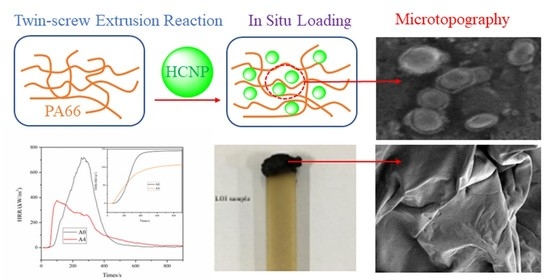
Thermal Stabilities and Flame Retardancy of Polyamide 66 Prepared by In Situ Loading of Amino-Functionalized Polyphosphazene Microspheres
Abstract
1. Introduction
2. Results
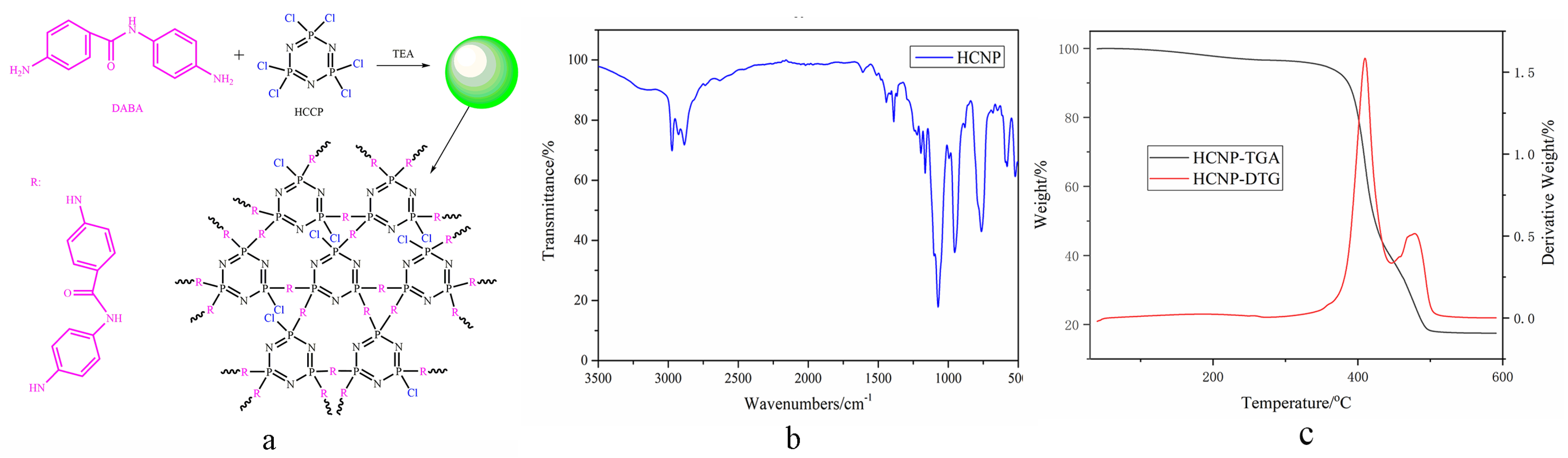
2.1. Characterization of HCNP
2.1.1. FTIR Analysis
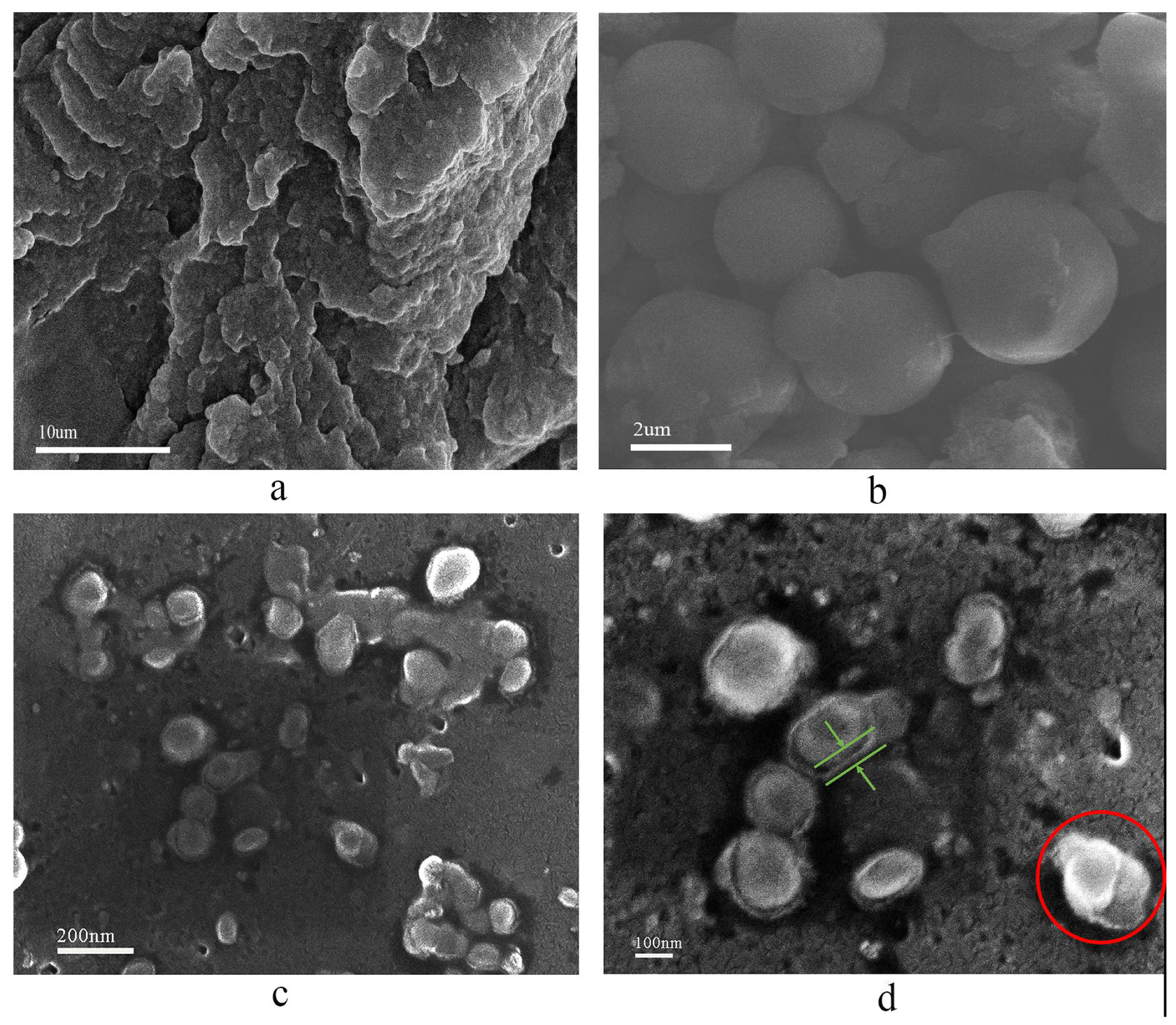
2.1.2. Microtopography of HCNP
2.2. LOI and UL-94 Test Results
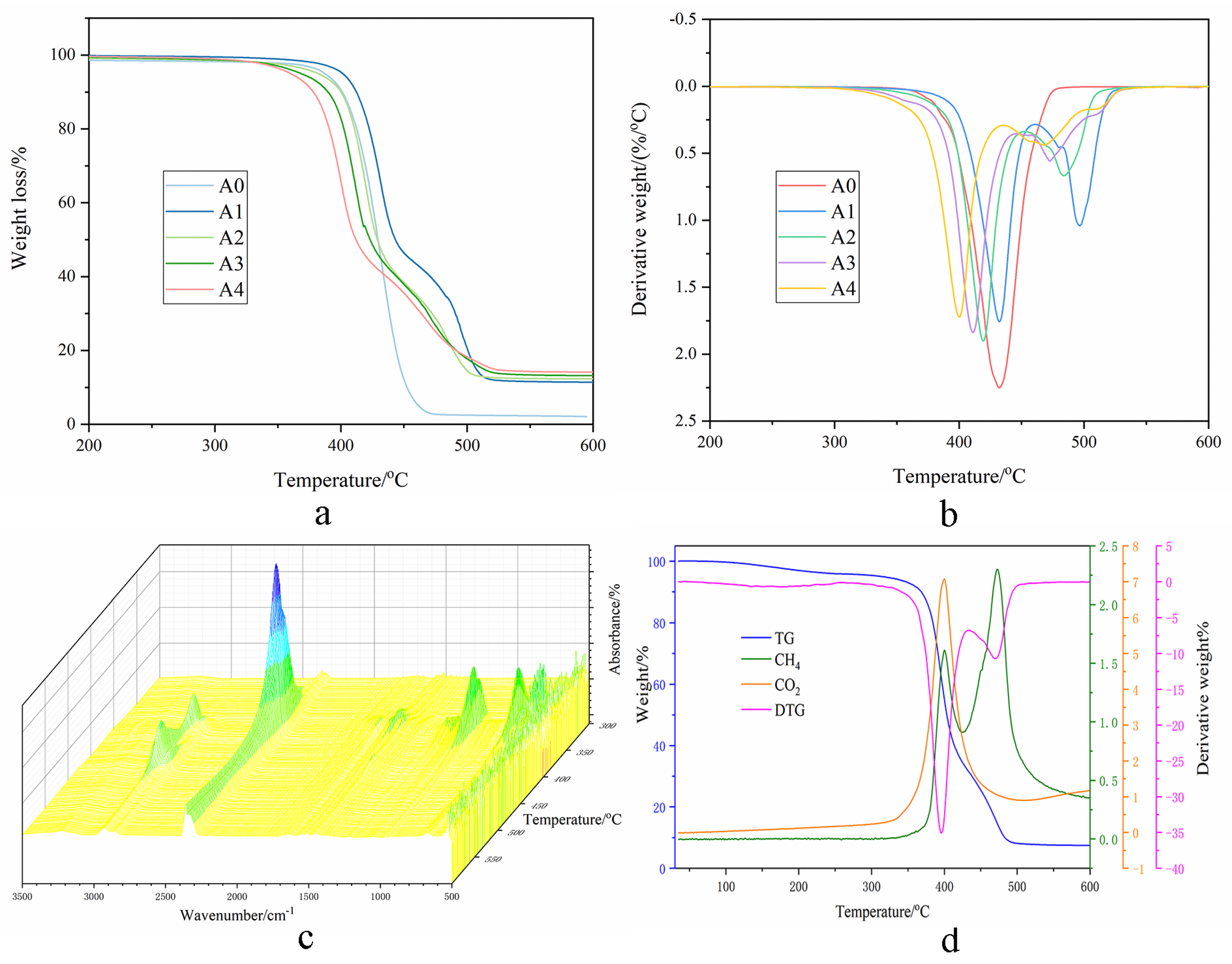
2.3. Thermal Stability of FR-PA66
2.3.1. TGA Analysis
2.3.2. TG-IR Analysis
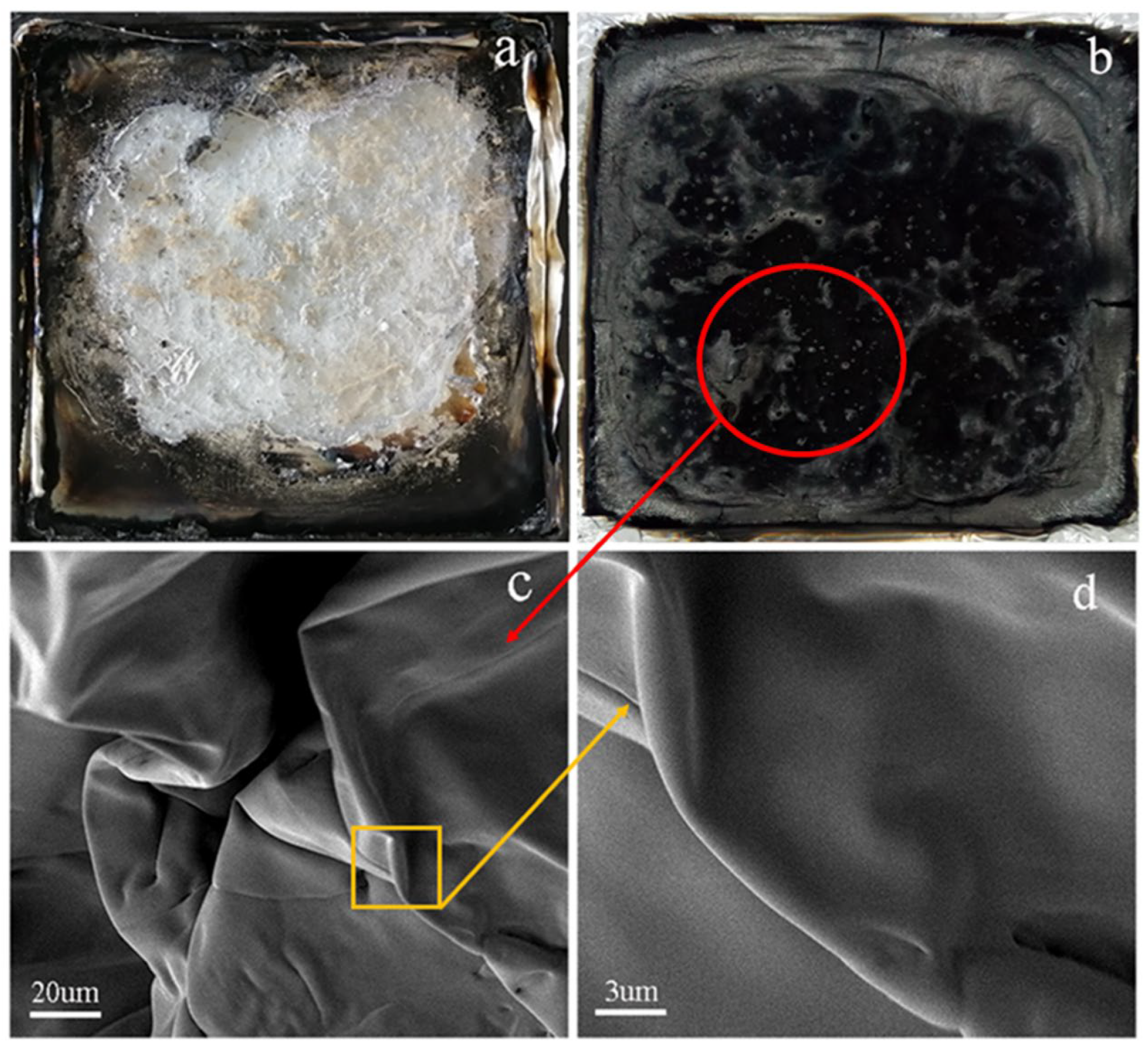
2.4. The Flame Retardancy of FR-PA66
2.5. Microtopography
3. Discussion
3.1. Thermal Stability of FR-PA66
3.2. Flame Retardancy of FR-PA66
4. Materials and Methods
4.1. Materials
4.2. Synthesis of HCNP
4.3. Preparation of FR-PA66
4.4. Measurement Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Lu, J.L.; Yang, H.Y.; Lang, J.Y.; Yang, H. Comparative Study on the Flame-Retardant Properties and Mechanical Properties of PA66 with Different Dicyclohexyl Hypophosphite Acid Metal Salts. Polymers 2019, 11, 1956. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.H.; Sun, J.J.; Yang, C.B.; Wu, J.; Ying, J.G.; Liu, P. Preparation and Technology of Flame Retardant Reinforced Toughening Nylon 66 Composites. J. Eng. Plas. Appl. 2012, 40, 49–53. [Google Scholar]
- Zhan, Z.S.; Shi, J.S.; Zhang, Y.; Zhang, Y.L.; Zhang, B.; Liu, W.B. The study on flame retardancy synergetic mechanism of magnesium oxide for PA66/AlPi composite. Mater. Res. Express 2019, 6, 115317. [Google Scholar] [CrossRef]
