Investigation of Transport Mechanism and Nanostructure of Nylon-6,6/PVA Blend Polymers
Abstract
1. Introduction
2. Positron Annihilation Spectroscopy
3. Materials and Methods
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harper, C.A. Modern Plastics Handbook; McGraw-Hill Education: New York, USA, 2000. [Google Scholar]
- Gürses, A. Introduction to Polymer-Clay Nanocomposites; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wang, Y.; Hou, D.F.; Ke, K.; Huang, Y.H.; Yan, Y.; Yang, W.; Yin, B.; Yang, M.B. Chemical resistant polyamide 6/polyketone composites with gradient encapsulation structure: An insight into the formation mechanism. Polymer 2021, 212, 123173–123182. [Google Scholar] [CrossRef]
- Saba, N.; Tahir, P.M.; Jawaid, M. A Review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Nguyen-Tran, H.D.; Hoang, V.T.; Do, V.T.; Chun, D.M.; Yum, Y.J. Effect of multiwalled carbon nanotubes on the mechanical properties of carbon fiber-reinforced, polyamide-6/polypropylene composites for lightweight automotive parts. Materials 2018, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Lu, J.; Zhao, J.; Wei, L.; Liu, T.; Zhao, L.; Park, C.B. Rheological and foaming behaviors of long-chain branched polyamide 6 with controlled branch length. Polymer 2021, 224, 123730–123739. [Google Scholar] [CrossRef]
- Dorr, D.; Kuhn, U.; Altstadt, V. Rheological study of gelation and crosslinking in chemical modified polyamide 12 using a multiwave technique. Polymers 2020, 12, 855. [Google Scholar] [CrossRef]
- Satheeskumar, S.; Kanagaraj, G. Experimental investigation on tribological behaviours of PA6, PA6-reinforced Al2O3 and PA6-reinforced graphite polymer composites. Bull. Mater. Sci. 2016, 39, 1467–1481. [Google Scholar] [CrossRef]
- Cai, J.; Wirasaputra, A.; Zhu, Y.; Liu, S.; Zhou, Y.; Zhang, C.; Zhao, J. The flame retardancy and rheological properties of PA6/MCA modified by DOPO-based chain extender. RSC Adv. 2017, 7, 19593–19603. [Google Scholar] [CrossRef]
- Finch, C.A. Polyvinyl Alcohol: Developments. A Comprehensive Reference; Wiley: New York, USA, 1992; pp. 12–18. [Google Scholar]
- Yeh, J.T.; Xu, P.; Tsai, F.C. Blending properties of poly(vinyl alcohol) and nylon 6-clay nanocomposite blends. J. Mater. Sci. 2007, 42, 6590–6599. [Google Scholar] [CrossRef]
- Cui, L.; Yeh, J.T.; Wang, K.; Fu, Q. Miscibility and isothermal crystallization behavior of polyamide 6/poly(vinyl alcohol) blend. J. Polym. Sci. Polym. Phys. Ed. 2008, 46, 1360–1368. [Google Scholar] [CrossRef]
- Cui, L.; Fu, Q.; Chang, C.J.; Yeh, J.T. The effect of poly(vinyl alcohol) hydrolysis on the properties of its blends with nylon 6. Polym. Eng. Sci. 2009, 49, 1553–1561. [Google Scholar] [CrossRef]
- Koulouri, E.G.; Kallitsis, J.K. Miscibility behavior of poly(vinyl alcohol)/Nylon 6 blends and their reactive blending with Poly(ethylene-co-ethyl acrylate). Polymer 1998, 39, 2373–2379. [Google Scholar] [CrossRef]
- Usuki, A.; Hasegawa, N.; Kato, M.; Kobayashi, S. Polymer-clay nanocomposites inorganic polymeric nanocomposites and membranes. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–24. [Google Scholar]
- Aiman, E.A.; Hasan, A.A.; Ismael, A. Miscibility, Crystallinity and morphology of polymer blends of polyamide-6/Poly (β-hydroxybutyrate). Jordan J. Chem. 2006, 1, 155–170. [Google Scholar]
- Khairy, Y.; Elsaeedy, H.I.; Mohammed, M.I.; Zahran, H.Y.; Yahia, I.S. Anomalous behaviour of the electrical properties for PVA/TiO2 nanocomposite polymeric films. Polym. Bull. 2000, 77, 6255–6269. [Google Scholar] [CrossRef]
- Li, J.; Yin, C.; Zhou, Y.; Li, G.; Yeh, J.T.; He, C. Positron annihilation studies of the free volumes in Nylon12/PVA films treated by supercritical carbon dioxide. Acta Phys. Pol. A 2017, 132, 1552–1555. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kinomura, A.; Kazama, S.; Inoue, K.; Toyama, T.; Nagai, Y.; Haraya, K.; Mohamed, H.F.M.; O’Rouke, B.E.; Oshima, N.; et al. Hole size distributions in cardo-based polymer membranes deduced from the lifetimes of ortho-positronium. J. Phys. Conf. Ser. 2016, 674, 012017. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Abdel-Hady, E.E.; Ohira, A. Per-fluorinated sulfonic acid/PTFE copolymer studied by positron annihilation lifetime and gas permeation techniques. J. Phys. Conf. Ser. 2015, 618, 012031. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kazama, K.S.; Inoue, T.; Toyama, Y.; Nagai, K.H.; Mohamed, H.F.M.; O’Rouke, B.E.; Oshima, N.; Kinomura, A.; Suzuki, R. Positron annihilation in cardo-based polymer membranes. J. Phys. Chem. B. 2014, 118, 6007–6014. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Kuroda, S.; Kobayashi, Y.; Oshima, N.; Suzuki, R.; Ohira, A. Possible presence of hydrophilic SO3H nanoclusters on the surface of dry ultrathin Nafion films: A positron annihilation study. Phys. Chem. Chem. Phys. 2013, 15, 1518–1525. [Google Scholar] [CrossRef]
- Hamdy, F.M.M.; Kobayashi, Y.; Kuroda, C.S.; Ohira, A. Impact of heating on the structure of perfluorinated polymer electrolyte membranes: A positron annihilation study. Macromol. Chem. Phys. 2011, 212, 708–714. [Google Scholar]
- Abdel-Hady, E.E. Positron annihilation lifetime study of irradiated and deformed low density polyethylene. Polym. Deg. Stab. 2003, 80, 363–368. [Google Scholar] [CrossRef]
- Hamdy, F.M.M.; Kobayashi, Y.; Kuroda, C.S.; Takimoto, N.; Ohira, A. Free volume, oxygen permeability, and uniaxial compression storage modulus of hydrated biphenol-based sulfonated poly(arylene ether sulfone). J. Mem. Sci. 2010, 360, 84–89. [Google Scholar]
- Al-Qaradawia, I.Y.; Britton, D.T.; Abdel-Hady, E.E.; Abdulmalika, D.A.; Al-Shobakia, M.A.; Minanib, E. Positron annihilation studies of the effect of gamma irradiation dose in polymers. Radiat. Phys. Chem. 2003, 68, 457–461. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Ito, Y.; El-Sayed, A.M.; Abdel-Hady, E.E. Positron annihilation in polyvinyl alcohol doped with CuCl2. Polymer 1996, 37, 1529–1533. [Google Scholar] [CrossRef]
- Abdel-Hady, E.E.; Mohamed, H.F.M. Microstructure changes of poly (vinyl chloride) investigated by positron annihilation techniques. Polym. Degr. Stab. 2002, 77, 449–456. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Abdel-Hady, E.E.; Mohamed, S.S. Temperature dependence of the free volume in polytetrafluoroethylene studied by positron annihilation spectroscopy. Radiat. Phys. Chem. 2007, 76, 160–164. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; El-Aziz, N.S.A. Study on polystyrene via positron annihilation lifetime and Doppler broadened techniques. Polymer 2001, 42, 8013–8017. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Ito, K.; Kobayashi, Y.; Takimoto, N.; Takeoka, Y.; Ohira, A. Free volume and permeabilities of O2 and H2 in Nafion membranes for polymer electrolyte fuel cells. Polymer 2008, 49, 3091–3097. [Google Scholar] [CrossRef]
- Shariff, G.; Sathyanarayana, P.M.; Thimmegowda, M.C.; Ashalatha, M.B.; Ramani, R.; Avasthi, D.K.; Ranganathaiah, C. Influence of ion-irradiation on the free volume controlled diffusion process in polycarbonateâ—A positron lifetime study. Polymer 2002, 43, 2819–2826. [Google Scholar]
