Research Progress in Energy Based on Polyphosphazene Materials in the Past Ten Years
Abstract
1. Introduction
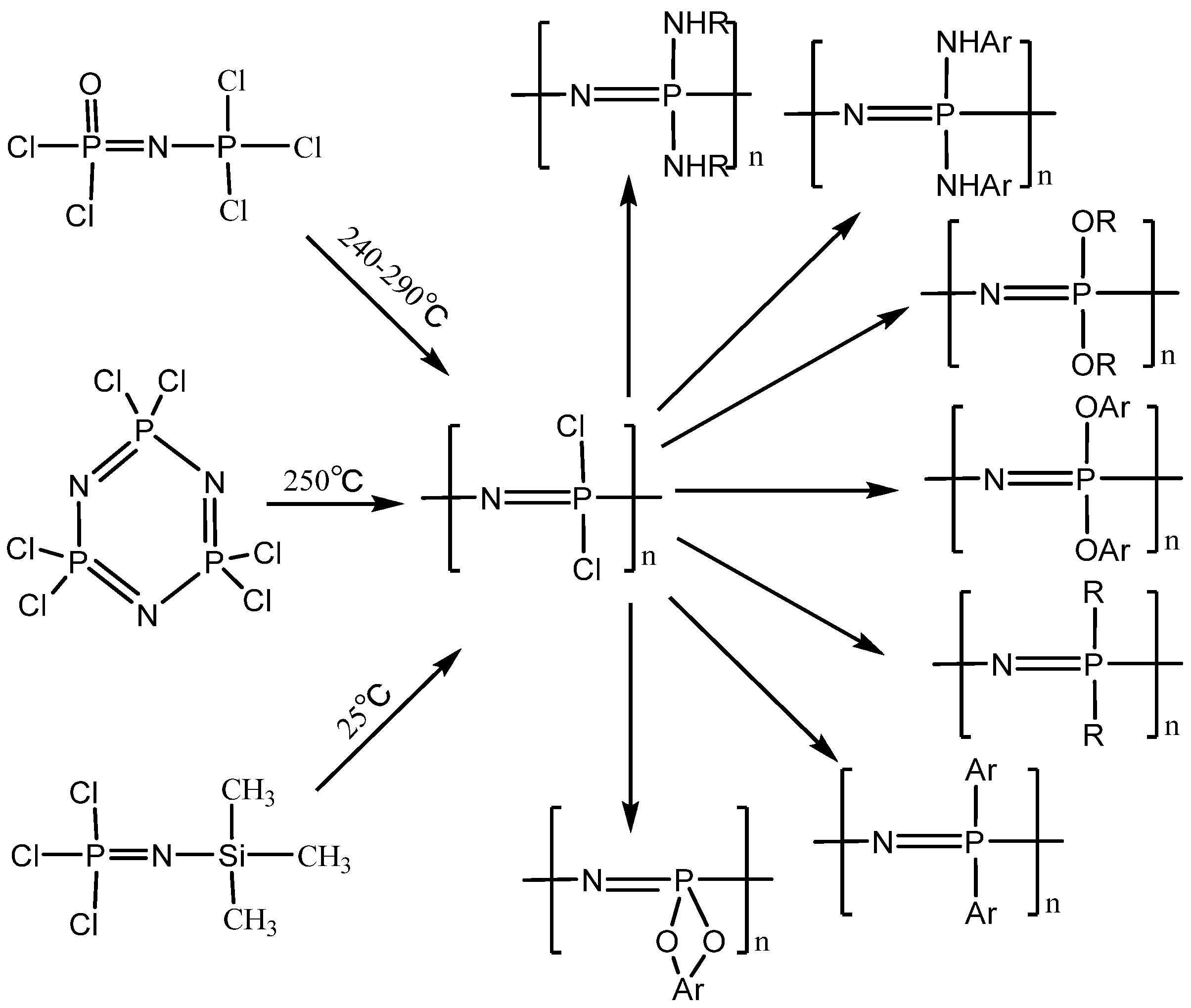
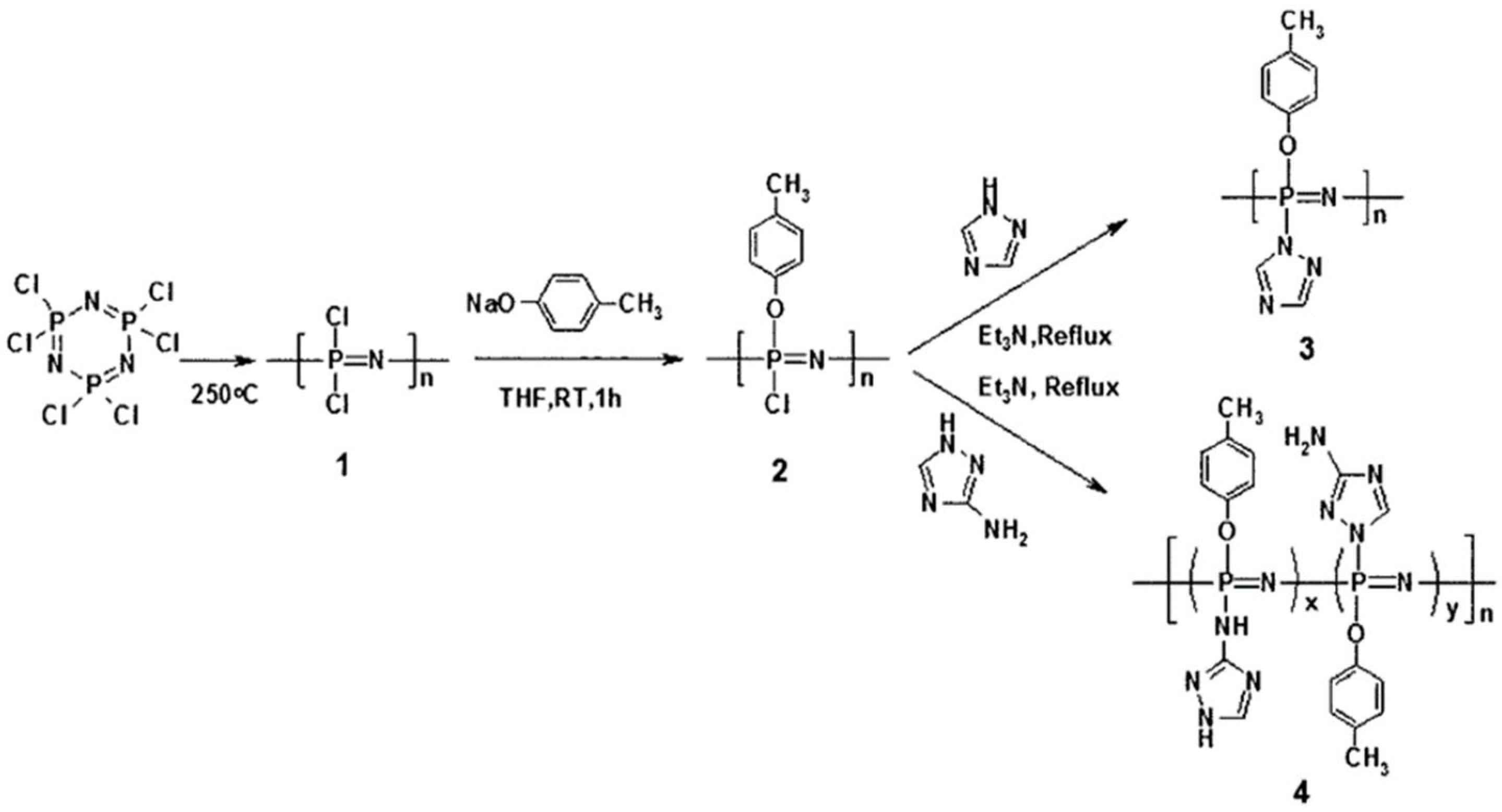
2. The Synthesis of Polyphosphazenes
3. The Application of Polyphosphazenes in Electrochemistry
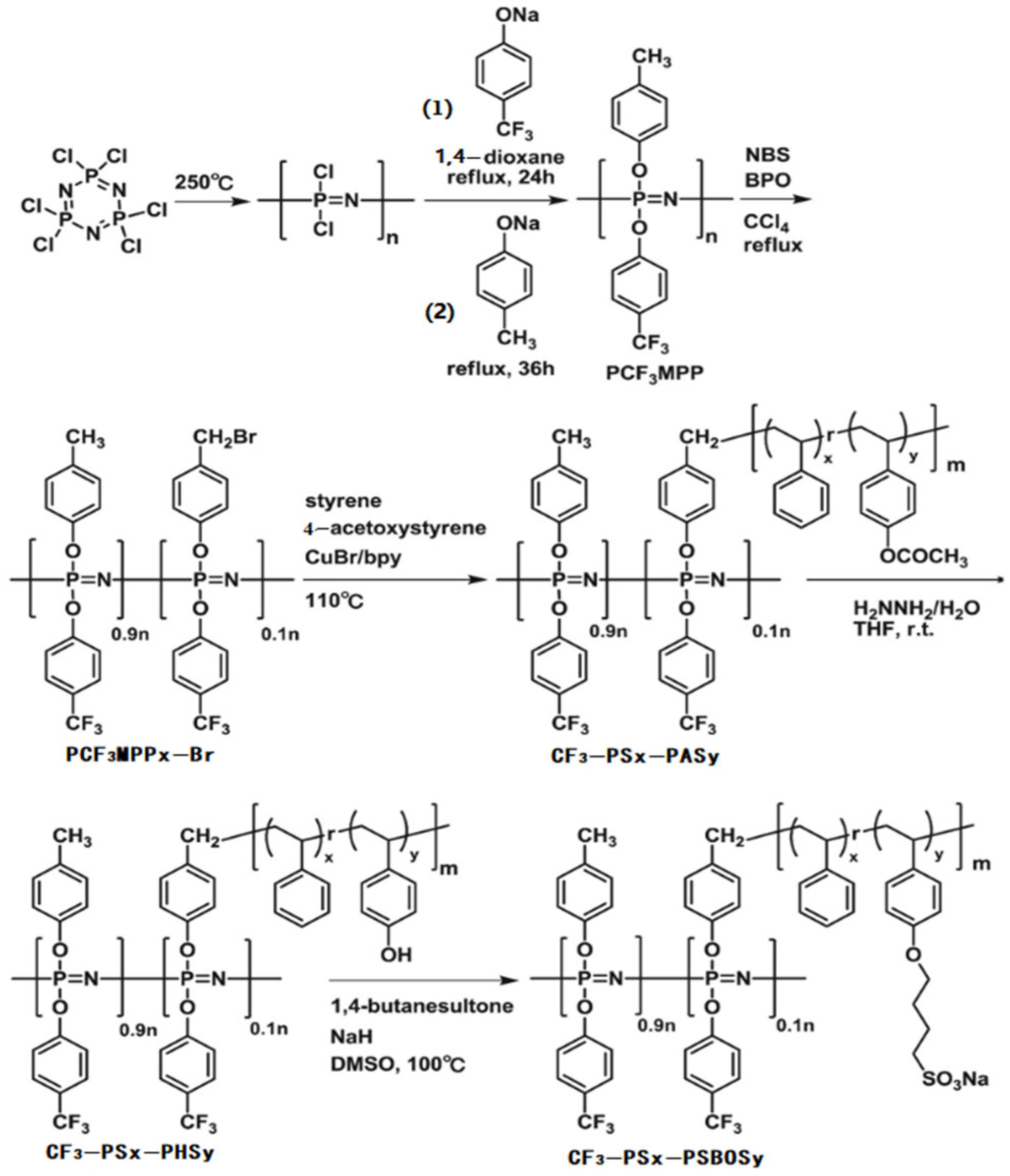
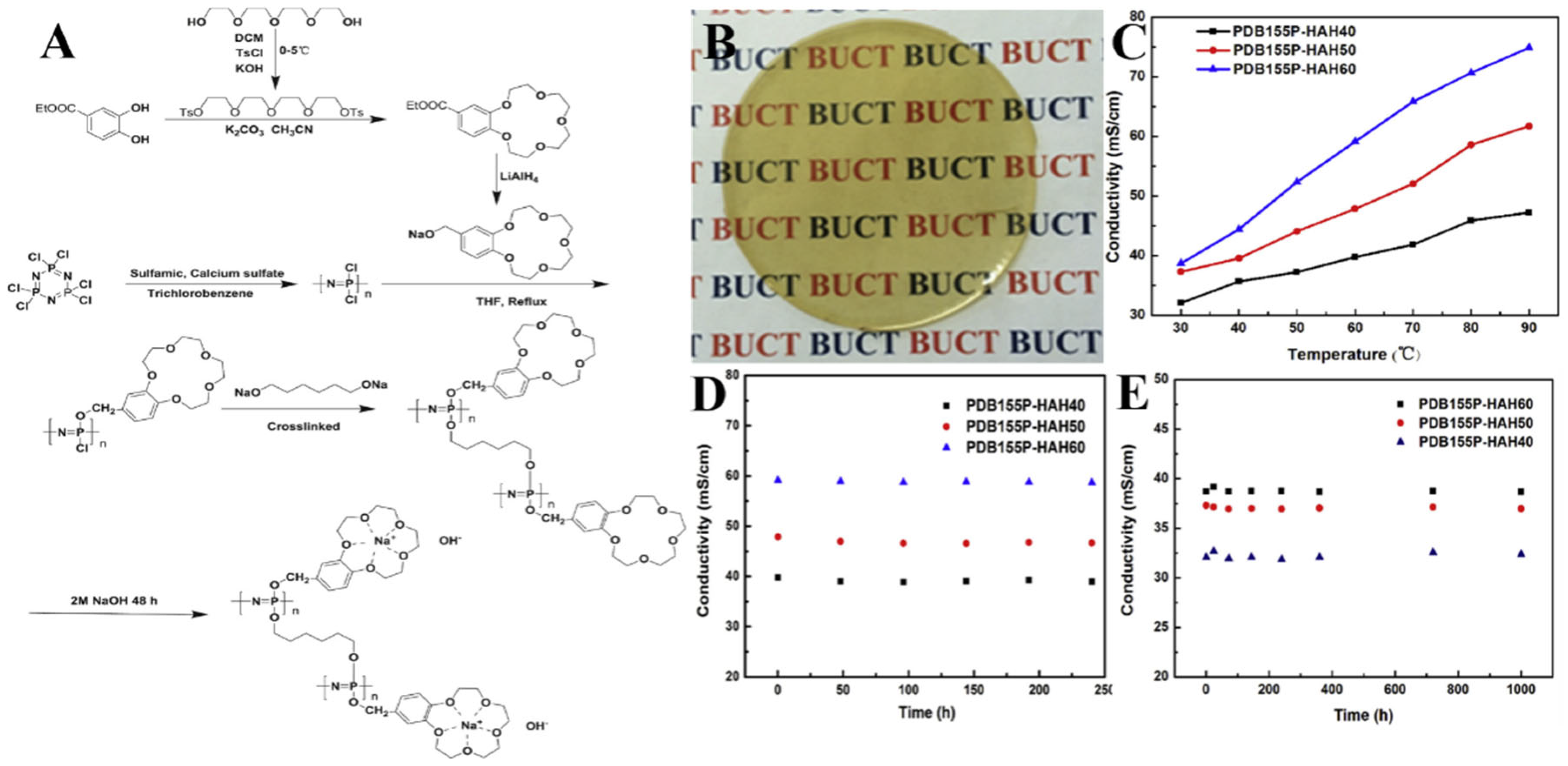
3.1. Fuel Cells
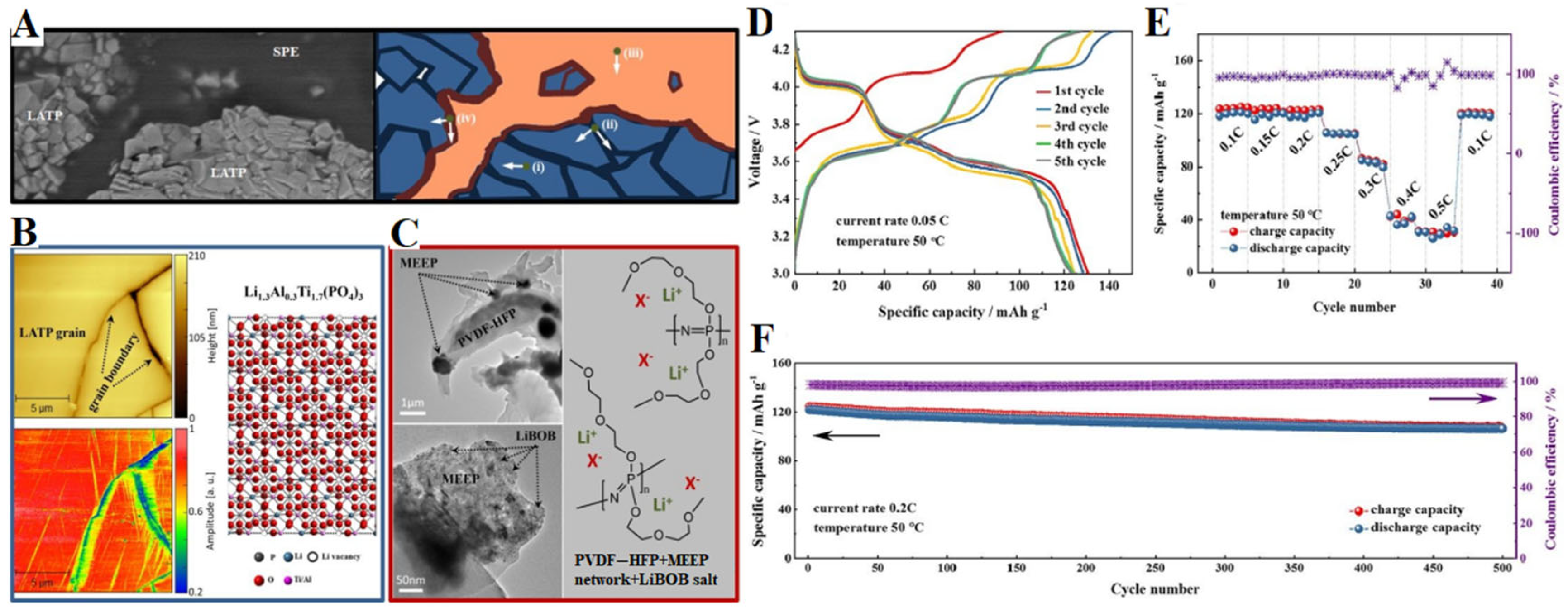
3.2. Li-Ion Batteries
3.3. Other Energy Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.X.; Wang, Y.J.; Chen, L.; Yang, Y.Q.; Li, X. Carbon emission of energy consumption of the electric vehicle development scenario. Environ. Sci. Pollut. Res. 2021, 28, 42401. [Google Scholar] [CrossRef] [PubMed]
- Gil-García, I.C.; García-Cascales, M.S.; Dagher, H.; Molina-García, A. Electric Vehicle and Renewable Energy Sources: Motor Fusion in the Energy Transition from a Multi-Indicator Perspective. Sustainability 2021, 13, 3430. [Google Scholar] [CrossRef]
- Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The Latest Trends in Electric Vehicles Batteries. Molecules 2021, 26, 3188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhou, Y.S.; Tian, Q.; Liao, X.Y.; Zhang, F.T. A Novel Method for Estimating State–of–Charge in Power Batteries for Electric Vehicles. Int. J. Precis. Eng. Man. 2019, 20, 845. [Google Scholar] [CrossRef]
- Rotondi, V.; Kashyap, R.; Pesando, L.M.; Spinelli, S.; Billari, F.C. Leveraging mobile phones to attain sustainable development. Proc. Natl. Acad. Sci. USA 2020, 117, 13413. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Pan, D. Solutions for the problems of silicon–carbon anode materials for lithium–ion batteries. R. Soc. Open Sci. 2018, 5, 172370. [Google Scholar] [CrossRef]
- Kim, G.Y.; Park, S.R.; Yu, J.S. Design and characteristics of low–resistance lithium-ion battery pack and its fast charging method for smart phones. Int. J. Energy Res. 2021, 45, 17631. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Z.C.; Dudney, N.J.; Liang, C.D. Lithium Superionic Sulfide Cathode for All–Solid Lithium–Sulfur Batteries. ACS Nano 2013, 7, 2829. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.D.; Tao, P.; Song, C.Y.; Wu, J.B.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium–ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653. [Google Scholar] [CrossRef]
- Yan, L.; Huang, J.H.; Guo, Z.W.; Dong, X.L.; Wang, Z.; Wang, Y.G. Solid–State Proton Battery Operated at Ultralow Temperature. ACS Energy Lett. 2020, 5, 685. [Google Scholar] [CrossRef]
- Zakaria, Z.; Shaari, N.; Kamarudin, S.K.; Bahru, R.; Musa, M.T. A review of progressive advanced polymer nanohybrid membrane in fuel cell application. Int. J. Energ. Res. 2020, 44, 8255. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect Engineering on Electrode Materials for Rechargeable Batteries. Adv. Mater. 2020, 32, e1905923. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, X.M. Nonflammable pseudoconcentrated electrolytes for batteries. Curr. Opin. Electrochem. 2021, 30, 100783. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.H.; Kim, J.H.; Lee, S.Y.; Lanceros–Mendez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Allcock, H.R.; Laurencin, C.T. Generational biodegradable and regenerative polyphosphazene polymers and their blends with poly (lactic–co–glycolic acid). Prog. Polym. Sci. 2019, 98, 101146. [Google Scholar] [CrossRef] [PubMed]
- Strasser, P.; Teasdale, I. Main–Chain Phosphorus–Containing Polymers for Therapeutic Applications. Molecules 2020, 25, 1716. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R. Generation of structural diversity in polyphosphazenes. Appl. Organomet. Chem. 2013, 27, 620. [Google Scholar] [CrossRef]
- Gutru, R.; Peera, S.G.; Bhat, S.D.; Sahu, A.K. Synthesis of sulfonated poly(bis(phenoxy)phosphazene) based blend membranes and its effect as electrolyte in fuel cells. Solid. State Ion. 2016, 296, 127. [Google Scholar] [CrossRef]
- Tong, C.Y.; Tian, Z.C.; Chen, C.; Li, Z.J.; Modzelewski, T.; Allcock, H.R. Synthesis and Characterization of Trifluoroethoxy Polyphosphazenes Containing Polyhedral Oligomeric Silsesquioxane (POSS) Side Groups. Macromolecules 2016, 49, 1313. [Google Scholar] [CrossRef]
- Mehmood, S.; Wang, L.; Yu, H.J.; Haq, F.; Fahad, S.; Bilal-ul-Amin; Uddin, M.A.; Haroon, M. Recent Progress on the Preparation of Cyclomatrix–Polyphosphazene Based Micro/Nanospheres and Their Application for Drug Release. ChemistrySelect 2020, 5, 5939. [Google Scholar] [CrossRef]