- Zhan, Z.S.; Li, B.; Xu, M.J.; Guo, Z.H. Synergistic effects of nano-silica on aluminum diethylphosphinate /polyamide 66 system for fire retardancy. High. Perform. Polym. 2016, 28, 140–146. [Google Scholar] [CrossRef]
- Luo, D.; Duan, W.F.; Liu, Y.; Chen, N.; Wang, Q. Melamine cyanurate surface treated by nylon of low molecular weight to prepare flame-retardant polyamide 66 with high flowability. Fire Mater. 2019, 43, 323–331. [Google Scholar] [CrossRef]
- Guo, Z.; Bao, M.; Ni, X.Y. The synthesis of meltable and highly thermostabletriazine-DOPO flame retardant and its application in PA66. Polym. Advan. Technol. 2021, 32, 815–828. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Current Practice and Recent Commercial Developments in Flame retardant of Polyamides. J. Fire Sci. 2004, 22, 251–264. [Google Scholar] [CrossRef]
- Levchik, S.V.; Levchik, G.F.; Balabanovich, A.I.; Weil, E.D.; Klatt, M. Phosphorus oxynitride: A thermally stable fire retardant additive for polyamide 6 and poly(butylene terephthalate). Macromol. Mater. Eng. 1999, 264, 48–55. [Google Scholar] [CrossRef]
- Jiang, P.; Gu, X.Y.; Zhang, S.; Wu, S.D.; Zhao, Q.; Hu, Z.W. Synthesis, Characterization, and Utilization of a Novel Phosphorus/Nitrogen-Containing Flame retardant. Ind. Eng. Chem. Res. 2015, 54, 2974–2982. [Google Scholar] [CrossRef]