- Mohamed, H.F.M.; Kobayashi, Y.; Kuroda, C.S.; Ohira, A. Effects of ion-exchange on free volume and oxygen permeation in Nafion for fuel cells. J. Phys. Chem. B. 2009, 113, 2247–2252. [Google Scholar] [CrossRef]
- Mogensen, O.E. Positron Annihilation in Chemistry; Springer Science & Business Media: Berlin/Heidelberg, German, 2012; p. 58. [Google Scholar]
- Jean, Y.C.; Mallon, P.E.; Schrader, D.M. Principles and Applications of Positron and Positronium Chemistry; World Scientific: London, UK, 2003; p. 258. [Google Scholar]
- Olsen, J.V.; Kirkegaard, P.; Pedersen, N.J.; Eldrup, M. PALSfit: A new program for the evaluation of positron lifetime spectra. Phys. Stat. Sol. C 2007, 4, 4004–4006. [Google Scholar] [CrossRef]
- Jean, Y.C. Positron annihilation in polymers. Mater. Sci. Forum 1995, 175, 59–70. [Google Scholar] [CrossRef]
- Kansy, J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nucl. Instrum. Methods. Phys. Res. A 1996, 374, 235–244. [Google Scholar] [CrossRef]
- Dlubek, G.; Supej, M.; Bondarenko, V.; Pionteck, J.; Pompe, G.; Krause-Rehberg, R.; Emri, I. Ortho-positronium lifetime distribution analyzed with MELT and LT and free volume in poly (ε-caprolactone) during glass transition, melting, and crystallization. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 3077–3088. [Google Scholar] [CrossRef]
- Giebel, D.; Kansy, J. A new version of LT program for positron lifetime spectra analysis. Mater. Sci. Forum 2011, 666, 138–141. [Google Scholar] [CrossRef]
- Jean, Y.C. Positron annihilation spectroscopy for chemical analysis: A novel probe for microstructural analysis of polymers. Microchem. J. 1990, 42, 72–102. [Google Scholar] [CrossRef]
- Tao, S.J. Positronium annihilation in molecular substances. J. Chem. Phys. 1972, 56, 5499–5510. [Google Scholar] [CrossRef]
- Eldrup, M.; Lightbody, D.; Sherwood, J.N. The temperature dependence of positron lifetimes in solid pivalic acid. J. Chem. Phys. 1981, 63, 51–58. [Google Scholar] [CrossRef]
- Nakanishi, H.; Wang, S.J.; Jean, Y.C. Positron Annihilation Studies of Fluids; World Science: Singapore, 1988; p. 292. [Google Scholar]
- Wang, Y.Y.; Nakanishi, H.; Jean, Y.C.; Sandreczki, T.C. Positron annihilation in amine-cured epoxy polymers—Pressure dependence. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 1431–1441. [Google Scholar] [CrossRef]
- Peng, Z.L.; Wang, B.; Li, S.Q.; Wang, S.J. Free volume and ionic conductivity of poly (ether urethane)-LiClO4 polymeric electrolyte studied by positron annihilation. J. App. Phys. 1995, 77, 334–338. [Google Scholar] [CrossRef]
- Hmamm, M.F.M.; Zedan, I.T.; Mohamed, H.F.M.; Hanafy, T.A.; Bekheet, A.E. Study of the nanostructure of free volume and ionic conductivity of polyvinyl alcohol doped with NaI. Polym. Adv. Tech. 2021, 32, 173–182. [Google Scholar] [CrossRef]
- Okamoto, K.I.; Tanaka, K.; Katsube, M.; Sueoka, O.; Ito, Y. Positronium formation in various polyimides. Rad. Phys. Chem. 1993, 41, 497–502. [Google Scholar] [CrossRef]
- Kirkegaard, P.; Olsen, J.V.; Eldrup, M.M. PALSfit3: A Software Package for Analysing Positron Lifetime Spectra; Technical University of Denmark: Lyngby, Denmark, 2017. [Google Scholar]
- Ito, K.; Oka, T.; Kobayashi, Y.; Shirai, Y.; Wada, K.; Matsumoto, M.; Fujinami, M.; Hirade, T.; Honda, Y.; Hosomi, H.; et al. Interlaboratory comparison of positron annihilation lifetime measurements for syntheti fused silica and polycarbonate. J. Appl. Phys. 2008, 104, 026102. [Google Scholar] [CrossRef]
- Ito, K.; Oka, T.; Kobayashi, Y.; Shirai, Y.; Wada, K.; Matsumoto, M.; Fujinami, M.; Hirade, T.; Honda, Y.; Hosomi, H.; et al. Interlaboratory comparison of positron annihilation lifetime measurements. Mater. Sci. Forum. 2009, 607, 248–250. [Google Scholar] [CrossRef]
- Gao, F.; Mei, B.; Xu, X.; Ren, J.; Zhao, D.; Zhang, Z.; Wang, Z.; Wu, Y.; Liu, X.; Zhang, Y. Rational design of ZnMn2O4 nanoparticles on carbon nanotubes for high-rate and durable aqueous zinc-ion batteries. Chem. Eng. J. 2022, 448, 137742. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Z.; Ren, J.; Xu, Y.; Xu, X.; Zhou, J.; Gao, F.; Tang, H.; Liu, S.; Wang, Z.; et al. Fe2VO4 nanoparticles on rGO as anode material for high-rate and durable lithium and sodium ion batteries. Chem. Eng. J. 2023, 451, 138882. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhao, D.; Ren, J.; Liu, S.; Tang, H.; Xu, P.; Gao, F.; Yue, X.; Yang, H.; et al. Core–shell Co2VO4/carbon composite anode for highly stable and fast-charging sodium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 55020–55028. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.J.; Lin, C.T. Electrochemical performance of an air-breathing direct methanol fuel cell using poly (vinyl alcohol)/hydroxyapatite composite polymer membrane. J. Power Sources 2008, 177, 40–49. [Google Scholar] [CrossRef]
- Parın, F.N.; Aydemir, Ç.İ.; Taner, G.; Yıldırım, K. Co-electrospun-electrosprayed PVA/folic acid nanofibers for transdermal drug delivery: Preparation, characterization, and in vitro cytocompatibility. J. Ind. Text. 2021, 51, 1323S–1347S. [Google Scholar] [CrossRef]
- Rianjanu, A.; Kusumaatmaja, A.; Suyono, E.A.; Triyana, K. Solvent vapor treatment improves mechanical strength of electrospun polyvinyl alcohol nanofibers. Heliyon 2018, 4, 00592. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, U.S.; Jeong, K.U.; Seo, Y.A.; Park, S.J.; Kim, H.Y. Preparation and characterization of poly (vinyl alcohol) nanofiber mats crosslinked with blocked isocyanate prepolymer. Polym. Int. 2010, 59, 1683–1689. [Google Scholar] [CrossRef]
- Kim, J.R.; Kim, S.H. Eco-friendly acaricidal effects of Nylon 66 nanofibers via grafted clove bud oil-loaded capsules on house dust mites. Nanomaterials 2017, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, H.H.; Metzger, G. A new analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Gupta, S.; Pramanik, A.K.; Kailath, A.; Mishra, T.; Guha, A.; Nayar, S.; Sinha, A. Composition dependent structural modulations in transparent poly(vinyl alcohol) hydrogels. Colloids Surf. B Biointerfaces 2009, 74, 186–190. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Ávila-Orta, C.A.; Gamero-Melo, P.; Reyes-Rodríguez, P.Y.; Quiñones-Jurado, Z.V.; Cadenas-Pliego, G.; Bartolo-Pérez, P.; Soriano-Corral, F.; Covarrubias-Gordillo, C. Composites based on nylon 6/clinoptilolite by ultrasound-assisted extrusion for enhanced flame retardant and mechanical properties. Polym. Bull. 2022, 79, 1803–1819. [Google Scholar] [CrossRef]
- Farias-Aguilar, J.C.; Ramírez-Moreno, M.J.; Téllez-Jurado, L.; Balmori-Ramírez, H. Low pressure and low temperature synthesis of polyamide-6 (PA6) using Na0 as catalyst. Mater. Lett. 2014, 136, 388–392. [Google Scholar] [CrossRef]
- Bunn, C.W.; Garner, E.V.; Bragg, W.L. The crystal structures of two polyamides (‘nylons’). Proceedings of the Royal Society of London. Series A. Math. Phys. Sci. 1947, 189, 39–68. [Google Scholar]
- Fityk. Available online: https://fityk.nieto.pl/ (accessed on 1 March 2020).
