Alkyl Chain Engineering of Low Bandgap Non-Fullerene Acceptors for High-Performance Organic Solar Cells: Branched vs. Linear Alkyl Side Chains
Abstract
:1. Introduction
2. Results and Discussion
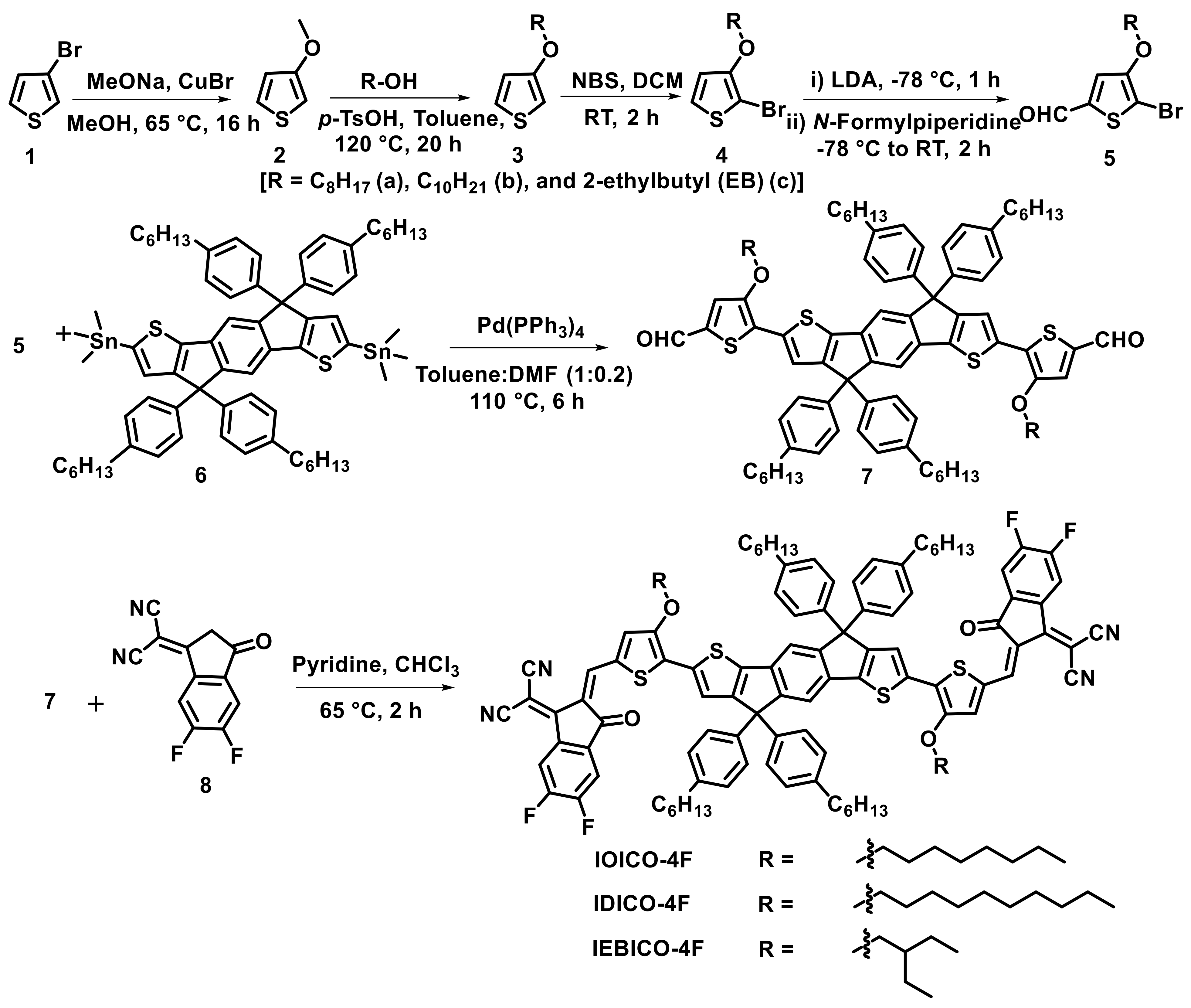
2.1. Synthesis of NFAs
2.2. Optical and Electrochemical Properties
2.3. Thermal Properties
2.4. Photovoltaic Properties
2.5. Morphological Characterization
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inganäs, O. Organic photovoltaics over three decades. Adv. Mater. 2018, 30, 1800388. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Liu, W.; Zhu, X. n-Type molecular photovoltaic materials: Design strategies and device applications. J. Am. Chem. Soc. 2020, 142, 11613–11628. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019, 48, 1596–1625. [Google Scholar] [CrossRef] [PubMed]
- Armin, A.; Li, W.; Sandberg, O.J.; Xiao, Z.; Ding, L.; Nelson, J.; Neher, D.; Vandewal, K.; Shoaee, S.; Wang, T.; et al. A history and perspective of non-fullerene electron acceptors for organic solar cells. Adv. Energy Mater. 2021, 11, 2003570. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Yuan, J.; Leclerc, M.; Zou, Y.; Li, Y. Low-bandgap non-fullerene acceptors enabling high-performance organic solar cells. ACS Energy Lett. 2021, 6, 598–608. [Google Scholar] [CrossRef]
- Mishra, A. Material perceptions and advances in molecular heteroacenes for organic solar cells. Energy Environ. Sci. 2020, 13, 4738–4793. [Google Scholar] [CrossRef]
- Wan, X.; Li, C.; Zhang, M.; Chen, Y. Acceptor-donor-acceptor type molecules for high performance organic photovoltaics-chemistry and mechanism. Chem. Soc. Rev. 2020, 49, 2828–2842. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photon. 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Cheng, P.; Zhao, X.; Zhan, X. Perylene Diimide-Based Oligomers and Polymers for Organic Optoelectronics. Acc. Mater. Res. 2022, 3, 309–318. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G.; et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J.; et al. Single-junction organic photovoltaic cell with 19% efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef]