- Yu, X.H.; Tang, X.Y.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R.; Kugel, R.L. Synthesis of High Polymeric Alkoxy and Aryloxyphosphonitriles. J. Am. Chem. Soc. 1965, 87, 4216. [Google Scholar] [CrossRef]
- Allcock, H.R.; Kugel, R.L.; Valan, K.J. Phosphonitrilic Compounds. VI. High Molecular Weight Poly (alkoxy and aryloxyphosphazenes). Inorg. Chem. 1966, 5, 1709. [Google Scholar] [CrossRef]
- Honeyman, C.H.; Manners, I.; Morrissey, C.T.; Allcock, H.R. Ambient-Temperature Synthesis of Poly (Dichlorophosphazene) with Molecular-Weight Control. J. Am. Chem. Soc. 1995, 117, 7035. [Google Scholar] [CrossRef]
- Allcock, H.R.; Reeves, S.D.; Nelson, J.M.; Crane, C.A.; Manners, I. Polyphosphazene block copolymers via the controlled cationic, ambient temperature polymerization of phosphoranimines. Macromolecules 1997, 30, 2213. [Google Scholar] [CrossRef]
- Gates, D.P.; Allcock, H.R. Chemistry and Applications of Polyphosphazenes. Von Harry R. Allcock. Angew. Chem. 2010, 42, 4570. [Google Scholar] [CrossRef]
- Allcock, H.R. Recent developments in polyphosphazene materials science. Curr. Opin. Solid State Mater. Sci. 2006, 10, 231. [Google Scholar] [CrossRef]
- Potts, M.K.; Hagnauer, G.L.; Sennett, M.S.; Davies, G. Monomer concentration effects on the kinetics and mechanism of the boron trichloride catalyzed solution polymerization of hexachlorocyclotriphosphazene. Macromolecules 1989, 22, 4235. [Google Scholar] [CrossRef]
- Dhalluin, G.; Dejaeger, R.; Chambrette, J.P.; Potin, P. Synthesis of Poly (Dichlorophosphazenes) from Cl3p=Np(O)Cl2.1. Kinetics and Reaction-Mechanism. Macromolecules 1992, 25, 1254. [Google Scholar] [CrossRef]
- Zhang, L.W.; Fu, H.B.; Zhu, Y.F. Efficient TiO2 photocatalysts from surface hybridization of TiO2 particles with graphite-like carbon. Adv. Funct. Mater. 2008, 18, 2180–2189. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, M.J.; Shim, W.G.; Lee, J.W.; Kim, T.Y.; Lee, D.H.; Moon, H. Adsorption and photocatalytic decomposition of organic molecules on carbon-coated TiO2. J. Mater. Sci. 2008, 43, 6486–6494. [Google Scholar] [CrossRef]
- Fu, J.W.; Huang, X.B.; Huang, Y.W.; Zhang, J.W.; Tang, X.Z. One–pot noncovalent method to functionalize multi-walled carbon nanotubes using cyclomatrix-type polyphosphazenes. Chem. Commun 2009, 9, 1049–1051. [Google Scholar] [CrossRef]
- Fu, J.; Huang, X.; Huang, Y.; Pan, Y.; Zhu, Y.; Tang, X. Preparation of Silver Nanocables Wrapped with Highly Cross-Linked Organic-Inorganic Hybrid Polyphosphazenes via a Hard–Template Approach. J. Phys. Chem. C 2008, 112, 16840–16844. [Google Scholar] [CrossRef]
- Gao, P.; Fu, J.; Yang, J.; Lv, R.; Wang, J.; Nuli, Y.; Tang, X. Microporous carbon coated silicon core/shell nanocomposite via in situ polymerization for advanced Li-ion battery anode material. Phys. Chem. Chem. Phys. 2009, 11, 11101–11105. [Google Scholar] [CrossRef]
- Carriedo, G.A.; García Alonso, F.J.; Gómez–Elipe, P.; Fidalgo, J.I.; García Álvarez, J.L.; Presa–Soto, A. A Simplified and Convenient Laboratory–Scale Preparation of 14N or 15N High Molecular Weight Poly (dichlorophosphazene) Directly from PCl5. Chem.–Eur. J. 2003, 9, 3833. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Chen, J.; Chen, Z.; Huang, X.; Tang, X. Controlled fabrication of uniform hollow core porous shell carbon spheres by the pyrolysis of core/shell polystyrene/cross–linked polyphosphazene composites. Chem. Commun. 2010, 46, 6563–6565. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Meng, L.; Lu, Q.; Fu, J.; Huang, X. Superparamagnetic submicro–megranates: Fe(3)O(4) nanoparticles coated with highly cross-linked organic/inorganic hybrids. Chem. Commun 2009, 42, 6370–6372. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, L.; Feng, X.; Zhang, X.; Lu, Q. One-pot synthesis of highly magnetically sensitive nanochains coated with a highly cross-linked and biocompatible polymer. Angew. Chem. Int. Ed. Engl. 2010, 49, 8476–8479. [Google Scholar] [CrossRef]
- Reddy, E.P.; Davydov, L.; Smirniotis, P. TiO2–loaded zeolites and mesoporous materials in the sonophotocatalytic decomposition of aqueous organic pollutants: The role of the support. Appl. Catal. B Environ. 2003, 42, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Fan, X. Grafting of amine-capped cross-linked polyphosphazenes onto carbon fiber surfaces: A novel coupling agent for fiber reinforced composites. RSC Adv. 2014, 4, 12198. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Kang, X.; Fu, J.; Zhu, Y.; Tang, X. Synthesis and Characterization of Novel Polyurethane Composites Based on Hybrid Inorganic/Organic Phosphazene-Containing Microspheres. Macromol. Mater. Eng. 2009, 294, 605–610. [Google Scholar] [CrossRef]
