Aminosilane-Functionalized Zeolite Y in Pebax Mixed Matrix Hollow Fiber Membranes for CO2/CH4 Separation
Abstract
1. Introduction
2. Materials and Methods
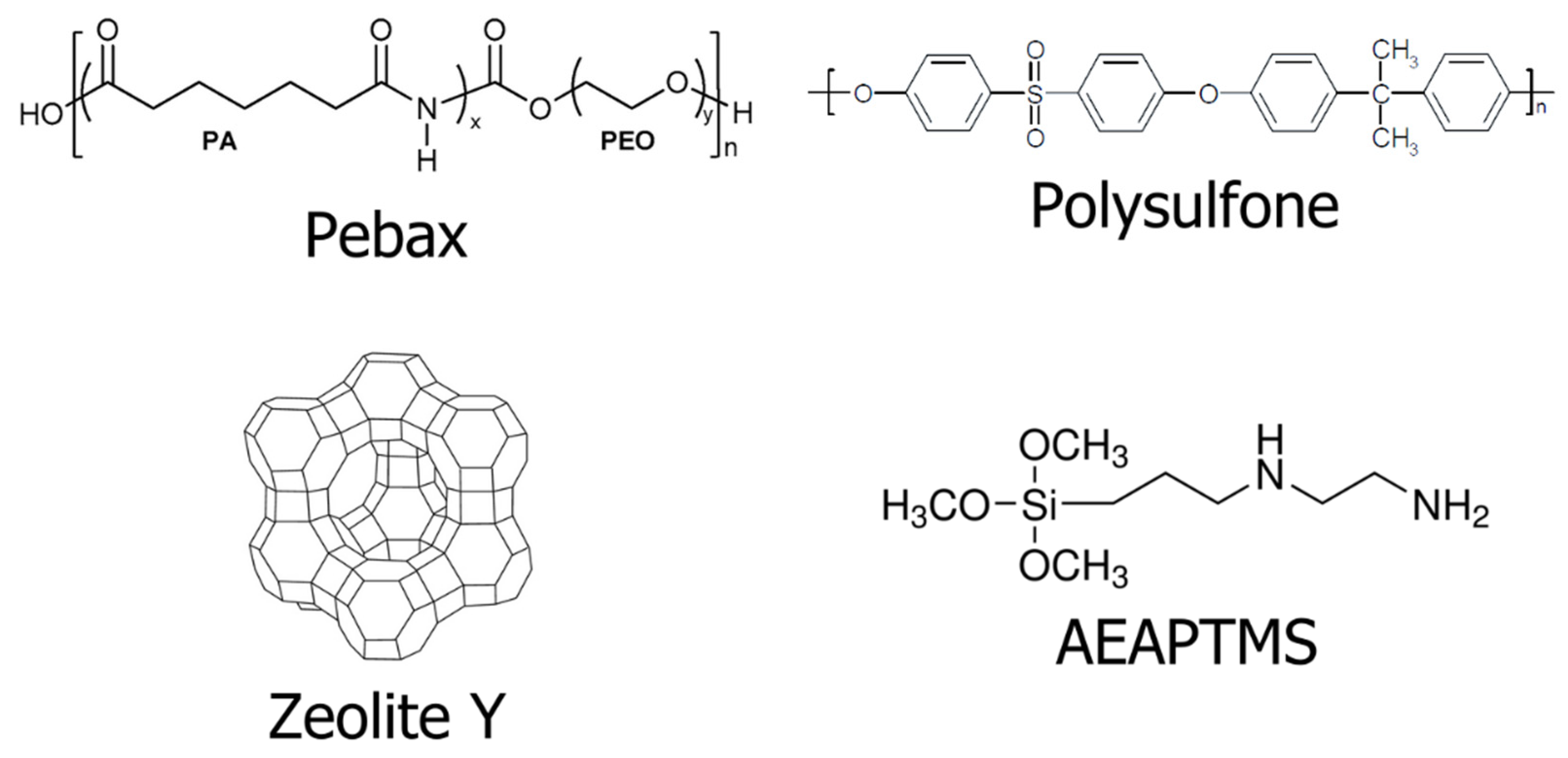
2.1. Materials
2.2. Zeolite Y Surface Modification
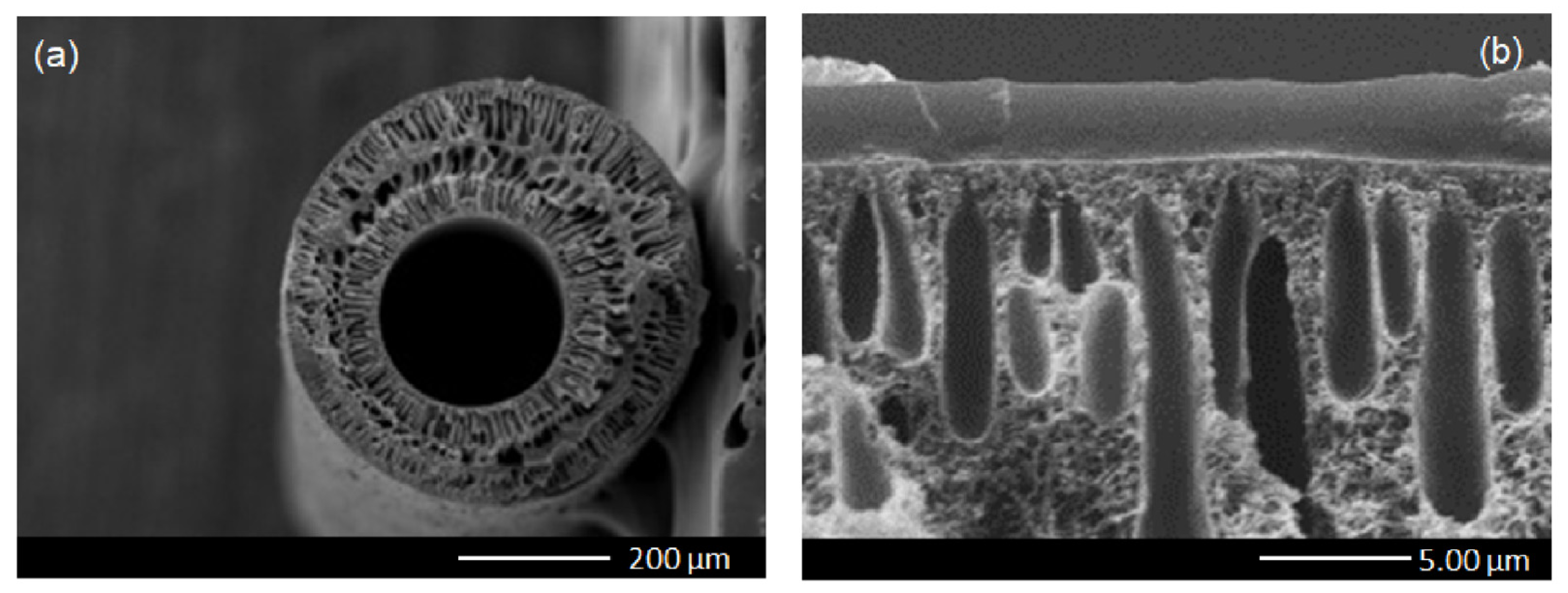
2.3. Fabrication of the Hollow Fiber Support
2.4. Fabrication of the Selective Layer
2.5. Characterization of Filler and Membranes
2.6. Membrane Performance Test
3. Results
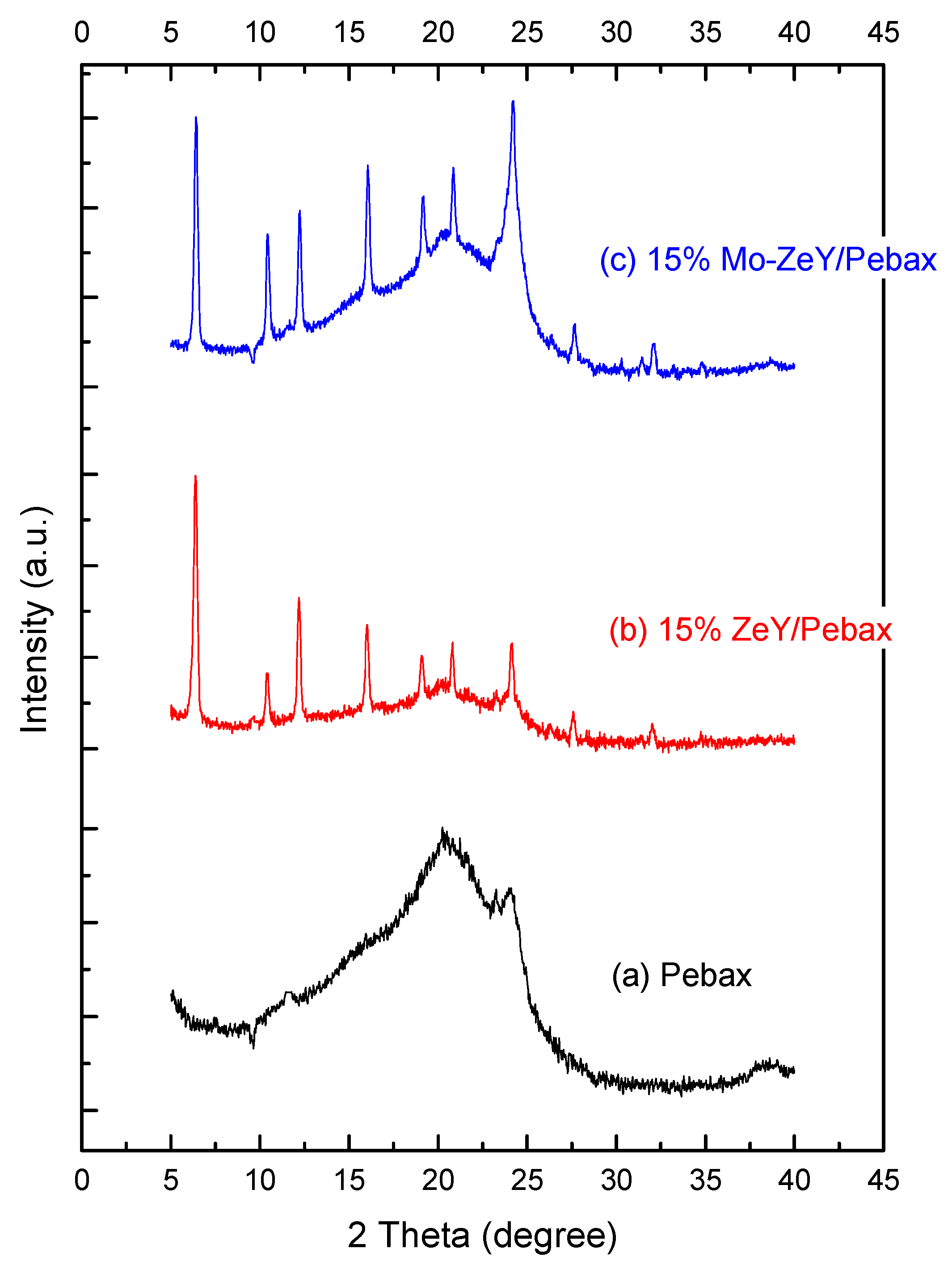
3.1. Characterization of Fillers
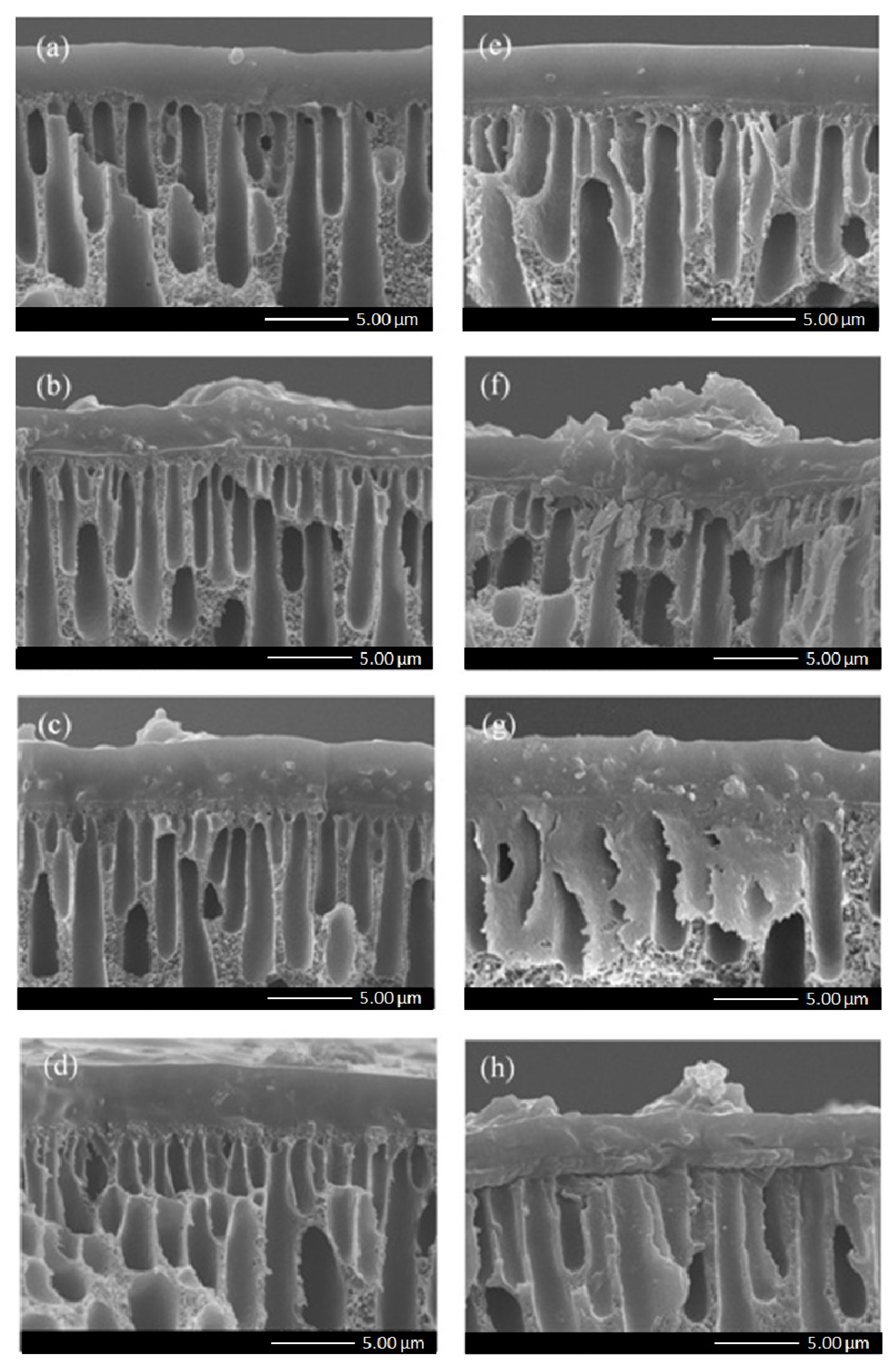
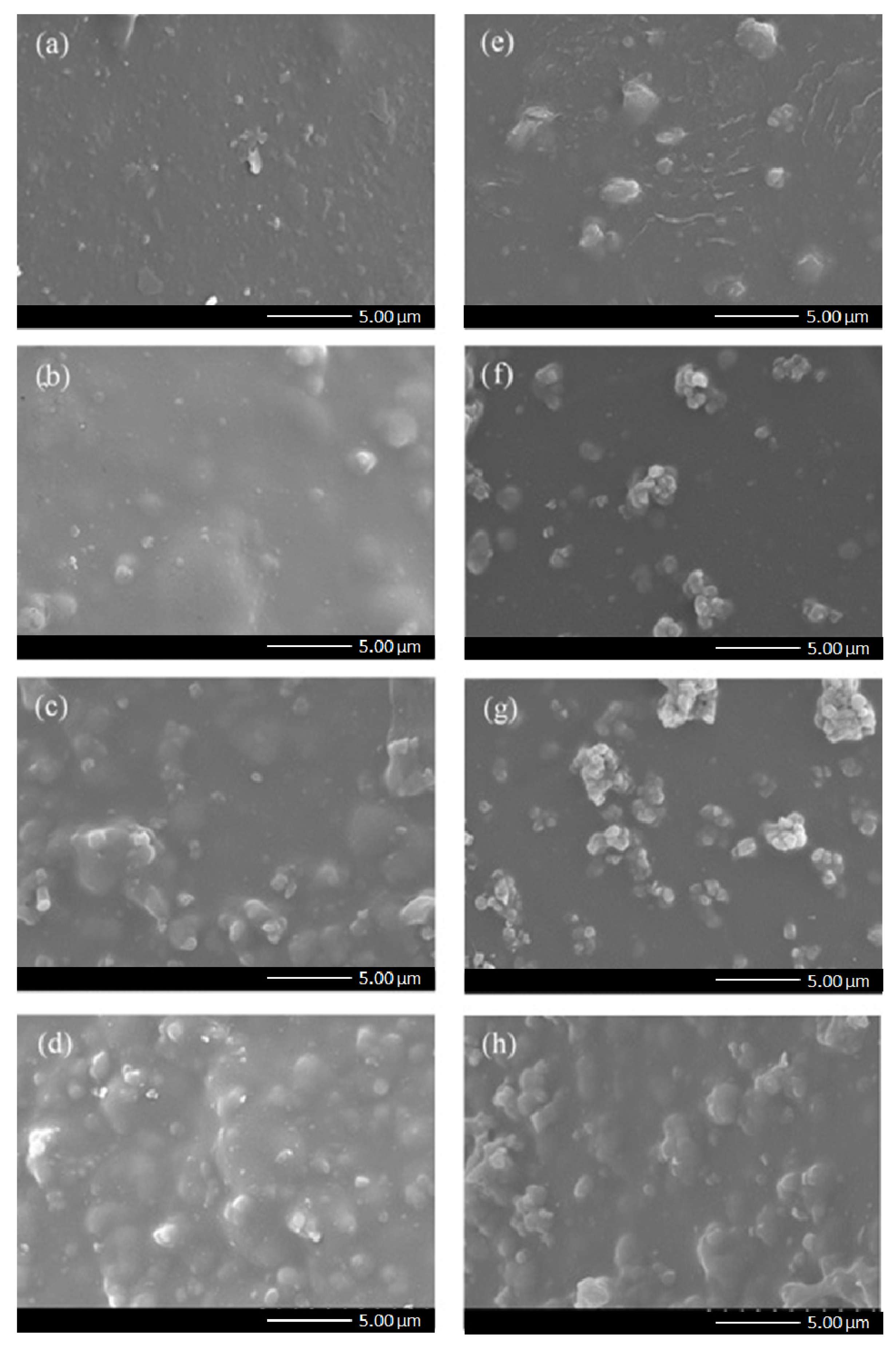
3.2. Membrane Characterization
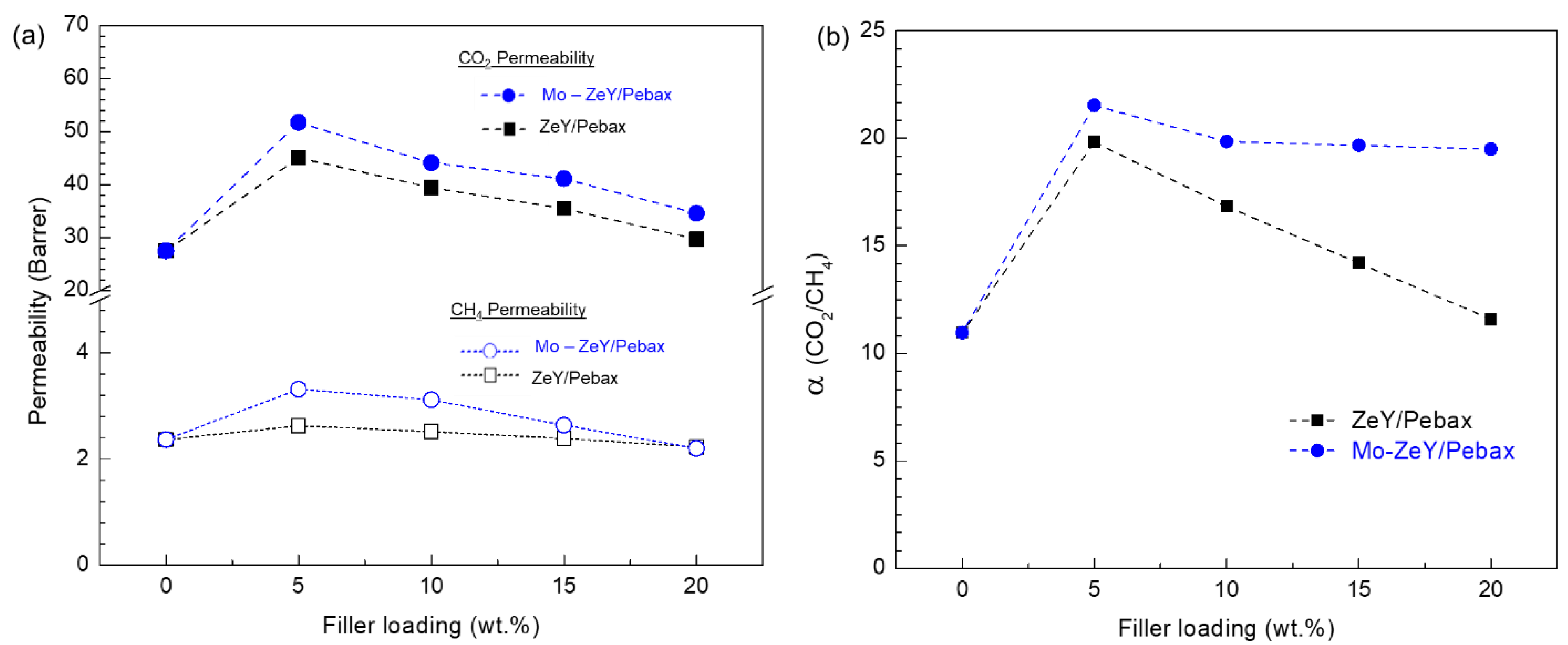
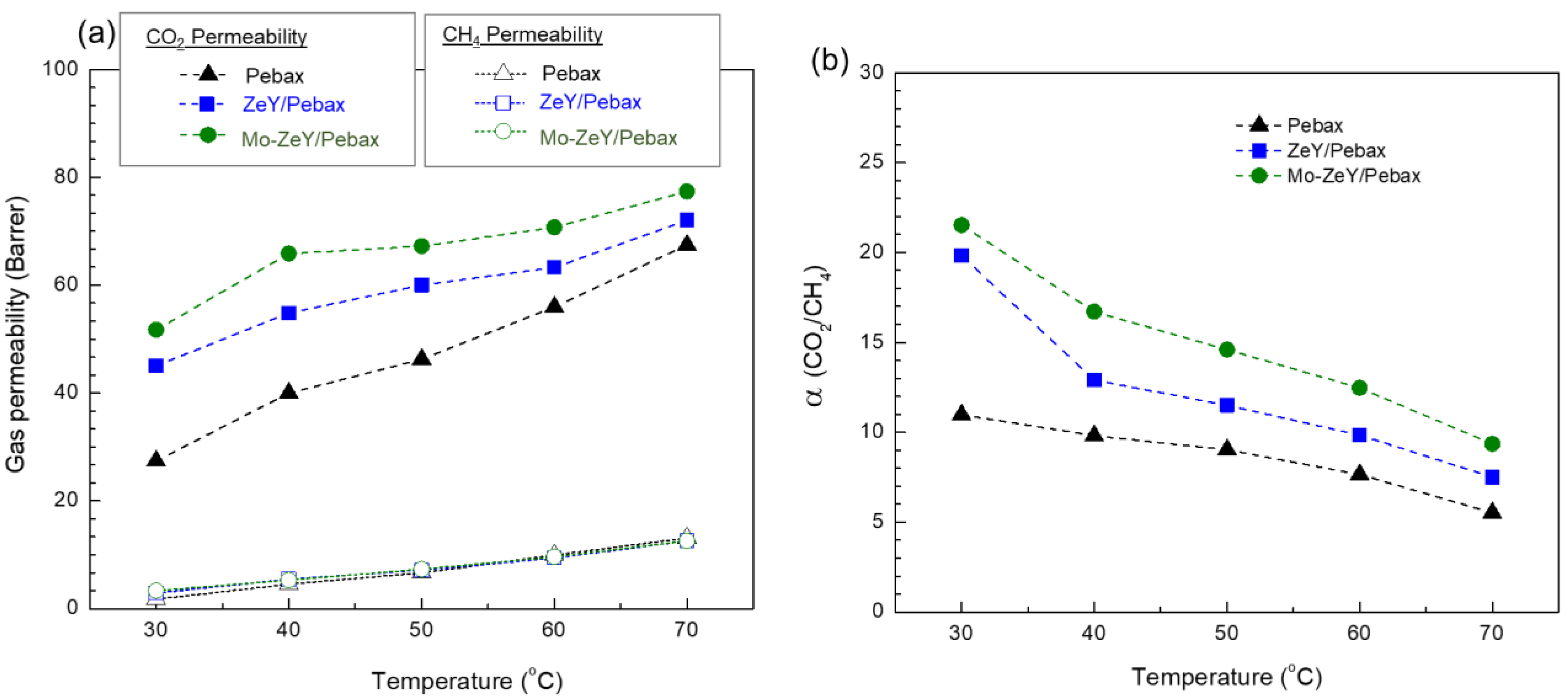
3.3. Gas Separation Performance of Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholz, M.; Melin, T.; Wessling, M. Transforming biogas into biomethane using membrane technology. Renew. Sust. Energy Rev. 2013, 17, 199–212. [Google Scholar] [CrossRef]
- Jeon, Y.-W.; Lee, D.-H. Gas membranes for CO2/CH4 (biogas) separation: A review. Environ. Eng. Sci. 2015, 32, 71–85. [Google Scholar] [CrossRef]
- Ozturk, B.; Demirciyeva, F. Comparison of biogas upgrading performances of different mixed matrix membranes. Chem. Eng. J. 2013, 222, 209–217. [Google Scholar] [CrossRef]
