Study on the Effectiveness of Two Biopolymer Coagulants on Turbidity and Chemical Oxygen Demand Removal in Urban Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Biopolymer Coagulants Production and Characterization
2.3. Experimental Design and Jar Test
2.4. Water Physicochemical Characterization
2.5. Statistical Analysis
3. Results and Discussion
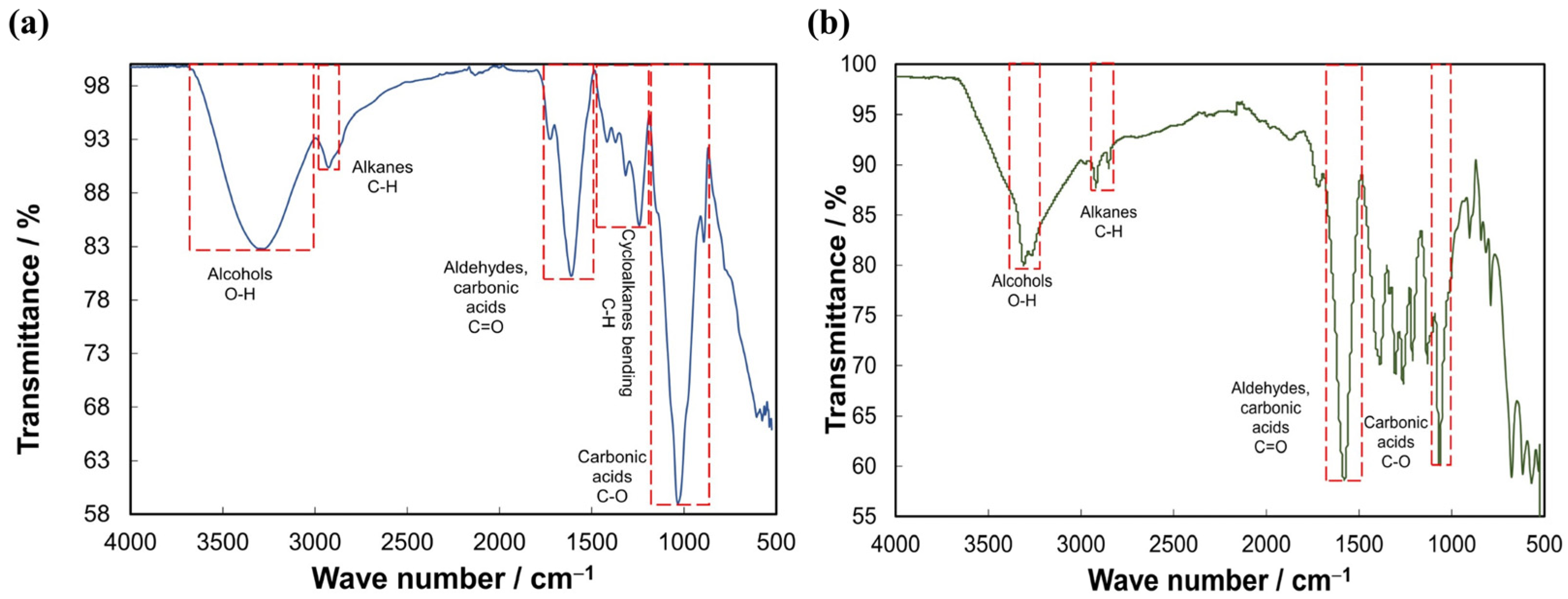
3.1. Characterization of Produced Biopolymer Coagulants
3.2. Physicochemical Parameters of Urban Wastewater before and after Coagulation Treatment
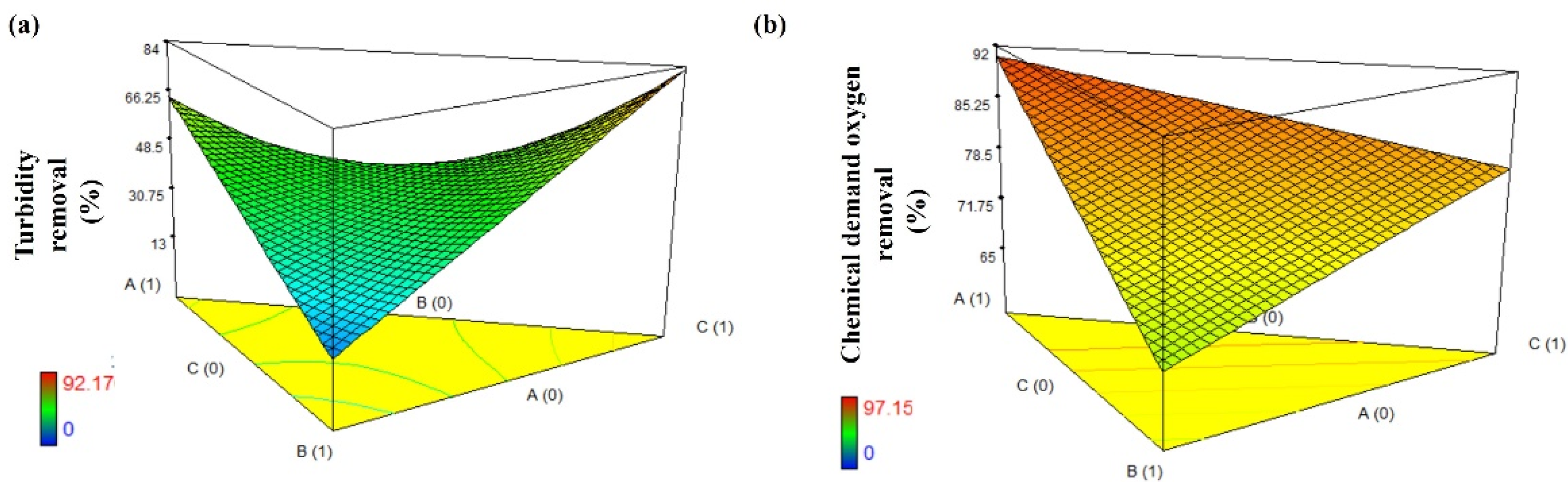
3.3. ANOVA and Optimization Analysis Results
3.4. Effectiveness of the Biopolymer Coagulants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Water Scarcity—One of the Greatest Challenges of Our Time. Available online: https://www.fao.org/fao-stories/article/en/c/1185405/ (accessed on 25 August 2022).
- World Health Organization. Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 29 August 2022).
- United Nations Development Programme. Sustainable Development Goals. Available online: https://www.undp.org/sustainable-development-goals?utm_source=EN&utm_medium=GSR&utm_content=US_UNDP_PaidSearch_Brand_English&utm_campaign=CENTRAL&c_src=CENTRAL&c_src2=GSR&gclid=Cj0KCQjw9ZGYBhCEARIsAEUXITVeLM8WQ6UnLErYFmkOBA6Ed8iFuKEBBT29Nmj0CcRP4iwFp0UzSXgaAtIkEALw_wcB (accessed on 29 August 2022).
- Ang, W.L.; Mohammad, A.W. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Jiang, J.-Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.K.; Ismail, N.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Bin Omar, M.A.; Mohd Zin, N.S.B.; Mohd Salleh, S.N.A.B. A review on performance of chemical, natural and composite coagulant. Int. J. Eng. Technol. 2018, 7, 56–60. [Google Scholar]
- Turunen, J.; Karppinen, A.; Ihme, R. Effectiveness of biopolymer coagulants in agricultural wastewater treatment at two contrasting levels of pollution. SN Appl. Sci. 2019, 1, 210. [Google Scholar] [CrossRef]
