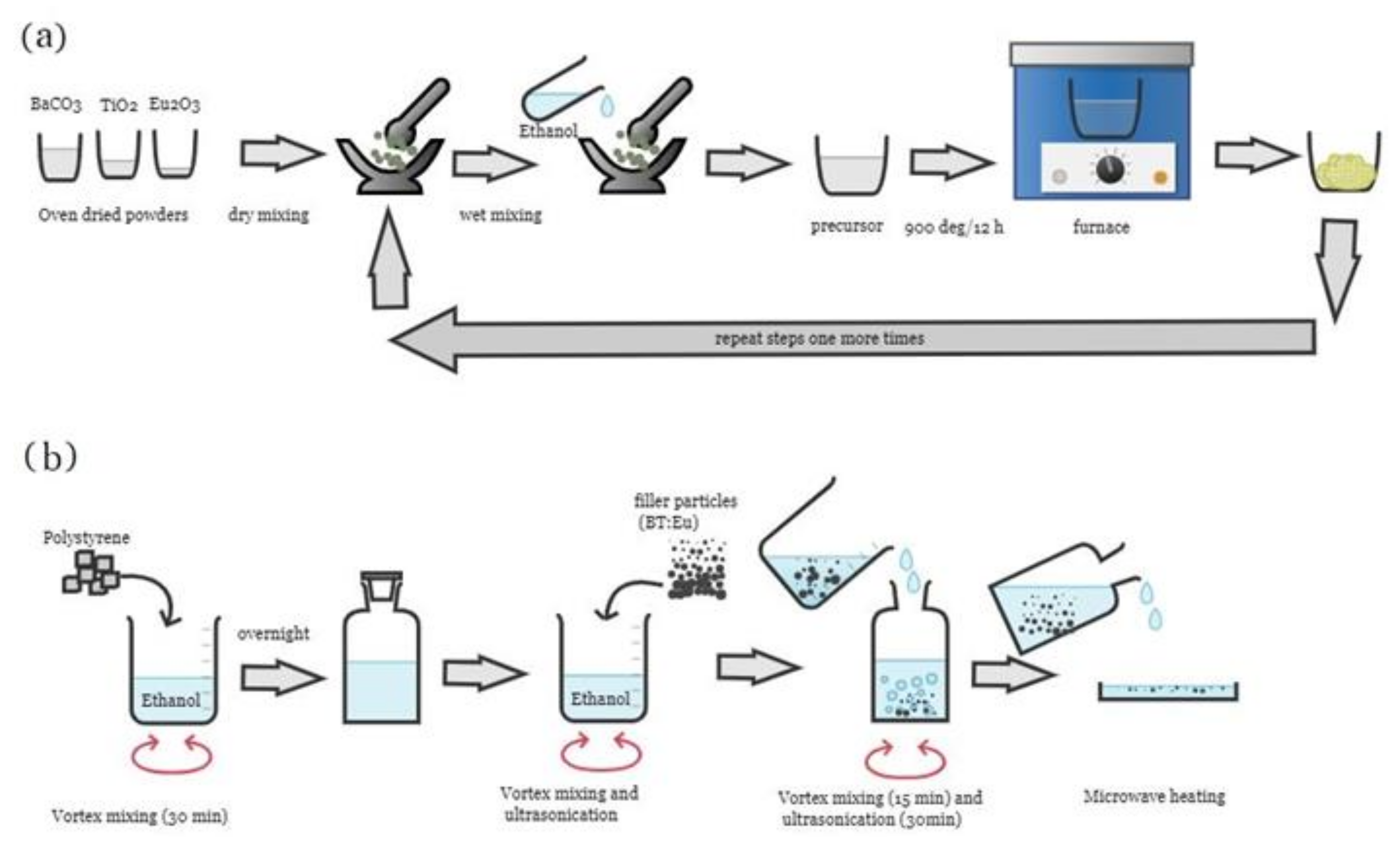
2. Experimental Details
2.1. Materials Used
2.2. Thermogravimetric Analysis
2.3. Characterizations
3. Theoretical Aspects
3.1. Lattice Parameters
3.2. Tolerance Factor
4. Results and Discussion
4.1. X-Ray Diffraction Analysis
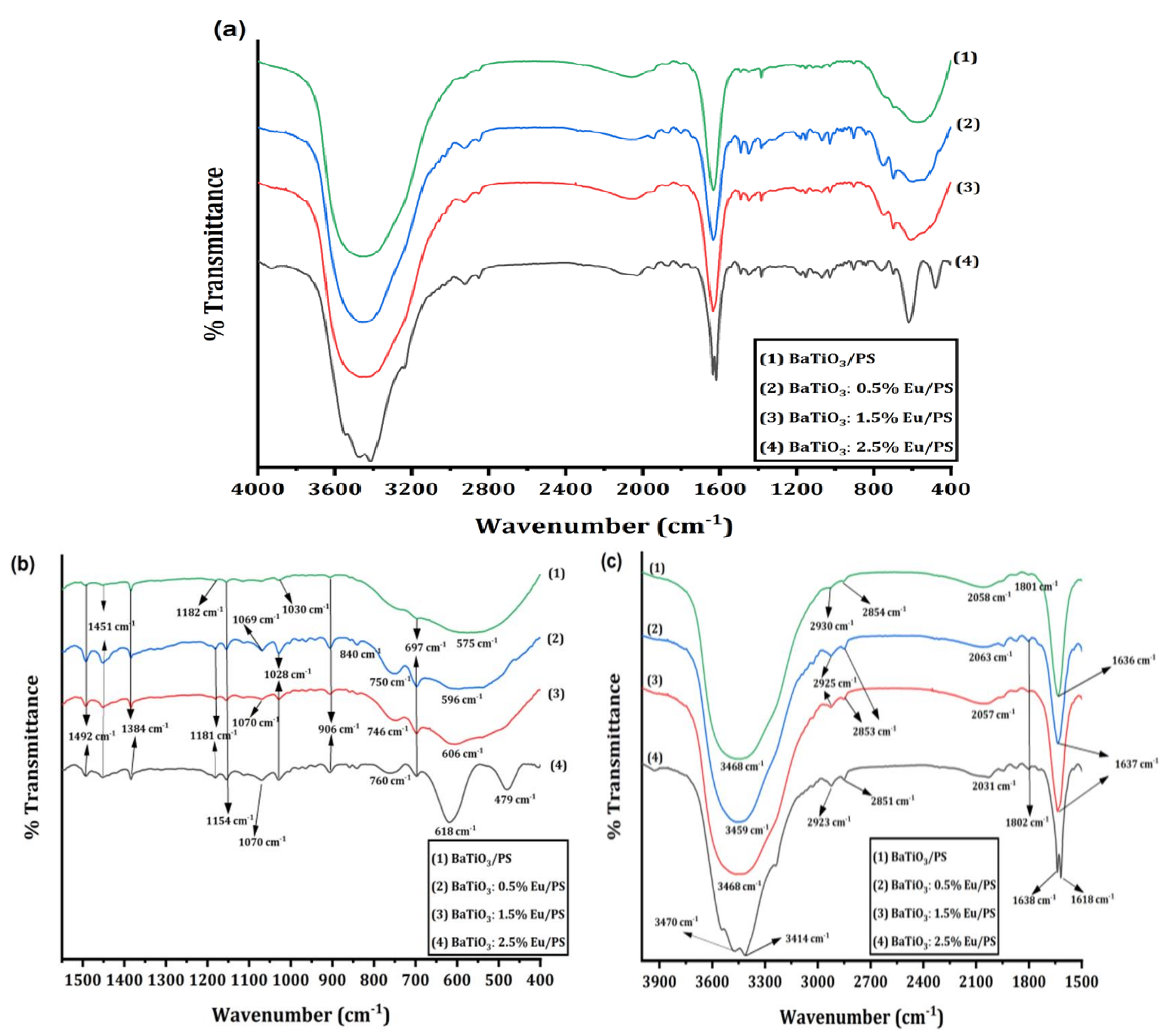
4.2. Fourier-Transform Infrared (FTIR) Analysis
4.3. Morphological Study
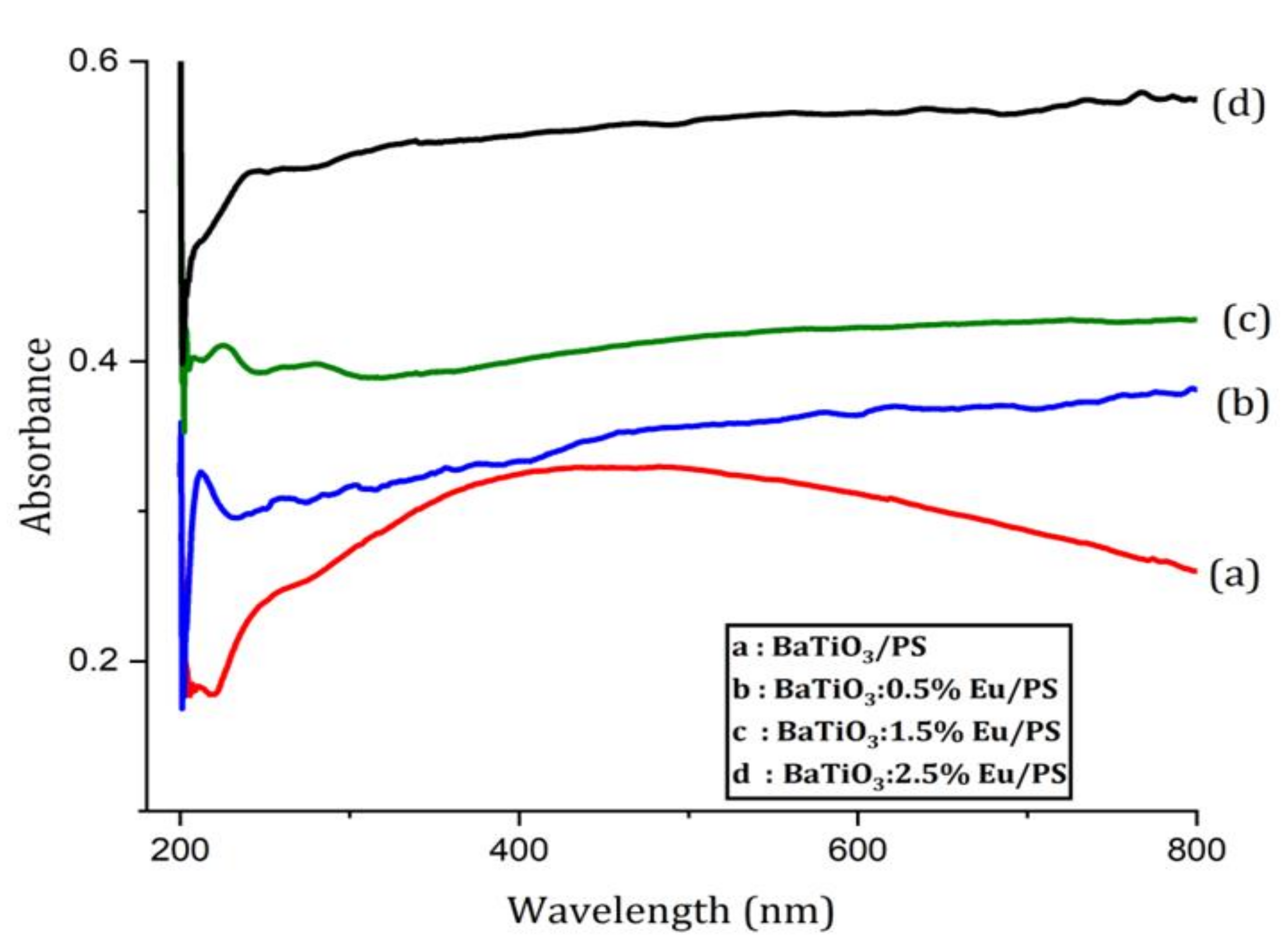
4.4. UV-Visible Absorption Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, T.; Chen, H.; Yi, K.; Wang, J.; Xu, P.; Chu, B. Synergistic effect of barium titanate and insulating fillers on dielectric performance of sandwich-structured PLA composites. Compos. Commun. 2022, 29, 101027. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Zhu, Y.; Jiang, P.; Huang, X. Tailoring the polarity of polymer shell on BaTiO3 nanoparticle surface for improved energy storage performance of dielectric polymer nanocomposites. Chin. Chem. Lett. 2021, 32, 2229–2232. [Google Scholar] [CrossRef]