- Issaoui, M.; Limousy, L. Low-cost ceramic membranes: Synthesis, classifications, and applications. Comptes Rendus Chim. 2019, 22, 175–187. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Kim, D.; Seo, J. Preparation and characterization of poly(ether-block-amide)/polyethylene glycol composite films with temperature-dependent permeation. Polymers 2018, 10, 225. [Google Scholar] [CrossRef]
- Bernardo, P.; Clarizia, G. Enhancing gas permeation properties of Pebax®1657 membranes via polysorbate nonionic surfactants doping. Polymers 2020, 12, 253. [Google Scholar] [CrossRef]
- Embaye, A.S.; Martínez-Izquierdo, L.; Malankowska, M.; Téllez, C.; Coronas, J. Poly(ether-block-amide) copolymer membranes in CO2 separation applications. Energy Fuels 2021, 35, 17085–17102. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Kargari, A.; Nasernejad, B.; Amooghin, A.E.; Omidkhah, M. A novel Co2+ exchanged zeolite Y/cellulose acetate mixed matrix membrane for CO2/N2 separation. J. Taiwan Inst. Chem. Eng. 2016, 60, 403–413. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Wang, B.; Sun, C.; Chakraborty, S.; Ramasubramanian, K.; Dutta, P.K.; Ho, W.H.W. Multilayer polymer/zeolite Y composite membrane structure for CO2 capture from flue gas. J. Membr. Sci. 2016, 498, 1–13. [Google Scholar] [CrossRef]
- Amooghin, A.E.; Omidkhah, M.; Kargari, A. Enhanced CO2 transport properties of membranes by embedding nano-porous zeolite particles into Matrimid® 5218 matrix. RSC Adv. 2015, 5, 8552–8565. [Google Scholar] [CrossRef]
