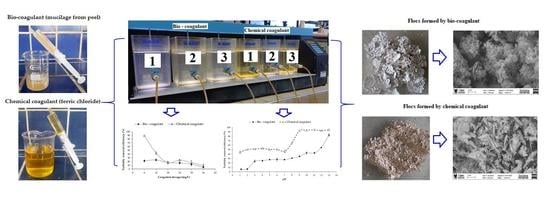
Evaluation of Turbidity and Color Removal in Water Treatment: A Comparative Study between Opuntia ficus-indica Fruit Peel Mucilage and FeCl3
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Synthetic Turbid Water
2.2. OFI Mucilage Biocoagulant
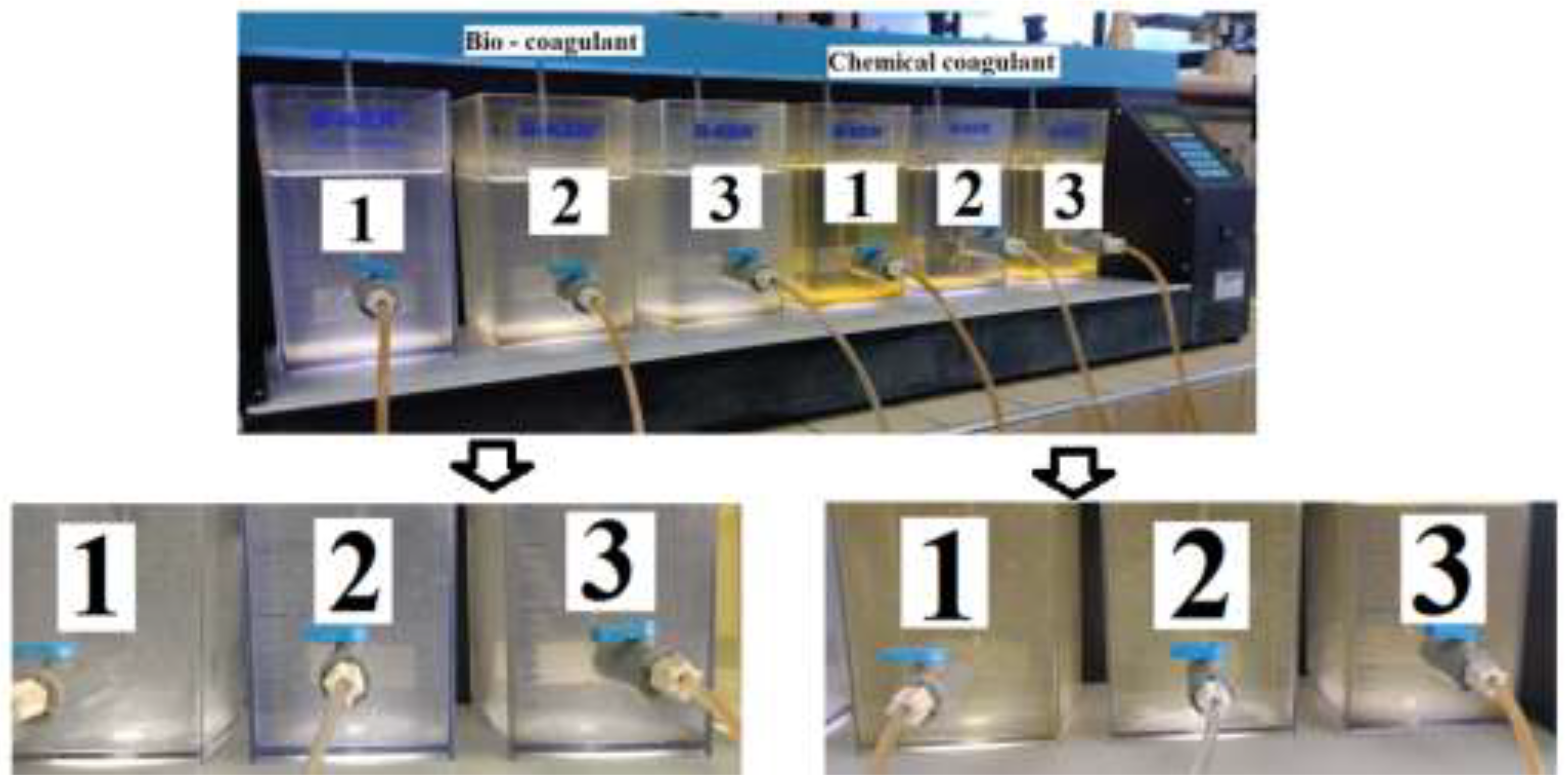
2.3. Coagulation/Flocculation Process
2.4. Floc Characterization
2.4.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4.2. Zeta Potential
2.4.3. Scanning Electron Microscopy (SEM)
2.5. Statistical Analysis
3. Results and Discussion
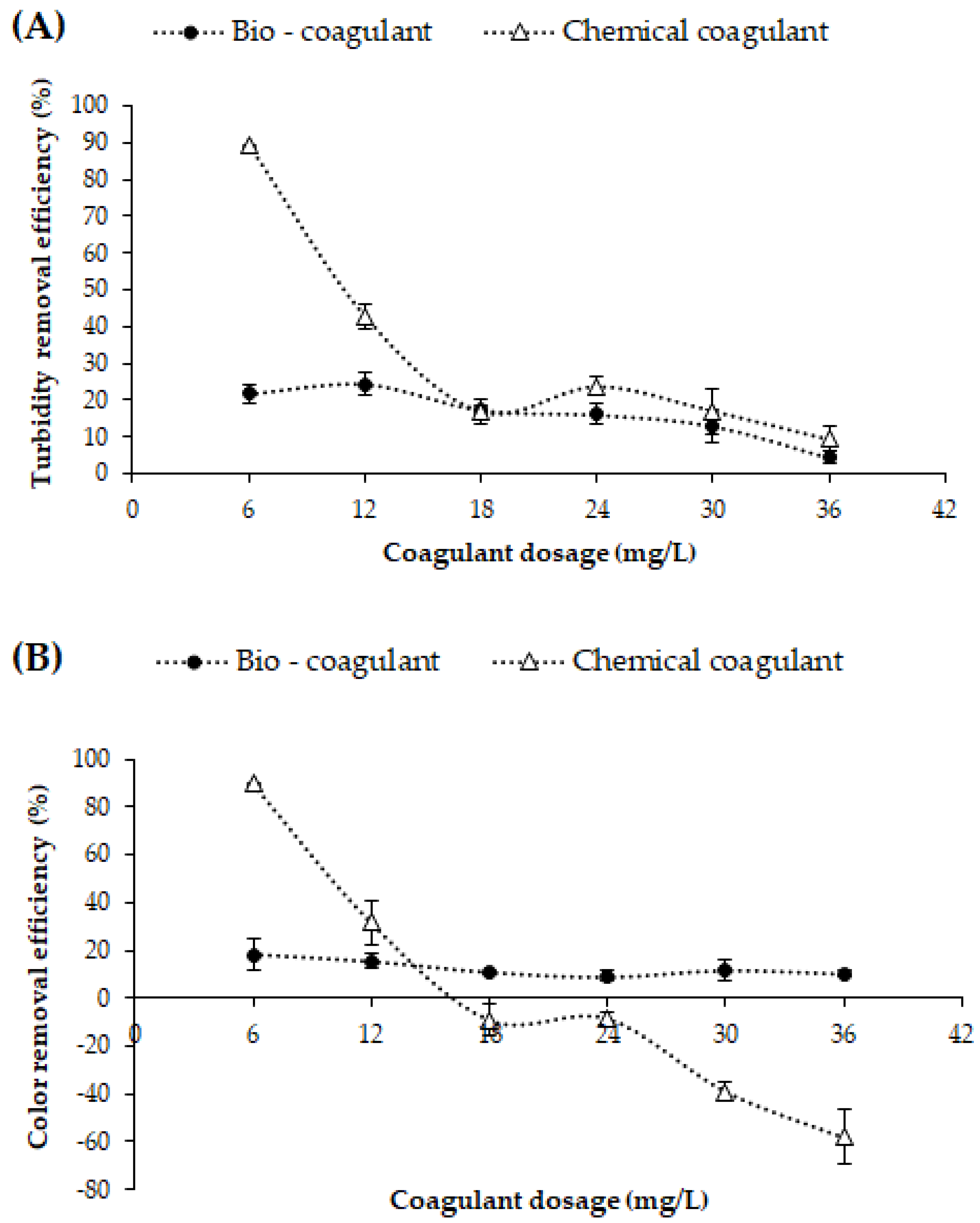
3.1. Dose-Dependent Coagulation Efficiency
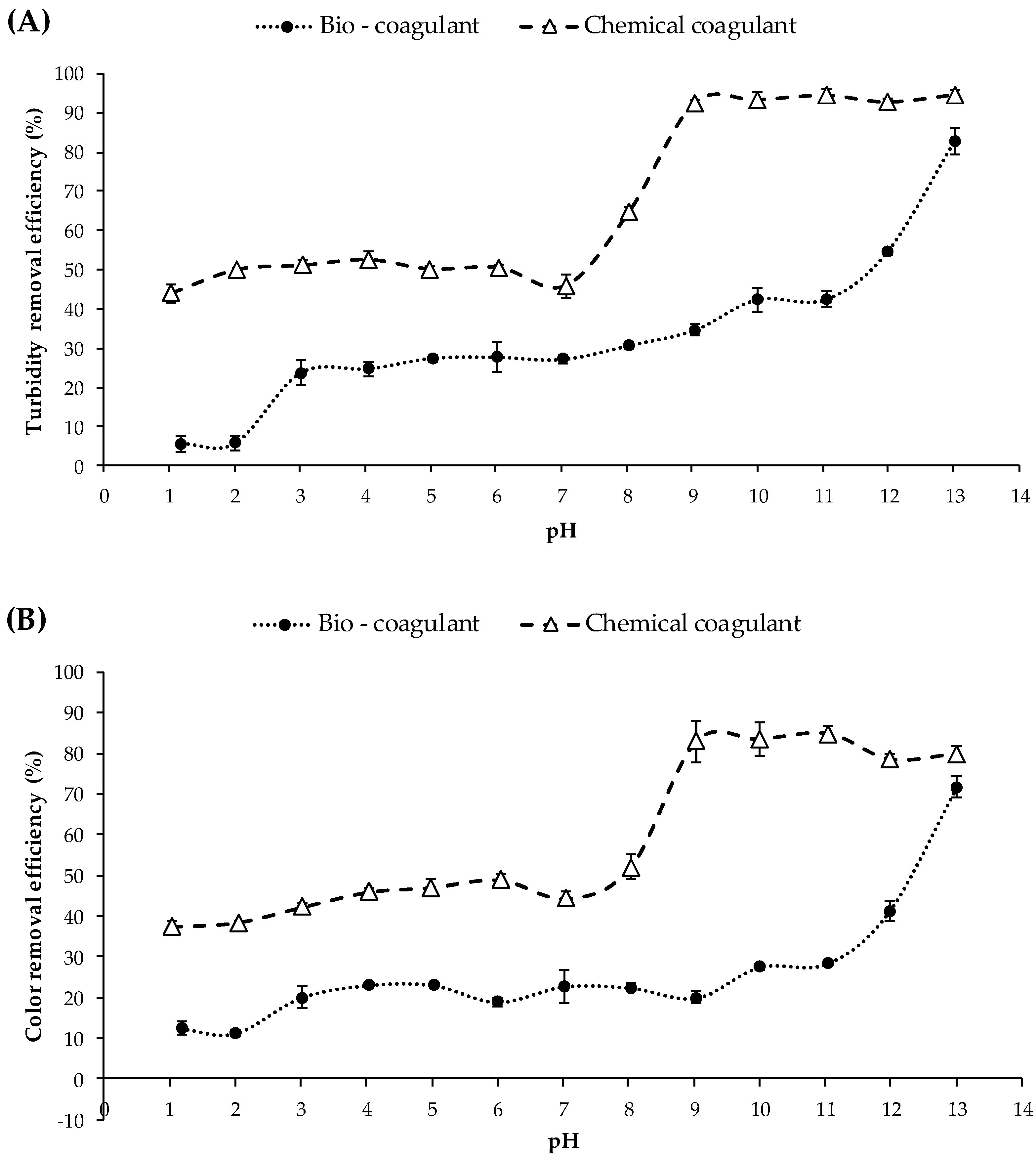