- Saleem, M.; Bachmann, R.T. A contemporary review on plant-based coagulants for applications in water treatment. J. Ind. Eng. Chem. 2019, 72, 281e297. [Google Scholar] [CrossRef]
- Hassan, K.F.; Obeid, S.H. Efficiency of fungi suspension spores as Biocoagulants for suspended solid sedimentation in wastewater. Int. J. Sci. Eng. Res. 2016, 7, 578–585. [Google Scholar]
- Abdul Hamid, S.H.; Lananan, F.; Din, W.N.S.; Lam, S.S.; Khatoon, H.; Endut, A.; Jusoh, A. Harvesting microalgae, Chlorella sp. by bio-flocculation of Moringa oleifera seed derivatives from aquaculture wastewater phytoremediation. Int. Biodeterior. Biodegrad. 2014, 95, 270–275. [Google Scholar] [CrossRef]
- Keogh, M.B.; Elmusharaf, K.; Borde, P.; McGuigan, K.G. Evaluation of the natural coagulant Moringa oleifera as a pretreatment for SODIS in contaminated turbid water. Sol. Energy 2017, 158, 448–454. [Google Scholar] [CrossRef]
- Jadhav, M.V.; Mahajan, Y.S. Investigation of the performance of chitosan as a coagulant for flocculation of local clay suspensions of different turbidities. KSCE J. Civ. Eng. 2013, 17, 328–334. [Google Scholar] [CrossRef]
- Othmani, B.; Rasteiro, M.G.; Khadhraoui, M. Toward green technology: A review on some efficient model plant-based coagulants/flocculants for freshwater and wastewater remediation. Clean Technol. Environ. Policy 2020, 22, 1025–1040. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Tang, X.; Jiang, J.; He, Q.; Xiong, Z.; Zheng, H. Biopolymer-based flocculants: A review of recent technologies. Environ. Sci. Pollut. Res. Int. 2021, 28, 46934–46963. [Google Scholar] [CrossRef]
- Graham, N.; Gang, F.; Fowler, G.; Watts, M. Characterisation and coagulation performance of a tannin-based cationic polymer: A preliminary assessment. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 327, 9–16. [Google Scholar] [CrossRef]
- Guerrero, P.C.; Majure, L.C.; Cornejo-Romero, A.; Hernández-Hernández, T. Phylogenetic relationships and evolutionary trends in the Cactus family. J. Hered. 2019, 110, 4–21. [Google Scholar] [CrossRef]
