Preparation of Anti-Bacterial Cellulose Nanofibrils (CNFs) from Bamboo Pulp in a Reactable Citric Acid–Choline Chloride Deep Eutectic Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNFs and Composite Films
2.3. Characterization of CNFs
2.4. Characterization of Composite Films
2.5. Anti-Bacterial Activate Determination of CNFs and Composite Films
3. Results and Discussion
3.1. Structural Characteristics of CNFs
3.2. Opacity of Films
3.3. Thermal Stabilities of Composite Films
3.4. Mechanical Properties of Composite Films
3.5. Anti-Bacterial Activate of CNFs and Composite Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5566. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.J.; Acharya, B.; Bissessur, R. Isolation of nanocrystalline cellulose from tuicates. J. Environ. Chem. Eng. 2018, 6, 4408–4412. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, M.; Jafari, S.M.; van de Ven, T.G.M. A review on surface-functionalized cellulosic nanostructures as biocompatible antibacterial materials. Nano-Micro Lett. 2020, 12, 73. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.; Tam, K. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.S.; Zheng, T.; Liu, W.; Zhang, M.; Liu, H.Y.; Zhang, X.Y.; Si, C.L. Lignin-containing cellulose nanomaterials: Preparation and applications. Green Chem. 2021, 23, 9723–9746. [Google Scholar] [CrossRef]
- Tan, Y.T.; Chua, A.S.M.; Ngoh, G.C. Deep eutectic solvent for lignocellulosic biomass fractionation and the subsequent conversion to bio-based products—A review. Bioresour. Technol. 2020, 297, 122522. [Google Scholar] [CrossRef]