- Chen, D.; Cui, Y.; Wang, X.; Tang, X. Polyphosphazene/low-density polyethylene blends: Miscibility and flame-retardance studies. J. Appl. Polym. Sci. 2002, 86, 709–714. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Y.; Li, J.; Liu, L.; Huang, X.; Zhang, J.; Kang, X.; Tang, X. Novel polyurethane networks based on hybrid inorganic/organic phosphazene–containing nanotubes with surface active hydroxyl groups. Polym. Adv. Technol. 2012, 23, 1–7. [Google Scholar] [CrossRef]
- Li, S.; Qiu, S.; Yu, B.; Tang, G.; Xing, W.; Hu, Y. POSS–functionalized polyphosphazene nanotube: Preparation and effective reinforcement on UV–curable epoxy acrylate nanocomposite coatings. RSC Adv. 2016, 6, 3025–3031. [Google Scholar] [CrossRef]
- Gu, X.; Huang, X.; Wei, H.; Tang, X. Synthesis of novel epoxy–group modified phosphazene–containing nanotube and its reinforcing effect in epoxy resin. Eur. Polym. J. 2011, 47, 903–910. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Zhu, K.J. Novel blends of poly[bis(glycine ethyl ester) phosphazene] and polyesters or polyanhydrides: Compatibility and degradation characteristics in vitro. Polym. Int. 2000, 49, 1283–1288. [Google Scholar] [CrossRef]
- Pan, Y.; Tang, X.; Zhu, L.; Huang, Y. Synthesis and characterization of a new high-Tg photorefractive material. Eur. Polym. J. 2007, 43, 1091–1095. [Google Scholar] [CrossRef]
- Pan, Y.; Tang, X. Synthesis and characterization of azobenzene-containing side-chain photorefractive polymers. Eur. Polym. J. 2008, 44, 408–414. [Google Scholar] [CrossRef]
- Ouadah, A.; Luo, T.; Gao, S.; Zhu, C. Controlling the degree of sulfonation and its impact on hybrid cross–linked network based polyphosphazene grafted butylphenoxy as proton exchange membrane. Int. J. Hydrogen Energy 2018, 43, 15466–15480. [Google Scholar] [CrossRef]
- Chen, Y.N.; Li, Z.M.; Chen, N.J.; Zhang, Y.J.; Wang, F.H.; Zhu, H. Preparation and characterization of cross-linked polyphosphazene-crown ether membranes for alkaline fuel cells. Electrochim. Acta 2017, 258, 311. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, F.; Wu, L. Heteroatoms-doped 3D carbon nanosphere cages embedded with MoS2 for lithium-ion battery. Electrochim. Acta 2020, 332, 135490. [Google Scholar]
- Wang, M.; Fu, J.; Zhang, Y.; Chen, Z.; Wang, M.; Zhu, J.; Cui, W.; Zhang, J.; Xu, Q. Removal of Rhodamine B, a Cationic Dye from Aqueous Solution Using Poly(cyclotriphosphazene–co–4,4′–sulfonyldiphenol) Nanotubes. J. Macromol. Sci. Part A 2015, 52, 105–113. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Fu, J.; Wang, M.; Wang, X.; Han, R.; Xu, Q. Adsorption of methylene blue onto poly(cyclotriphosphazene–co–4,4′–sulfonyldiphenol) nanotubes: Kinetics, isotherm and thermodynamics analysis. J. Hazard. Mater. 2014, 273, 263–271. [Google Scholar] [CrossRef]
- Wei, W.; Lu, R.; Ye, W.; Sun, J.; Zhu, Y.; Luo, J.; Liu, X. Liquid Marbles Stabilized by Fluorine–Bearing Cyclomatrix Polyphosphazene Particles and Their Application as High-Efficiency Miniature Reactors. Langmuir 2016, 32, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Pearse, A.J.; Schmitt, T.E.; Fuller, E.J.; El-Gabaly, F.; Lin, C.-F.; Gerasopoulos, K.; Kozen, A.C.; Talin, A.A.; Rubloff, G.; Gregorczyk, K.E. Nanoscale Solid State Batteries Enabled by Thermal Atomic Layer Deposition of a Lithium Polyphosphazene Solid State Electrolyte. Chem. Mater. 2017, 29, 3740–3753. [Google Scholar] [CrossRef]
- Jankowsky, S.; Hiller, M.M.; Fromm, O.; Winter, M.; Wiemhöfer, H.D. Enhanced Lithium–Ion Transport in Polyphosphazene based Gel Polymer Electrolytes. Electrochim. Acta 2015, 155, 364–371. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Y.; Shen, G.; Zhang, S. Recent progress in fluorinated electrolytes for improving the performance of Li–S batteries. J. Energy Chem. 2020, 41, 149–170. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kang, Y.; Kim, D.-W. Lithium polymer cell assembled by in situ chemical cross–linking of ionic liquid electrolyte with phosphazene-based cross-linking agent. Electrochim. Acta 2013, 89, 359–364. [Google Scholar] [CrossRef]
