Fabrication of Bio-Based Film Comprising Metal Oxide Nanoparticles Loaded Chitosan for Wound Dressing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication CS Thin Film Containing Metal Oxide Hybrid Nanocomposites
2.3. Characterizations
Examination of Films Morphology
2.4. Contact Angle
2.5. In Vitro Cell Viability Tests
2.6. Antibacterial Test
3. Results and Discussion
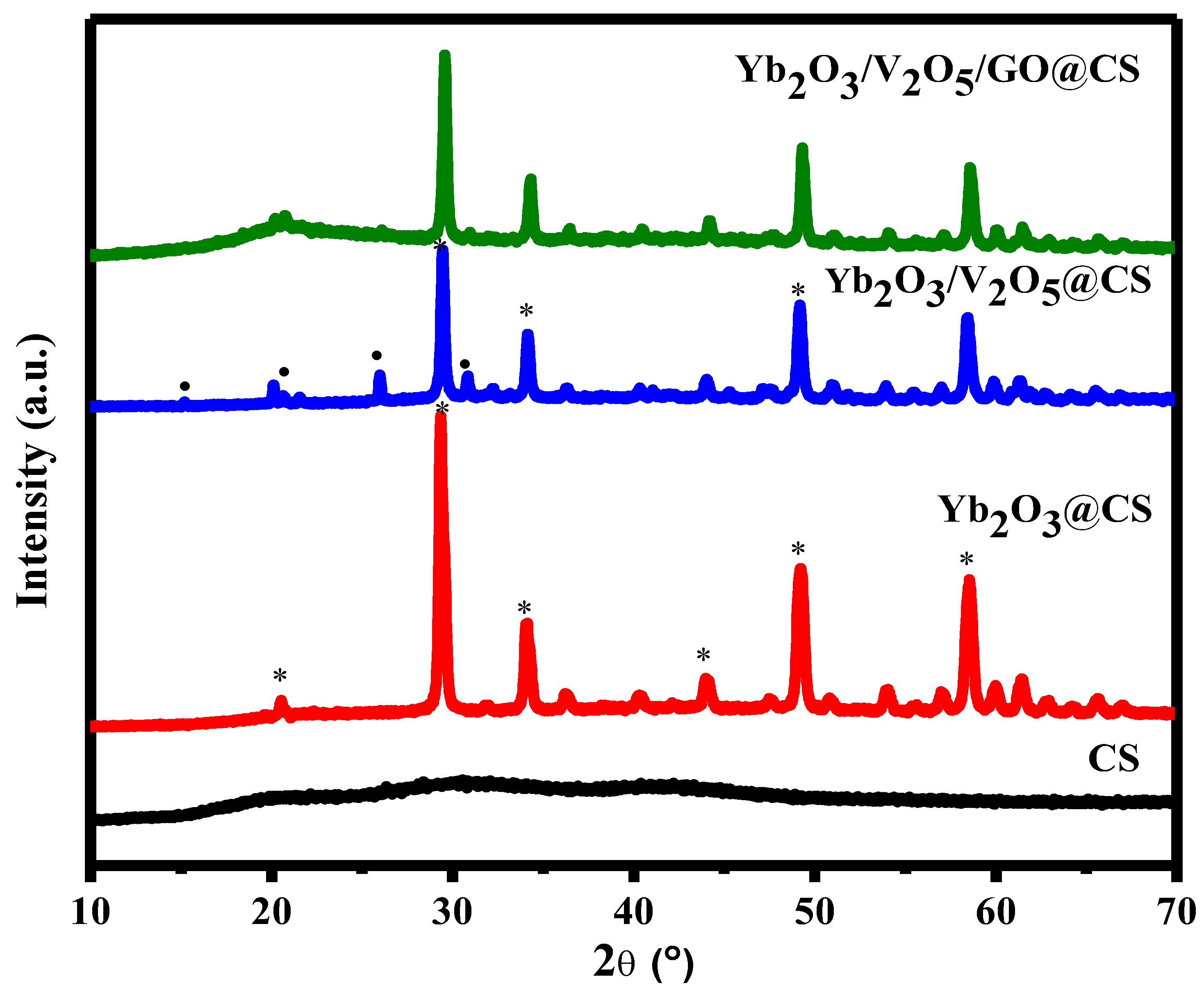
3.1. XRD
3.2. FTIR
3.3. Surface Morphology
3.4. EDX
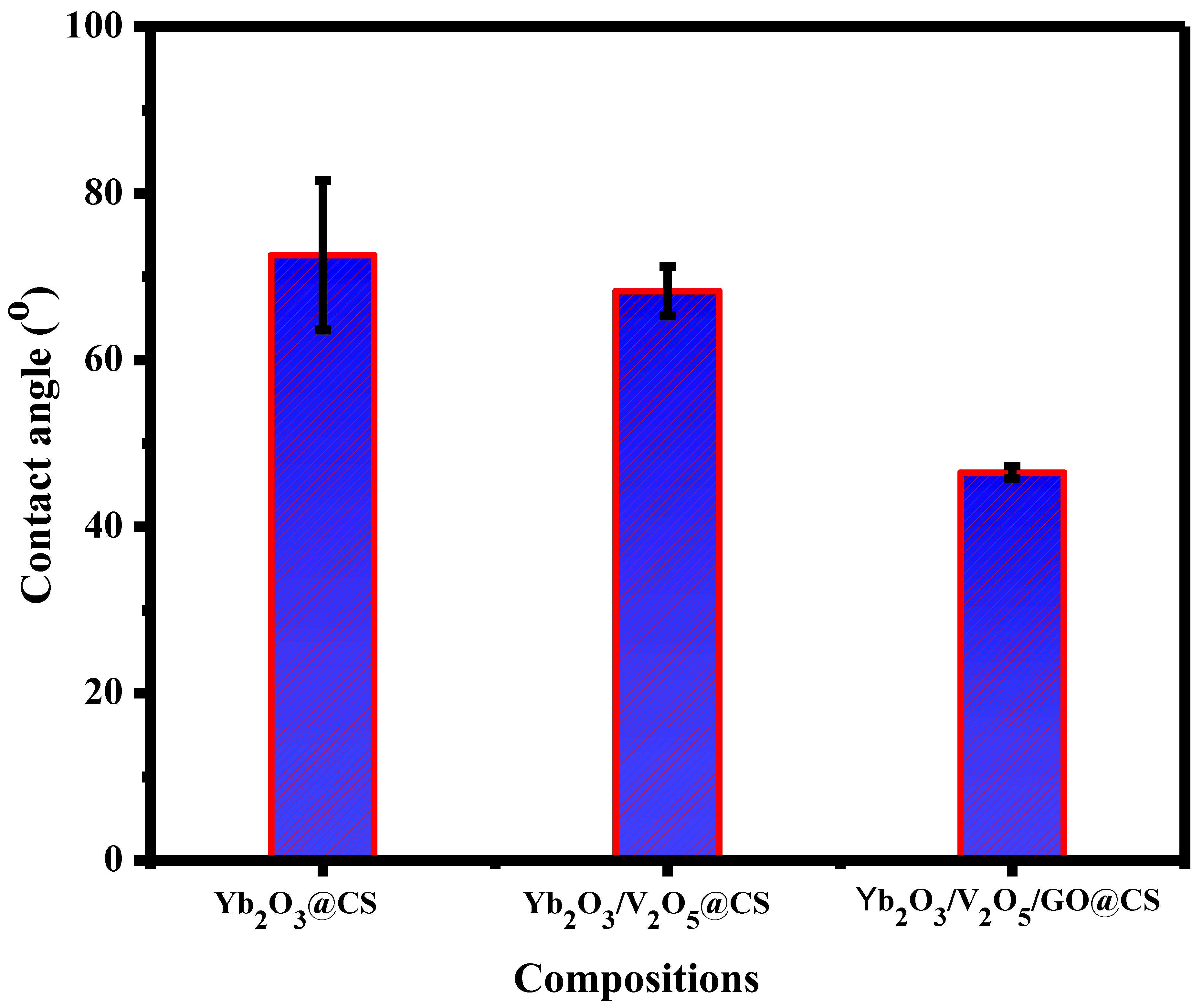
3.5. Contact Angle
3.6. Thermal Stability
3.7. Cell Viability
3.8. Antibacterial Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loo, H.L.; Goh, B.H.; Lee, L.-H.; Chuah, L.H. Application of chitosan-based nanoparticles in skin wound healing. Asian. J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef]
- Prakash, J.; Venkataprasanna, K.S.; Bharath, G.; Banat, F.; Niranjan, R.; Venkatasubbu, G.D. In-vitro evaluation of electrospun cellulose acetate nanofiber containing Graphene oxide/TiO2/Curcumin for wound healing application. Colloids. Surf. A Physicochem. Eng. Asp. 2021, 627, 127166. [Google Scholar] [CrossRef]
- Soliman, M.; Sadek, A.A.; Abdelhamid, H.N.; Hussein, K. Graphene oxide-cellulose nanocomposite accelerates skin wound healing. Res. Vet. Sci. 2021, 137, 262–273. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Amorim, M.T.P. New method to produce poly(vinyl alcohol)/cellulose acetate films with improved antibacterial action. Mater. Today Proc. 2020, 31, S269–S272. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Wang, L.; Yan, X.; Ma, D.; Liu, Z.; Liu, X. Sesamol incorporated cellulose acetate-zein composite nanofiber membrane: An efficient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef]
- Gonçalves, M.M.; Lobsinger, K.L.; Carneiro, J.; Picheth, G.F.; Pires, C.; Saul, C.K.; Maluf, D.F.; Pontarolo, R. Morphological study of electrospun chitosan/poly(vinyl alcohol)/glycerol nanofibres for skin care applications. Int. J. Biol. Macromol. 2022, 194, 172–178. [Google Scholar] [CrossRef]