- Amooghin, A.E.; Omidkhah, M.; Kargari, A. The effects of aminosilane grafting on NaY zeolite-Matrimid®5218 mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2015, 490, 364–379. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Hua, K.; Deng, M. Poly(amide-6-b-ethylene oxide)/SAPO-34 mixed matrix membrane for CO2 separation. J. Energy Chem. 2014, 23, 227–234. [Google Scholar] [CrossRef]
- Sharmila, T.K.B.; Sasi, S.; Suja, N.R.; Beegum, P.M.S.; Thachil, E.T. A comparative investigation of aminosilane/ethylene diamine-functionalized graphene epoxy nanocomposites with commercial and chemically reduced graphene: Static and dynamic mechanical properties. Emergent Mater. 2019, 2, 371–386. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, H.; Muradi, G.; Zhang, B. Tuning the multi-scale structure of mixed-matrix membranes for upgrading CO2 separation performances. Sep. Purif. Technol. 2022, 293, 121118. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Khoo, C.P.; Leo, C.P.; Ahmad, A.L. The effects of solvents on the modification of SAPO-34 zeolite using 3-aminopropyl trimethoxy silane for the preparation of asymmetric polysulfone mixed matrix membrane in the application of CO2 separation. Microporous Mesoporous Mater. 2014, 92, 52–59. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.A.; Moura, P.A.S.; Azevedo, D.C.S.; Rodríguez-Castellón, E. Assessing CO2 adsorption on amino-functionalized mesocellular foams synthesized at different aging temperatures. Front. Chem. 2020, 8, 591766. