Morphological, Mechanical, and Antimicrobial Properties of PBAT/Poly(methyl methacrylate-co-maleic anhydride)–SiO2 Composite Films for Food Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of SiO2 Nanoparticles (NPs)
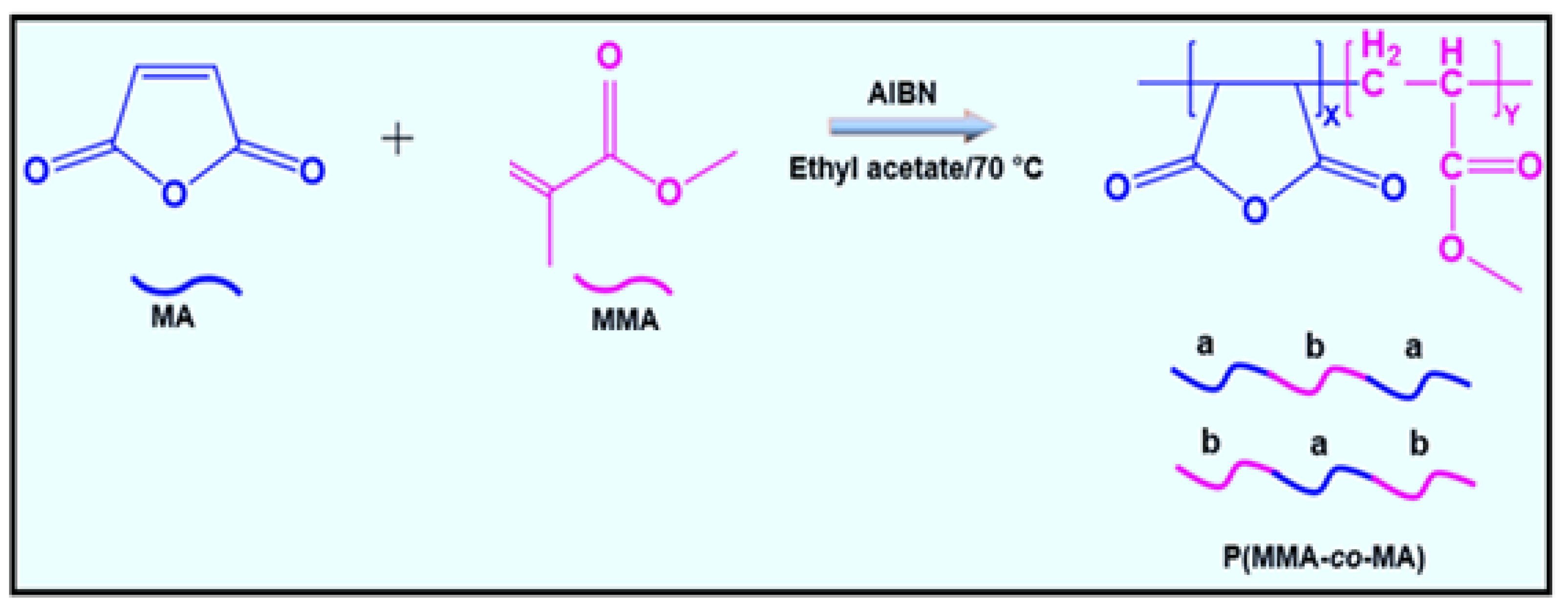
2.3. Synthesis of Poly(methyl methacrylate-co-maleic anhydride)
2.4. Purification of P(MMA-co-MA) Copolymer
2.5. Fabrication of PBAT/P(MMA-co-MA)–SiO2 Composites
2.6. Characterization
2.6.1. Structural Characterization
2.6.2. Morphological Studies
2.6.3. Thermal Characterization
2.6.4. Mechanical Strength Measurements
2.6.5. Barrier Properties
2.6.6. Water Contact Angle Measurements
2.6.7. Antimicrobial Activities
2.6.8. Statistical Analysis
3. Results and Discussion
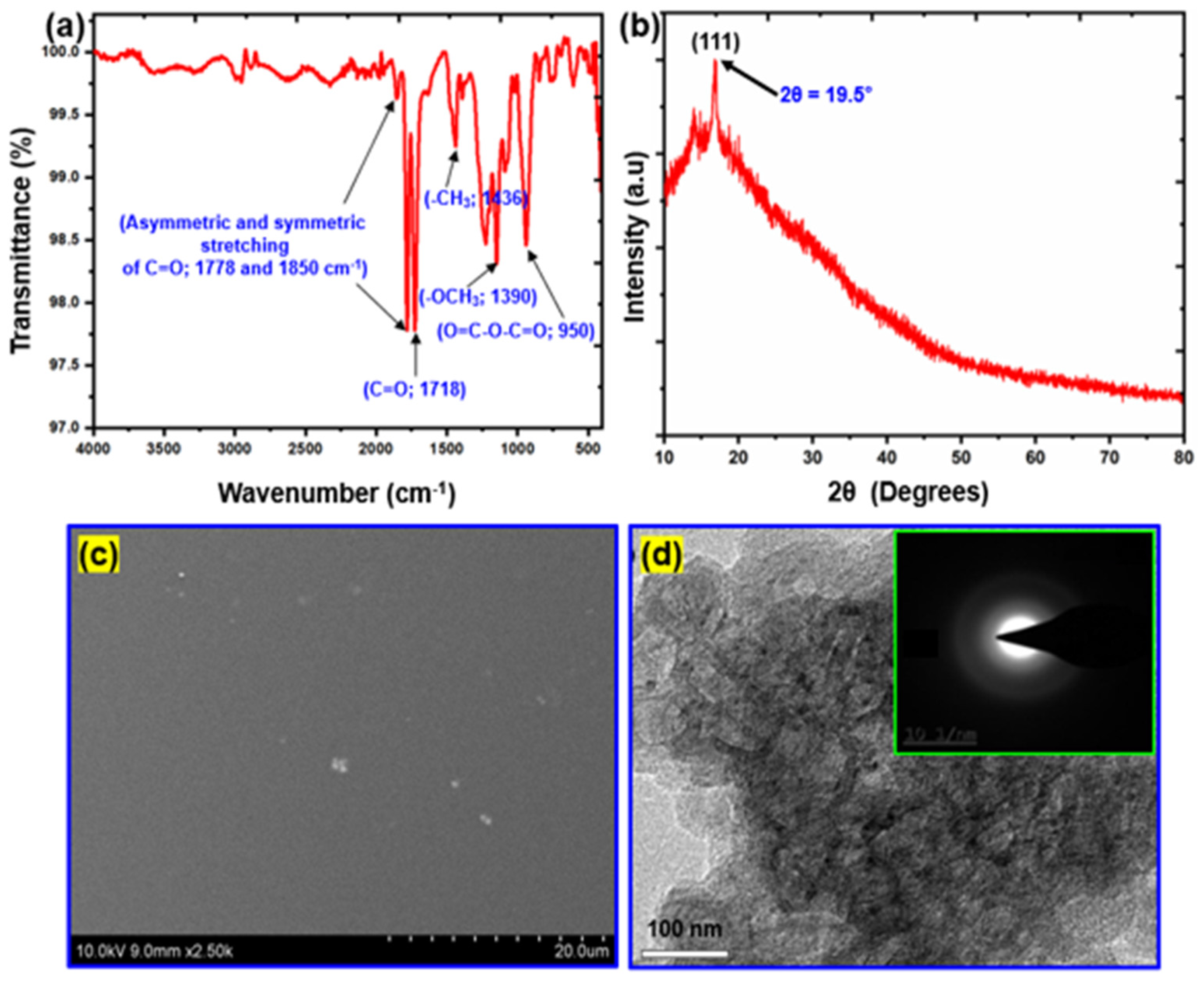
3.1. Characterization of SiO2 Nanoparticles
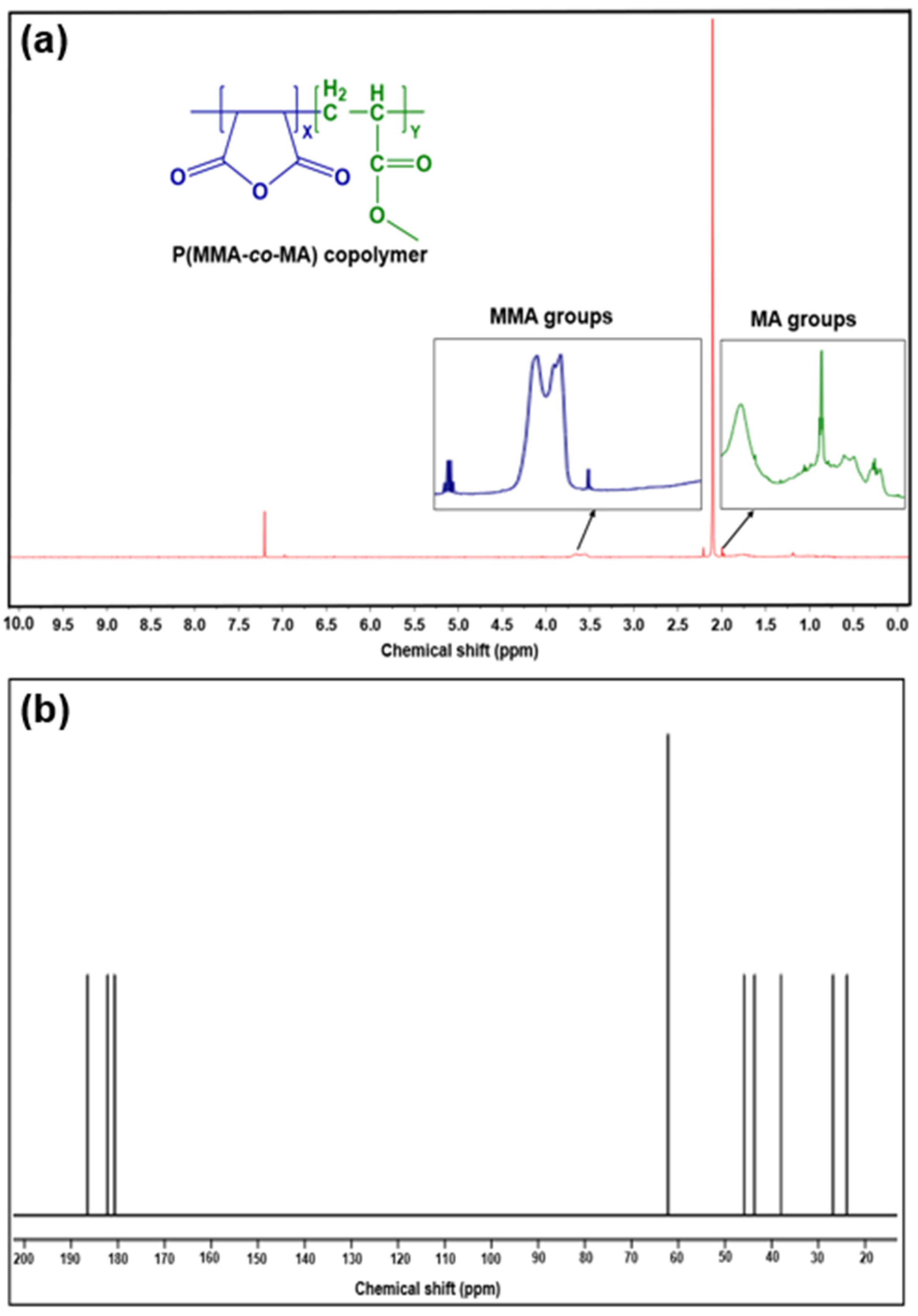
3.2. Characterization of P(MMA-co-MA) Copolymer
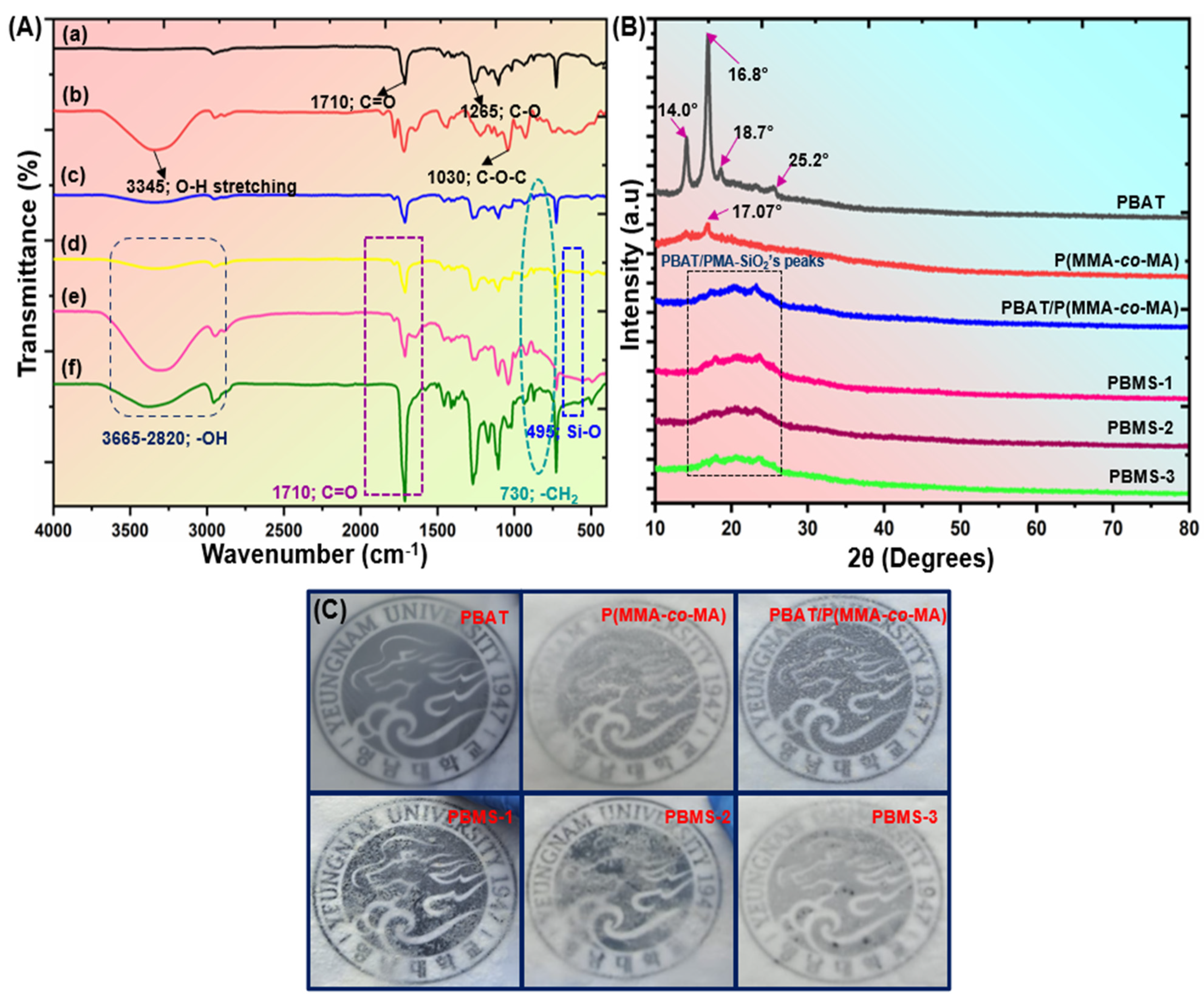
3.3. Characterization of PBAT/P(MMA-co-MA)–SiO2 Composites
3.4. Thermal Properties of PBAT/P(MMA-co-MA)–SiO2 Composite Films
3.4.1. Thermogravimetric Analysis (TGA)
3.4.2. Differential Scanning Calorimetry (DSC)
3.5. Mechanical Strength of PBAT/P(MMA-co-MA)–SiO2 Composite Films
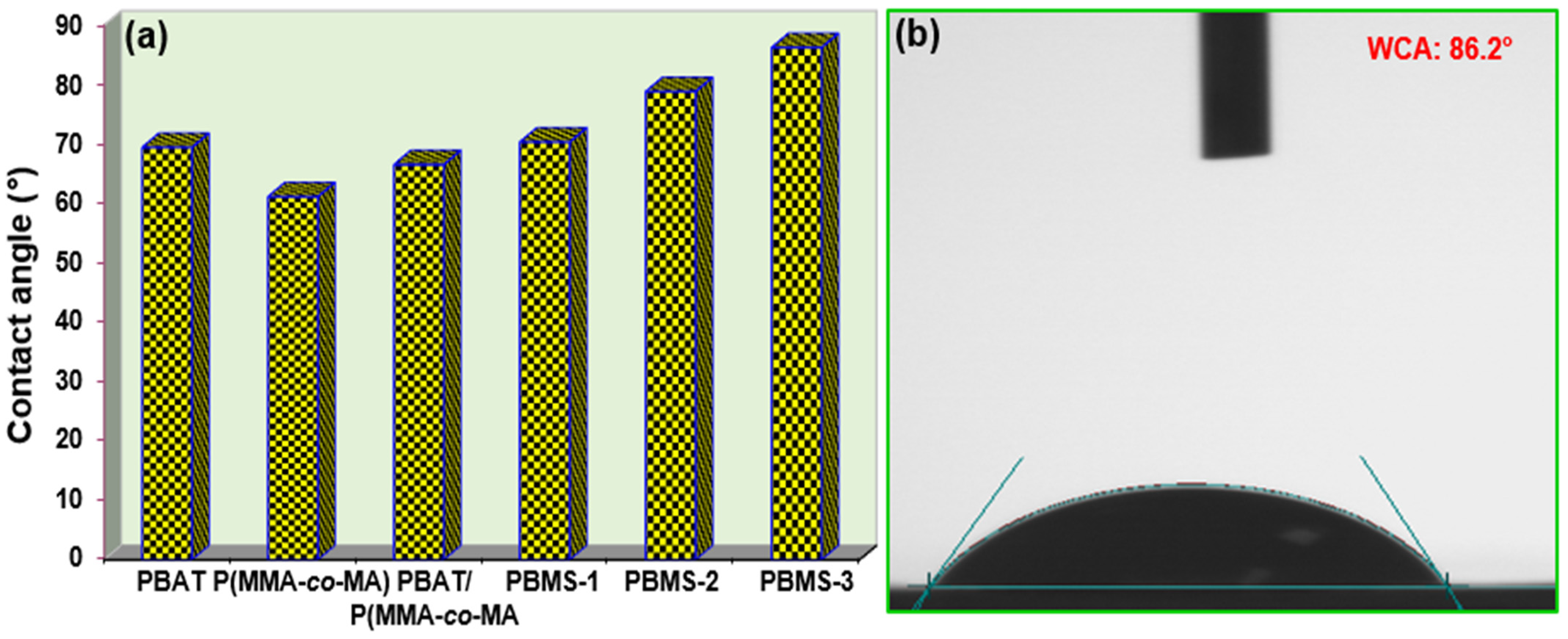
3.6. Water Contact Angle
3.7. Barrier Properties
3.8. Antimicrobial Activities of PBAT/P(MMA-co-MA)–SiO2 Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, Ó.L.; Pereira, R.N.; Cerqueira, M.A.; Martins, J.R.; Teixeira, J.A.; Malcata, F.X.; Vicente, A.A. Bio-based nanocomposites for food packaging and their effect in food quality and safety. In Food Packaging and Preservation; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 271–306. [Google Scholar] [CrossRef]
- Coppola, G.; Gaudio, M.T.; Lopresto, C.G.; Calabro, V.; Curcio, S.; Chakraborty, S. Bioplastic from renewable biomass: A facile solution for a greener environment. Earth Syst. Environ. 2021, 5, 231–251. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Cabedo, L.; Feijoo, J.L.; Villanueva, M.P.; Lagar’on, J.M.; Gimenez, E. Optimization of biodegradable nanocomposites based on PLA/PCL blends for food packaging applications. Macromol. Symp. 2006, 233, 191–197. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Ha, C.S. Tensile, water barrier and antimicrobial properties of PLA/nanoclay composite films. LWT Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Popa, I.; Offenberg, H.; Beldie, C.; Uglea, C.V. Benzocaine modified maleic anhydride copolymers-I. Synthesis and characterization of benzocaine modified poly(maleic anhydride-co-vinyl acetate), poly(maleic anhydride-co-methyl methacrylate) and poly(maleic anhydride-co-styrene). Eur. Polym. J. 1997, 33, 1511–1514. [Google Scholar] [CrossRef]
- Wallach, J.A.; Huang, S.J. Copolymers of itaconic anhydride and methacrylate-terminated poly(lactic acid) macromonomers. Biomacromolecules 2000, 1, 174–179. [Google Scholar] [CrossRef]
- Jeon, I.; Lee, S.W.; Jho, J.Y. Compatibilizing effect of poly(methyl methacrylate-co-maleic anhydride) on the morphology and mechanical properties of polyketone/polycarbonate blends. Macromol. Res. 2019, 27, 821–826. [Google Scholar] [CrossRef]
- Bunkerd, R.; Molloy, R.; Punyodom, W.; Somsunan, R. Reactive blending of poly(L-lactide) and chemically-modified starch grafted with a maleic anhydride-methyl methacrylate copolymer. Macromol. Symp. 2015, 354, 340–346. [Google Scholar] [CrossRef]
- Xu, J.; Shi, W.; Ming, G.; Fei, Y.; Yu, F. Preparation of poly(methyl methacrylate-co-maleic anhydride)/SiO2-TiO2 hybrid materials and their thermo- and photodegradation behaviors. J. Appl. Polym. Sci. 2005, 97, 1714–1724. [Google Scholar] [CrossRef]
- Becker, D.; Hage, E.J.; Pessan, L.A. Synthesis and characterization of poly(methyl methacrylate-co-maleic anhydride) copolymers and its potential as a compatibilizer for amorphous polyamide blends. J. Appl. Polym. Sci. 2007, 106, 3248–3252. [Google Scholar] [CrossRef]
- Xiao, L.; Li, Z.; Dong, J.; Liu, L.; Lei, S.; Zhang, X.; Zhang, H.; Ao, Y.; Dong, A. Fabrication of poly(methyl methacrylate-co-maleic anhydride) copolymers and their kinetic analysis of the thermal degradation. Colloid Polym. Sci. 2015, 293, 2807–2813. [Google Scholar] [CrossRef]