3.2. Effect of pH on Coagulation Efficiency
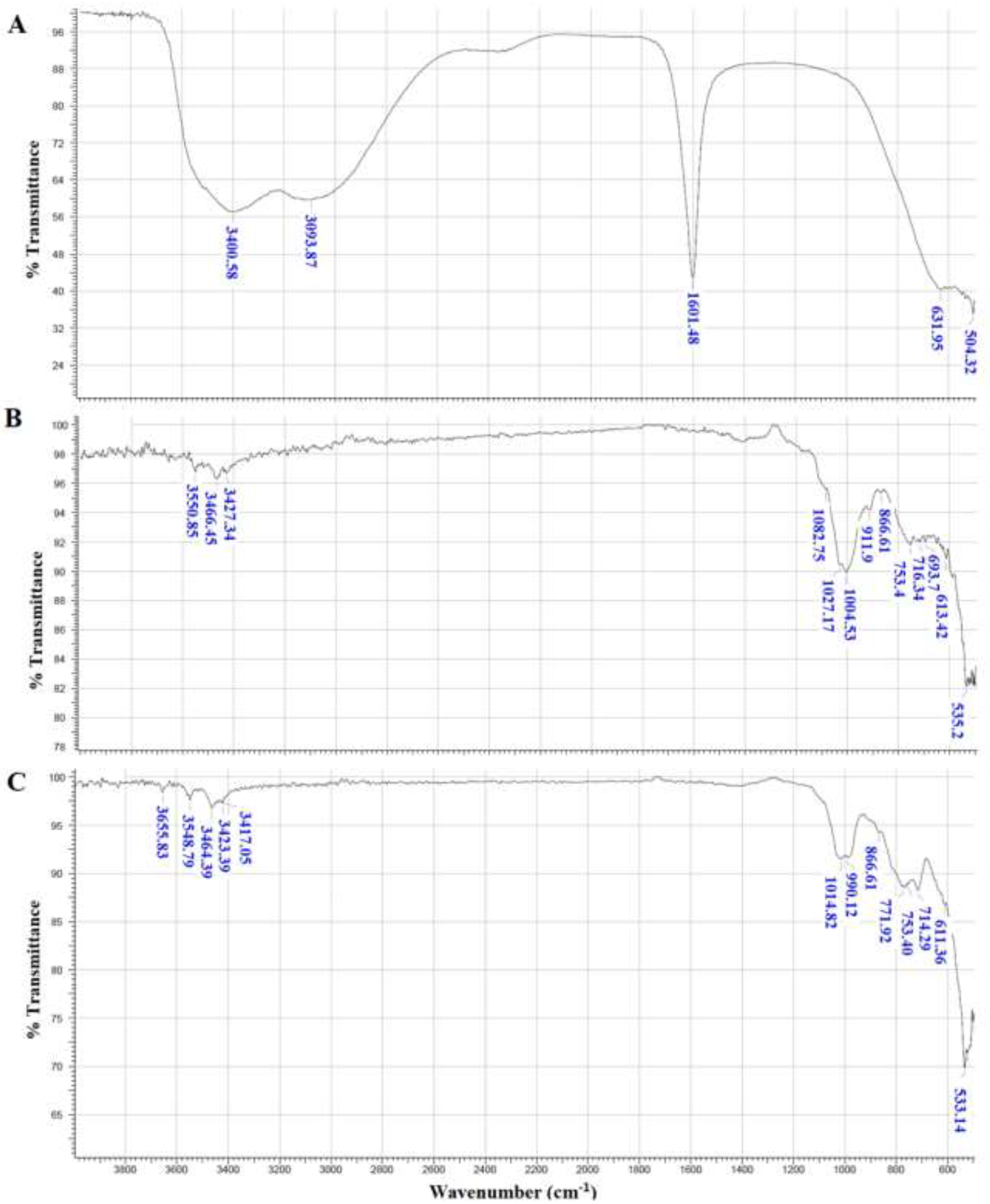
3.3. FTIR Analysis of Dried Flocs
3.4. Zeta Potential of Flocs
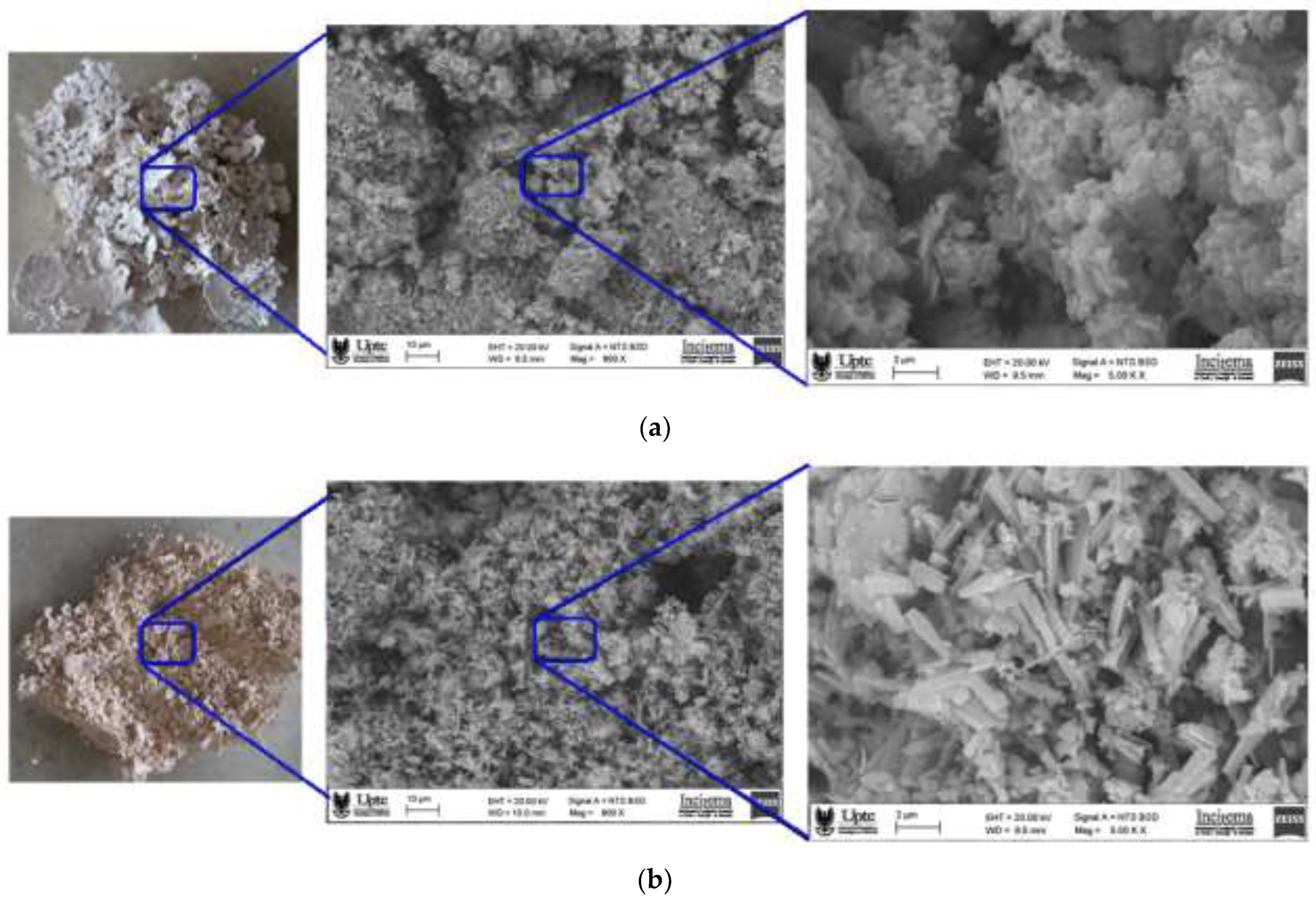
3.5. Microscopic Morphology of Dried Flocs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleem, M.; Bachmann, R.T. A contemporary review on plant-based coagulants for applications in water treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Okoro, B.U.; Sharifi, S.; Mike, A.; Jesson, M.A.; Bridgeman, J. Natural organic matter (NOM) and turbidity removal by plant-based coagulants: A review. J. Environ. Chem. Eng. 2021, 9, 106588. [Google Scholar] [CrossRef]