- Ma, C.; Sun, J.; Li, B.; Feng, Y.; Sun, Y.; Xiang, L.; Wu, B.; Xiao, L.; Liu, B.; Petrovskii, V. Ultra-strong bio-glue from genetically engineered polypeptides. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Pan, M.; Weng, Z.; Liu, J. Effect of positive bias on properties of chitosan coating prepared on micro-arc oxidation surface of Ti–6Al–4V alloy by electrophoretic deposition. Mater. Chem. Phys. 2022, 275, 125257. [Google Scholar] [CrossRef]
- Rojas, D.; Espeso, J.; Fernández, L.R.; Barquín, L.F. Heat capacity of nanocrystalline Yb2O3. Ceram. Int. 2022, 48, 879–886. [Google Scholar] [CrossRef]
- Kose, E.; Aydogu, S. The structural, optical properties, surface and electrical properties of vanadium pentoxide (V2O5) films produced at different substrate temperatures. Optik 2022, 268, 169775. [Google Scholar] [CrossRef]
- Oh, H.; Son, D.; Lee, J.; Kim, M.; Sung, D.; Lee, H.; Choi, W. Reactive oxygen species scavenging nanofibers with chitosan-stabilized Prussian blue nanoparticles for enhanced wound healing efficacy. Int. J. Biol. Macromol. 2022, 219, 835–843. [Google Scholar] [CrossRef]
- Kisielewska, A.; Spilarewicz-Stanek, K.; Cichomski, M.; Kozłowski, W.; Piwoński, I. The role of graphene oxide and its reduced form in the in situ photocatalytic growth of silver nanoparticles on graphene-TiO2 nanocomposites. Appl. Surf. Sci. 2022, 576, 151759. [Google Scholar] [CrossRef]
- Khabnadideh, S.; Mirzaei, E.; Amiri-Zirtol, L. L-arginine modified graphene oxide: A novel heterogeneous catalyst for synthesis of benzo[b]pyrans and pyrano[3,2-c]chromenes. J. Mol. Struct. 2022, 1261, 132934. [Google Scholar] [CrossRef]
- Biswas, H.S.; Bala, S.; Kundu, A.K.; Saha, I.; Poddar, S.; Sarkar, S.; Mandal, P. Tuned synthesis and designed characterization of graphene oxide thin film. Inorg. Chem. Commun. 2022, 139, 109356. [Google Scholar] [CrossRef]
- Kisielewska, A.; Spilarewicz-Stanek, K.; Cichomski, M.; Kozłowski, W.; Piwoński, I. Tribological properties of defect-rich molybdenum disulfide/graphene oxide composite coating under various environments. Vacuum 2022, 202, 111125. [Google Scholar]