- Liu, J.; Song, X.; Zhou, L.; Wang, S.; Song, W.; Liu, W.; Long, H.; Zhou, L.; Wu, H.; Feng, C.; et al. Fluorinated phosphazene derivative—A promising electrolyte additive for high voltage lithium ion batteries: From electrochemical performance to corrosion mechanism. Nano Energy 2018, 46, 404–414. [Google Scholar] [CrossRef]
- Zhou, M.; Qin, C.; Liu, Z.; Feng, L.; Su, X.; Chen, Y.; Xia, L.; Xia, Y.; Liu, Z. Enhanced high voltage cyclability of LiCoO2 cathode by adopting poly[bis–(ethoxyethoxyethoxy)phosphazene] with flame–retardant property as an electrolyte additive for lithium-ion batteries. Appl. Surf. Sci. 2017, 403, 260–266. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wei, H.; Fu, J.; Huang, Y.; Tang, X. Enhanced electrochemical properties of polyethylene oxide–based composite solid polymer electrolytes with porous inorganic–organic hybrid polyphosphazene nanotubes as fillers. J. Solid State Electrochem. 2012, 16, 101–107. [Google Scholar] [CrossRef]
- Gao, S.; Xu, H.; Luo, T.; Guo, Y.; Li, Z.; Ouadah, A.; Zhang, Y.; Zhang, Z.; Zhu, C. Novel proton conducting membranes based on cross-linked sulfonated polyphosphazenes and poly(ether ether ketone). J. Membr. Sci. 2017, 536, 1–10. [Google Scholar] [CrossRef]
- Jankowsky, S.; Hiller, M.M.; Wiemhöfer, H.D. Preparation and electrochemical performance of polyphosphazene based salt-in-polymer electrolyte membranes for lithium ion batteries. J. Power Sources 2014, 253, 256–262. [Google Scholar] [CrossRef]
- Prasanna, D.; Selvaraj, V. Development of non–covalent ternary polymer–CNT composites as a novel supporting material for electrooxidation of glycerol. RSC Adv. 2015, 5, 98822–98833. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Z.; Chen, X.; Oyama, M. AuPd bimetallic nanoparticles decorated on graphene nanosheets: Their green synthesis, growth mechanism and high catalytic ability in 4–nitrophenol reduction. J. Mater. Chem. A 2014, 2, 5668–5674. [Google Scholar] [CrossRef]
- Amin, A.M.; Wang, L.; Wang, J.; Yu, H.; Huo, J.; Gao, J.; Xiao, A. Recent Research Progress in the Synthesis of Polyphosphazene and Their Applications. Des. Monomers Polym. 2012, 12, 357–375. [Google Scholar] [CrossRef]
- Fu, J.; Huang, X.; Huang, Y.; Zhu, L.; Zhu, Y.; Tang, X. Facile Preparation of Branched Phosphazene-Containing Nanotubes via an in situ Template Approach. Macromol. Mater. Eng. 2008, 293, 173–177. [Google Scholar] [CrossRef]
- Zhang, S.; Ali, S.; Kong, Y.; Ma, H.; Zhang, L.; Wu, Z.; Wu, D. Evolution of chemical structure of polydichlorophosphazene in various solvents and ways to prolong its stability. J. Mol. Liq. 2017, 225, 536–543. [Google Scholar] [CrossRef]
- Ullah, R.S.; Wang, L.; Yu, H.; Abbasi, N.M.; Akram, M.; ul-Abdin, Z.; Saleem, M.; Haroon, M.; Khan, R.U. Synthesis of polyphosphazenes with different side groups and various tactics for drug delivery. RSC Adv. 2017, 7, 23363–23391. [Google Scholar] [CrossRef]
- Allcock, H.R. The expanding field of polyphosphazene high polymers. Dalton Trans. 2016, 45, 1856–1862. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wei, H.; Fu, J.; Huang, Y.; Tang, X. Effect of surface modified porous inorganic–organic hybrid polyphosphazene nanotubes on the properties of polyethylene oxide based solid polymer electrolytes. Electrochim. Acta 2010, 55, 5966–5974. [Google Scholar] [CrossRef]
- Wei, W.; Huang, X.; Tao, Y.; Chen, K.; Tang, X. Enhancement of the electrocapacitive performance of manganese dioxide by introducing a microporous carbon spheres network. Phys. Chem. Chem. Phys. 2012, 14, 5966–5972. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Li, Y.; Wang, H.-G.; Lv, X.; Li, Y.; Xu, D.; Jiang, Y.; Meng, Y. Multi–heteroatom–doped dual carbon–confined Fe3O4 nanospheres as high–capacity and long-life anode materials for lithium/sodium ion batteries. J. Colloid Interface Sci. 2020, 565, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zheng, D.; Zhao, M.; Chen, H.; Fan, X.; Gao, B.; Gu, L.; Guo, Y.; Qin, J.; Wei, J.; et al. Cross–Linked Polyphosphazene Hollow Nanosphere–Derived N/P–Doped Porous Carbon with Single Nonprecious Metal Atoms for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 14639–14646. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, H.; Liu, D.; Yan, C.; Zhu, Y. A facile one–pot fabrication of polyphosphazene microsphere/carbon fiber hybrid reinforcement and its effect on the interfacial adhesion of epoxy composites. Appl. Surf. Sci. 2017, 410, 530–539. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, S.; Han, T.; Hua, M.; He, S. New organic–inorganic hybrid polymers as Pickering emulsion stabilizers. Colloids Surf. A Physicochem. Eng. Asp. 2018, 542, 42–51. [Google Scholar] [CrossRef]