- Wang, J.; Jing, W.; Tian, H.; Liu, M.; Yan, H.; Bi, W.; Chen, D.D.Y. Investigation of deep eutectic solvent-based microwave-assisted extraction and efficient recovery of natural products. ACS Sustain. Chem. Eng. 2022, 8, 12080–12088. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-based antibacterial materials. Adv. Healthc. Mater. 2018, 7, 1800334. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zhang, J.H.; Zhao, P.; Wang, D.W.; Shi, Z.J.; Yang, J.; Yang, H.Y. Fabrication of cellulose nanocrystals (CNCs) in choline chloride-citric acid (ChCl-CA) solvent to lodge antimicrobial activity. Bioresource 2022, 17, 4347–4359. [Google Scholar] [CrossRef]
- Tanpichai, S.; Oksman, K. Crosslinked poly(vinyl alcohol) composite films with cellulose nanocrystals: Mechanical and thermal properties. J. Appl. Polym. Sci. 2018, 135, 45710. [Google Scholar] [CrossRef]
- Ling, Z.; Guo, Z.; Huang, C.X.; Yao, L.; Xu, F. Deconstruction of oriented crystalline cellulose by novel levulinic acid based deep eutectic solvents pretreatment for improved enzymatic accessibility. Bioresour. Technol. 2020, 305, 123025. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Du, H.S.; Liu, K.; Liu, H.Y.; Xie, H.X.; Si, C.L.; Pang, B.; Zhang, X.Y. Sustainable preparation of cellulose nanofibrils via choline chloride-citric acid deep eutectic solvent pretreatment combined with high-pressure homogenization. Carbohydr. Polym. 2021, 267, 118220. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Y.; Zhu, E.Q.; Wang, D.W.; Yang, J.; Yang, H.Y.; Shi, Z.J. Cationic functionalized bamboo fibers with spinnable and antibacterial properties prepared in chlorocholine chloride/urea deep eutectic solvent. Ind. Crop. Prod. 2022, 188, 115607. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Cao, X.W.; Liu, M.; Bi, W.T.; Lin, J.; Chen, D.D.Y. Direct carboxylation of cellulose in deep eutectic solvent and its adsorption behavior of methylene blue. Carbohydr. Polym. Technol. Appl. 2022, 4, 100222. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Guo, B.T.; Xia, Q.Q.; Meng, J.; Chen, W.S.; Liu, S.X.; Wang, Q.W.; Liu, Y.X.; Li, J.; Yu, H.P. Efficient cleavage of strong hydrogen bonds in cotton by deep eutectic solvents and facile fabrication of cellulose nanocrystals in high yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Chen, Y.Y.; Liu, J.M.; Ren, Z.C. Facile one-step extraction and oxidative carboxylation of cellulose nanocrystals through hydrothermal reaction by using mixed inorganic acids. Cellulose 2017, 24, 3243–3254. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, J.Y.; Lan, P.; Yang, H.Y.; Lu, J.L.; Wang, Z. Study on the dissolution mechanism of cellulose by ChCl-based deep eutectic solvents. Materials 2020, 13, 278. [Google Scholar] [CrossRef]
- Soleimanzadeh, H.; Mirzaee, F.; Samaneh, B.; Ahari1, Z.; Salari, D.; Olad, A.; Ostadrahimi, A. Optimization of cellulose extraction process from sugar beet pulp and preparation of its nanofbers with choline chloride–lactic acid deep eutectic solvents. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Shi, L.; Yang, J.H.; Zhang, H.B.; Chen, Y.M.; Yang, S.C.; Wu, C.; Zeng, H.; Yoshihito, O.; Zhang, Q. Carbon dots with high fluorescence quantum yield: The fluorescence originates from organic fluorophores. Nanoscale 2016, 8, 14374–14378. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, R.; Xu, X.Y.; Zhao, P.C.; Wong, D.S.H.; Chen, X.Y.; Chen, S.J.; Yan, X.H. Citrate-based fluorophores in polymeric matrix by easy and green in situ synthesis for full-band UV shielding and emissive transparent display. J. Mater. Sci. 2019, 54, 1236–1247. [Google Scholar] [CrossRef]