- Usawattanakul, N.; Torgbo, S.; Sukyai, P.; Khantayanuwong, S.; Puangsin, B.; Srichola, P. Development of Nanocomposite Film Comprising of polyvinyl alcohol (PVA) incorporated with bacterial cellulose nanocrystals and magnetite nanoparticles. Polymers 2021, 13, 1778. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rasheed, M.A.; Abdullah, O.G.; Ahmed, H.M. Polymer blending as a novel approach for tuning the SPR peaks of silver nanoparticles. Polymers 2017, 9, 486. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Rasheed, M.A. Structural and optical characterization of PVA: KMnO4 based solid polymer electrolyte. Results Phys. 2016, 6, 1103–1108. [Google Scholar] [CrossRef]
- Cagiao, M.E.; Ania, F.; Balta Calleja, F.J.; Hirami, M.; Shimomura, T. Structure–microhardness correlation in blends of Nylon 6/Nylon 66 monofilaments. J. App. Polym. Sci. 2000, 77, 636–643. [Google Scholar] [CrossRef]
- Tadokoro, H. Structure of Crystalline Polymer; John Wiley & Sons: New York, NY, USA, 1979. [Google Scholar]
- Ramasanta, L.J.; Hernandez, M.; Lopez-Manchaob, M.A.; Verdejo, R. Large-Surface-area BN nanosheets and their utilization in polymeric composites with improved thermal and dielectric properties. Nanoscale Res. Lett. 2011, 6, 508–513. [Google Scholar]
- Li, R.; Xiong, C.; Kuang, D.; Dong, L.; Lei, Y.; Yao, J.; Jiang, M.; Li, L. Polyamide 11/poly vinylidene fluoride) blends as novel flexible materials for capacitors. Macromol. Rapid Commun. 2008, 29, 1449–1454. [Google Scholar] [CrossRef]
- Ahmad, Z.; Atiq, S.; Abbas, S.K.; Ramay, S.M.; Riaz, S.; Naseem, S. Structural and complex impedance spectroscopic studies of Mg-substituted CoFe2O4. Ceram. Int. 2016, 42, 18271–18282. [Google Scholar] [CrossRef]
- Sahu, G.; Das, M.; Yadav, M.; Sahoo, B.P.; Tripathy, J. Dielectric relaxation behavior of silver nanoparticles and graphene oxide embedded poly (vinyl alcohol) nanocomposite film: An effect of ionic liquid and temperature. Polymers 2020, 12, 374. [Google Scholar] [CrossRef]
- Koops, C.G. On the dispersion of resistivity and dielectric constant of some semiconductors at audio frequencies. Phys. Rev. 1951, 83, 121. [Google Scholar] [CrossRef]