- Gao, M.; Fu, J.; Wang, M.; Wang, K.; Wang, S.; Wang, Z.; Chen, Z.; Xu, Q. A self–template and self–activation co–coupling green strategy to synthesize high surface area ternary–doped hollow carbon microspheres for high performance supercapacitors. J. Colloid Interface Sci. 2018, 524, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Ma, C.; Wang, X. Melamine-containing polyphosphazene wrapped ammonium polyphosphate: A novel multifunctional organic-inorganic hybrid flame retardant. J. Hazard. Mater. 2018, 344, 839–848. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Q.; Low, C.T.J.; Walton, R.I.; McNally, T.; Wan, C. Heteroatom–doped core/shell carbonaceous framework materials: Synthesis, characterization and electrochemical properties. New J. Chem. 2019, 43, 5632–5641. [Google Scholar] [CrossRef]
- Schmohl, S.; He, X.; Wiemhofer, H.D. Boron Trifluoride Anionic Side Groups in Polyphosphazene Based Polymer Electrolyte with Enhanced Interfacial Stability in Lithium Batteries. Polymers 2018, 10, 1350. [Google Scholar] [CrossRef]
- Yu, S.; Schmohl, S.; Liu, Z.; Hoffmeyer, M.; Schön, N.; Hausen, F.; Tempel, H.; Kungl, H.; Wiemhöfer, H.D.; Eichel, R.A. Insights into a layered hybrid solid electrolyte and its application in long lifespan high–voltage all–solid–state lithium batteries. J. Mater. Chem. A 2019, 7, 3882–3894. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Yin, G.P.; Wang, Z.B.; Gao, Y.Z. Proton exchange membrane fuel cell from low temperature to high temperature: Material challenges. J. Power Sources 2007, 167, 235. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Bodner, M.; Hacker, V. A Brief Review of Poly (Vinyl Alcohol)—Based Anion Exchange Membranes for Alkaline Fuel Cells. Polymers 2022, 14, 3565. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, M.; Zhang, S.; Wei, L.; Wang, F.; Wang, Z.; Wei, Y.; Han, K. Combined method to prepare core–shell structured catalyst for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2013, 38, 3323. [Google Scholar] [CrossRef]
- Park, H.; Kim, K.M.; Kim, H.; Kim, D.-K.; Won, Y.S.; Kim, S.-K. Electrodeposition-fabricated PtCu-alloy cathode catalysts for high-temperature proton exchange membrane fuel cells. Korean J. Chem. Eng. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Lyonnard, S.; Gebel, G. Neutrons for fuel cell membranes: Structure, sorption and transport properties. Eur. Phys. J.-Spec. Top. 2012, 213, 195. [Google Scholar] [CrossRef]
- Arcella, V.; Troglia, C.; Ghielmi, A. Hyflon ion membranes for fuel cells. Ind. Eng. Chem. Res. 2005, 44, 7646. [Google Scholar] [CrossRef]
- Wang, J.W.; Yue, Z.R.; Economy, J. Preparation of proton–conducting composite membranes from sulfonated poly (ether ether ketone) and polyacrylonitrile. J. Membr. Sci. 2007, 291, 210. [Google Scholar] [CrossRef]
- Roziere, J.; Jones, D.J. Non-fluorinated polymer materials for proton exchange membrane fuel cells. Annu. Rev. Mater. Res. 2003, 33, 503. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Solid polymer electrolyte membranes for fuel cell applications—A review. J. Membr. Sci. 2005, 259, 10. [Google Scholar] [CrossRef]
- Kerres, J.A. Blended and cross–linked ionomer membranes for application in membrane fuel cells. Fuel Cells 2005, 5, 230. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H.; Dahlan, K.Z.M. Single–step radiation induced grafting for preparation of proton exchange membranes for fuel cell. J. Membr. Sci. 2009, 339, 115. [Google Scholar] [CrossRef]
- Kaliaguine, S.; Mikhailenko, S.D.; Wang, K.P.; Xing, P.; Robertson, G.; Guiver, M. Properties of SPEEK based PEMs for fuel cell application. Catal. Today 2003, 82, 213. [Google Scholar] [CrossRef]
- Asensio, J.A.; Borros, S.; Gomez–Romero, P. Proton–conducting polymers based on benzimidazoles and sulfonated benzimidazoles. J. Polym. Sci. Pol. Chem. 2002, 40, 3703. [Google Scholar] [CrossRef]
- Yoshimura, K.; Iwasaki, K. Aromatic Polymer with Pendant Perfluoroalkyl Sulfonic Acid for Fuel Cell Applications. Macromolecules 2009, 42, 9302. [Google Scholar] [CrossRef]
- Tsui, E.M.; C ortalezzi, M.M.; Wiesner, M.R. Proton conductivity and methanol rejection by ceramic membranes derived from ferroxane and alumoxane precursors. J. Membr. Sci. 2007, 306, 8. [Google Scholar] [CrossRef]