- Dakka, S.M. TG/DTA/MS of poly(methyl methacrylate), the effect of particle size. J. Therm. Anal. Calorim. 2003, 74, 729–734. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Li, J. Synergistic flame retarded poly(methyl methacrylate) by nano-ZrO2 and triphenylphosphate. J. Therm. Anal. Calorim. 2011, 103, 741–746. [Google Scholar] [CrossRef]
- Pal, M.K.; Singh, B.; Guatam, J. Thermal stability and UV-shielding of polymethyl methacrylate and polystyrene modified with calcium carbonate nanoparticles. J. Therm. Anal. Calorim. 2012, 107, 85–96. [Google Scholar] [CrossRef]
- Lomonaco, D.; Maia, F.J.N.; Mazzetto, S.E. Thermal evaluation of cashew nutshell liquid as new bioadditives for poly(methyl methacrylate). J. Therm. Anal. Calorim. 2013, 111, 619–626. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Cauich-Rodriguez, J.V.; Herera-Kao, W.A.; Vazquez-Torres, H.; Marcos-Fernandez, A. Thermal degradation behavior of polymethacrylates containing side groups. Polym. Degrad. Stab. 2008, 93, 1891–1900. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The effect of polymerization conditions on the kinetics and mechanisms of thermal degradation of PMMA. Polym. Degrad. Stab. 2002, 77, 435–439. [Google Scholar] [CrossRef]
- Arshady, R. Suspension, emulsion, and dispersion polymerization: A methodological survey. Colloid Polym. Sci. 1992, 270, 717–732. [Google Scholar] [CrossRef]
- Fitzwater, S.; Chang, H.R.; Parker, H.Y.; Westmoreland, D.G. Propagating radical termination at high conversion in emulsion polymerization of MMA: Rate coefficient determination from ESR data. Macromolecules 1999, 32, 3183–3189. [Google Scholar] [CrossRef]
- Ishigami, A.; Watanabe, K.; Kurose, T.; Ito, H. Physical and morphological properties of tough and transparent PMMA-based blends modified with polyrotaxane. Polymers 2020, 12, 1790. [Google Scholar] [CrossRef]
- Mohamed, D.; Fourati, Y.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Blends of PBAT with plasticized starch for packaging applications: Mechanical properties, rheological behaviour and biodegradability. Ind. Crops Prod. 2020, 144, 112061. [Google Scholar] [CrossRef]
- Jiao, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar]
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. PBAT-based blends and composites. In Biodegradable Polymers, Blends and Composites; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 327–354. [Google Scholar] [CrossRef]