- Abral, H.; Mahardika, M.; Handayani, D.; Sugiarti, E.; Muslimin, A.N. Characterization of disintegrated bacterial cellulose nanofibers/PVA bionanocomposites prepared via ultrasonication. Int. J. Biol. Macromol. 2019, 135, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.M.; Ilyas, R.A. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2020, 81, 106186. [Google Scholar] [CrossRef]
- Das, A.M.; Ali, A.A.; Hazarika, M.P. Synthesis and characterization of cellulose acetate from rice husk: Eco-friendly condition. Carbohydr. Polym. 2014, 112, 342–349. [Google Scholar] [CrossRef]
- Sirviö, J.A. Fabrication of regenerated cellulose nanoparticles by mechanical disintegration of cellulose after dissolution and regeneration from a deep eutectic solvent. J. Mater. Chem. A 2019, 7, 755–763. [Google Scholar] [CrossRef]
- Ge, L.M.; Zhu, M.J.; Li, X.Y.; Xu, Y.B.; Ma, X.N.; Shi, R.; Li, D.F.; Mu, C.D. Development of active rosmarinic acid-gelatin biodegradable films with antioxidant and long-term antibacterial activities. Food Hydrocoll. 2018, 83, 308–316. [Google Scholar] [CrossRef]
- He, M.M.; Ou, F.Y.; Wu, Y.; Suna, X.D.; Chen, X.C.; Han Li, H.; Sun, D.; Zhang, L. Smart multi-layer PVA foam/CMC mesh dressing with integrated multi-functions for wound management and infection monitoring. Mater. Des. 2020, 194, 108913. [Google Scholar] [CrossRef]
- Kang, J.W.; Lee, H.Y.; Kang, D.H. Synergistic bactericidal effect of hot water with citric acid against Escherichia coli O157:H7 biofilm formed on stainless steel. Food Microbiol. 2021, 95, 103676. [Google Scholar] [CrossRef]