- Ding, Y.; Swann, D.J.; Sun, Q.; Stoliarov, I.S.; Kraemer, H.R. Development of a pyrolysis model for glass fiber reinforced polyamide 66 blended with red phosphorus: Relationship between flammability behavior and material composition. Compos. Part B-Eng. 2019, 176, 107263. [Google Scholar] [CrossRef]
- Sui, Y.L.; Sim, H.F.; Shao, W.D.; Zhang, C.L. Novel bioderived cross-linked polyphosphazene microspheres decorated with FeCo-layered double hydroxide as an all-in-one intumescent flame retardant for epoxy resin. Compos. Part B-Eng. 2022, 229, 109463. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Wu, W.; Xu, T.; Xu, H.; Zhong, Y.; Zhang, L.P.; Ma, Y.M.; Sui, X.F.; Wang, B.J.; Feng, X.L.; et al. Preparation and characterization of polyphosphazene-based flame retardants with different functional groups. Polym. Degrad. Stabil. 2022, 196, 109815. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.L.; Mu, X.W.; Zhou, M.T.; Cai, W.; Song, L.; Xing, W.Y.; Hu, Y. Polyphosphazenes-based flame retardants: A review. Compos. Part B-Eng. 2020, 202, 108397. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.L.; He, L.X.; Cai, W.; Chu, F.K.; Zhu, Y.L.; Jiang, X.; Song, L.; Hu, Y. Bifunctional linear polyphosphazene decorated by allyl groups: Synthesis and application as efficient flame-retardant and toughening agent of bismaleimide. Compos. Part B-Eng. 2022, 233, 109653. [Google Scholar] [CrossRef]
- Qiu, S.L.; Ma, C.; Wang, X.; Zhou, X.; Feng, X.M.; Yuen, R.K.K.; Hu, Y. Melamine-containing polyphosphazene wrapped ammonium polyphosphate: A novel multifunctional organic-inorganic hybrid flame retardant. J. Hazard. Mater. 2018, 344, 839–848. [Google Scholar] [CrossRef]