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Liang, W.; Wang, J.; Chen, Y. Effect of silane treatment on mechanical properties and thermal behavior of bamboo fibers reinforced polypropylene composites. J. Eng. Fibers Fabr. 2020, 15, 1558925020958195. [Google Scholar] [CrossRef]
- Ahmedzeki, N.S.; Yilmaz, S.; Al-Tabbakh, B.A. Synthesis and characterization of nanocrystalline zeolite Y. Al-Khawarizmi Eng. J. 2016, 12, 79–89. [Google Scholar]
- Sosa, N.; Chanlek, N.; Wittayakun, J. Facile ultrasound-assisted grafting of silica gel by aminopropyltriethoxysilane for aldol condensation of furfural and acetone. Ultrason. Sonochem. 2020, 62, 104857. [Google Scholar] [CrossRef]
- Mohd, N.H.; Ismail, N.F.H.; Zahari, J.I.; Fathilah, W.; Kargarzadeh, H.; Ramli, S.; Ahmad, I.; Yarmo, M.A.; Othaman, R. Effect of aminosilane modification on nanocrystalline cellulose properties. J. Nanomater. 2016, 2016, 4804271. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Kargari, A.; Nasernejad, B. Aminosilane-functionalization of a nanoporous Y-type zeolite for application in a cellulose acetate based mixed matrix membrane for CO2 separation. RSC Adv. 2014, 4, 63966–63976. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Fabrication of silanated zeolite T/6FDA-durene composite membranes for CO2/CH4 separation. J. Clean. Prod. 2017, 166, 1043–1058. [Google Scholar] [CrossRef]