- Atif, M.; Idrees, M.; Nadeem, M.; Siddique, M.; Ashraf, M.W. Investigation on the structural, dielectric and impedance analysis of manganese substituted cobalt ferrite i.e., Co1−xMnxFe2O4 (0.0≤ x≤ 0.4). RSC Adv. 2016, 6, 20876–20885. [Google Scholar] [CrossRef]
- Dyre, J.C.; and Schrøder, T.B. Universality of ac conduction in disordered solids. Rev. Modern Phys. 2000, 72, 873–892. [Google Scholar] [CrossRef]
- Rao, J.K.; Raizada, A.; Ganguly, D.; Mankad, M.M.; Satayanarayana, S.V.; Madhu, G.M. Investigation of structural and electrical properties of novel CuO–PVA nanocomposite films. J. Maters. Sci. 2015, 50, 7064–7074. [Google Scholar] [CrossRef]
- Ganaie, M.; Zulfequar, M. Ac conductivity measurement of Cd5Se95−xZnx chalcogenide semiconductor using correlated barrier hopping model. Acta Phys. Pol. A 2015, 128, 59–63. [Google Scholar] [CrossRef]
- Thakur, S.; Rai, R.; Bdikin, I.; Valente, M.A. Impedance and modulus spectroscopy characterization of Tb modified Bi0.8A0.1Pb0.1Fe0.9Ti0.1O3 ceramics. Mater. Res. 2016, 19, 1–8. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Uedono, A.; Li, Y.Y.; He, J.S. Compatibilization of metallocene polyethylene/polyamide blends with maleic anhydride studied by positron annihilation. Jpn. J. Appl. Phys. 2002, 41, 2146–2149. [Google Scholar] [CrossRef]