- Yan, C.; Qin, J.; Wang, Y.; Li, G.; Cheng, P. Emerging Strategies toward Mechanically Robust Organic Photovoltaics: Focus on Active Layer. Adv. Energy Mater. 2022, 12, 2201087. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Y.; Qin, M.; Li, J.; Wang, Y.; Qin, J.; Cheng, P. Towards High-Performance Organic Photovoltaics: The New Cooperation of Sequential Solution-Processing and Promising Non-fullerene Acceptors. Mater. Horiz. 2022, 9, 2097–2108. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, X. Fused-Ring Electron Acceptors for Photovoltaics and Beyond. Acc. Chem. Res. 2021, 54, 132–143. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, J.; Chen, Y.; Chai, G.; Xu, X.; Yu, L.; Ma, R.; Yu, H.; Liu, T.; Liu, P.; et al. Achieving efficient ternary organic solar cells using structurally similar non-fullerene acceptors with varying flanking side chains. Adv. Energy Mater. 2021, 11, 2100079. [Google Scholar] [CrossRef]
- Zhang, J.; Fujin, B.; Angunawela, I.; Xu, X.; Luo, S.; Li, C.; Chai, G.; Yu, H.; Chen, Y.; Hu, H.; et al. Alkyl-chain branching of non-fullerene acceptors flanking conjugated side groups toward highly efficient organic solar cells. Adv. Energy Mater. 2021, 11, 2102596. [Google Scholar] [CrossRef]
- Warnan, J.; Cabanetos, C.; Bude, R.; El Labban, A.; Li, L.; Beaujuge, P.M. Electron-deficient n-alkyloyl derivatives of thieno[3,4-c]pyrrole-4,6-dione yield efficient polymer solar cells with open-circuit voltages > 1 V. Chem. Mater. 2014, 26, 2829–2835. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.-G.; Bin, H.; Chen, S.; Gao, L.; Xue, L.; Yang, C.; Li, Y. Side-chain isomerization on an n-type organic semiconductor itic acceptor makes 11.77% high efficiency polymer solar cells. J. Am. Chem. Soc. 2016, 138, 15011–15018. [Google Scholar] [CrossRef]
- Aldrich, T.J.; Swick, S.M.; Melkonyan, F.S.; Marks, T.J. Enhancing indacenodithiophene acceptor crystallinity via substituent manipulation increases organic solar cell efficiency. Chem. Mater. 2017, 29, 10294–10298. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, S.; Liu, Y.; Hou, R.; Zhang, Z.; Xu, X.; Wu, Y.; Bo, Z. Effect of non-fullerene acceptors’ side chains on the morphology and photovoltaic performance of organic solar cells. ACS Appl. Mater. Interfaces 2017, 9, 33906–33912. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Hong, L.; Yao, H.; Wu, Z.; Cui, Y.; Zhang, T.; Xu, Y.; Yu, R.; Liao, Q.; Gao, B.; Xian, K.; et al. Eco-compatible solvent-processed organic photovoltaic cells with over 16% efficiency. Adv. Mater. 2019, 31, 1903441. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Wei, Q.; Lai, J.Y.L.; Peng, Z.; Kim, H.K.; Yuan, J.; Ye, L.; Ade, H.; Zou, Y.; Yan, H. Alkyl chain tuning of small molecule acceptors for efficient organic solar cells. Joule 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Yao, H.; Chen, Y.; Qin, Y.; Yu, R.; Cui, Y.; Yang, B.; Li, S.; Zhang, K.; Hou, J. Design and synthesis of a low bandgap small molecule acceptor for efficient polymer solar cells. Adv. Mater. 2016, 28, 8283–8287. [Google Scholar] [CrossRef]