- Tawakkoly, B.; Alizadehdakhel, A.; Dorosti, F. Evaluation of COD and turbidity removal from compost leachate wastewater using Salvia hispanica as a natural coagulant. Ind. Crops Prod. 2019, 137, 323–331. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Cifuentes, G.R.; Gómez Castaño, J.A. Use of Opuntia ficus-indica Fruit Peel as a Novel Source of Mucilage with Coagulant Physicochemical/Molecular Characteristics. Polymers 2022, 14, 3832. [Google Scholar] [CrossRef]
- Daverey, A.; Tiwari, N.; Dutta, K. Utilization of extracts of Musa paradisiac (banana) peels and Dolichos lablab (Indian bean) seeds as low-cost natural coagulants for turbidity removal from water. Environ. Sci. Pollut. Res. 2019, 26, 34177–34183. [Google Scholar] [CrossRef]
- Afolabi, F.O.; Musonge, P.; Bakare, B.F. Evaluation of lead (II) removal from wastewater using banana peels: Optimization study. Pol. J. Environ. Stud. 2021, 30, 1487–1496. [Google Scholar] [CrossRef]
- Hodúr, C.; Bellahsen, N.; Mikó, E.; Nagypál, V.; Šereš, Z.; Kertész, S. The adsorption of ammonium nitrogen from milking parlor wastewater using pomegranate peel powder for sustainable water, resources, and waste management. Sustainability 2020, 12, 4880. [Google Scholar] [CrossRef]
- Ibisi, N.E.; Asoluka, C.A. Use of agro-waste (Musa paradisiaca peels) as a sustainable biosorbent for toxic metal ions removal from contaminated water. Chem. Int. 2018, 4, 52. [Google Scholar]