- Vichi, F.M.; Colomer, M.T.; Anderson, M.A. Nanopore ceramic membranes as novel electrolytes for proton exchange membranes. Electrochem. Solid-State Lett. 1999, 2, 313. [Google Scholar] [CrossRef]
- Vichi, F.M.; Tejedor–Tejedor, M.I.; Anderson, M.A. Effect of pore–wall chemistry on proton conductivity in mesoporous titanium dioxide. Chem. Mater. 2000, 12, 1762. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Sasmito, A.P.; Shamim, T. Advances in proton exchange membrane fuel cell with dead–end anode operation: A review. Appl. Energy 2019, 252, 113416. [Google Scholar] [CrossRef]
- Wang, C.G.; Li, Z.F.; Sun, P.; Pei, H.C.; Yin, X.Y. Preparation and Properties of Covalently Crosslinked Polybenzimidazole High Temperature Proton Exchange Membranes Doped with High Sulfonated Polyphosphazene. J. Electrochem. Soc. 2020, 167, 104517. [Google Scholar] [CrossRef]
- Hacivelioglu, F.; Ozden, S.; Celik, S.U.; Yesilot, S.; Kilic, A.; Bozkurt, A. Azole substituted polyphosphazenes as nonhumidified proton conducting membranes. J. Mater. Chem. 2011, 21, 1020. [Google Scholar] [CrossRef]
- Luo, T.W.; Zhang, Y.X.; Xu, H.L.; Zhang, Z.Y.; Fu, F.Y.; Gao, S.T.; Ouadah, A.; Dong, Y.; Wang, S.; Zhu, C.J. Highly conductive proton exchange membranes from sulfonated polyphosphazene-graft-copolystyrenes doped with sulfonated single–walled carbon nanotubes. J. Membr. Sci. 2016, 514, 527. [Google Scholar] [CrossRef]
- Han, H.S.; Ma, H.M.; Yu, J.H.; Zhu, H.; Wang, Z.M. Preparation and performance of novel tetraphenylphosphonium–functionalized polyphosphazene membranes for alkaline fuel cells. Eur. Polym. J. 2019, 114, 109. [Google Scholar] [CrossRef]
- Yan, X.M.; Gu, S.; He, G.H.; Wu, X.M.; Zheng, W.J.; Ruan, X.H. Quaternary phosphonium–functionalized poly (ether ether ketone) as highly conductive and alkali–stable hydroxide exchange membrane for fuel cells. J. Membr. Sci. 2014, 466, 220. [Google Scholar] [CrossRef]
- Wang, L.D.; Liu, Y.; Wang, J.Y. Crosslinked anion exchange membrane with improved membrane stability and conductivity for alkaline fuel cells. J. Appl. Polym. Sci. 2019, 136, 48169. [Google Scholar] [CrossRef]
- Liu, F.H.; Lin, C.X.; Hu, E.N.; Yang, Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Anion exchange membranes with well–developed conductive channels: Effect of the functional groups. J. Membr. Sci. 2018, 564, 298. [Google Scholar] [CrossRef]
- Ran, J.; Ding, L.; Yu, D.B.; Zhang, X.; Hu, M.; Wu, L.; Xu, T.W. A novel strategy to construct highly conductive and stabilized anionic channels by fluorocarbon grafted polymers. J. Membr. Sci. 2018, 549, 631. [Google Scholar] [CrossRef]
- Pan, J.; Han, J.J.; Zhu, L.; Hickner, M.A. Cationic Side–Chain Attachment to Poly (Phenylene Oxide) Backbones for Chemically Stable and Conductive Anion Exchange Membranes. Chem. Mater. 2017, 29, 5321. [Google Scholar] [CrossRef]
- Pan, Z.F.; An, L.; Zhao, T.S.; Tang, Z.K. Advances and challenges in alkaline anion exchange membrane fuel cells. Prog. Energy Combust. Sci. 2018, 66, 141. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, E.J.; Han, J.; Shin, D.W.; Kim, Y.S.; Bae, C. Poly(terphenylene) Anion Exchange Membranes: The Effect of Backbone Structure on Morphology and Membrane Property. ACS Macro Lett. 2017, 6, 566. [Google Scholar] [CrossRef]
- Pham, T.H.; Olsson, J.S.; Jannasch, P. N–Spirocyclic Quaternary Ammonium Ionenes for Anion–Exchange Membranes. J. Am. Chem. Soc. 2017, 139, 2888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zimudzi, T.J.; Wang, Y.; Yu, X.; Pan, J.; Han, J.; Kushner, D.I.; Zhuang, L.; Hickner, M.A. Mechanically Robust Anion Exchange Membranes via Long Hydrophilic Cross–Linkers. Macromolecules 2017, 50, 2329. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.Y. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629. [Google Scholar] [CrossRef]
- Li, Z.; He, G.W.; Li, Z.Y.; Zhang, Y.; Zhao, J.; Xu, M.Z.; Xu, S.M.; Jiang, Z.Y. Enhancing the hydroxide conductivity of imidazolium-functionalized polysulfone by incorporating organic microsphere with ionic brushes. J. Membr. Sci. 2018, 554, 6. [Google Scholar] [CrossRef]
- Khan, A.; Sherazi, T.A.; Khan, Y.; Li, S.H.; Naqvi, S.A.R.; Cui, Z.L. Fabrication and characterization of polysulfone/modified nanocarbon black composite antifouling ultrafiltration membranes. J. Membr. Sci. 2018, 554, 71. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.X.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131. [Google Scholar] [CrossRef]