- Pan, H.; Li, F.; Liu, Y.; Zhang, Q.; Wang, M.; Lan, S.; Zheng, Y.; Ma, J.; Gu, L.; Shen, Y.; et al. Ultrahigh–energy density lead-free dielectric films via polymorphic nanodomain design. Science 2019, 365, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Wang, Z.; Li, Y.; Wu, D.; Xue, Y. Tunable BaxSr1-xTiO3 nanoparticles induced high energy storage density in layer-structured asymmetric polymer-based nanocomposites. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Chen, W. A brief review of Ba (Ti0. 8Zr0. 2) O3-(Ba0. 7Ca0. 3) TiO3 based lead-free piezoelectric ceramics: Past, present and future perspectives. J. Phys. Chem. Solids 2018, 114, 207–219. [Google Scholar] [CrossRef]
- Yu, S.; Ding, C.; Liu, Y.; Liu, Y.; Zhang, Y.; Luo, H.; Zhang, D.; Chen, S. Enhanced breakdown strength and energy density over a broad temperature range in polyimide dielectrics using oxidized MXenes filler. J. Power Sources 2022, 535, 231415. [Google Scholar] [CrossRef]
- Tang, X.; Din, C.; Yu, S.; Liu, Y.; Luo, H.; Zhang, D.; Chen, S. Synthesis of dielectric polystyrene via one-step nitration reaction for large-scale energy storage. Chem. Eng. J. 2022, 446, 137281. [Google Scholar] [CrossRef]
- Piana, F.; Cacciotti, I.; Šlouf, M.; Nanni, F.; Pfleger, J. One-pot preparation of surface-functionalized barium titanate nanoparticles for high-K polystyrene composite films prepared via floating method. J. Mater. Sci. 2018, 53, 11343–11354. [Google Scholar] [CrossRef]
- Ding, C.; Tang, X.; Yu, S.; Chen, S.; Liu, Z.; Luo, H.; Zhang, D. Concurrently enhanced dielectric properties and energy density in poly(vinylidene fluoride)-based core–shell BaTiO3 nanocomposites via constructing a polar and rigid polymer interfacial layer. J. Mater. Chem. C 2022, 10, 6323–6333. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, B.; Jin, Y.; Zhang, H.; Qiao, Y.; Zhang, Z. 3D segregated architecture BaTiO3/polystyrene composites with enhanced dielectric constant fabricated via hot pressing core–shell polystyrene@BaTiO3 composite microspheres. J. Mater. Sci. Mater. Electron. 2020, 31, 3101–3110. [Google Scholar] [CrossRef]
- Shireesha, G.; Manjunatha, C.; Jain, A.; Radhakrishna, M.C. Influence of Cd0. 99Eu0. 01SiO3 nanoparticles concentration on Cd0. 99Eu0. 01SiO3/PVDF nanocomposite films. Mater. Today Proc. 2018, 5, 21162–21174. [Google Scholar] [CrossRef]
- Pithan, C.; Katsu, H.; Waser, R. Defect chemistry of donor-doped BaTiO3 with BaO-excess for reduction resistant PTCR thermistor applications–redox-behaviour. Phys. Chem. Chem. Phys. 2020, 22, 8219–8232. [Google Scholar] [CrossRef] [PubMed]
- Characterization of Minerals, Metals, and Materials 2018, SpringerLink. Available online: https://link.springer.com/book/10.1007/978-3-319-72484-3 (accessed on 20 September 2022).