- Oyewo, O.A.; Onyango, M.S.; Wolkersdorfer, C. Application of banana peels nanosorbent for the removal of radioactive minerals from real mine water. J. Environ. Radioact. 2016, 164, 369–376. [Google Scholar] [CrossRef]
- Amaya-CruzIz, D.M.; Pérez-Ramírez, I.F.; Delgado-García, J.; Mondragón-Jacobo, C.; Dector-Espinoza, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef]
- Gheribi, R.; Habibi, Y.; Khwaldia, K. Prickly pear peels as a valuable resource of added-value polysaccharide: Study of structural, functional and film forming properties. J. Biol. Macromol. 2019, 126, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Sendra, E.; Carbonell-Barrachina, A.; Legua, P.; Hernández, F. Fatty acid profile of fruits (pulp and peel) and cladodes (young and old) of prickly pear [Opuntia ficus-indica (L.) Mill.] from six Spanish cultivars. J. Food Compos. Anal. 2019, 84, 103294. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Sáenz, C.; Sepúlveda, E. Opuntia spp. Mucilage’s: A Functional Component with Industrial Perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar]
- Lim, K.S.; Sethu, V.; Selvarajoo, A. Natural plant materials as coagulant and flocculants for the treatment of palm oil mill effluent. Mater. Today Proc. 2022, 48, 871–887. [Google Scholar] [CrossRef]
- APHA, 2012; WPCF, Standard Methods For the Examination of Water and Wastewater. American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2012.