- Bernardino-Nicanor, A.; Mancera-Castro, P.; Ramírez-Ortíz, M.E.; Acosta-García, G.; González-Cruz, L. Quality of the parenchymatous tissue of Opuntia and its use in the development of set yogurt. Int. J. Gastron. Food Sci. 2021, 24, 100344. [Google Scholar] [CrossRef]
- Gomes Honório, I.C.; Bertoni, B.W.; de Campos Telles, M.P.; dos Santos Braga, R.; de Castro França, S.; da Silva Coppede, J.; Conde Correa, V.S.; Felizola Diniz Filho, J.A.; Soares Pereira, A.M. Genetic and chemical diversity of Uncaria tomentosa (Willd. ex. Schult.) DC. in the Brazilian Amazon. PLoS ONE 2017, 12, e0177103. [Google Scholar]
- Rojas-Sandoval, J. Invasive Species Compendium, Dolichandra unguis-cati (Cat’s Claw Creeper). Available online: https://www.cabi.org/isc/datasheet/9159 (accessed on 1 September 2022).
- Custodio, R. Información de Salud Natural, Plantas Medicinales, Uña de Gato. Información de Salud Natural. Available online: https://www.casapia.com/blog/plantas-medicinales/plantas-medicinales-utiles-en-el-tratamiento-de-afecciones-osteoarticulares-articulo-informativo.html (accessed on 29 August 2022).
- Sáenz, C.; Berger, H.; Corrales García, J.; Galletti, L.; García de Cortázar, V.; Higuera, I.; Mondragón, C.; Rodríguez-Félix, A.; Sepúlveda, E.; Varnero, M.T. Boletín de Servicios Agrícolas de la FAO 162, Utilización Agroindustrial del Nopal. Available online: https://repositorio.uchile.cl/bitstream/handle/2250/120301/Utilizacion-agroindustrial-del-nopal.pdf?sequence=1 (accessed on 1 September 2022).
- Secretaría de Economía. Norma Mexicana NMX-AA-030/2-SCFI-2011 Análisis de Agua—Determinación de la Demanda Química de Oxígeno en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba—Parte 2—Determinación del Índice de la Demanda Química de Oxígeno—Método de Tubo Sellado a Pequeña Escala. Available online: https://www.gob.mx/cms/uploads/attachment/file/166775/NMX-AA-030-2-SCFI-2011.pdf (accessed on 19 September 2022).