- Fuentes, S.; Espinoza-González, R.; Rosales, M.; León, J. Effects of Eu3+ on the morphological, structural and optical properties of BaTiO3@ ZnO: Eu nanoparticles. J. Alloys Compd. 2020, 846, 156452. [Google Scholar] [CrossRef]
- Alshoaibi, A.; Saber, O.; Ahmed, F. Enhancement of optical activity and properties of barium titanium oxides to be active in sunlight through using hollandite phase instead of perovskite phase. Crystals 2021, 11, 550. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hussein, S.; Hussein, A.M.; Saeed, S.R. Optical characteristics of polystyrene based solid polymer composites: Effect of metallic copper powder. Int. J. Met. 2013, 2013, 123657. [Google Scholar] [CrossRef]
- Khan, S.; Humera, N.; Niaz, S.; Riaz, S.; Atiq, S.; Naseem, S. Simultaneous normal–Anomalous dielectric dispersion and room temperature ferroelectricity in CBD perovskite BaTiO3 thin films. J. Mater. Res. Technol. 2020, 9, 11439–11452. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, S.; Dalela, B.; Kumar, S.; Alvi, P.; Dalela, S. Defects and oxygen vacancies tailored structural and optical properties in CeO2 nanoparticles doped with Sm3+ cation. J. Alloys Compd. 2018, 752, 520–531. [Google Scholar] [CrossRef]
- Ye, C.; Yang, J.; Yao, L.; Chen, N. Regularities of formation and lattice distortion of perovskite-type compounds. Chin. Sci. Bull. 2002, 47, 458–460. [Google Scholar] [CrossRef]
- Moreira, R.L.; Dias, A. Comment on Prediction of lattice constant in cubic perovskites. J. Phys. Chem. Solids 2007, 68, 1617–1622. [Google Scholar] [CrossRef]