- Freitas, T.K.F.S.; Oliveira, V.M.; de Souzaa, M.T.F.; Geraldino, H.C.L.; Almeida, V.C.; Fávaro, S.L.; Garcia, J.C. Optimization of coagulation-flocculation process for treatment of industrial textile wastewater using okra (A. esculentus) mucilage as natural coagulant. Ind. Crops Prod. 2015, 76, 538–544. [Google Scholar] [CrossRef]
- Tan, K.L.; Lim, K.Y.; Chow, Y.N.; Foo, K.Y.; Liew, Y.S.; Desa, S.M.; Yahaya, N.K.E.M.; Noh, M.N.M. Facile preparation of rice husk-derived green coagulant via water-based heatless and salt-free technique for the effective treatment of urban and agricultural runoffs. Ind. Crops Prod. 2022, 178, 114547. [Google Scholar] [CrossRef]
- Yin, C.Y. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef]
- Feng, L.; Liu, J.; Xu, C.; Lu, W.; Li, D.; Zhao, C.; Liu, B.; Li, X.; Khan, S.; Zheng, H.; et al. Better understanding the polymerization kinetics of ultrasonic-template method and new insight on sludge floc characteristics research. Sci. Total Environ. 2019, 689, 546–556. [Google Scholar] [CrossRef]
- Shin, J.Y.; Spinette, R.F.; O’Melia, C.R. Stoichiometry of coagulation revisited. Environ. Sci. Technol. 2008, 42, 2582–2589. [Google Scholar] [CrossRef]
- Wang, J.-P.; Chen, Y.-Z.; Wang, Y.; Yuan, S.-J.; Yu, H.-Q. Optimization of the coagulation-flocculation process for pulp mill wastewater treatment using a combination of uniform design and response surface methodology. Water Res. 2011, 45, 5633–5640. [Google Scholar] [CrossRef]
- Vargas-Solano, S.V.; Rodríguez-González, F.; Martínez-Velarde, R.; Morales-García, S.S.; Jonathan, M.P. Removal of heavy metals present in water from the Yautepec River Morelos Mexico, using Opuntia ficus-indica mucilage. Environ. Adv. 2022, 7, 100160. [Google Scholar] [CrossRef]
- Azamzam, A.A.; Rafatullah, M.; Yahya, E.B.; Ahmad, M.I.; Lalung, J.; Alam, M.; Siddiqui, M.R. Enhancing the Efficiency of Banana Peel Bio-Coagulant in Turbid and River Water Treatment Applications. Water 2022, 14, 2473. [Google Scholar] [CrossRef]
- Chong, K.H.; Kiew, P.L. Potential of banana peels as bio-flocculant for water clarification. Prog. Energy Environ. 2017, 1, 47–56. [Google Scholar]
- Wan, J.; Chakraborty, T.; Xu, C.; Ray, M.B. Treatment train for tailings pond water using Opuntia ficus-indica as coagulant. Sep. Purif. Rev. 2019, 211, 455–488. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y.; Chai, S.-P. A comprehensive study on coagulant performance and floc characterization of natural Cassia obtusifolia seed gum in treatment of raw pulp and paper mill effluent. Ind. Crops Prod. 2014, 61, 317–324. [Google Scholar] [CrossRef]
- Zhou, L.; Han, Y.; Li, W.; Zhu, Y. Study on polymer-bridging flocculation performance of ultrafine specular hematite ore and its high gradient magnetic separation behavior: Description of floc microstructure and flocculation mechanism. Sep. Purif. Technol. 2021, 276, 119304. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.-Y.; Xu, X.-M.; Xu, W.-Y.; Xu, G.-Y. Characterization of floc size, strength and structure in various aluminum coagulants treatment. J. Colloid Interface Sci. 2009, 332, 354–359. [Google Scholar] [CrossRef]