- Movaffagh, J.; Khatib, M.; Bazzaz, B.S.F.; Taherzadeh, Z.; Hashemi, M.; Moghaddam, A.S.; Tabatabaee, S.A.; Azizzadeh, M.; Jirofti, N. Evaluation of wound-healing efficiency of a functional Chitosan/Aloe vera hydrogel on the improvement of re-epithelialization in full thickness wound model of rat. J. Tissue Viability 2022, 31, 649–656. [Google Scholar] [CrossRef]
- Alharthi, S.S.; Gomathi, T.; Joseph, J.J.; Rakshavi, J.; Florence, J.A.K.; Sudha, P.N.; Rajakumar, G.; Thiruvengadam, M. Biological activities of chitosan-salicylaldehyde schiff base assisted silver nanoparticles. J. King Saud. Univ.-Sci. 2022, 34, 102177. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Chaudhary, G. Tuning of structural, optical and toxicological properties of Gd3+ doped Yb2O3 nanoparticles. Ceram. Int. 2019, 45, 19307–19315. [Google Scholar] [CrossRef]
- Gorokhova, E.; Dymshits, O.; Venevtsev, I.; Basyrova, L.; Alekseeva, I.; Khubetsov, A.; Baranovd, M.; Tsentera, M.; Zhiline, A.; Loiko, P. ZnO—Yb2O3 composite optical ceramics: Synthesis, structure and spectral-luminescent properties. J. Eur. Ceram. Soc. 2022, 42, 616–630. [Google Scholar] [CrossRef]
- Shankar, V.U.; Govindarajan, D.; Christuraj, P.; Salethraj, M.J.; Johanson, F.J.; Raja, M.D. Enhanced the electrochemical properties of Ni doped V2O5 as a electrode material for supercapacitor applications. Mater. Today Proc. 2022, 50, 2675–2678. [Google Scholar] [CrossRef]
- Teaima, M.; Elasaly, M.; Omar, S.; El-Nabarawi, M.; Shoueir, K. Wound healing activities of polyurethane modified chitosan nanofibers loaded with different concentrations of linezolid in an experimental model of diabetes. J. Drug Deliv. Sci. Technol. 2022, 67, 102982. [Google Scholar] [CrossRef]
- Tian, T.; Ruan, J.; Zhang, J.; Zhao, C.-X.; Chen, D.; Shan, J. Nanocarrier-based tumor-targeting drug delivery systems for hepatocellular carcinoma treatments: Enhanced therapeutic efficacy and reduced drug toxicity. J. Biomed. Nanotechnol. 2022, 18, 660–676. [Google Scholar] [CrossRef]
- Algharib, S.A.; Dawood, A.; Zhou, K.; Chen, D.; Li, C.; Meng, K.; Zhang, A.; Luo, W.; Ahmed, S.; Huang, L.; et al. Preparation of chitosan nanoparticles by ionotropic gelation technique: Effects of formulation parameters and in vitro characterization. J. Mol. Struct. 2022, 1252, 132129. [Google Scholar] [CrossRef]