- Vu, H.-T.; Harth, F.M.; Wilde, N. Silylated zeolites with enhance hydrothermal stability for the aqueous-phase hydrogenation of levulinic acid to gamma-valerolactone. Front. Chem. 2018, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Esteban Florez, F.L.; Trofimov, A.A.; Ievlev, A.; Qian, S.; Rondinone, A.J.; Khajotia, S.S. Advanced characterization of surface-modified nanoparticles and nanofilled antibacterial dental adhesive resins. Sci. Rep. 2020, 10, 9811. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Shilton, S.J.; Dunkin, I.R.; Gallivan, S.L. Direct measurement of rheologically induced molecular orientation in gas separation hollow fibre membranes and effects on selectivity. J. Membr. Sci. 1997, 126, 133–137. [Google Scholar] [CrossRef]
- Zarshenas, K.; Raisi, A.; Aroujalian, A. Mixed matrix membrane of nano-zeolite NaX/poly (ether-block-amide) for gas separation applications. J. Membr. Sci. 2016, 510, 270–283. [Google Scholar] [CrossRef]
- Norahim, N.; Faungnawakij, K.; Quitain, A.T.; Klaysom, C. Composite membranes of graphene oxide for CO2/CH4 separation. J. Chem. Technol. 2019, 94, 2783–2791. [Google Scholar]
- Li, M.; Zhang, X.; Zeng, S.; Bai, L.; Gao, H.; Deng, J.; Yang, Q.; Zhang, S. Pebax-based composite membranes with high gas transport properties enhanced by ionic liquids for CO2 separation. RSC Adv. 2017, 7, 6422–6431. [Google Scholar] [CrossRef]