- Burford, T.; William, R.; Samy, M. Biodegradable poly(butylene adipate-co-terephthalate) (PBAT). In Physical Sciences Reviews; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Zhang, M.; Diao, X.; Jin, Y.; Weng, Y. Preparation and characterization of biodegradable blends of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and poly(butylene adipate-co-terephthalate). J. Polym. Eng. 2016, 36, 473–480. [Google Scholar] [CrossRef]
- Sargsyan, A.; Tonoyan, A.; Davtyan, S.; Schick, C. The amount of immobilized polymer in PMMA/SiO2 nanocomposites determined from calorimetric data. Eur. Polym. J. 2007, 43, 3113–3127. [Google Scholar] [CrossRef]
- Priestley, R.D.; Rittigstein, P.; Broadbelt, L.J.; Fukao, K.; Torkelson, J.M. Evidence for the molecular-scale origin of the suppression of physical ageing in confined polymer: Fluorescence and dielectric spectroscopy studies of polymer–silica nanocomposites. J. Phys. Condens. Matter 2007, 19, 205120–205132. [Google Scholar] [CrossRef]
- Chrissafs, K.; Paraskevopoulos, K.M.; Pavlidou, E.; Bikiaris, D. Thermal degradation mechanism of HDPE nanocomposites containing fumed silica nanoparticles. Thermochim. Acta 2009, 485, 65–71. [Google Scholar] [CrossRef]
- Voronin, E.F.; Gun’ko, V.M.; Guzenko, N.V.; Pakhlov, E.M.; Nosach, L.V.; Leboda, R.; Skubiszewska-Zieba, J.; Malysheva, M.L.; Borysenko, M.V.; Chuiko, A.A. Interaction of poly(ethylene oxide) with fumed silica. J. Colloid Interface Sci. 2004, 279, 326–340. [Google Scholar] [CrossRef]
- Chrissafs, K.; Paraskevopoulos, K.M.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal and dynamic mechanical behavior of bionanocomposites: Fumed silica nanoparticles dispersed in poly(vinyl pyrrolidone), chitosan, and poly(vinyl alcohol). J. Appl. Polym. Sci. 2008, 110, 1739–1749. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.J.; Jang, J. Effect of silica nanofillers on isothermal crystallization of poly(vinyl alcohol): In-situ ATR-FTIR study. Polym. Test. 2008, 27, 360–367. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Ansari, S.; Estevez, L.; Hayrapetyan, S.; Giannelis, E.P.; Lai, H.M. Preparation and properties of biodegradable starch-clay nanocomposites. Carbohydr. Polym. 2010, 79, 391–396. [Google Scholar] [CrossRef]