- Braim, F.S.; Ab Razak, N.N.A.N.; Aziz, A.A.; Ismael, L.Q.; Sodipo, B.K. Ultrasound assisted chitosan coated iron oxide nanoparticles: Influence of ultrasonic irradiation on the crystallinity, stability, toxicity and magnetization of the functionalized nanoparticles. Ultrason. Sonochemistry 2022, 88, 106072. [Google Scholar] [CrossRef]
- Tien, N.; Geng, T.; Heyward, C.; Reseland, J.; Lyngstadaas, S.; Blaker, J.; Haugen, H. Solution blow spinning of highly deacetylated chitosan nanofiber scaffolds for dermal wound healing. Biomater. Adv. 2022, 137, 212871. [Google Scholar] [CrossRef]
- Munawar, T.; Mukhtar, F.; Nadeem, M.S.; Riaz, M.; Rahman, M.N.U.; Mahmood, K.; Hasan, M.; Arshad, M.; Hussain, F.; Hussain, A.; et al. Novel photocatalyst and antibacterial agent; direct dual Z-scheme ZnO–CeO2-Yb2O3 heterostructured nanocomposite. Solid. State Sci. 2020, 109, 106446. [Google Scholar] [CrossRef]
- Unal, F.; Kaya, F. Modelling of relation between synthesis parameters and average crystallite size of Yb2O3 nanoparticles using Box-Behnken design. Ceram. Int. 2020, 46, 26800–26808. [Google Scholar] [CrossRef]
- Jayalakshmi, T.; Harini, R.; Nagaraju, G. Lithium ion battery performance of micro and nano-size V2O5 cathode materials. Mater. Today Proc. 2022, 65, 200–206. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, J.; Zhang, Y.; Li, C.; Wei, Z.; Shen, J.; Li, J.; Wang, F.; Han, B.; Chen, D. Engineering DNA-Guided Hydroxyapatite Bulk Materials with High Stiffness and Outstanding Antimicrobial Ability for Dental Inlay Applications. Adv. Mater. 2022, 34, 2202180. [Google Scholar] [CrossRef]
- Istiqomah, A.; Utami, M.R.; Firdaus, M.; Suryanti, V.; Kusumaningsih, T. Antibacterial chitosan-Dioscorea alata starch film enriched with essential oils optimally prepared by following response surface methodology. Food Biosci. 2022, 46, 101603. [Google Scholar] [CrossRef]
- Amin, P.; Shojaei, A.; Hamzehlouyan, T. ZIF-8/Chitosan hybrid nanoparticles with tunable morphologies as superior adsorbents towards both anionic and cationic dyes for a broad range of acidic and basic environments. Microporous Mesoporous Mater. 2022, 343, 112149. [Google Scholar] [CrossRef]