- Li, C.R.; Wang, G.H.; Yu, D.B.; Sheng, F.M.; Shehzad, M.A.; He, T.Y.; Xu, T.T.; Ren, X.M.; Cao, M.; Wu, B.; et al. Cross-linked anion exchange membranes with hydrophobic side–chains for anion separation. J. Membr. Sci. 2019, 581, 150. [Google Scholar] [CrossRef]
- Guan, Q.B.; Yuan, L.; Zhang, Y.; Gu, A.J.; Liang, G.Z. Tailoring the structure of aligned carbon nanotube bundle by reactive polymer for strengthening its surface interaction with thermosets and the excellent properties of the hybrid thermosets. Appl. Surf. Sci. 2018, 439, 638. [Google Scholar] [CrossRef]
- Wu, Q.X.; Pan, Z.F.; An, L. Recent advances in alkali-doped polybenzimidazole membranes for fuel cell applications. Renew. Sust. Energy Rev. 2018, 89, 168. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, H.; Jana, T.; Scanlon, E.; Chen, R.; Choe, E.W.; Ramanathan, L.S.; Yu, S.; Benicewicz, B.C. Synthesis and characterization of pyridine–based polybenzimidazoles for high temperature polymer electrolyte membrane fuel cell applications. Fuel Cells 2005, 5, 287. [Google Scholar] [CrossRef]
- Jheng, L.C.; Hsu, S.L.C.; Lin, B.Y.; Hsu, Y.L. Quaternized polybenzimidazoles with imidazolium cation moieties for anion exchange membrane fuel cells. J. Membr. Sci. 2014, 460, 160. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.N.; Zhang, J.; Lu, S.F.; Xiang, Y. Effect of side chain on the electrochemical performance of poly (ether ether ketone) based anion-exchange membrane: A molecular dynamics study. J. Membr. Sci. 2020, 605, 118105. [Google Scholar] [CrossRef]
- Yan, X.M.; Gu, S.; He, G.H.; Wu, X.M.; Benziger, J. Imidazolium–functionalized poly (ether ether ketone) as membrane and electrode ionomer for low–temperature alkaline membrane direct methanol fuel cell. J. Power Sources 2014, 250, 90. [Google Scholar] [CrossRef]
- Luther, T.A.; Stewart, F.F.; Budzien, J.L.; LaViolette, R.A.; Bauer, W.F.; Harrup, M.K.; Allen, C.W.; Elayan, A. On the Mechanism of Ion Transport through Polyphosphazene Solid Polymer Electrolytes: NMR, IR, and Raman Spectroscopic Studies and Computational Analysis of 15N-Labeled Polyphosphazenes. J. Phys. Chem. B 2003, 107, 3168. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, H.; Li, R.; Chen, N.; Han, K. Layered double hydroxide–polyphosphazene–based ionomer hybrid membranes with electric field–aligned domains for hydroxide transport. J. Mater. Chem. A 2014, 2, 8376–8385. [Google Scholar] [CrossRef]
- Dar, S.U.; Din, M.A.U.; Hameed, M.U.; Ali, S.; Akram, R.; Wu, Z.P.; Wu, D.Z. Oxygen reduction reaction of (C–PCTNB@CNTs): A nitrogen and phosphorus dual-doped carbon electro–catalyst derived from polyphosphazenes. J. Power Sources 2018, 373, 61. [Google Scholar] [CrossRef]
- Yu, K.; Lin, Y.; Fan, J.C.; Li, Q.X.; Shi, P.H.; Xu, Q.J.; Min, Y.L. Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction. Catalysts 2019, 9, 114. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, F.; Jiang, Z.; Liu, T.; Fei, Y.; Kuang, T.; Zhong, M.; Liu, X.; Song, S. MoS2 nanosheets uniformly grown on polyphosphazene–derived carbon nanospheres for lithium-ion batteries. Surf. Interfaces 2021, 24, 101034. [Google Scholar] [CrossRef]
- Zhang, C.L.; Song, A.G.; Yuan, P.L.; Wang, Q.; Wang, P.; Zhang, S.L.; Cao, G.Q.; Hu, J.H. Amorphous carbon shell on Si particles fabricated by carbonizing of polyphosphazene and enhanced performance as lithium ion battery anode. Mater. Lett. 2016, 171, 63. [Google Scholar] [CrossRef]
- Dufek, E.J.; Stone, M.L.; Jamison, D.K.; Stewart, F.F.; Gering, K.L.; Petkovic, L.M.; Wilson, A.D.; Harrup, M.K.; Rollins, H.W. Hybrid phosphazene anodes for energy storage applications. J. Power Sources 2014, 267, 347. [Google Scholar] [CrossRef]
- Pappas, G.S.; Ferrari, S.; Huang, X.; Bhagat, R.; Haddleton, D.M.; Wan, C. Heteroatom Doped–Carbon Nanospheres as Anodes in Lithium Ion Batteries. Materials 2016, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Wemyss, A.M.; Brown, O.B.; Huang, Q.Y.; Wan, C.Y. Structure and electrochemical properties of hierarchically porous carbon nanomaterials derived from hybrid ZIF–8/ZIF–67 bi–MOF coated cyclomatrix poly(organophosphazene) nanospheres. New J. Chem. 2020, 44, 4353. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, R.; Yin, C.; Li, Z.; Wu, W.; Wu, M. In situ growth of polyphosphazene nanoparticles on graphene sheets as a highly stable nanocomposite for metal–free lithium anodes. RSC Adv. 2016, 6, 62005. [Google Scholar] [CrossRef]