- Glenn, G.; Klamczynski, A.; Ludvik, C.; Chiou, B.S.; Shed, I.; Shey, U.; William, O.; Delilah, W. In situ lamination of starch-based baked foam packaging with degradable films. Packag. Technol. Sci. 2007, 20, 77–85. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. ZnO/PBAT nanocomposite films: Investigation on the mechanical and biological activity for food packaging. Polym. Adv. Technol. 2017, 28, 20–27. [Google Scholar] [CrossRef]
- Muthuraj, R.; Manjusri, M.; Mohanty, A.K. Biodegradable poly(butylene succinate) and poly(butylene adipate-co-terephthalate) blends: Reactive extrusion and performance evaluation. J. Polym. Environ. 2014, 22, 336–349. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. Poly(butylene adipate-co-terephthalate) bionanocomposites: Effect of SnO2 NPs on mechanical, thermal, morphological, and antimicrobial activity. Adv. Compos. Hybrid Mater. 2018, 1, 731–740. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical recycling of packaging plastics: A review. Macromol. Rapid Commun. 2021, 42, e2000415. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, X.; Liu, Z.; Xiang, Y.; Zheng, X. Inter-component synergetic corrosion inhibition mechanism of Passiflora edulia Sims shell extract for mild steel in pickling solution: Experimental, DFT and reactive dynamics investigations. Sustain. Chem. Pharm. 2022, 29, 100821. [Google Scholar] [CrossRef]
- Rabiee, H.; Ge, L.; Zhang, X.; Hu, S.; Li, M.; Yuan, Z. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: A review. Energy Environ. Sci. 2021, 14, 1959–2008. [Google Scholar] [CrossRef]
- Zhou, S.; Zhai, X.; Zhang, R.; Wang, W.; Lim, L.-T.; Hou, H. High-throughput fabrication of antibacterial Starch/PBAT/AgNPs@SiO2 films for food packaging. Nanomaterials 2021, 11, 3062. [Google Scholar] [CrossRef]
- Thiyagu, T.T.; Gokilakrishnan, G.; Uvaraja, V.C.; Maridurai, T.; Arun Prakash, V.R. Effect of SiO2/TiO2 and ZnO nanoparticle on cardanol oil compatibilized PLA/PBAT biocomposite packaging film. Silicon 2022, 14, 3795–3808. [Google Scholar] [CrossRef]