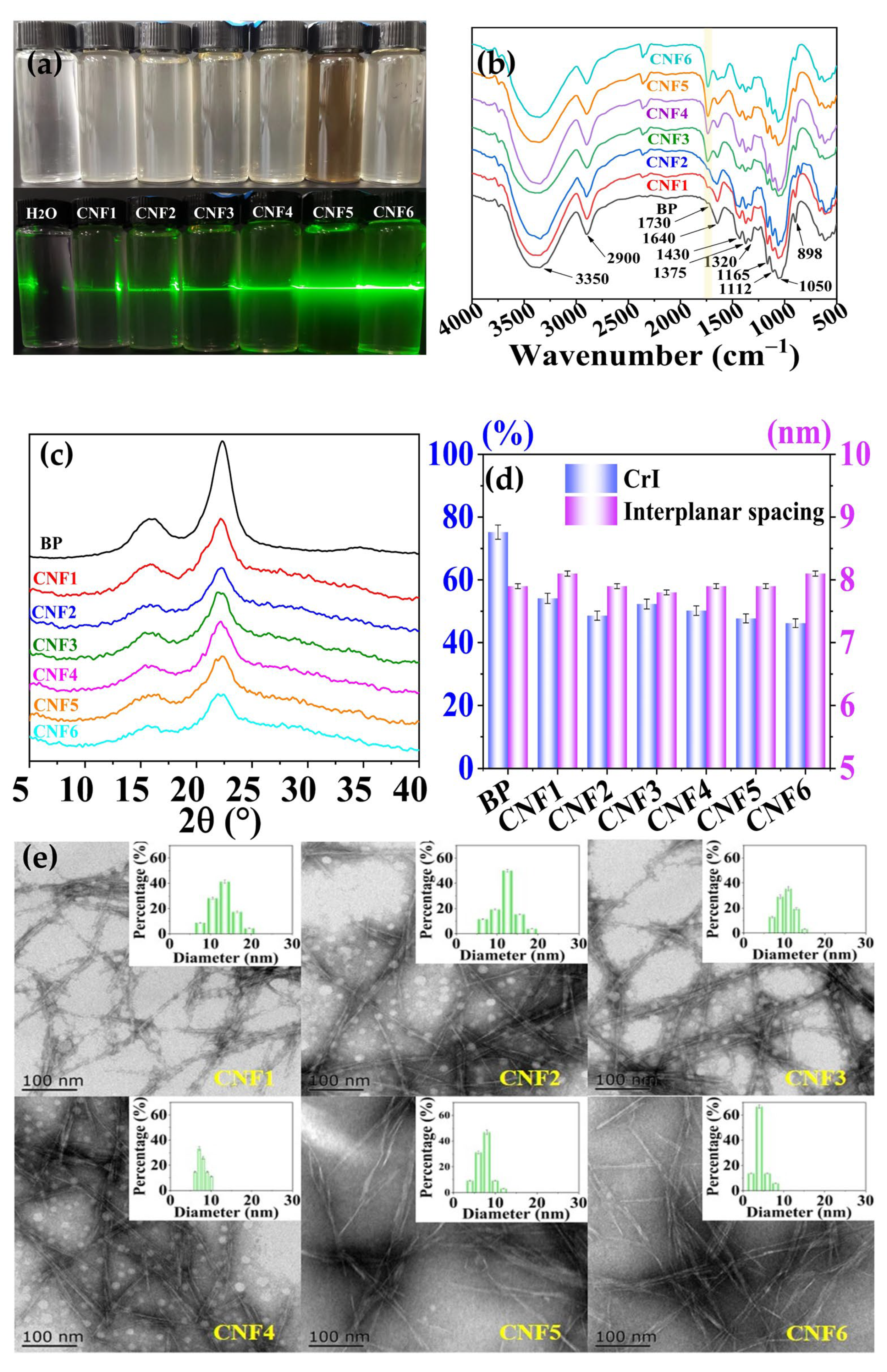


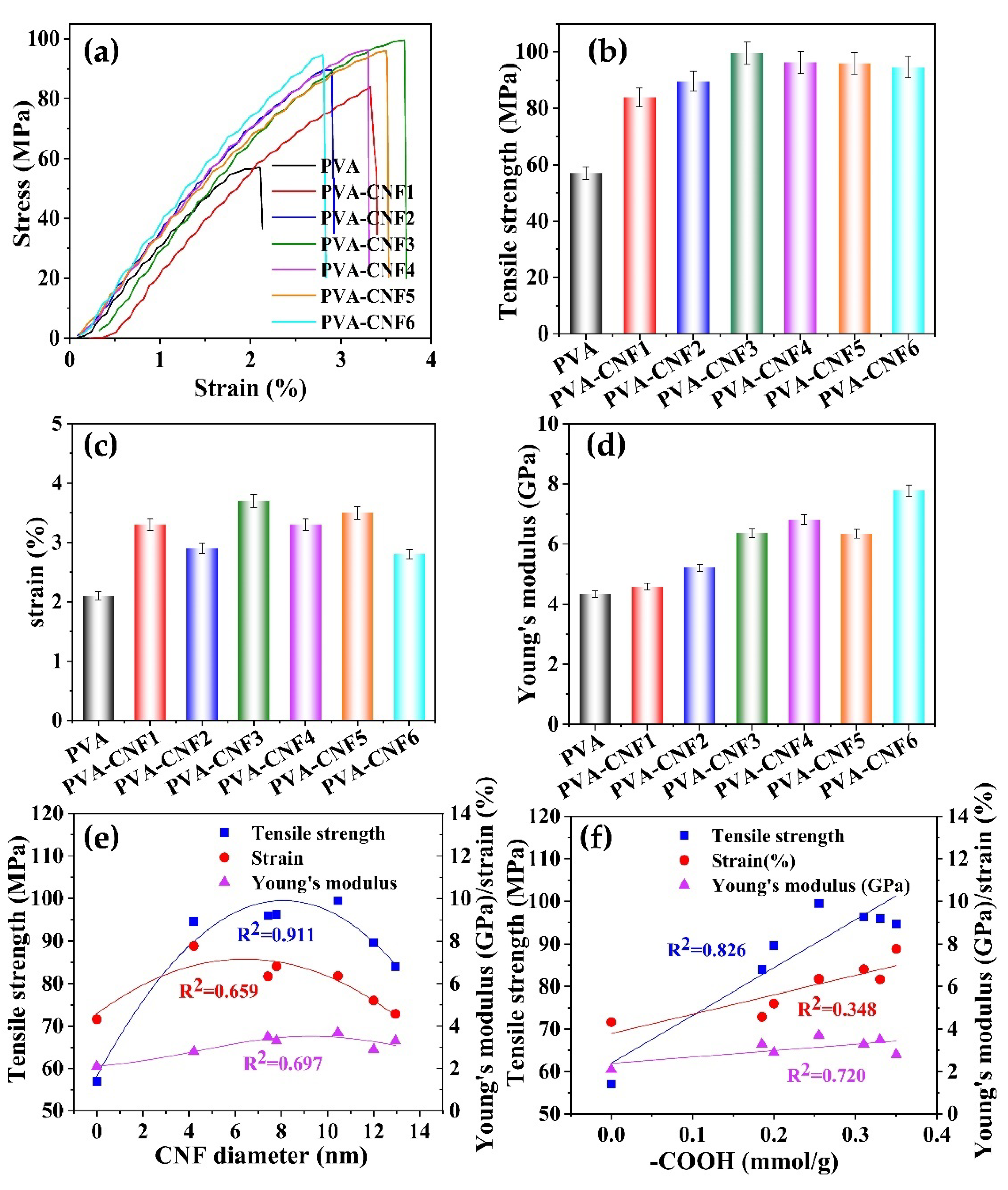
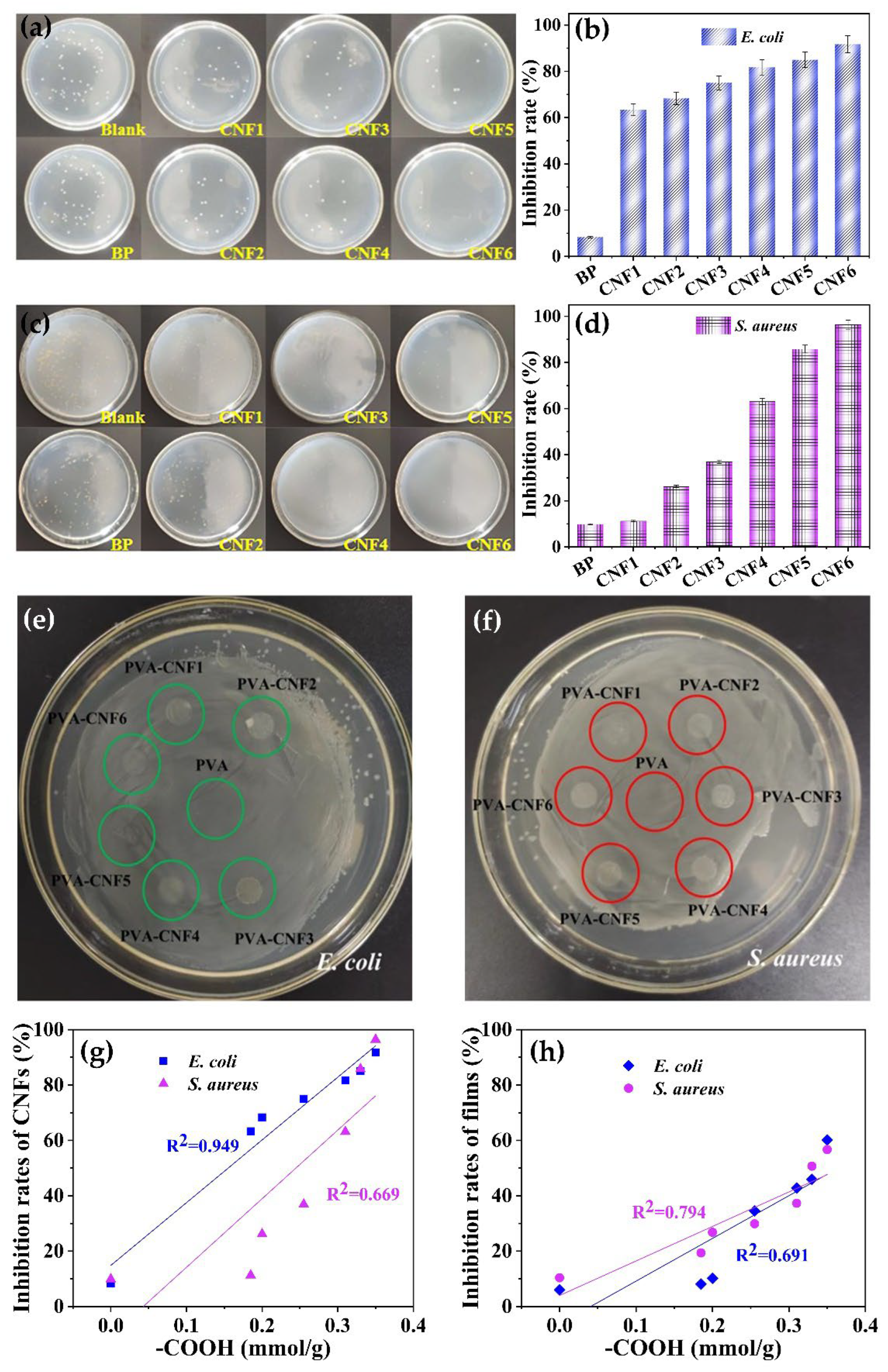
| CNFs | Temperature (°C) | Time (h) | Yield (%) | −COOH (mmol/g) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| BP | Non detectable | Non detectable | |||
| CNF1 | 100 | 12 | 84.5 | 0.19 | −6.24 |
| CNF2 | 100 | 24 | 84.1 | 0.20 | −7.24 |
| CNF3 | 120 | 12 | 81.2 | 0.26 | −14.1 |
| CNF4 | 120 | 24 | 76.0 | 0.31 | −16.9 |
| CNF5 | 140 | 12 | 71.2 | 0.33 | −16.1 |
| CNF6 | 140 | 24 | 66.6 | 0.35 | −16.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhang, J.; Wang, D.; Shi, Z.; Yang, J.; Yang, H. Preparation of Anti-Bacterial Cellulose Nanofibrils (CNFs) from Bamboo Pulp in a Reactable Citric Acid–Choline Chloride Deep Eutectic Solvent. Polymers 2023, 15, 148. https://doi.org/10.3390/polym15010148
Zhu Y, Zhang J, Wang D, Shi Z, Yang J, Yang H. Preparation of Anti-Bacterial Cellulose Nanofibrils (CNFs) from Bamboo Pulp in a Reactable Citric Acid–Choline Chloride Deep Eutectic Solvent. Polymers. 2023; 15(1):148. https://doi.org/10.3390/polym15010148
Chicago/Turabian StyleZhu, Yuanchen, Jinhui Zhang, Dawei Wang, Zhengjun Shi, Jing Yang, and Haiyan Yang. 2023. "Preparation of Anti-Bacterial Cellulose Nanofibrils (CNFs) from Bamboo Pulp in a Reactable Citric Acid–Choline Chloride Deep Eutectic Solvent" Polymers 15, no. 1: 148. https://doi.org/10.3390/polym15010148
APA StyleZhu, Y., Zhang, J., Wang, D., Shi, Z., Yang, J., & Yang, H. (2023). Preparation of Anti-Bacterial Cellulose Nanofibrils (CNFs) from Bamboo Pulp in a Reactable Citric Acid–Choline Chloride Deep Eutectic Solvent. Polymers, 15(1), 148. https://doi.org/10.3390/polym15010148