- Ghadimi, A.; Amirilargani, M.; Mohammadi, T.; Kasiri, N.; Sadatnia, B. Preparation of alloyed poly(ether block amide)/poly(ethylene glycol diacrylate) membranes for separation of CO2/H2 (syngas application). J. Membr. Sci. 2014, 458, 14–26. [Google Scholar] [CrossRef]
- Ito, Y.; Mohamed, H.F.M.; Seguchi, T.; Oshima, A. Vacancy spectroscopy of radiation cross-linked and degraded polytetrafluoroethylenes. Radiat. Phys. Chem. 1996, 48, 775–779. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Abdel-Hady, E.E.; Abdel-Moneim, M.M.Y.; Bakr, M.A.M.; Soliman, M.A.M.; Shehata, M.G.H.; Ismail, M.A.T. Effect of Al2O3 on nanostructure and ion transport properties of PVA/PEG/SSA polymer electrolyte membrane. Polymers 2022, 14, 4029. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Ohira, A.; Kobayashi, Y. Free volume and oxygen permeability in polymers related to polymer electrolyte fuel cells. Mater. Sci. Forum 2009, 607, 58–60. [Google Scholar] [CrossRef]
- Varghese, A.M.; Karanikolos, G.N. CO2 capture adsorbents functionalized by amine-bearing polymers: A review. Int. J. Greenh. Gas Control 2020, 96, 103005. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Enhanced gas separation performance using mixed matrix membranes containing zeolite T and 6FDA-durene polyimide. J. Membr. Sci. 2017, 525, 175–186. [Google Scholar] [CrossRef]