- Okaiyeto, K.; Nwodo, U.U.; Okoli, S.A.; Mabinya, L.V.; Okoh, A.I. Implications for public health demands alternatives to inorganic and synthetic flocculants: Bioflocculants as important candidates. MicrobiologyOpen 2016, 5, 177–211. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S. Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment—A review. J. Water Process Eng. 2021, 42, 102096. [Google Scholar] [CrossRef]
- Chang, R. Fisicoquímica, 3rd ed.; McGraw-Hill/Interamericana: Ciudad de Mexico, Mexico, 2008; pp. 724–725. [Google Scholar]
- Skoog, D.A.; Holler, F.J.; Nieman, T.A. Principios de Análisis Instrumental, 6th ed.; Cengage Learning: Ciudad de Mexico, Mexico, 2008; pp. 461–463. [Google Scholar]
- Bernardino-Nicanor, A.; Montañez-Soto, J.L.; Conde-Barajas, E.; Negrete-Rodríguez, M.D.L.L.X.; Teniente-Martínez, G.; Vargas-León, E.A.; Juarez-Goiz, J.M.S.; Acosta-García, G.; González-Cruz, L. Spectroscopic and structural analyses of Opuntia Robusta mucilage and its potential as an edible coating. Coat 2018, 8, 466. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality. Available online: https://apps.who.int/iris/bitstream/handle/10665/204411/9789241547611_eng.pdf?sequence=1 (accessed on 29 August 2022).
- United States Environmental Protection Agency. Water: Monitoring & Assessment, 5.9 Conductivity. Available online: https://archive.epa.gov/water/archive/web/html/vms59.html (accessed on 29 August 2022).
- Secretaría de Medio Ambiente y Recursos Naturales. Norma Oficial Mexicana NOM-001-SEMARNAT-2021, Que Establece los Límites Permisibles de Contaminantes en las Descargas de Aguas Residuales en Cuerpos Receptores Propiedad de la Nación. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5645374&fecha=11/03/2022 (accessed on 29 August 2022).
- Mwewa, B.; Stopić, S.; Ndlovu, S.; Simate, G.S.; Xakalashe, B.; Friedrich, B. Synthesis of Poly-Alumino-Ferric Sulphate Coagulant from Acid Mine Drainage by Precipitation. Metals 2019, 9, 1166. [Google Scholar] [CrossRef]
- Secretaría de Salud. Norma Oficial Mexicana NOM-127-SSA1-2021, Agua Para Uso y Consumo Humano, Límites Permisibles de la Calidad del Agua. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5650705&fecha=02/05/2022 (accessed on 29 August 2022).
- Osode, A.N.; Okoh, A.I. Impact of Discharged Wastewater Final Effluent on the Physicochemical Qualities of a Receiving Watershed in a Suburban Community of the Eastern Cape Province. Clean Soil Air Water 2009, 37, 938–944. [Google Scholar] [CrossRef]
- Marobhe, N.J.; Dalhammar, G.; Gunaratna, K. Simple and Rapid Methods for Purification and Characterization of Active Coagulants from the Seeds of Vigna unguiculata and Parkinsonia aculeata. Environ. Technol. 2007, 28, 671–681. [Google Scholar] [CrossRef]
- Febrina, W.; Mesra, T.; Hendra. Optimum Dosage of Coagulant and Flocculant on Sea Water Purification Process. IOP Conf. Ser. Earth Environ. Sci. 2020, 469, 012023. [Google Scholar] [CrossRef]