- Wang, C.L.; Wang, S.J.; Zheng, W.G.; Qi, Z.N. Positron annihilation study on PP/EPDM polymer blend. Phys. Status Solidi A 1994, 141, 253–260. [Google Scholar] [CrossRef]
- Sood, R.; Kulkarni, M.G.; Dutta, A.; Mashelkar, R.A. Viscosity of homogeneous polymer blends: An altered free-volume state model. Polym. Eng. Sci. 1988, 28, 20–31. [Google Scholar] [CrossRef]
- Liu, J.; Jean, Y.C.; Yang, H. Free-volume hole properties of polymer blends probed by positron annihilation spectroscopy: Miscibility. Macromolecules 1995, 28, 5774–5779. [Google Scholar] [CrossRef]
- Haragirimana, A.; Ingabire, P.B.; Zhu, Y.; Lu, Y.; Li, N.; Hu, Z.; Chen, S. Four-polymer blend proton exchange membranes derived from sulfonated poly (aryl ether sulfone)s with various sulfonation degrees for application in fuel cells. J. Membrain Sci. 2019, 583, 209–219. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Abdel-Hady, E.E.; Abdel-Moneim, M.M.Y.; Bakr, M.A.M.; Soliman, M.A.M.; Shehata, M.G.H.; Ismail, M.A.T. Effect of Al2O3 on nanostructure and ion transport properties of PVA/PEG/SSA polymer electrolyte membrane. Polymers 2022, 14, 4029. [Google Scholar] [CrossRef]