- Dubey, R.S.; Rajesh, Y.B.R.D.; More, M.A. Synthesis and characterization of SiO2 nanoparticles via sol-gel method 725 for industrial applications. Mater. Today Proc. 2015, 2, 3575–3579. [Google Scholar]
- Venkatesan, R.; Rajeswari, N. Preparation, mechanical and antimicrobial properties of SiO2/poly(butylene adipate-co-terephthalate) films for active food packaging. Silicon 2019, 11, 2233–2239. [Google Scholar] [CrossRef]
- Nasirtabrizi, M.H.; Ziaei, Z.M.; Jadid, A.P.; Fatin, L.Z. Synthesis and chemical modification of maleic anhydride copolymers with phthalimide groups. Int. J. Ind. Chem. 2013, 4, 11. [Google Scholar] [CrossRef]
- Venkatesan, R.; Zhang, Y.; Che, G. Preparation of poly(butylene adipate-co-terephthalate)/ZnSnO3 composites with enhanced antimicrobial activity. Compos. Commun. 2020, 22, 100469. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. 2012, 2012, 132424. [Google Scholar] [CrossRef]
- Gao, Z.; Dong, X.; Li, L.; Ren, J. Novel two-dimensional silicon dioxide with in-plane negative poisson’s ratio. Nano Lett. 2017, 17, 772–777. [Google Scholar] [CrossRef]
- Kim, T.G.; An, G.S.; Han, J.S.; Hur, J.U.; Park, B.G.; Choi, S.C. Synthesis of size controlled spherical silica nanoparticles via sol-gel process within hydrophilic solvent. J. Korean Ceram. Soc. 2017, 54, 49–54. [Google Scholar] [CrossRef]
- Zhan, P.; Chen, J.; Zheng, A.; Huang, T.; Shi, H.; Wei, D.; Xu, X.; Guan, Y. Preparation of methyl methacrylate-maleic anhydride copolymers via reactive extrusion by regulating the trommsdorff effect. Mater. Res. Express. 2019, 6, 025315. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. Nanosilica-reinforced poly(butylene adipate-co-terephthalate) nanocomposites: Preparation, characterization and properties. Polym. Bull. 2019, 76, 4785–4801. [Google Scholar] [CrossRef]
- Moustsfa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT bionanocomposites with antimicrobial natural rosin for green packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef] [PubMed]
- Zehetmeyer, G.; Meira, S.M.M.; Scheibel, J.M.; de Oliveira RV, B.; Brandelli, A.; Soares, R.M.D. Influence of melt processing on biodegradable nisin-PBAT films intended for active food packaging applications. J. Appl. Polym. Sci. 2015, 133, 43212. [Google Scholar] [CrossRef]