- Gandiwa, B.I.; Moyo, L.B.; Ncube, S.; Mamvura, T.A.; Hlabangana, N. Optimisation of using a blend of plant based natural and synthetic coagulants for water treatment: (Moringa Oleifera-Cactus Opuntia-Alum Blend). S. Afr. J. Chem. Eng. 2020, 34, 158–164. [Google Scholar] [CrossRef]
- Batista Magalhães, E.R.; Fonseca de Menezes, N.N.; Leite Silva, F.; Alves Garrido, J.W.; dos Santos Bezerra Sousa, M.A.; Silvino dos Santos, E. Effect of oil extraction on the composition, structure, and coagulant effect of Moringa oleifera seeds. J. Clean. Prod. 2021, 279, 123902. [Google Scholar] [CrossRef]
- Dhivya, S.; Ramesh, S.T.; Gandhimathi, R.; Nidheesh, P.V. Performance of Natural Coagulant Extracted from Plantago ovata Seed for the Treatment of Turbid Water. Water Air Soil Pollut. 2017, 228, 423. [Google Scholar] [CrossRef]
- Ayangunna, R.R.; Giwa, S.O.; Giwa, A. Coagulation-Flocculation Treatment of Industrial Wastewater Using Tamarind Seed Powder. Int. J. Chemtech Res. 2016, 9, 771–780. [Google Scholar]
- Kakoi, B.; Kaluli, J.W.; Ndiba, P.; Thiong’o, G. Banana pith as a natural coagulant for polluted river water. Ecol. Eng. 2016, 95, 699–705. [Google Scholar] [CrossRef]
- Abidin, Z.Z.; Ismail, N.; Yunus, R.; Ahamad, I.S.; Idris, A. A preliminary study on Jatropha curcas as coagulant in wastewater treatment. Environ. Technol. 2011, 32, 71–77. [Google Scholar] [CrossRef]
- Carpinteyro-Urban, S.; Vaca, M.; Torres, L.G. Can Vegetal Biopolymers Work as Coagulant-Flocculant Aids in the Treatment of High-Load Cosmetic Industrial Wastewaters? Water Air Soil Pollut. 2012, 223, 4925–4936. [Google Scholar] [CrossRef]
- Mohd-Salleh, S.N.A.; Mohd-Zin, N.S.; Othman, N. A review of Wastewater Treatment using Natural Material and Its Potential as Aid and Composite Coagulant. Sains Malays. 2019, 48, 155–164. [Google Scholar] [CrossRef]
- Yonge, D, A Comparison of Aluminum and Iron-based Coagulants for Treatment of Surface Water in Sarasota County, Florida. Available online: https://stars.library.ucf.edu/etd/2435 (accessed on 22 September 2022).
- Henrique Silva, S.; Oliveira Neves, I.C.; Leite Oliveira, N.; Freitas de Oliveira, A.C.; Teixeira Lago, A.M.; de Oliveira Giarola, T.M.; Vilela de Resende, J. Extraction processes and characterization of the mucilage obtained from green fruits of Pereskia aculeata Miller. Ind. Crops Prod. 2019, 140, 111716. [Google Scholar] [CrossRef]
| Trial Number | Components’ Proportion Values | Dose (mg/L) | ||
|---|---|---|---|---|
| Uncaria tomentosa Biopolymer Coagulant | Opuntia robusta Biopolymer Coagulant | Aluminum Sulfate | ||
| 1 | 0.00 | 0.00 | 1.00 | 800 |
| 2 | 0.67 | 0.33 | 0.00 | 200 |
| 3 | 0.67 | 0.33 | 0.00 | 800 |
| 4 | 0.67 | 0.00 | 0.33 | 200 |
| 5 | 0.67 | 0.00 | 0.33 | 800 |
| 6 | 0.00 | 0.67 | 0.33 | 200 |
| 7 | 0.00 | 0.67 | 0.33 | 800 |
| 8 | 0.33 | 0.33 | 0.33 | 200 |
| 9 | 0.33 | 0.33 | 0.33 | 800 |
| 10 | 0.33 | 0.67 | 0.00 | 200 |
| 11 | 0.33 | 0.67 | 0.00 | 800 |
| 12 | 0.33 | 0.00 | 0.67 | 200 |
| 13 | 0.33 | 0.00 | 0.67 | 800 |
| 14 | 0.00 | 0.33 | 0.67 | 200 |
| 15 | 0.00 | 0.33 | 0.67 | 800 |
| 16 | 1.00 | 0.00 | 0.00 | 200 |