- Ubic, R.; Tolman, K.; Talley, K.; Joshi, B.; Schmidt, J.; Faulkner, E.; Owens, J.; Papac, M.; Garland, A.; Rumrill, C.; et al. Lattice-constant prediction and effect of vacancies in aliovalently doped perovskites. J. Alloys Compd. 2015, 644, 982–995. [Google Scholar] [CrossRef]
- Ubic, R. Revised method for the prediction of lattice constants in cubic and pseudocubic perovskites. J. Am. Ceram. Soc. 2007, 90, 3326–3330. [Google Scholar] [CrossRef]
- Smith, E.; Škapin, S.; Ubic, R. Correlative models for oxygen vacancies in perovskites. J. Alloys Compd. 2020, 836, 155475. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Padalia, D.; Bisht, G.; Johri, U.; Asokan, K. Fabrication and characterization of cerium doped barium titanate/PMMA nanocomposites. Solid State Sci. 2013, 19, 122–129. [Google Scholar] [CrossRef]
- Kumari, S.; Padalia, D.; Kumar, U.; Kumari, R. Investigation of structural properties of pure and Ce-doped barium titanate. Ionics 2022, 28, 4401–4411. [Google Scholar] [CrossRef]
- Pyykkö, P.; Atsumi, M. Molecular Single-Bond Covalent Radii for Elements 1-118. Chem. Eur. J. 2009, 15, 186–197. [Google Scholar] [CrossRef]
- Shaalan, N.; Hamad, D.; Abdel-Latief, A.; Abdel-Rahim, M. Preparation of quantum size of tin oxide: Structural and physical characterization. Prog. Nat. Sci. Mater. Int. 2016, 26, 145–151. [Google Scholar] [CrossRef]
- García-Hernández, M.; Chadeyron, G.; Boyer, D.; García-Murillo, A.; Carrillo-Romo, F.; Mahiou, R. Hydrothermal Synthesis and Characterization of Europium-doped Barium Titanate Nanocrystallites. Nano-Micro Lett. 2013, 5, 57–65. [Google Scholar] [CrossRef]