- Yao, H.; Cui, Y.; Yu, R.; Gao, B.; Zhang, H.; Hou, J. Design, synthesis, and photovoltaic characterization of a small molecular acceptor with an ultra-narrow band gap. Angew. Chem. Int. Ed. 2017, 56, 3045–3049. [Google Scholar] [CrossRef]
- Gao, W.; An, Q.; Hao, M.; Sun, R.; Yuan, J.; Zhang, F.; Ma, W.; Min, J.; Yang, C. Thick-film organic solar cells achieving over 11% efficiency and nearly 70% fill factor at thickness over 400 nm. Adv. Funct. Mater. 2020, 30, 1908336. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Z.; Xu, Y.; Wang, J.; Wang, Y.; Zhang, S.; Hou, J. Quantifying Voc loss induced by alkyl pendants of acceptors in organic solar cells. J. Mater. Chem. C 2020, 8, 12568–12577. [Google Scholar] [CrossRef]





| NFA | Absorption (nm) | εmax,solution (M−1·cm−1) | εmax,film (cm−1) | λOnset(film) (nm) | Egopt (eV) | Energy Levels (eV) | EgCV(eV) | ||
|---|---|---|---|---|---|---|---|---|---|
| Solution | Film | HOMO | LUMO | ||||||
| IEBICO-4F | 804 | 854 | 1.8 × 105 | 0.81 × 105 | 970 | 1.28 | −5.37 | −3.98 | 1.39 |
| IEHICO-4F | 807 | 860 | 1.6 × 105 | 0.93 × 105 | 969 | 1.28 | −5.41 | −4.05 | 1.36 |
| IOICO-4F | 805 | 858 | 1.5 × 105 | 0.93 × 105 | 961 | 1.29 | −5.40 | −3.99 | 1.41 |
| IDICO-4F | 806 | 853 | 1.8 × 105 | 0.93 × 105 | 962 | 1.29 | −5.41 | −3.99 | 1.42 |
| Active Layer | VOC | JSC | Calculated JSC | FF | PCE |
|---|---|---|---|---|---|
| (V) | (mA/cm2) | (mA/cm2) | (%) | (%) | |
| PTB7-Th:IEBICO-4F | 0.736 | 22.22 | 22.16 | 64.94 | 10.63 |
| (0.730 ± 0.004) | (21.55 ± 0.19) | (64.21 ± 1.55) | (10.11 ± 0.30) | ||
| PTB7-Th:IEHICO-4F | 0.726 | 22.20 | 21.29 | 66.06 | 10.64 |
| (0.724 ± 0.004) | (21.85 ± 0.27) | (63.89 ± 1.72) | (10.11 ± 0.38) | ||
| PTB7-Th:IOICO-4F | 0.718 | 21.50 | 21.03 | 64.38 | 9.93 |
| (0.715 ± 0.003) | (21.20 ± 0.18) | (63.09 ± 1.59) | (9.56 ± 0.29) | ||
| PTB7-Th:IDICO-4F | 0.737 | 21.10 | 20.31 | 57.76 | 8.98 |
| (0.731 ± 0.006) | (20.13 ± 0.58) | (54.57 ± 3.71) | (8.04 ± 0.75) |
| Sample | Sector | Location | d Spacing | Amplitude | Area | FWHM | CL |
|---|---|---|---|---|---|---|---|
| (Å−1) | (Å) | (Å−1) | (nm) | ||||
| PTB7-Th | OOP (010) | 1.616 | 3.89 | 1431 | 752 | 0.387 | 1.46 |
| IP (100) | 0.262 | 23.98 | 2133 | 314 | 0.104 | 5.44 | |
| IEBICO-4F | OOP (100) | 0.352 | 17.85 | 2576 | 290 | 0.096 | 5.92 |