| 17 | 1.00 | 0.00 | 0.00 | 800 |
| 18 | 0.00 | 1.00 | 0.00 | 200 |
| 19 | 0.00 | 1.00 | 0.00 | 800 |
| 20 | 0.00 | 0.00 | 1.00 | 200 |
| 21 | 0.50 | 0.50 | 0.00 | 500 |
| 22 | 0.00 | 0.00 | 1.00 | 500 |
| 23 | 1.00 | 0.00 | 0.00 | 500 |
| 24 | 0.00 | 1.00 | 0.00 | 500 |
| 25 | 0.50 | 0.00 | 0.50 | 500 |
| 26 | 0.00 | 0.67 | 0.33 | 200 |
| 27 | 0.00 | 0.67 | 0.33 | 800 |
| 28 | 0.33 | 0.33 | 0.33 | 200 |
| 29 | 0.33 | 0.33 | 0.33 | 800 |
| 30 | 0.00 | 0.33 | 0.67 | 200 |
| Parameter | Obtained Value | Reference Value | Unit |
|---|---|---|---|
| Temperature | 10.00 ± 0.50 | <25 a | °C |
| pH | 7.71 ± 0.02 | 6.5–8.5 a | - |
| Electrical conductivity | 1256 ± 1.00 | 150–500 b | µS/cm |
| Turbidity | 56.1 ± 0.50 | <5 a | NTU |
| Chemical oxygen demand | 163.1 ± 2.00 | 60–150 c | mg/L |
| Trial Number | Doses (mg/L) | Change Values (Final−Initial) | Turbidity Removal (%) | Chemical Oxygen Demand Removal (%) | ||
|---|---|---|---|---|---|---|
| Temperature (°C) | pH | Electrical Conductivity (µS) | ||||
| 1 | 800 | 0.0 | −1.2 | +1030.5 | 88.5 | 87.2 |
| 2 | 200 | −0.5 | −0.1 | +620.8 | 24.9 | 61.1 |
| 3 | 800 | 0.0 | −0.3 | +671.1 | 0.0 | 0.0 |
| 4 | 200 | −0.5 | −0.3 | +691.6 | 58.9 | 72.7 |
| 5 | 800 | −0.5 | −0.7 | +723.2 | 32.6 | 63.3 |
| 6 | 200 | −0.5 | −0.3 | +691.6 | 39.5 | 67.1 |
| 7 | 800 | 0.0 | −0.7 | +712.1 | 88.1 | 83.0 |
| 8 | 200 | 0.0 | −0.4 | +694.4 | 42.5 | 69.7 |
| 9 | 800 | 0.0 | −0.7 | +754.9 | 17.7 | 51.2 |
| 10 | 200 | 0.0 | −0.2 | +632.0 | 38.9 | 66.3 |
| 11 | 800 | 0.0 | −0.3 | +661.8 | 1.6 | 34.5 |
| 12 | 200 | +0.5 | −0.3 | +680.4 | 52.1 | 70.0 |
| 13 | 800 | +0.5 | −1.2 | +591.0 | 0.0 | 0.0 |
| 14 | 200 | 0.0 | −0.6 | +696.8 | 67.6 | 72.7 |
| 15 | 800 | 0.0 | −1.1 | +916.9 | 20.0 | 58.4 |
| 16 | 200 | −0.5 | −0.2 | +600.4 | 17.2 | 39.4 |
| 17 | 800 | 0.0 | −0.6 | +680.4 | 0.0 | 0.0 |
| 18 | 200 | 0.0 | −0.1 | +691.6 | 42.3 | 69.6 |
| 19 | 800 | −0.5 | −0.2 | +658.1 | 0.00 | 0.0 |
| 20 | 200 | 0.0 | −0.6 | +704.6 | 97.2 | 97.2 |
| 21 | 500 | 0.0 | −0.2 | +650.6 | 28.3 | 62.9 |
| 22 | 500 | 0.0 | −0.9 | +916.9 | 88.6 | 92.6 |
| 23 | 500 | 0.0 | −0.3 | +669.3 | 15.2 | 37.4 |
| 24 | 500 | −0.5 | −0.2 | +643.2 | 39.2 | 64.9 |
| 25 | 500 | 0.0 | −0.6 | +706.5 | 7.9 | 36.2 |
| 26 | 200 | 0.0 | −0.3 | +680.4 | 40.1 | 67.8 |
| 27 | 800 | 0.0 | −0.7 | +712.1 | 0.0 | 0.0 |
| 28 | 200 | 0.0 | −0.4 | +695.3 | 43.0 | 69.5 |
| 29 | 800 | 0.0 | −0.8 | +827.5 | 18.4 | 47.5 |
| 30 | 200 | 0.0 | −0.6 | +695.3 | 67.2 | 72.6 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 14,996.75 | 4 | 3749.19 | 9.70 | <0.0001 |
| Linear Mixture | 9722.79 | 2 | 4861.39 | 12.58 | 0.0002 |
| A × C | 2207.85 | 1 | 2207.85 | 5.71 | 0.0247 |
| A × D | 3066.12 | 1 | 3066.12 | 7.93 | 0.0093 |
| Residual | 9661.22 | 25 | 386.45 | ||
| Lack of Fit | 5781.05 | 20 | 289.05 | 0.37 | 0.9484 |
| Pure Error | 3880.17 | 5 | 776.03 | ||
| Cor Total | 24,657.97 | 29 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 12,185.39 | 4 | 3046.35 | 6.48 | 0.0010 |