- Qiu, S.L.; Hu, Y.X.; Shi, Y.Q.; Hou, Y.B.; Kan, Y.C.; Chu, F.K.; Sheng, H.B.; Yuen, R.K.K.; Xing, W.Y. In situ growth of polyphosphazene particles on molybdenum disulfide nanosheets for flame retardant and friction application. Compos. Part A-Appl. Sci. Manuf. 2018, 114, 407–417. [Google Scholar] [CrossRef]
- Zhou, M.T.; Zeng, D.S.; Qiu, S.L.; Zhou, X.; Cheng, L.; Xu, Z.M.; Xing, W.Y.; Hu, Y. Amino-functionalized carbon nanotubes/polyphosphazene hybrids for improving the fire safety and mechanical properties of epoxy. Prog. Nat. Sci.-Mater. Int. 2022, 32, 179–189. [Google Scholar] [CrossRef]
- Dong, L.P.; Huang, S.C.; Li, Y.M.; Deng, C.; Wang, Y.Z. A Novel Linear-Chain Polyamide Charring Agent for the Fire Safety of Noncharring Polyolefin. Ind. Eng. Chem. Res. 2016, 55, 7132–7141. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, Q.; Zhang, S.; Gu, X.Y.; Hu, Z.W.; Xu, G.Z. Flammability and Char Formation of Polyamide 66 Fabric: Chemical Grafting versus Pad-Dry Process. Ind. Eng. Chem. Res. 2015, 54, 6085–6092. [Google Scholar] [CrossRef]
- Schartel, B.; Kunze, R.; Neubert, D. Red phosphorus-controlled decomposition for fire retardant PA 66. J. Appl. Polym. Sci. 2002, 83, 2060–2071. [Google Scholar] [CrossRef]
- Guan, X.Y.; Zheng, G.Q.; Dai, K.; Liu, C.; Yan, X.R.; Shen, C.Y.; Guo, Z.H. Carbon Nanotubes-Adsorbed Electrospun PA66 Nanofiber Bundles with Improved Conductivity and Robust Flexibility. ACS Appl. Mater. Inter. 2016, 8, 14150–14159. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, B.; Xing, J.; Sun, M.M.; Zhang, X.G.; Li, J.H.; Wang, L.; Liu, C.Z. A facile approach to synthesize in situ functionalized graphene oxide/epoxy resin nanocomposites: Mechanical and thermal properties. J. Mater. Sci. 2019, 54, 13973–13989. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.L.; Shao, X.M.; Jiang, L.; Huang, K.; Zhao, S. Synergistic effects of a highly effective intumescent flame retardant based on tannic acid functionalized graphene on the flame retardant and smoke suppression properties of natural rubber. Compos. Part A-Appl. Sci. Manuf. 2020, 129, 105715. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Y.; Hu, X. Synthesis of a novel phosphorus-containing polymer and its application in amino intumescent fire resistant coating. Prog. Orga. Coat. 2013, 76, 188–193. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.S.; Zhang, Z.; Wang, Q. Preparation of ammonium polyphosphate and dye co-intercalated LDH/polypropylene composites with enhanced flame retardant and UV resistance properties. Chemosphere 2021, 277, 130370. [Google Scholar] [CrossRef]


| Samples | PA66/wt% | HCNP/wt% | LOI | UL94 |
|---|---|---|---|---|
| A0 | 100 | 0 | 24 | V-2 |
| A1 | 97 | 3 | 25 | V-2 |
| A2 | 95 | 5 | 26 | V-2 |
| A3 | 93 | 7 | 28.5 | V-1 |
| A4 | 91 | 9 | 30 | V-0 |
| Samples | Ti/°C | Tmax1/°C | Tmax2/°C | Residue%/(Tmax1) | Residue% (Final) |
|---|---|---|---|---|---|
| A0 | 387 | 432 | - | 39.7 | 2.1 |