- Venkatesan, R.; Surya, S.; Raorane, C.J.; Raj, V.; Kim, S.-C. Hydrophilic composites of chitosan with almond gum: Characterization and mechanical, and antimicrobial activity for compostable food packaging. Antibiotics 2022, 11, 1502. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, A.F.; Riquelme, S.; Montoya, L.F.; Sánchez-Sanhueza, G.; Medinam, C.; Rojas, D.; Salazar, F.; Sanhueza, J.P.; Meléndrez, M.F. Influence of the concentration of copper nanoparticles on the thermo-mechanical and antibacterial properties of nanocomposites based on poly(butylene adipate-co-terephthalate). Polym. Compos. 2019, 40, 1870–1882. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Riquelme, S.A.; Sánchez-Sanhueza, G.; Medina, C.; Solís-Pomar, F.; Rojas, D.; Montalba, C.; Melendrez, M.F.; Pérez-Tijerina, E. Comparative study of the antimicrobial effect of nanocomposites and composite based on poly(butylene adipate-co-terephthalate) using Cu and Cu/Cu2O nanoparticles and CuSO4. Nanoscale Res. Lett. 2019, 14, 158. [Google Scholar] [CrossRef]
- Venkatesan, R.; Vanaraj, R.; Alagumalai, K.; Asrafali, S.P.; Raorane, C.J.; Raj, V.; Kim, S.-C. Thermoplastic starch composites reinforced with functionalized POSS: Fabrication, characterization, and evolution of mechanical, thermal and biological activities. Antibiotics 2022, 11, 1425. [Google Scholar] [CrossRef]

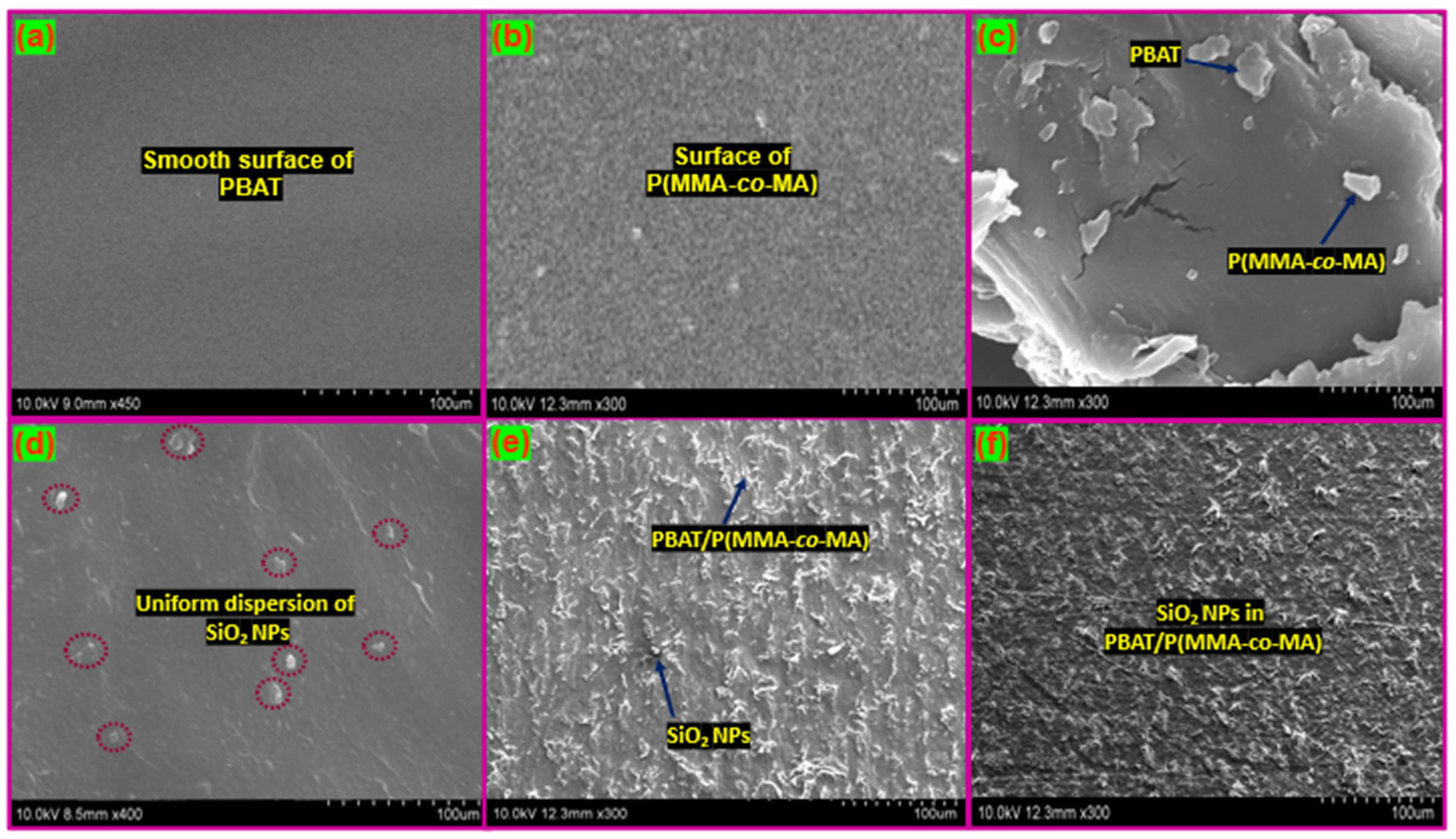
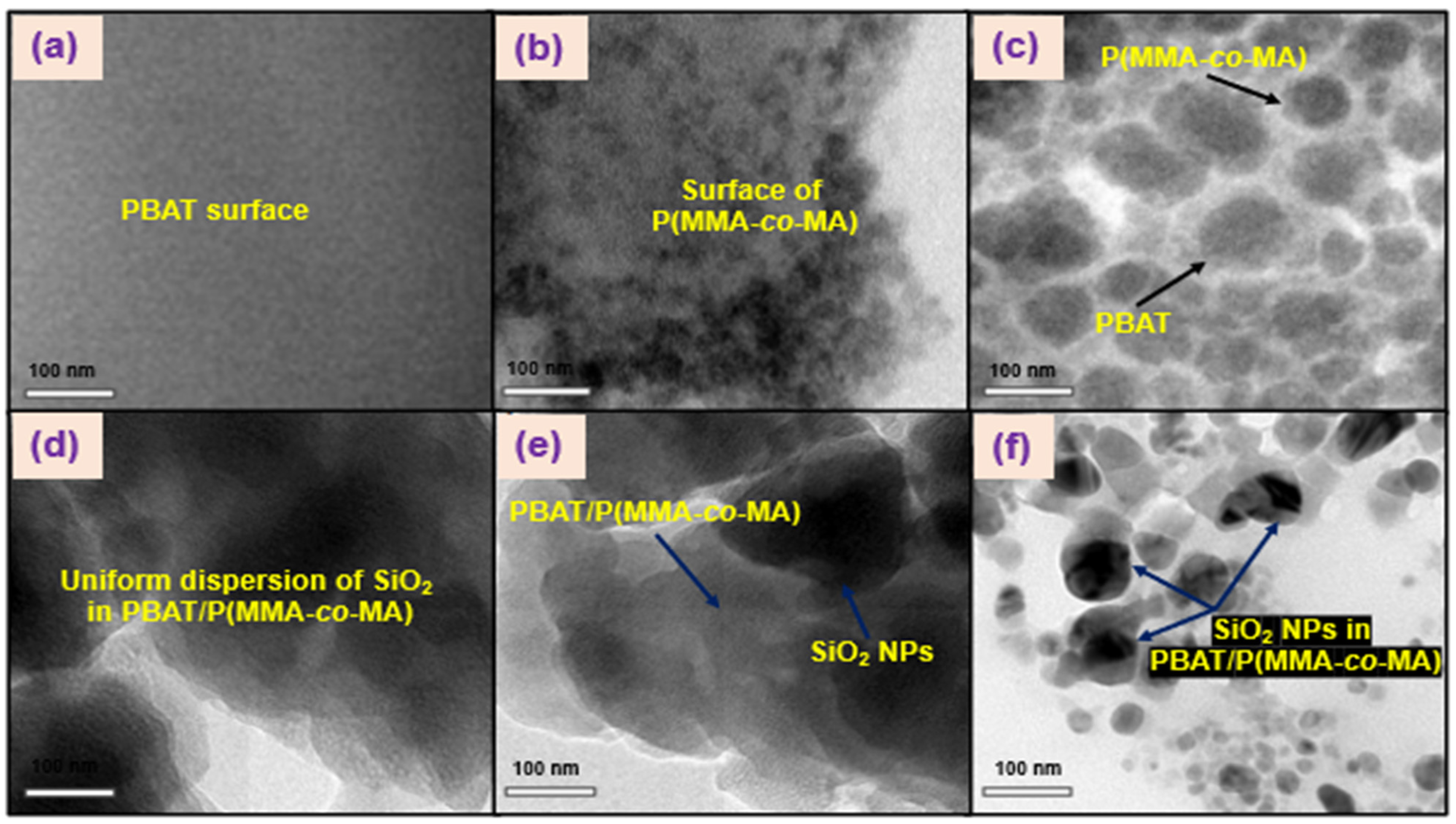
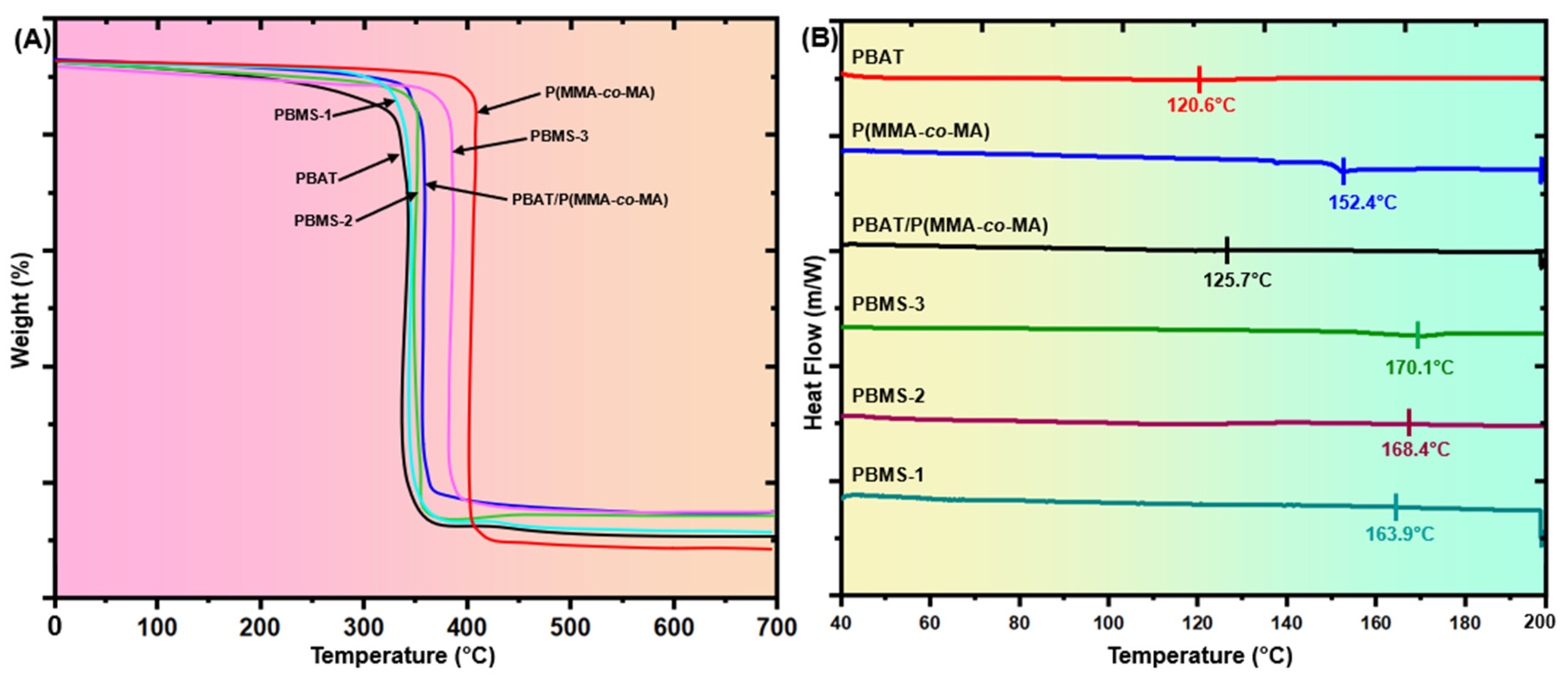
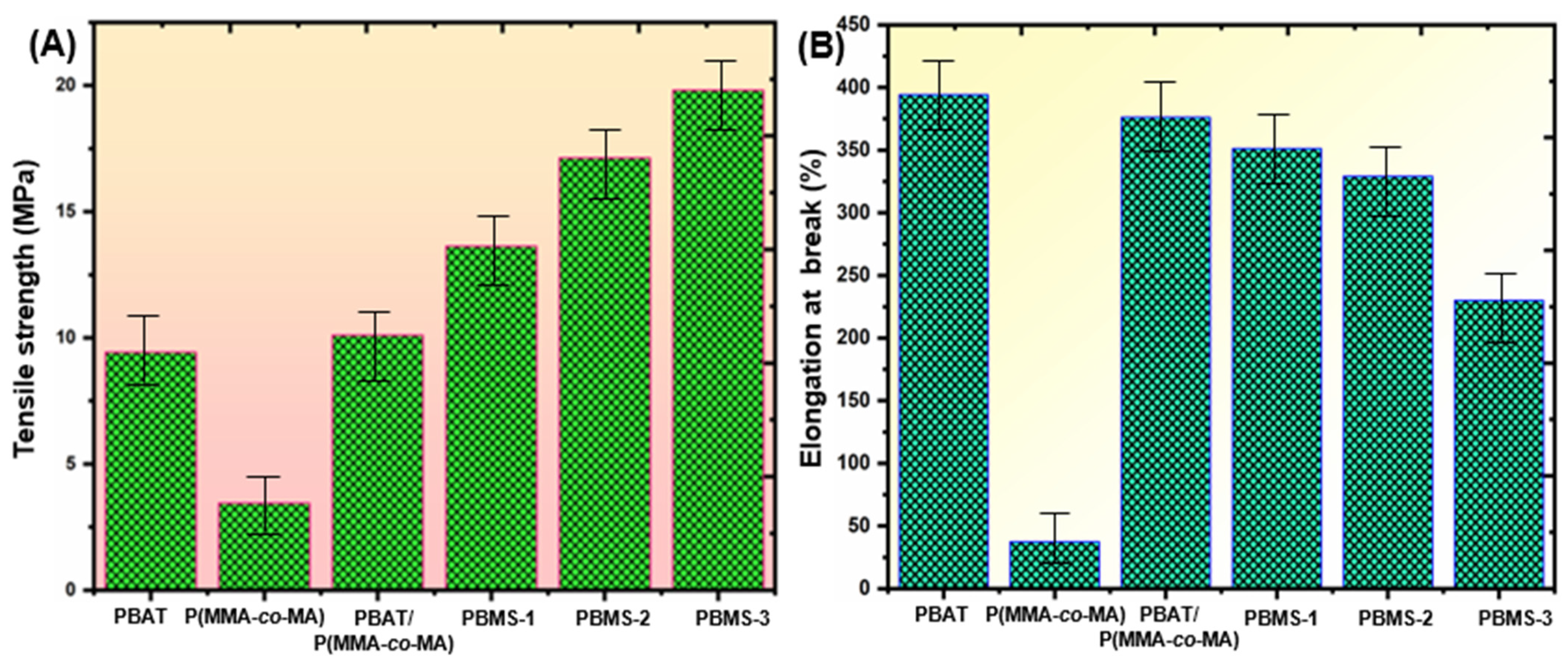

| Samples | PBAT (wt.%) | P(MMA-co-MA) (wt.%) | SiO2 NPs (wt.%) |
|---|---|---|---|
| PBAT | 100.0 | - | - |
| P(MMA-co-MA) | - | 100.0 | - |
| PBAT/P(MMA-co-MA) | 70.0 | 30.0 | - |
| PBMS-1 | 70.0 | 30.0 | 1.0 |
| PBMS-2 | 70.0 | 30.0 | 3.0 |
| PBMS-3 | 70.0 | 30.0 | 5.0 |
| Samples | TGA | DSC | |||
|---|---|---|---|---|---|
| Initial Degradation Temperature (°C) a | Final Degradation Temperature (°C) b | Ash Content (%) c | Tg (°C) | Tm (°C) | |
| PBAT | 322.3 | 358.2 | 3.59 | 49.3 | 120.6 |
| P(MMA-co-MA) | 395.0 | 420.6 | 2.56 | 53.9 | 152.4 |