- Chae, D.W.; Kim, B.C. Characterization on polystyrene/zinc oxide nanocomposites prepared from solution mixing. Polym. Adv. Technol. 2005, 16, 846–850. [Google Scholar] [CrossRef]
- Chen, Q.; Hong, R.; Feng, W. Preparation and characterization of composites from Ba0.5Sr0.5TiO3 and polystyrene. J. Alloys Compd. 2014, 609, 274–283. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.M.; Dannoun, E.M.A.; Aziz, S.B.; Brza, M.A.; Abdulwahid, R.T.; Hussen, S.A.; Rostam, S.; Mustafa, D.M.T.; Muhammad, D.S. Steps toward the band gap identification in polystyrene based solid polymer nanocomposites integrated with tin titanate nanoparticles. Polymers 2020, 12, 2320. [Google Scholar] [CrossRef] [PubMed]
- Ramakanth, S.; Raju, K.C.J. Band gap narrowing in BaTiO3 nanoparticles facilitated by multiple mechanisms. J. Appl. Phys. 2014, 115, 173507. [Google Scholar] [CrossRef]


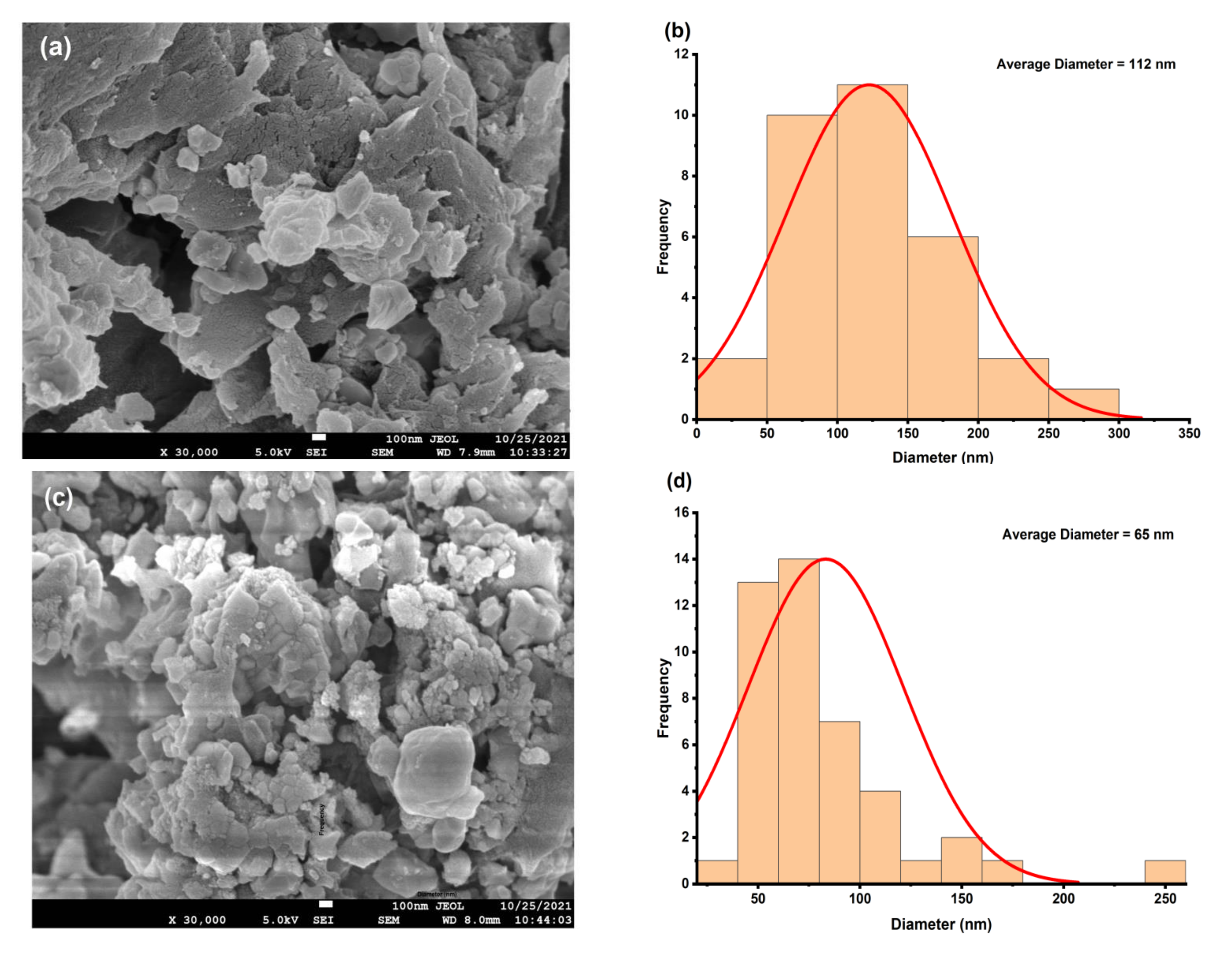
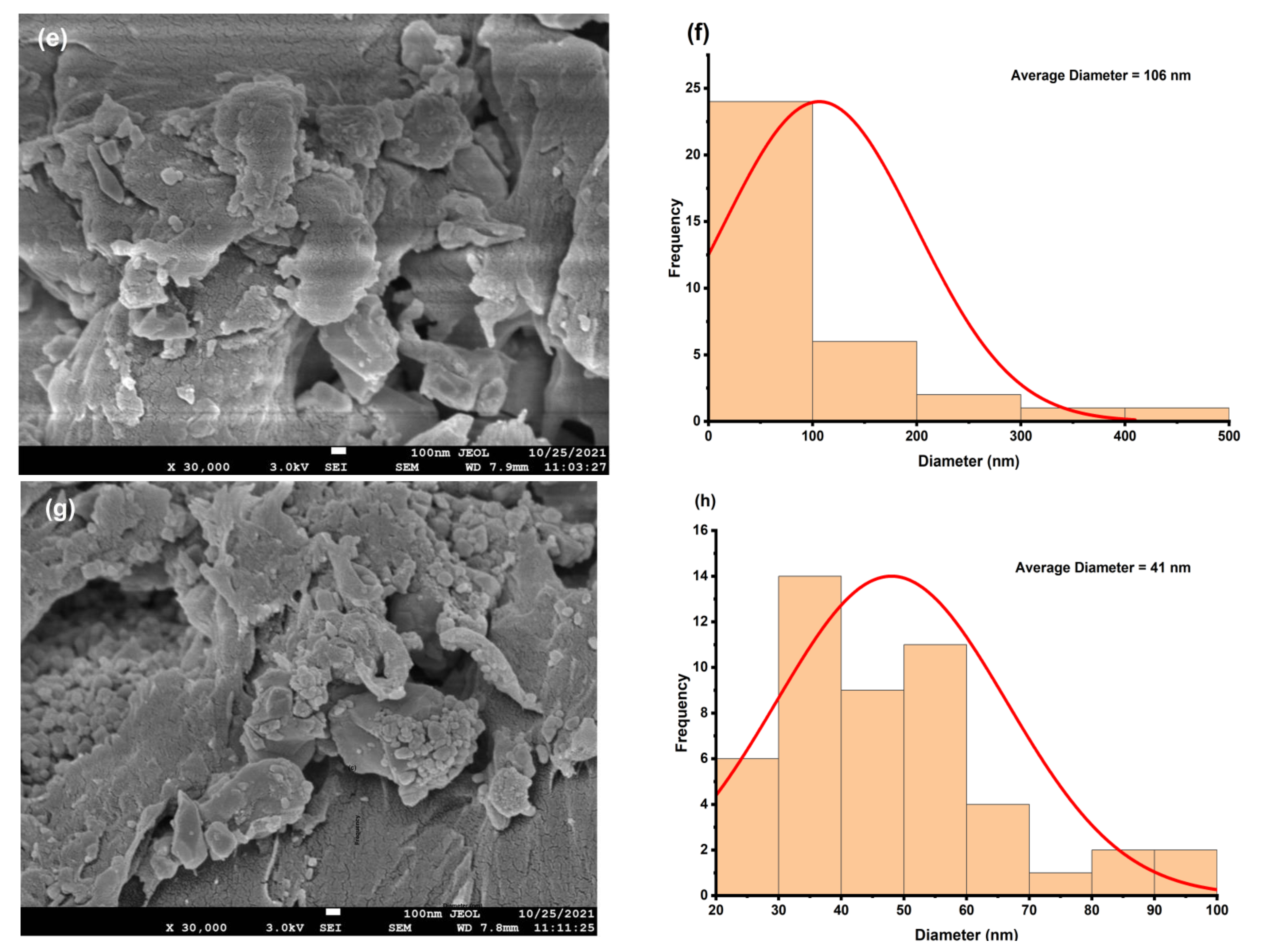

| Composition | D (Scherrer) nm | D (SSP) nm | d (FESEM) nm | n | δ × 10−3 (SSP, nm−2) | ε × 10−2 | Eg (eV) |
|---|---|---|---|---|---|---|---|
| x = 0.000 | 20.16 | 20.63 | 112 | 71.49515 | 2.35 | 1.47 | 4.35 |
| x = 0.005 | 27.61 | 12.61 | 65 | 16.45934 | 6.28 | 2.38 | 4.33 |
| x = 0.015 | 17.89 | 18.63 | 106 | 53.21427 | 2.88 | 1.41 | 4.29 |
| x = 0.025 | 19.58 | 16.76 | 41 | 38.40016 | 3.56 | 1.56 | 3.45 |
| Composition | (Equation (4)) | (Equation (5)) | (Equation (6)) | in nm | (Equation (7)) | t1 (Equation (8)) | t2 (Equation (9)) |
|---|---|---|---|---|---|---|---|
| x = 0.000 | 3.99737 | 3.99922 | 4.00704 | 4.00559 | 1.07061 | 1.0449 | 0.97614 |