| Linear Mixture | 5200.90 | 2 | 2600.45 | 5.53 | 0.0103 |
| A × D | 3720.72 | 1 | 3720.72 | 7.91 | 0.0094 |
| B × D | 1548.06 | 1 | 1548.06 | 3.29 | 0.0816 |
| Residual | 11,756.12 | 25 | 470.24 | ||
| Lack of Fit | 8307.86 | 20 | 415.39 | 0.60 | 0.8098 |
| Pure Error | 3448.27 | 5 | 689.65 | ||
| Cor Total | 23,941.51 | 29 |
| Biopolymer Coagulant Source | Water Type | Doses Used (mg/L) | Effectiveness in the Removal of Pollutants | Reference |
|---|---|---|---|---|
| Opuntia robusta cladodes | Urban wastewater | 10 | 68.7% of turbidity 86.1% of chemical oxygen demand | This study |
| Uncaria tomentosa leaves | Urban wastewater | 200 | 17.2% of turbidity 39.4% of chemical oxygen demand | This study |
| Moringa Oleifera | Synthetic turbid Wastewater | 82.43% of oil and grease | [38] | |
| Moringa Oleifera | Drinking water | 200 | 85% of turbidity | [12] |
| Acacia mearnsii tannin | Agricultural wastewater | 5–8 | 70% of total phosphorous 82% of turbidity | [8] |
| Solanum tuberosum starch | Agricultural wastewater | 1–2 | 80% of total phosphorous 82% of turbidity | [8] |
| Crustacean shells chitosan | Agricultural wastewater | 5–10 | 95% of total phosphorous 98% of turbidity | [8] |
| Plantago ovata seeds | Turbid water | 50 | >80% of turbidity | [39] |
| Tamarind seeds | Detergent wastewater | 400 | 97% of turbidity 39% of chemical oxygen demand | [40] |
| Banana pith | River water | 100 | 98.5% of turbidity 54.3% of chemical oxygen demand 96.0% of suspended solids | [41] |
| Jatropha curcas seeds | Kaolin synthetic water | 120 | >96 of turbidity | [42] |
| Opuntia mucilage | Cosmetic Wastewater | 150 | 50% of turbidity 38% of chemical oxygen demand | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilera Flores, M.M.; Valdivia Cabral, G.I.; Medellín Castillo, N.A.; Ávila Vázquez, V.; Sánchez Mata, O.; García Torres, J. Study on the Effectiveness of Two Biopolymer Coagulants on Turbidity and Chemical Oxygen Demand Removal in Urban Wastewater. Polymers 2023, 15, 37. https://doi.org/10.3390/polym15010037
Aguilera Flores MM, Valdivia Cabral GI, Medellín Castillo NA, Ávila Vázquez V, Sánchez Mata O, García Torres J. Study on the Effectiveness of Two Biopolymer Coagulants on Turbidity and Chemical Oxygen Demand Removal in Urban Wastewater. Polymers. 2023; 15(1):37. https://doi.org/10.3390/polym15010037
Chicago/Turabian StyleAguilera Flores, Miguel Mauricio, Gloria Itzel Valdivia Cabral, Nahum Andrés Medellín Castillo, Verónica Ávila Vázquez, Omar Sánchez Mata, and Jésica García Torres. 2023. "Study on the Effectiveness of Two Biopolymer Coagulants on Turbidity and Chemical Oxygen Demand Removal in Urban Wastewater" Polymers 15, no. 1: 37. https://doi.org/10.3390/polym15010037
APA StyleAguilera Flores, M. M., Valdivia Cabral, G. I., Medellín Castillo, N. A., Ávila Vázquez, V., Sánchez Mata, O., & García Torres, J. (2023). Study on the Effectiveness of Two Biopolymer Coagulants on Turbidity and Chemical Oxygen Demand Removal in Urban Wastewater. Polymers, 15(1), 37. https://doi.org/10.3390/polym15010037