- Yesilot, S.; Hacivelioglu, F.; Kucukkoylu, S.; Celik, K.B.; Sayan, G.; Demir–Cakan, R. Design, Synthesis, and Characterization of Polyphosphazene Bearing Stable Nitroxide Radicals as Cathode–Active Materials in Li-Ion Batteries. Macromol. Chem. Phys. 2017, 218. [Google Scholar] [CrossRef]
- Yesilot, S.; Hacivelioglu, F.; Kucukkoylu, S.; Demir, E.; Celik, K.B.; Demir–Cakan, R. A novel polyphosphazene with nitroxide radical side groups as cathode–active material in Li–ion batteries. Polym. Adv. Technol. 2019, 30, 2977. [Google Scholar] [CrossRef]
- Wang, J.M.; Ge, B.C.; Li, H.; Yang, M.; Wang, J.; Liu, D.; Fernandez, C.; Chen, X.B.; Peng, Q.M. Challenges and progresses of lithium-metal batteries. Chem. Eng. J. 2021, 420, 129739. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Dar, S.U.; Zhao, Y.; Akram, R.; Zhang, X.; Jin, S.; Wu, Z.; Wu, D. Polyphosphazene-derived heteroatoms-doped carbon materials for supercapacitor electrodes. Carbon 2018, 129, 420–427. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Yang, Y.; Liu, W.; Qiu, M.; Abbas, Y.; Wu, Z.; Wu, D. Heteroatoms doped carbons derived from crosslinked polyphosphazenes for supercapacitor electrodes. Electrochim. Acta 2019, 328, 135064. [Google Scholar] [CrossRef]
- Rangom, Y.; Tang, X.; Nazar, L.F. Carbon Nanotube–Based Supercapacitors with Excellent ac Line Filtering and Rate Capability via Improved Interfacial Impedance. ACS Nano 2015, 9, 7248–7255. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, S.; Abbas, Y.; Zhang, C.; Liu, W.; Wu, Z.; Dai, S.; Zhang, T. Heteroatom–doped ultrahigh specific area carbons from hybrid polymers with promising capacitive performance. J. Power Sources 2020, 478, 228761. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, S.; Abbas, Y.; Liu, W.; Zhang, Y.; Wu, Z.; Xu, B. Structurally designed heterochain polymer derived porous carbons with high surface area for high-performance supercapacitors. Appl. Surf. Sci. 2020, 530, 147296. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Z.; Chao, L.; Wu, X.; Lei, X.; Chang, Z.; Sun, X. Synthesis of hierarchical porous N-doped sandwich-type carbon composites as high-performance supercapacitor electrodes. J. Mater. Chem. A 2015, 3, 3667–3675. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible–Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050. [Google Scholar] [CrossRef] [PubMed]
- Hailegnaw, B.; Poscher, V.; Ulbricht, C.; Seelajaroen, H.; Teasdale, I.; Salinas, Y.; Sariciftci, N.S.; Scharber, M.C. Improving the Performance of Perovskite Solar Cells using a Polyphosphazene Interfacing Layer. Phys. Status Solidi A 2019, 216. [Google Scholar] [CrossRef]
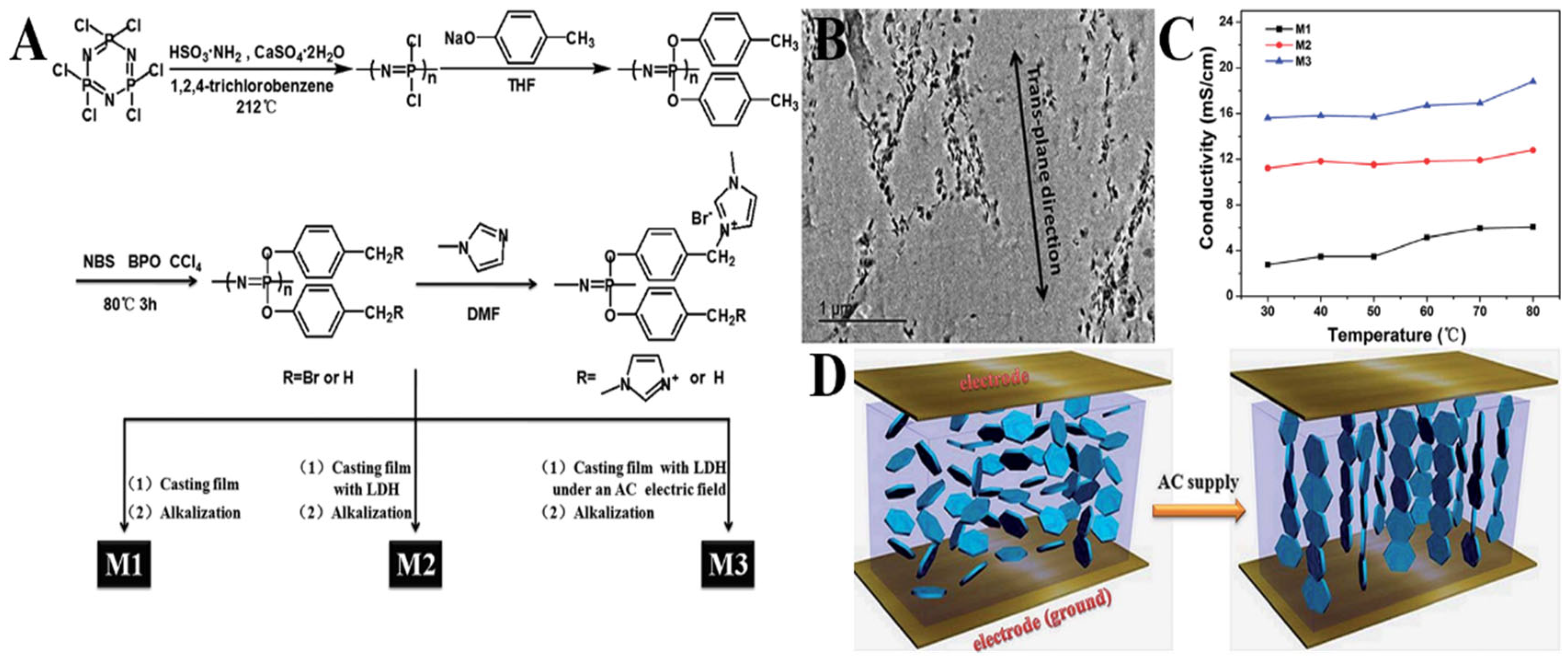
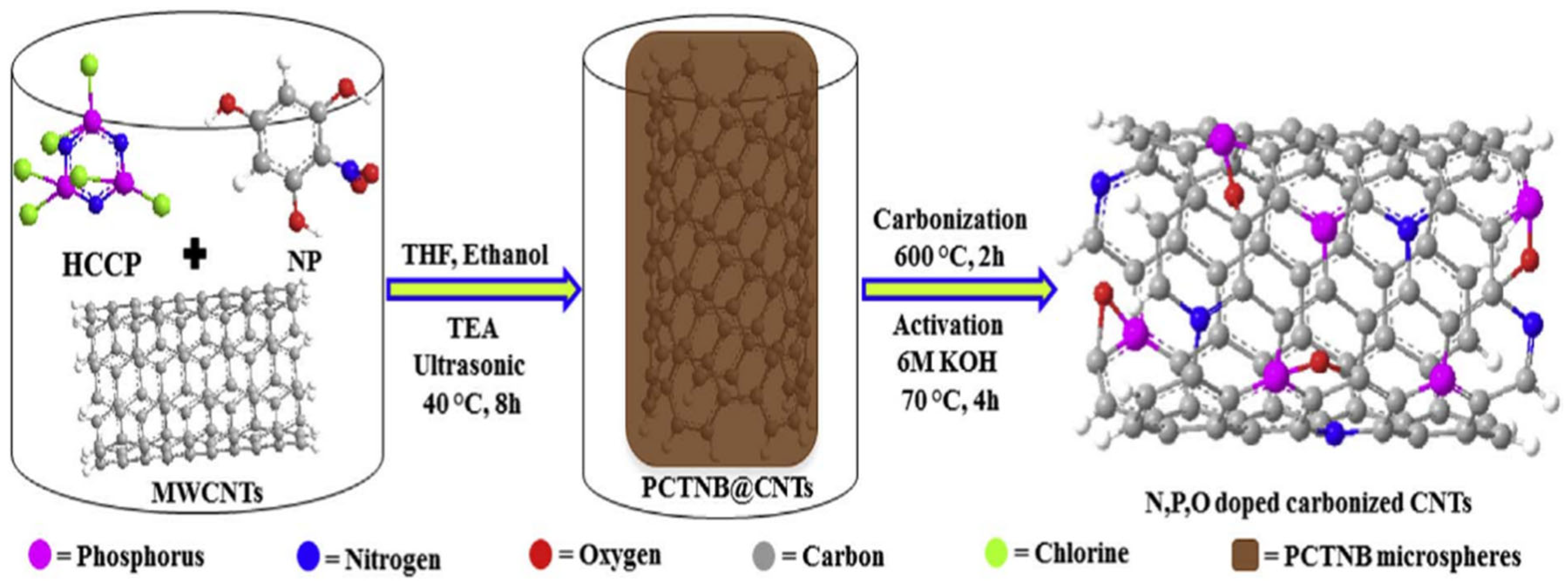
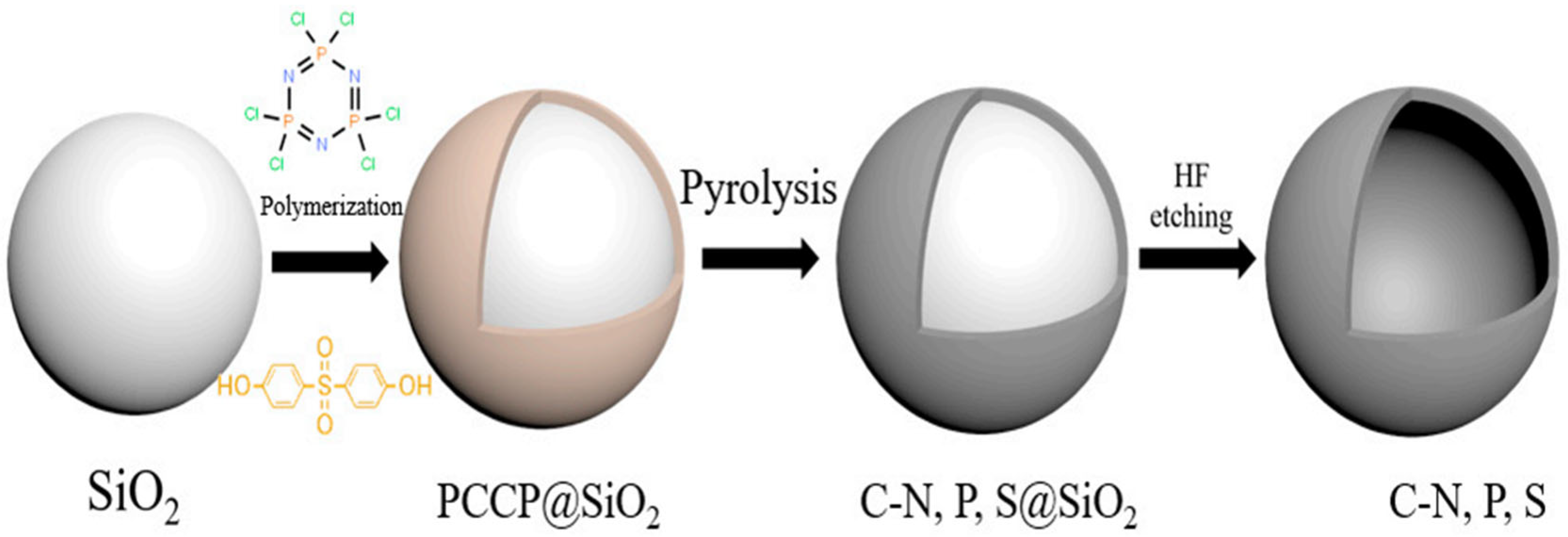
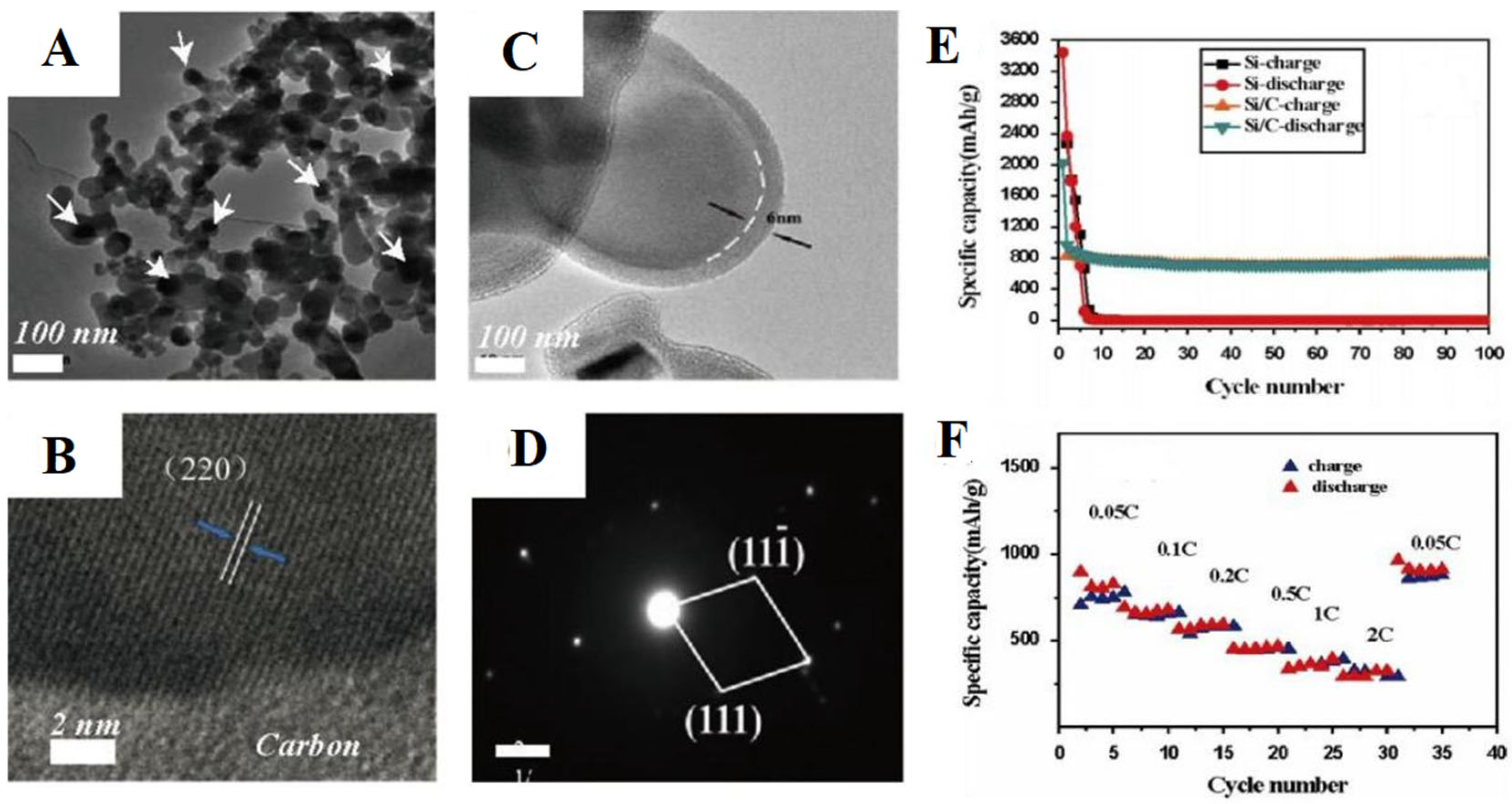



| PPNs Ion-Exchange Membranes | Ionic-Conductivity | Reference |
|---|---|---|
| SPOP–SPEEK | 0.132 S/cm (70 °C) | [18] |
| M4–p-BMPP–PSx–PSBSy–4-BP | 0.51 S/cm (25 °C) | [49] |
| PDB155P–HAH60 | 0.786 S/cm (90 °C) | [50] |
| SPEEK:SCNTB1:2 | 0.132 S/cm (25 °C) | [62] |
| PVDF-g–PSSA-0.65 | 0.114 S/cm (25 °C) | [92] |
| mPBI–TGIG–SPOP | 0.143 S/cm (180 °C) | [100] |
| PMPCP | 0.041 S/cm (130 °C) | [101] |
| CF3–PSx–PSBOSy–SCNT | 0.55 S/cm (140 °C) | [102] |
| PPMPP-3-40% | 0.223 S/cm (80 °C) | [103] |
| PEEK–QPOHs | 0.089 S/cm (60 °C) | [104] |
| AEM-2 | 0.047 S/cm (60 °C) | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Jiang, Z.; Chen, F.; Kuang, T.; Zhou, D.; Meng, F. Research Progress in Energy Based on Polyphosphazene Materials in the Past Ten Years. Polymers 2023, 15, 15. https://doi.org/10.3390/polym15010015
Zhou Z, Jiang Z, Chen F, Kuang T, Zhou D, Meng F. Research Progress in Energy Based on Polyphosphazene Materials in the Past Ten Years. Polymers. 2023; 15(1):15. https://doi.org/10.3390/polym15010015
Chicago/Turabian StyleZhou, Zeping, Zhen Jiang, Feng Chen, Tairong Kuang, Dapeng Zhou, and Fuliang Meng. 2023. "Research Progress in Energy Based on Polyphosphazene Materials in the Past Ten Years" Polymers 15, no. 1: 15. https://doi.org/10.3390/polym15010015
APA StyleZhou, Z., Jiang, Z., Chen, F., Kuang, T., Zhou, D., & Meng, F. (2023). Research Progress in Energy Based on Polyphosphazene Materials in the Past Ten Years. Polymers, 15(1), 15. https://doi.org/10.3390/polym15010015