- Mirbahoush, S.M.; Chaibakhsh, N.; Moradi-Shoeili, Z. Highly efficient removal of surfactant from industrial effluents using flaxseed mucilage in coagulation/photo-Fenton oxidation process. Chemosphere 2019, 231, 51–59. [Google Scholar] [CrossRef]
- Chua, S.-C.; Malek, M.A.; Chong, F.-K.; Sujarwo, W.; Ho, Y.-C. Red lentil (Lens culinaris) extract as a novel natural coagulant for turbidity reduction: An evaluation, characterization and performance optimization study. Water 2019, 11, 1686. [Google Scholar] [CrossRef]
- Lim, B.-C.; Lim, J.-W.; Ho, Y.-C. Garden Cress Mucilage as A Potential Emerging Biopolymer for Improving Turbidity Removal in Water Treatment. Process Saf. Environ. Prot. 2018, 119, 233–241. [Google Scholar] [CrossRef]
- Choudhary, M.; Ray, M.B.; Neogi, S. Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water. Sep. Purif. Technol. 2019, 209, 714–724. [Google Scholar] [CrossRef]
- Lanan, F.A.B.M.; Selvarajoo, A.; Sethu, V.; Arumugasamy, S.K. Utilisation of natural plant-based fenugreek (Trigonella foenum-graecum) coagulant and okra (Abelmoschus escluentus) flocculant for palm oil mill effluent (POME) treatment. J. Environ. Chem. Eng. 2021, 9, 104667. [Google Scholar] [CrossRef]
- Zong, Y.; Li, Y.; Jin, X.; Shang, Y.; Jin, P.; Wang, X.C. Enhanced phosphate removal by coral reef-like flocs: Coagulation performance and mechanisms. Sep. Purif. Technol. 2022, 299, 121690. [Google Scholar] [CrossRef]
- Nharingo, T.; Moyo, M. Application of Opuntia ficus-indica in bioremediation of wastewaters. A critical review. J. Environ. Manag. 2016, 166, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Shak, K.P.Y.; Wu, T.Y. Coagulation–flocculation treatment of high-strength agro-industrial wastewater using natural Cassia obtusifolia seed gum: Treatment efficiencies and flocs characterization. Chem. Eng. J. 2014, 256, 293–305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Gómez Castaño, J.A.; Cifuentes, G.R. Evaluation of Turbidity and Color Removal in Water Treatment: A Comparative Study between Opuntia ficus-indica Fruit Peel Mucilage and FeCl3. Polymers 2023, 15, 217. https://doi.org/10.3390/polym15010217
Otálora MC, Wilches-Torres A, Lara CR, Gómez Castaño JA, Cifuentes GR. Evaluation of Turbidity and Color Removal in Water Treatment: A Comparative Study between Opuntia ficus-indica Fruit Peel Mucilage and FeCl3. Polymers. 2023; 15(1):217. https://doi.org/10.3390/polym15010217
Chicago/Turabian StyleOtálora, Maria Carolina, Andrea Wilches-Torres, Carlos Rafael Lara, Jovanny A. Gómez Castaño, and Gabriel Ricardo Cifuentes. 2023. "Evaluation of Turbidity and Color Removal in Water Treatment: A Comparative Study between Opuntia ficus-indica Fruit Peel Mucilage and FeCl3" Polymers 15, no. 1: 217. https://doi.org/10.3390/polym15010217
APA StyleOtálora, M. C., Wilches-Torres, A., Lara, C. R., Gómez Castaño, J. A., & Cifuentes, G. R. (2023). Evaluation of Turbidity and Color Removal in Water Treatment: A Comparative Study between Opuntia ficus-indica Fruit Peel Mucilage and FeCl3. Polymers, 15(1), 217. https://doi.org/10.3390/polym15010217