- Askadskii, A.A.; Popova, M.; Matseevich, T.A.; Kurskaya, E. The influence of the degree of crystallinity on the glass transition temperature of polymers. Adv. Mat. Res. 2013, 864, 751–754. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H.; et al. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Membr. Sci. 2019, 570–571, 343–354. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. Permeability and diffusivity of CO2 in glassy polymers with and without plasticization. J. Membr. Sci. 2013, 435, 176–185. [Google Scholar] [CrossRef]
- Ismail, A.F.; Lorna, W. Penetrant-induced plasticization phenomenon in glassy polymers for gas separation membrane. Sep. Purif. Technol. 2002, 27, 173–194. [Google Scholar] [CrossRef]
- Salestan, S.K.; Rahimpour, A.; Abedini, R. Experimental and theoretical studies of biopolymers on the efficient CO2/CH4 separation of thin-film Pebax®1657 membrane. Chem. Eng. Process. Process Intensif. 2021, 163, 108366. [Google Scholar] [CrossRef]
- Khalkhali, M.; Ghorbani, A.; Bayati, B. Study of adsorption and diffusion of methyl mercaptan and methane on FAU zeolite using molecular simulation. Polyhedron 2019, 171, 403–410. [Google Scholar] [CrossRef]
- Zhang, J.; Burke, N.; Zhang, S.; Liu, K.; Pervukhina, M. Thermodynamic analysis of molecular simulations of CO2 and CH4 adsorption in FAU zeolites. Chem. Eng. Sci. 2014, 113, 54–61. [Google Scholar] [CrossRef]
- Habibiannejad, S.; Aroujalian, A.; Raisi, A. Pebax-1657 mixed matrix membrane containing surface modified multi-walled carbon nanotubes for gas separation. RSC Adv. 2016, 6, 79563–79577. [Google Scholar] [CrossRef]