| x = 0.005 | 3.99569 | 3.99689 | 4.00440 | 3.99488 | 1.06727 | 1.0446 | 0.97875 |
| x = 0.015 | 3.99233 | 3.99222 | 3.99911 | 3.99144 | 1.06445 | 1.04257 | 0.9796 |
| x = 0.025 | 3.98896 | 3.98756 | 3.99382 | 4.00334 | 1.06034 | 1.03546 | 0.97668 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, U.; Padalia, D.; Bhandari, P.; Kumar, P.; Ranakoti, L.; Singh, T.; Lendvai, L. Fabrication of Europium-Doped Barium Titanate/Polystyrene Polymer Nanocomposites Using Ultrasonication-Assisted Method: Structural and Optical Properties. Polymers 2022, 14, 4664. https://doi.org/10.3390/polym14214664
Kumar U, Padalia D, Bhandari P, Kumar P, Ranakoti L, Singh T, Lendvai L. Fabrication of Europium-Doped Barium Titanate/Polystyrene Polymer Nanocomposites Using Ultrasonication-Assisted Method: Structural and Optical Properties. Polymers. 2022; 14(21):4664. https://doi.org/10.3390/polym14214664
Chicago/Turabian StyleKumar, Umesh, Diwakar Padalia, Prabhakar Bhandari, Pawan Kumar, Lalit Ranakoti, Tej Singh, and László Lendvai. 2022. "Fabrication of Europium-Doped Barium Titanate/Polystyrene Polymer Nanocomposites Using Ultrasonication-Assisted Method: Structural and Optical Properties" Polymers 14, no. 21: 4664. https://doi.org/10.3390/polym14214664
APA StyleKumar, U., Padalia, D., Bhandari, P., Kumar, P., Ranakoti, L., Singh, T., & Lendvai, L. (2022). Fabrication of Europium-Doped Barium Titanate/Polystyrene Polymer Nanocomposites Using Ultrasonication-Assisted Method: Structural and Optical Properties. Polymers, 14(21), 4664. https://doi.org/10.3390/polym14214664