| IP (010) | 1.783 | 3.52 | 290 | 70 | 0.192 | 2.94 | |
| IEHICO-4F | OOP (010) | 1.821 | 3.45 | 2246 | 402 | 0.114 | 4.97 |
| IP (100) | 0.325 | 19.31 | 6032 | 310 | 0.041 | 13.64 | |
| IOICO-4F | OOP (100) | 0.370 | 16.97 | 8048 | 997 | 0.113 | 5.03 |
| IP (010) | 1.744 | 3.60 | 125 | 48 | 0.252 | 2.25 | |
| IDICO-4F | OOP (100) | 0.375 | 16.77 | 102859 | 3177 | 0.028 | 20.54 |
| IP (010) | 1.764 | 3.56 | 90 | 15 | 0.154 | 3.67 |
| Position (Å−1) | FWHM (Å−1) | d Spacing (Å) | CL (nm) | The Relative (010) Peak Intensity of NFA | The Intensity Ratio of NFA/Polymer | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polymer | NFA | Polymer | NFA | Polymer | NFA | Polymer | NFA | |||
| PTB7-Th: IEBICO-4F | 1.563 | 1.779 | 0.551 | 0.228 | 4.02 | 3.53 | 1.03 | 2.48 | 0.61 | 0.360 |
| PTB7-Th: IEHICO-4F | 1.596 | 1.784 | 0.521 | 0.180 | 3.94 | 3.52 | 1.09 | 3.15 | 0.48 | 0.287 |
| PTB7-Th: IOICO-4F | 1.592 | 1.795 | 0.450 | 0.175 | 3.95 | 3.50 | 1.26 | 3.24 | 1.00 | 0.595 |
| PTB7-Th: IDICO-4F | 1.566 | 1.754 | 0.495 | 0.223 | 4.01 | 3.58 | 1.14 | 2.53 | 0.66 | 0.547 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Raju, T.B.; Yeom, H.; Gopikrishna, P.; Kim, K.; Cho, H.W.; Moon, J.W.; Cho, J.H.; Kim, J.Y.; Kim, B. Alkyl Chain Engineering of Low Bandgap Non-Fullerene Acceptors for High-Performance Organic Solar Cells: Branched vs. Linear Alkyl Side Chains. Polymers 2022, 14, 3812. https://doi.org/10.3390/polym14183812
Lee Y, Raju TB, Yeom H, Gopikrishna P, Kim K, Cho HW, Moon JW, Cho JH, Kim JY, Kim B. Alkyl Chain Engineering of Low Bandgap Non-Fullerene Acceptors for High-Performance Organic Solar Cells: Branched vs. Linear Alkyl Side Chains. Polymers. 2022; 14(18):3812. https://doi.org/10.3390/polym14183812
Chicago/Turabian StyleLee, Youngwan, Telugu Bhim Raju, Hyerim Yeom, Peddaboodi Gopikrishna, Kwangmin Kim, Hye Won Cho, Jung Woo Moon, Jeong Ho Cho, Jin Young Kim, and BongSoo Kim. 2022. "Alkyl Chain Engineering of Low Bandgap Non-Fullerene Acceptors for High-Performance Organic Solar Cells: Branched vs. Linear Alkyl Side Chains" Polymers 14, no. 18: 3812. https://doi.org/10.3390/polym14183812
APA StyleLee, Y., Raju, T. B., Yeom, H., Gopikrishna, P., Kim, K., Cho, H. W., Moon, J. W., Cho, J. H., Kim, J. Y., & Kim, B. (2022). Alkyl Chain Engineering of Low Bandgap Non-Fullerene Acceptors for High-Performance Organic Solar Cells: Branched vs. Linear Alkyl Side Chains. Polymers, 14(18), 3812. https://doi.org/10.3390/polym14183812