| A1 | 401 | 432 | 497 | 62.8 | 11.4 |
| A2 | 382 | 419 | 484 | 66.0 | 12.3 |
| A3 | 368 | 411 | 473 | 67.4 | 13.2 |
| A4 | 360 | 400 | 467 | 67.5 | 14.1 |
| Samples | pkHRR/(kW/m2) | THR/(MJ/m2) | ML/% | TSR/(m2/m2) |
|---|---|---|---|---|
| A0 | 717.5 | 145.1 | 99.5 | 2667.0 |
| A4 | 373.7 | 106.7 | 92.5 | 944.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, W.; Lv, J.; Zhu, C.; Zhang, Y.; Cheng, Y.; Zeng, L.; Wang, L.; Liao, C. Thermal Stabilities and Flame Retardancy of Polyamide 66 Prepared by In Situ Loading of Amino-Functionalized Polyphosphazene Microspheres. Polymers 2023, 15, 218. https://doi.org/10.3390/polym15010218
Lv W, Lv J, Zhu C, Zhang Y, Cheng Y, Zeng L, Wang L, Liao C. Thermal Stabilities and Flame Retardancy of Polyamide 66 Prepared by In Situ Loading of Amino-Functionalized Polyphosphazene Microspheres. Polymers. 2023; 15(1):218. https://doi.org/10.3390/polym15010218
Chicago/Turabian StyleLv, Wenyan, Jun Lv, Cunbing Zhu, Ye Zhang, Yongli Cheng, Linghong Zeng, Lu Wang, and Changrong Liao. 2023. "Thermal Stabilities and Flame Retardancy of Polyamide 66 Prepared by In Situ Loading of Amino-Functionalized Polyphosphazene Microspheres" Polymers 15, no. 1: 218. https://doi.org/10.3390/polym15010218
APA StyleLv, W., Lv, J., Zhu, C., Zhang, Y., Cheng, Y., Zeng, L., Wang, L., & Liao, C. (2023). Thermal Stabilities and Flame Retardancy of Polyamide 66 Prepared by In Situ Loading of Amino-Functionalized Polyphosphazene Microspheres. Polymers, 15(1), 218. https://doi.org/10.3390/polym15010218