| CS | Yb2O3@CS | Yb2O3/V2O5@CS | Yb2O3/V2O5/ GO@CS | Assignment | Ref. |
|---|---|---|---|---|---|
| … | 472 | … | … | Yb–O | [27] |
| … | … | 558 | … | V–O–V bond | [29] |
| … | … | 611 | 613 | Yb–O | [27] |
| 658 | 654 | 657 | 656 | N–H | [22] |
| 894 | … | … | 893 | C–O–C | [23] |
| 994 | … | … | … | C–O–C | [24] |
| 1091 | … | … | … | C–O | [25] |
| 1152 | 1150 | 1152 | 1152 | C–O | [12] |
| 1334 | … | 1333 | 1333 | C–O–N group | [22] |
| 1407 | 1410 | … | 1407 | –OH group | [22] |
| … | 1512 | 1513 | 1513 | C–O bond | [28] |
| 1538 | … | … | … | Bending of -NH+3 | [26] |
| 1555 | 1554 | 1555 | 1554 | Amide II (N–H deformation) | [23] |
| … | 1649 | 1650 | 1651 | C–O | [28] |
| 2878 | 2882 | 2879 | 2878 | CH | [23] |
| … | … | 3120 | … | O–H | [27] |
| … | 3131 | … | … | O–H | [27] |
| … | … | 3175 | 3176 | O–H | [27] |
| … | … | 3375 | … | O–H | [27] |
| 3397 | … | … | 3399 | Amine -NH and OH | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almaieli, L.M.A.; Khalaf, M.M.; Gouda, M.; Elmushyakhi, A.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Fabrication of Bio-Based Film Comprising Metal Oxide Nanoparticles Loaded Chitosan for Wound Dressing Applications. Polymers 2023, 15, 211. https://doi.org/10.3390/polym15010211
Almaieli LMA, Khalaf MM, Gouda M, Elmushyakhi A, Abou Taleb MF, Abd El-Lateef HM. Fabrication of Bio-Based Film Comprising Metal Oxide Nanoparticles Loaded Chitosan for Wound Dressing Applications. Polymers. 2023; 15(1):211. https://doi.org/10.3390/polym15010211
Chicago/Turabian StyleAlmaieli, Latifah Mohammed Ali, Mai M. Khalaf, Mohamed Gouda, Abraham Elmushyakhi, Manal F. Abou Taleb, and Hany M. Abd El-Lateef. 2023. "Fabrication of Bio-Based Film Comprising Metal Oxide Nanoparticles Loaded Chitosan for Wound Dressing Applications" Polymers 15, no. 1: 211. https://doi.org/10.3390/polym15010211
APA StyleAlmaieli, L. M. A., Khalaf, M. M., Gouda, M., Elmushyakhi, A., Abou Taleb, M. F., & Abd El-Lateef, H. M. (2023). Fabrication of Bio-Based Film Comprising Metal Oxide Nanoparticles Loaded Chitosan for Wound Dressing Applications. Polymers, 15(1), 211. https://doi.org/10.3390/polym15010211