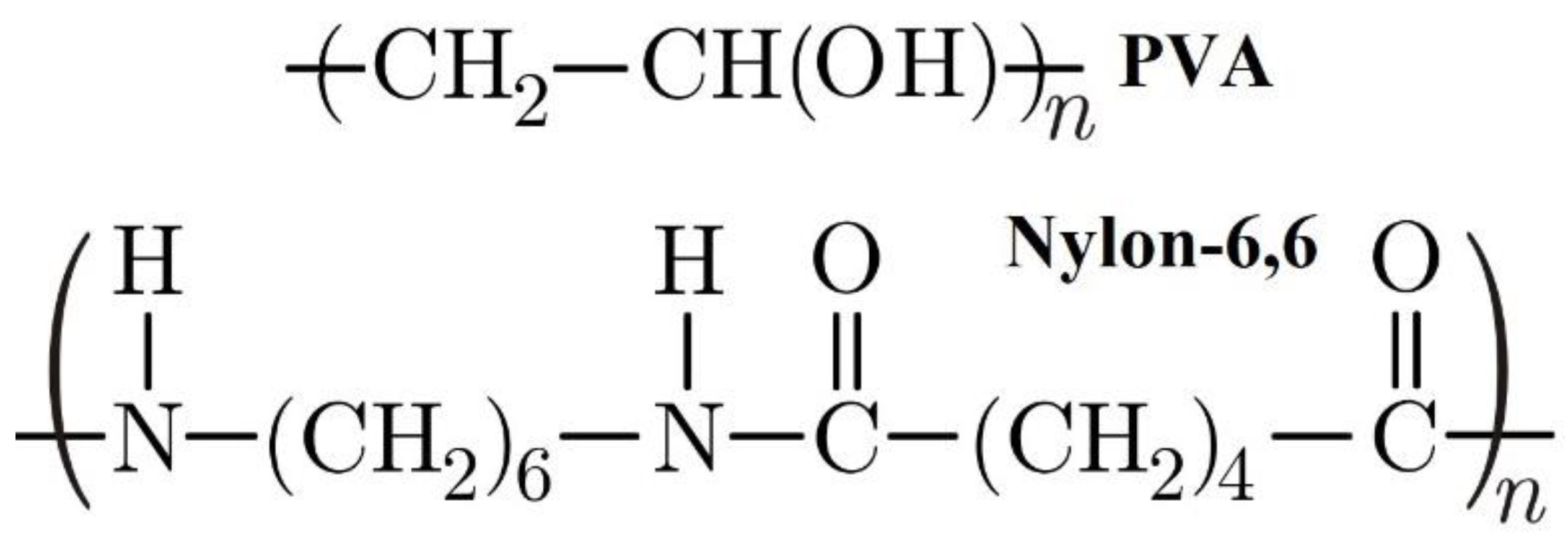
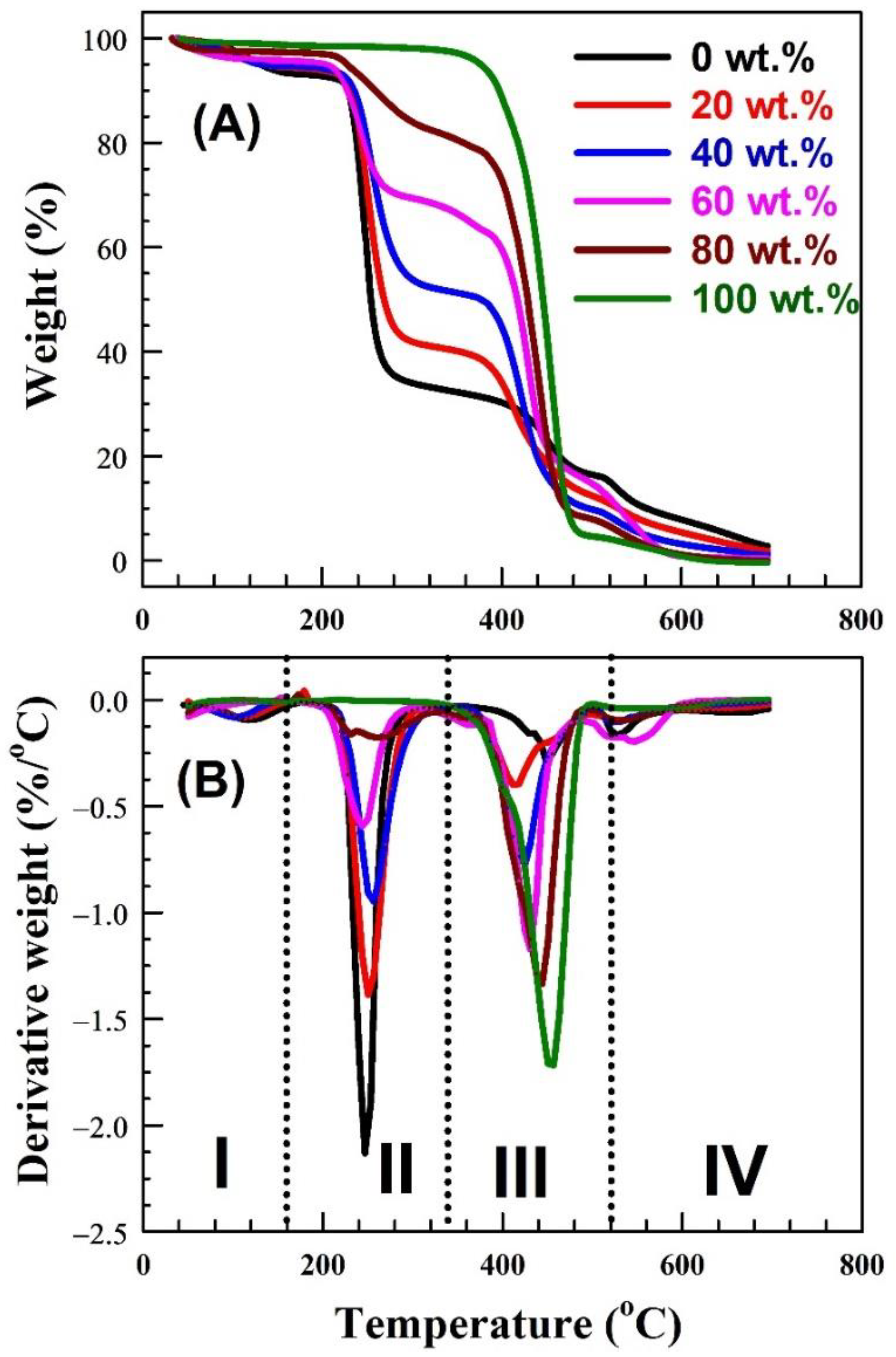
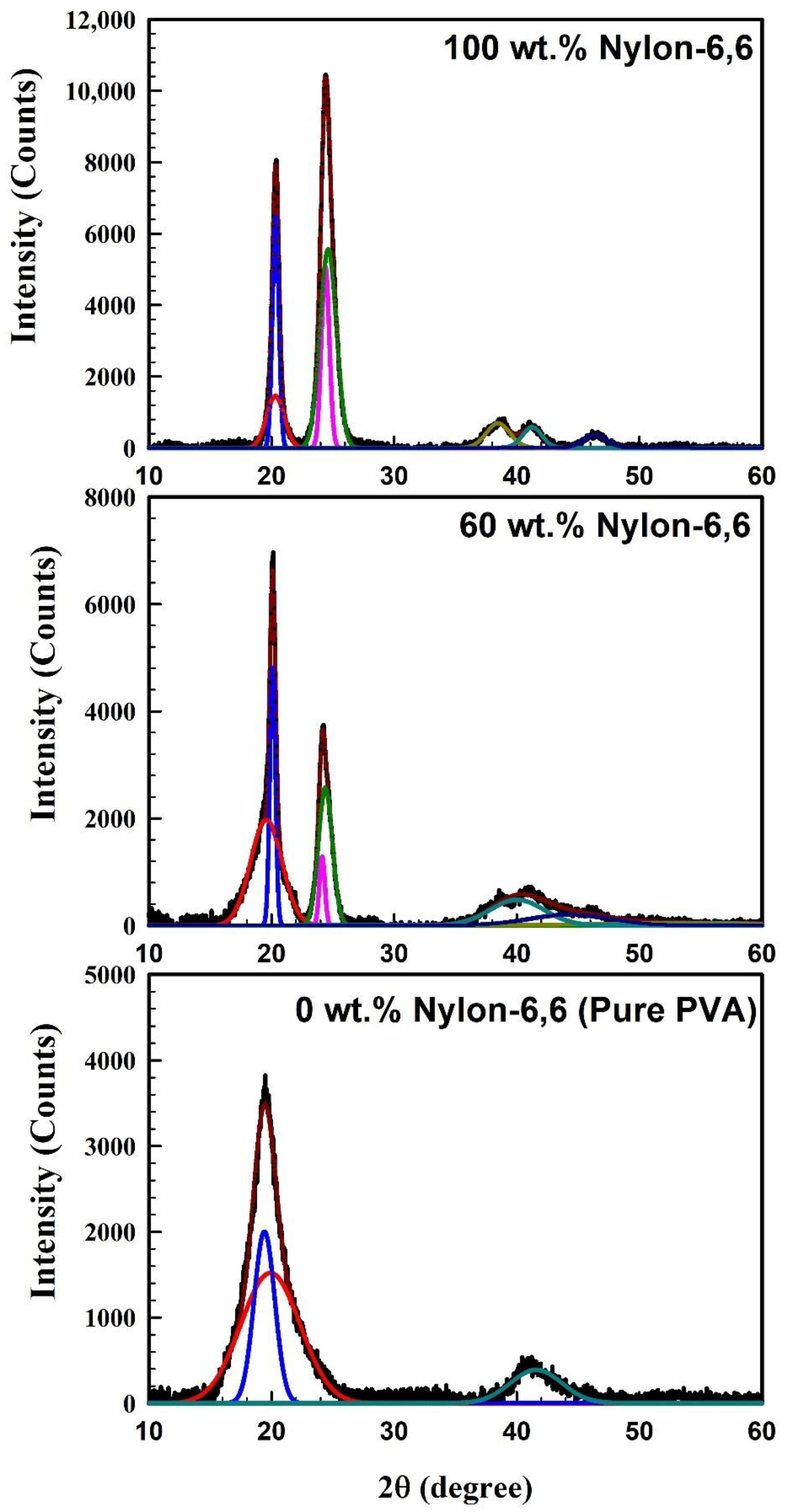
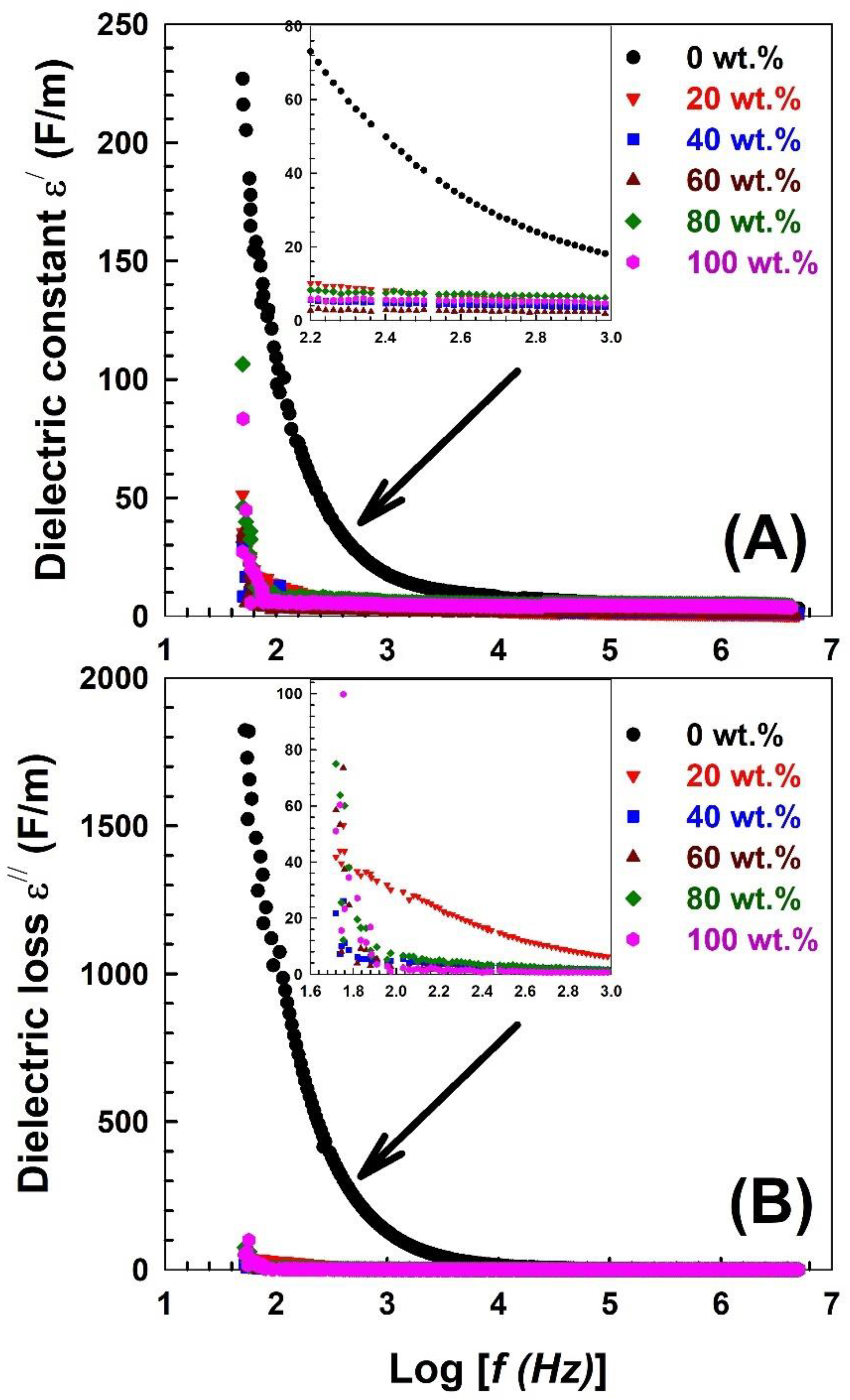
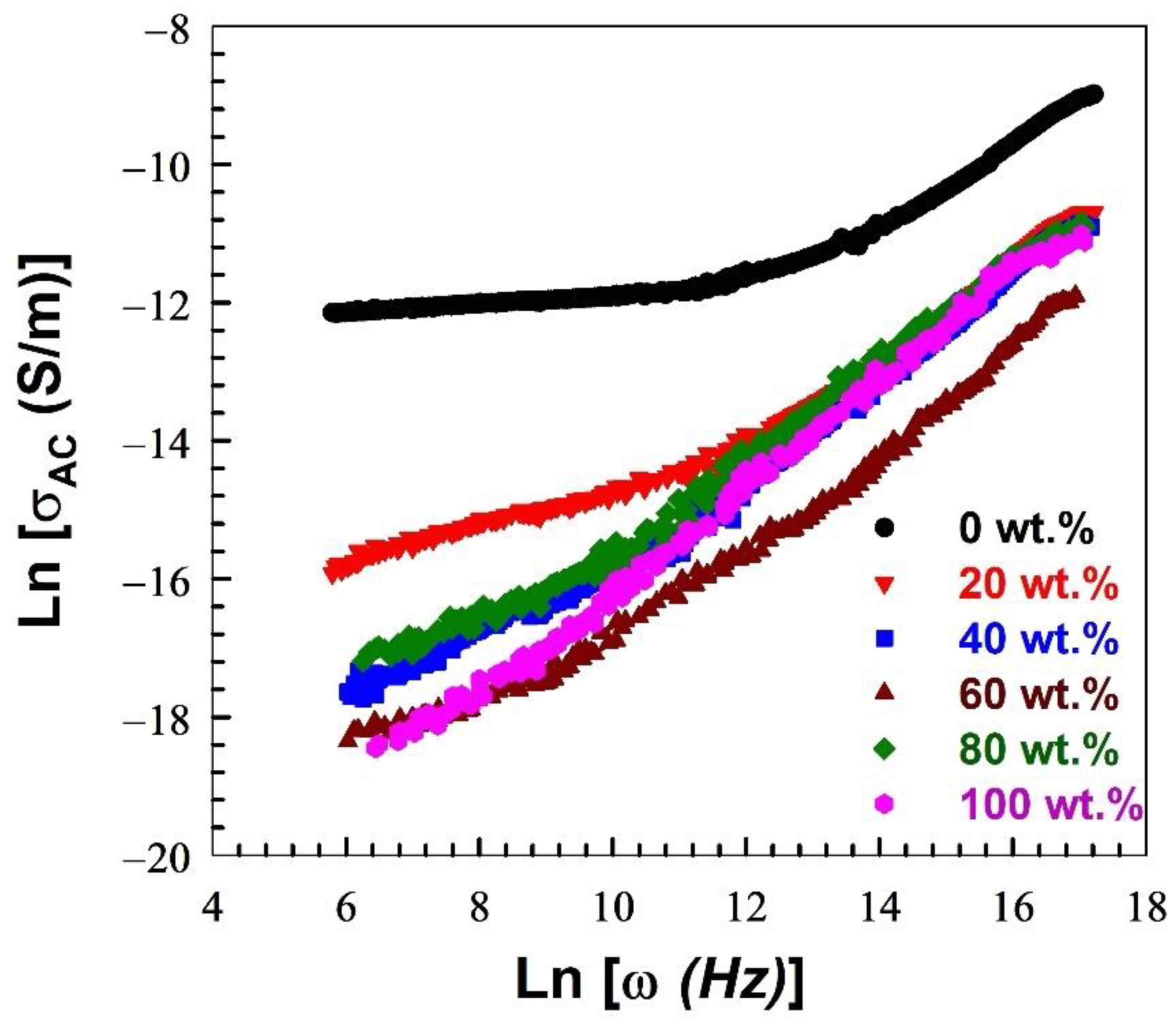
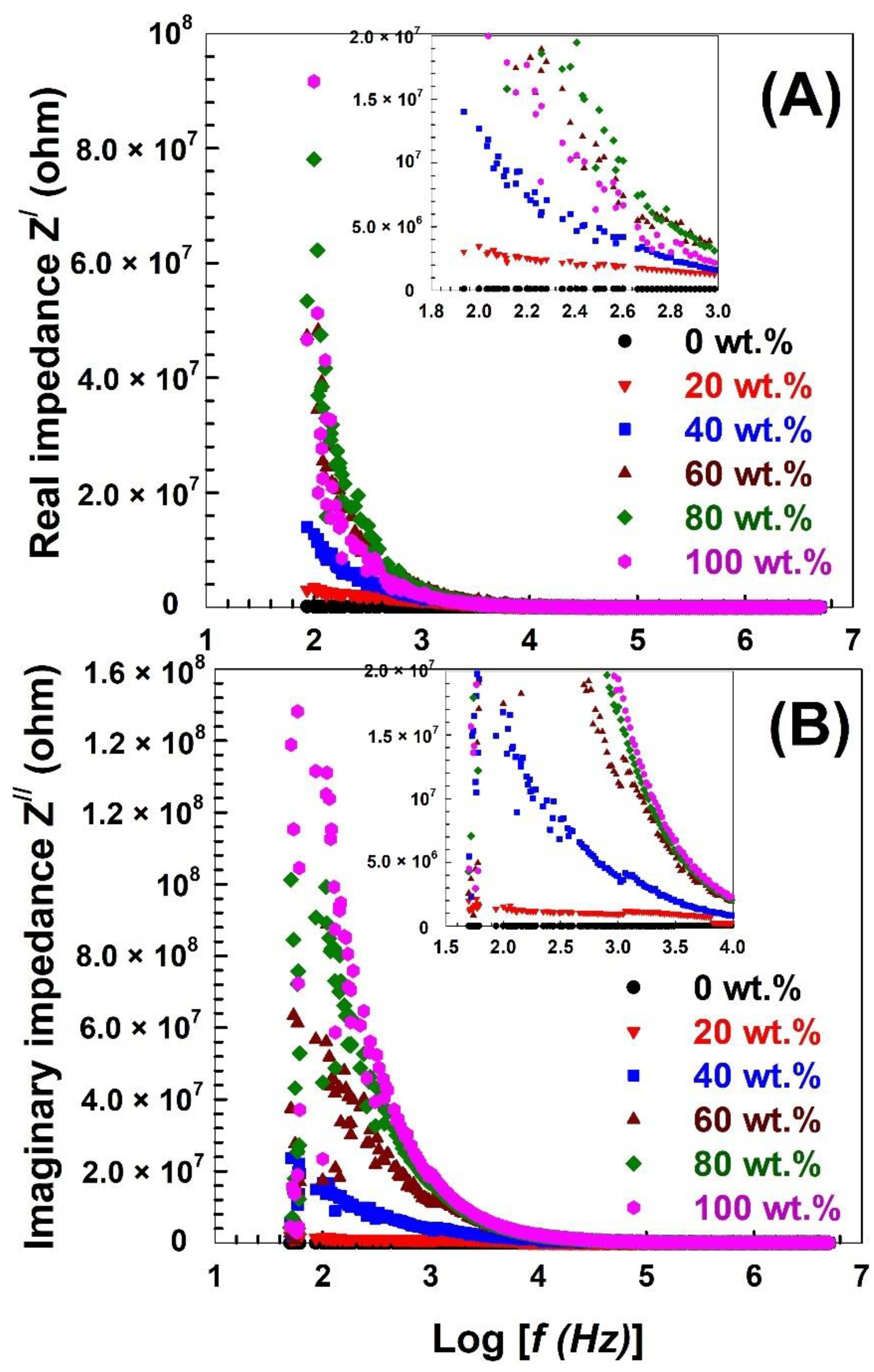
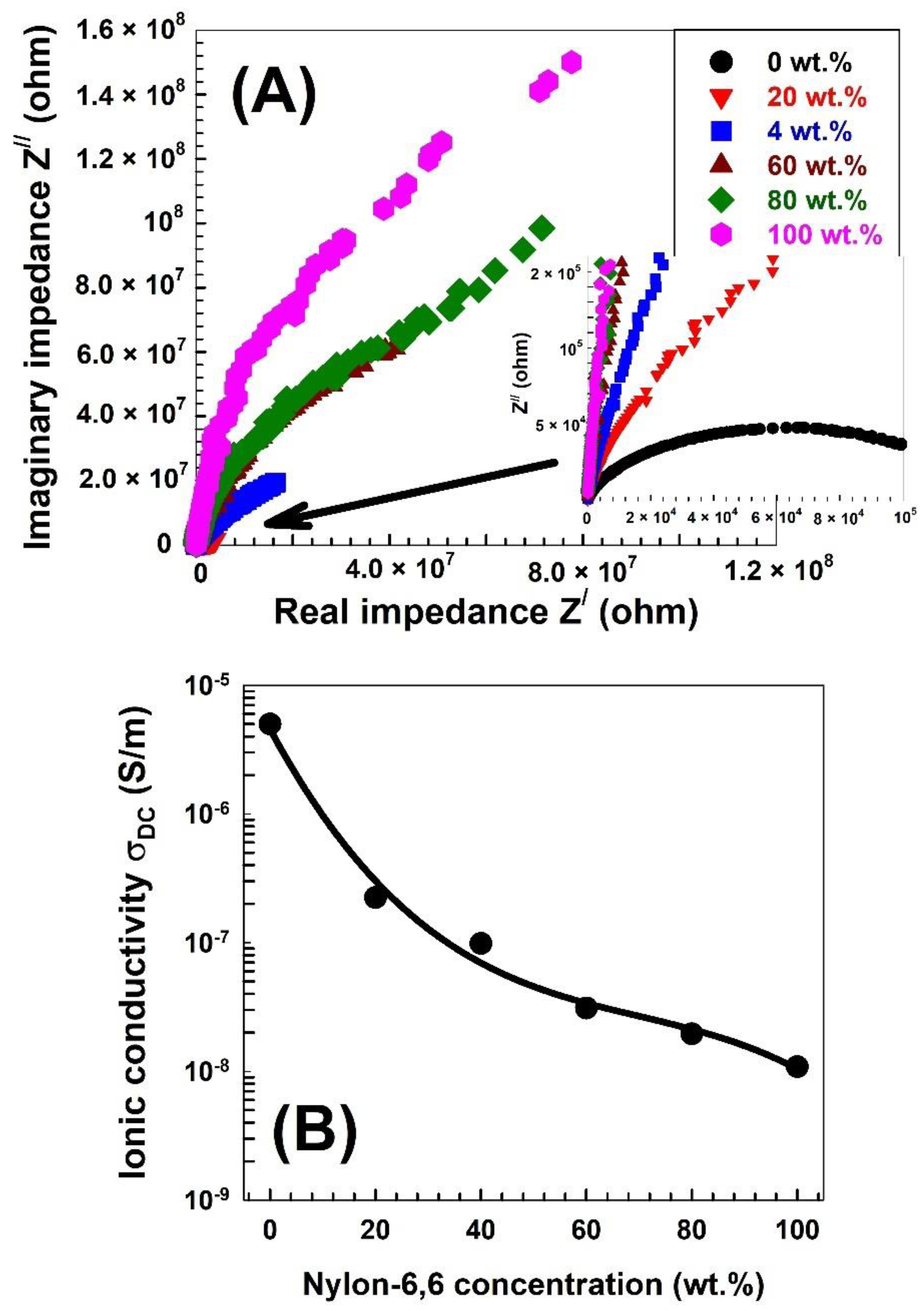
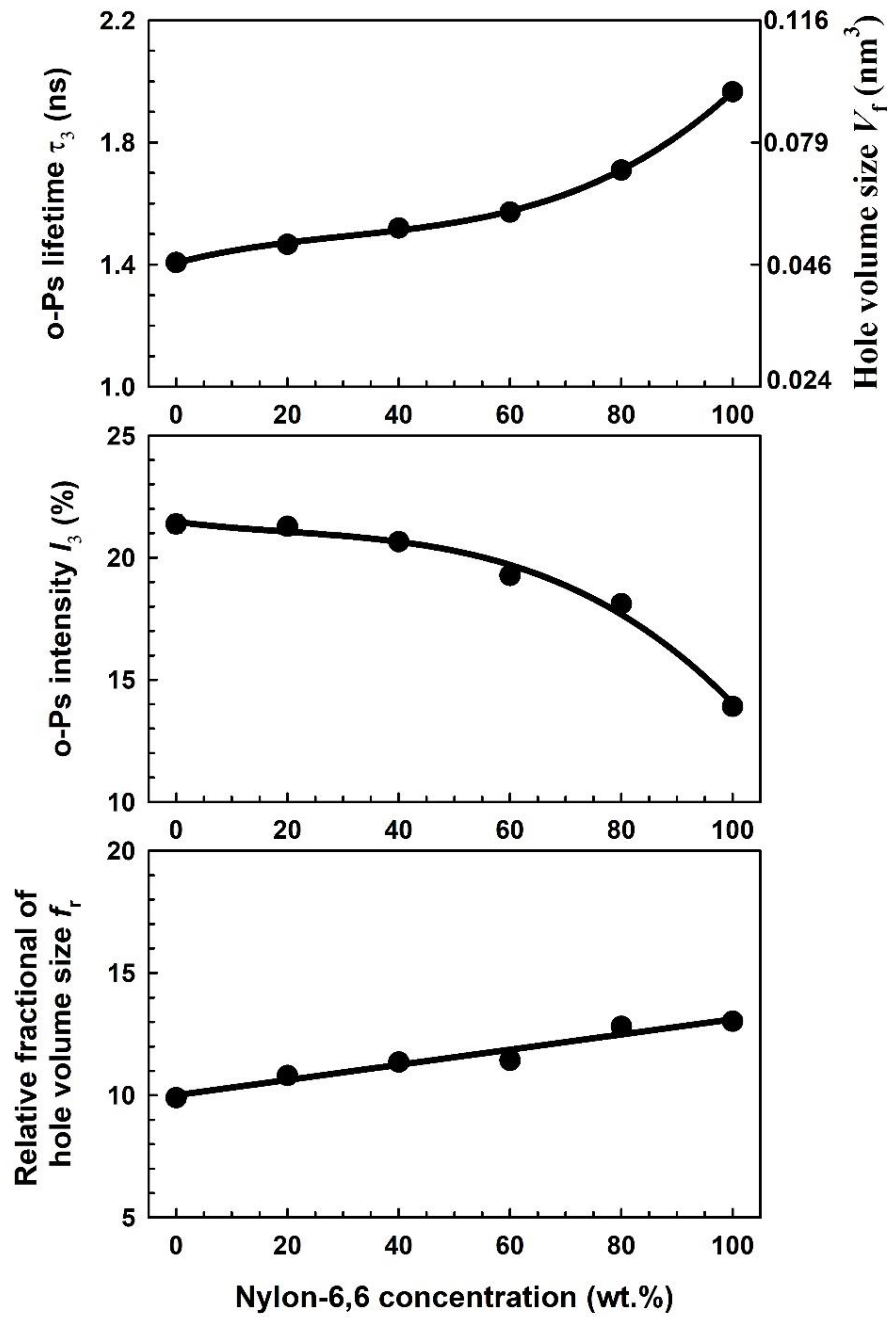
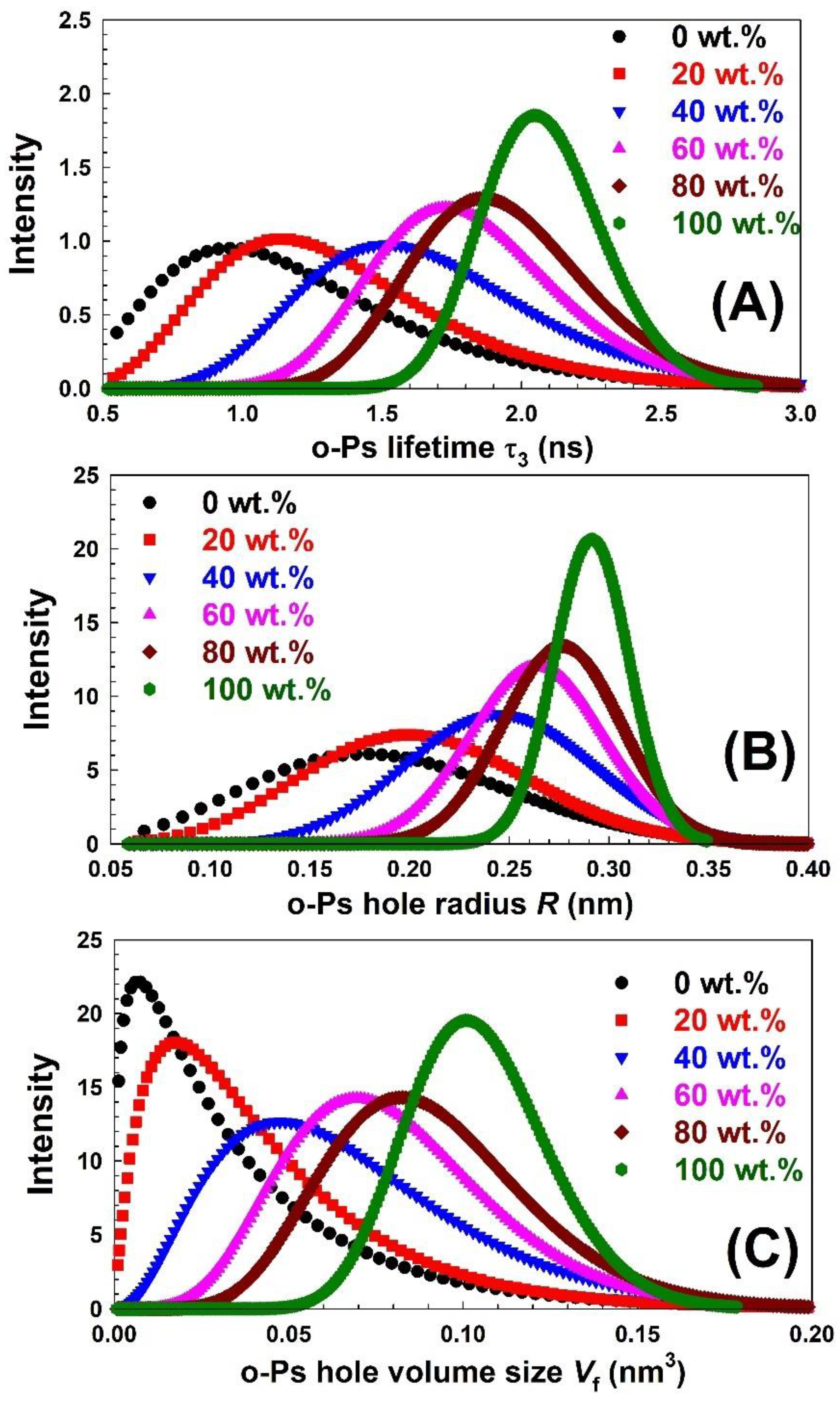
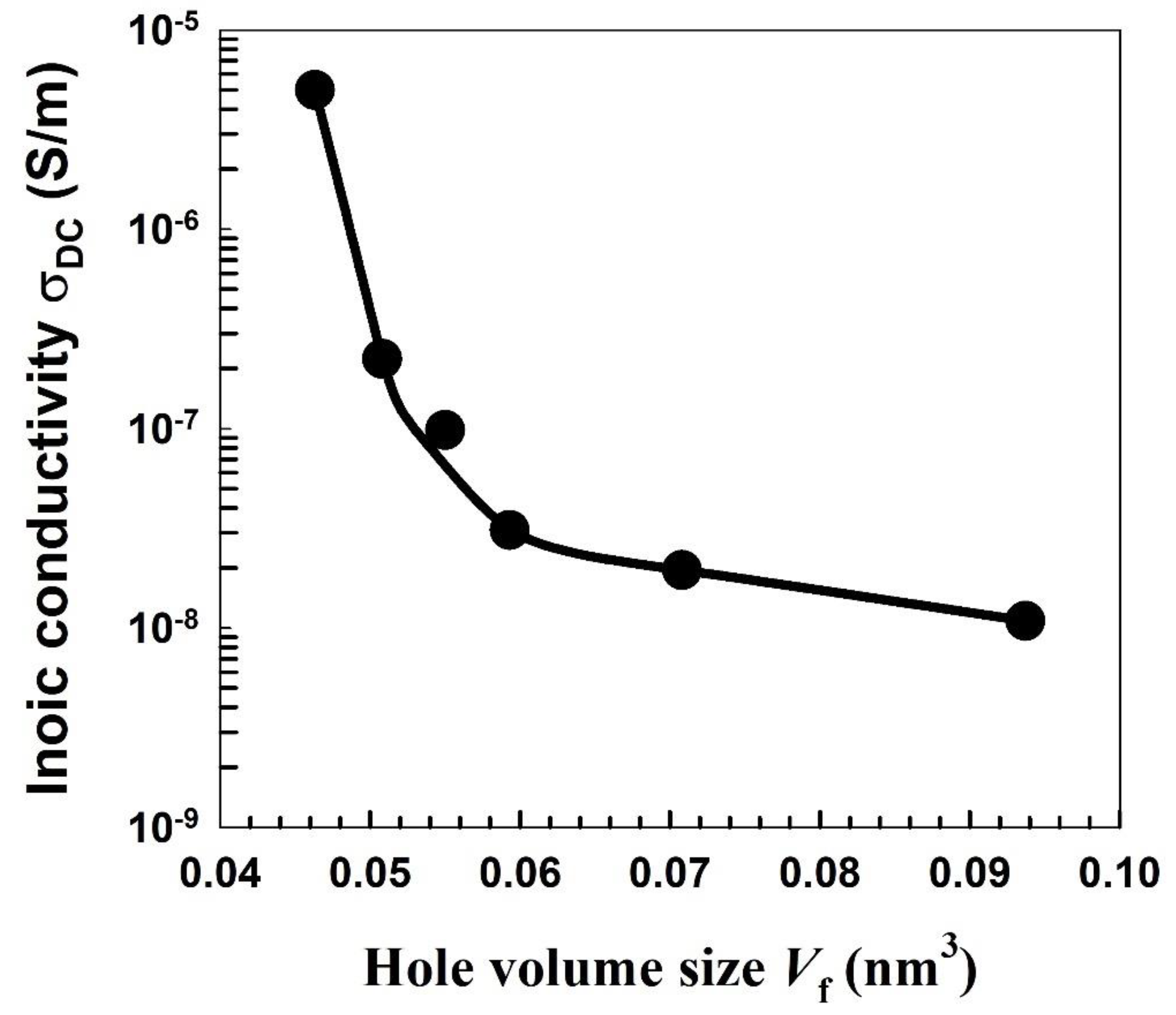
| Nylon-6,6 Concentration Blended with PVA (wt.%) | Activation Energy of PVA (J/Mole) At ~240 °C | Activation Energy of Nylon (J/Mole) At ~440 °C |
|---|---|---|
| 0 | 2.1 × 105 | ---- |
| 20 | 7.6 × 104 | 2.73 × 105 |
| 40 | 1.18 × 105 | 2.94 × 105 |
| 60 | 2.9 × 104 | 8.2 × 104 |
| 80 | 8.5 × 104 | 2.3 × 105 |
| 100 | ---- | 1.52 × 105 |
| Sample | Crystalline Peak | Amorphous Peak | ||||
|---|---|---|---|---|---|---|
| Peak Position | FWHM | Grain Size (nm) | Peak Position | FWHM | Grain Size (nm) | |
| Pure Nylon-6,6 | 20.341° | 0.610° | 13.82 | 20.319° | 1.678° | 5.03 |
| 24.399° | 0.762° | 11.14 | 24.628° | 1.587° | 5.35 | |