| PBAT/P(MMA-co-MA) | 368.4 | 385.2 | 1.68 | 56.0 | 125.7 |
| PBMS-1 | 348.1 | 359.4 | 1.13 | 52.1 | 163.9 |
| PBMS-2 | 364.9 | 379.0 | 1.41 | 60.7 | 168.4 |
| PBMS-3 | 382.5 | 403.1 | 2.06 | 65.2 | 170.1 |
| Samples | Oxygen Transmission Rate, (cc m−2 Per 24 h) | Water Vapor Transmission Rate, (g m−2 Per 24 h) |
|---|---|---|
| PBAT | 1137.2 ± 2.5 a | 127.1 ± 2.9 b |
| P(MMA-co-MA) | 860.2 ± 3.0 a | 41.0 ± 2.2 a |
| PBAT/P(MMA-co-MA) | 1095.6 ± 2.0 c | 82.9 ± 3.1 c |
| PBMS-1 | 824.1 ± 3.4 a | 63.2 ± 2.7 c |
| PBMS-2 | 589.3 ± 2.7 b | 41.0 ± 3.0 a |
| PBMS-3 | 318.9 ± 2.0 c | 26.3 ± 2.5 a |
| Strain | Zone of Inhibition in (mm) | |||
|---|---|---|---|---|
| PBAT/P(MMA-co-MA) | PBMS-1 | PBMS-2 | PBMS-3 | |
| S. aureus | - | 9.2 ± 3.84 c | 11.2 ± 1.85 a | 14.0 ± 2.63 b |
| E. coli | - | 10.6 ± 2.35 b | 14.1 ± 3.31 c | 17.9 ± 2.56 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, R.; Alagumalai, K.; Raorane, C.J.; Raj, V.; Shastri, D.; Kim, S.-C. Morphological, Mechanical, and Antimicrobial Properties of PBAT/Poly(methyl methacrylate-co-maleic anhydride)–SiO2 Composite Films for Food Packaging Applications. Polymers 2023, 15, 101. https://doi.org/10.3390/polym15010101
Venkatesan R, Alagumalai K, Raorane CJ, Raj V, Shastri D, Kim S-C. Morphological, Mechanical, and Antimicrobial Properties of PBAT/Poly(methyl methacrylate-co-maleic anhydride)–SiO2 Composite Films for Food Packaging Applications. Polymers. 2023; 15(1):101. https://doi.org/10.3390/polym15010101
Chicago/Turabian StyleVenkatesan, Raja, Krishnapandi Alagumalai, Chaitany Jayprakash Raorane, Vinit Raj, Divya Shastri, and Seong-Cheol Kim. 2023. "Morphological, Mechanical, and Antimicrobial Properties of PBAT/Poly(methyl methacrylate-co-maleic anhydride)–SiO2 Composite Films for Food Packaging Applications" Polymers 15, no. 1: 101. https://doi.org/10.3390/polym15010101
APA StyleVenkatesan, R., Alagumalai, K., Raorane, C. J., Raj, V., Shastri, D., & Kim, S.-C. (2023). Morphological, Mechanical, and Antimicrobial Properties of PBAT/Poly(methyl methacrylate-co-maleic anhydride)–SiO2 Composite Films for Food Packaging Applications. Polymers, 15(1), 101. https://doi.org/10.3390/polym15010101