| Pure PVA | 19.427° | 1.985° | 4.24 | 19.909° | 5.657° | 1.49 |
| Blend polymer 60 wt.% Nylon-6,6/40 wt.% PVA | 20.099° | 0.53° | 15.88 | 19.577° | 2.985° | 2.82 |
| 24.130° | 0.527° | 16.11 | 24.396° | 1.347° | 6.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, H.F.M.; Abdel-Hady, E.E.; Mohammed, W.M. Investigation of Transport Mechanism and Nanostructure of Nylon-6,6/PVA Blend Polymers. Polymers 2023, 15, 107. https://doi.org/10.3390/polym15010107
Mohamed HFM, Abdel-Hady EE, Mohammed WM. Investigation of Transport Mechanism and Nanostructure of Nylon-6,6/PVA Blend Polymers. Polymers. 2023; 15(1):107. https://doi.org/10.3390/polym15010107
Chicago/Turabian StyleMohamed, Hamdy F. M., Esam E. Abdel-Hady, and Wael M. Mohammed. 2023. "Investigation of Transport Mechanism and Nanostructure of Nylon-6,6/PVA Blend Polymers" Polymers 15, no. 1: 107. https://doi.org/10.3390/polym15010107
APA StyleMohamed, H. F. M., Abdel-Hady, E. E., & Mohammed, W. M. (2023). Investigation of Transport Mechanism and Nanostructure of Nylon-6,6/PVA Blend Polymers. Polymers, 15(1), 107. https://doi.org/10.3390/polym15010107