- Ito, Y.; Mohamed, H.F.M.; Tanaka, K.; Okamoto, K.; Lee, K. Sorption of CO2 in polymers observed by positron annihilation technique. J. Radioanal. Nucl. Chem. 1996, 211, 211–218. [Google Scholar] [CrossRef]
- Ebadi, R.; Maghsoudi, H.; Babaluo, A.A. Fabrication and characterization of Pebax-1657 mixed matrix membrane loaded with Si-CHA zeolite for CO2 separation from CH4. J. Nat. Gas Eng. 2021, 90, 103947. [Google Scholar] [CrossRef]
- Beiragh, H.H.; Omidkhah, M.; Abedini, R.; Khosravi, T.; Pakseresht, S. Synthesis and characterization of poly(ether-block-amide) mixed matrix membranes incorporated by nanoporous ZSM-5 particles for CO2/CH4 separation. Asia-Pac. J. Chem. Eng. 2016, 11, 522–532. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Liu, X.; Zhang, B. Mixed matrix membranes incorporated with aminosilane-functionalized SAPO-34 for upgrading CO2/CH4 separation performances. Ind. Eng. Chem. Res. 2021, 60, 13927–13937. [Google Scholar] [CrossRef]
- Maleh, M.S.; Raisi, A. CO2-philic moderate selective layer mixed matrix membranes containing surface functionalized NaX towards highly-efficient CO2 capture. RSC Adv. 2019, 9, 15542–15553. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Asghari, M.; Zargar, V. CO2/CH4 separation through a novel commercializable three-phase PEBA/PEG/NaX nanocomposite membrane. J. Ind. Eng. Chem. 2015, 23, 238–242. [Google Scholar] [CrossRef]
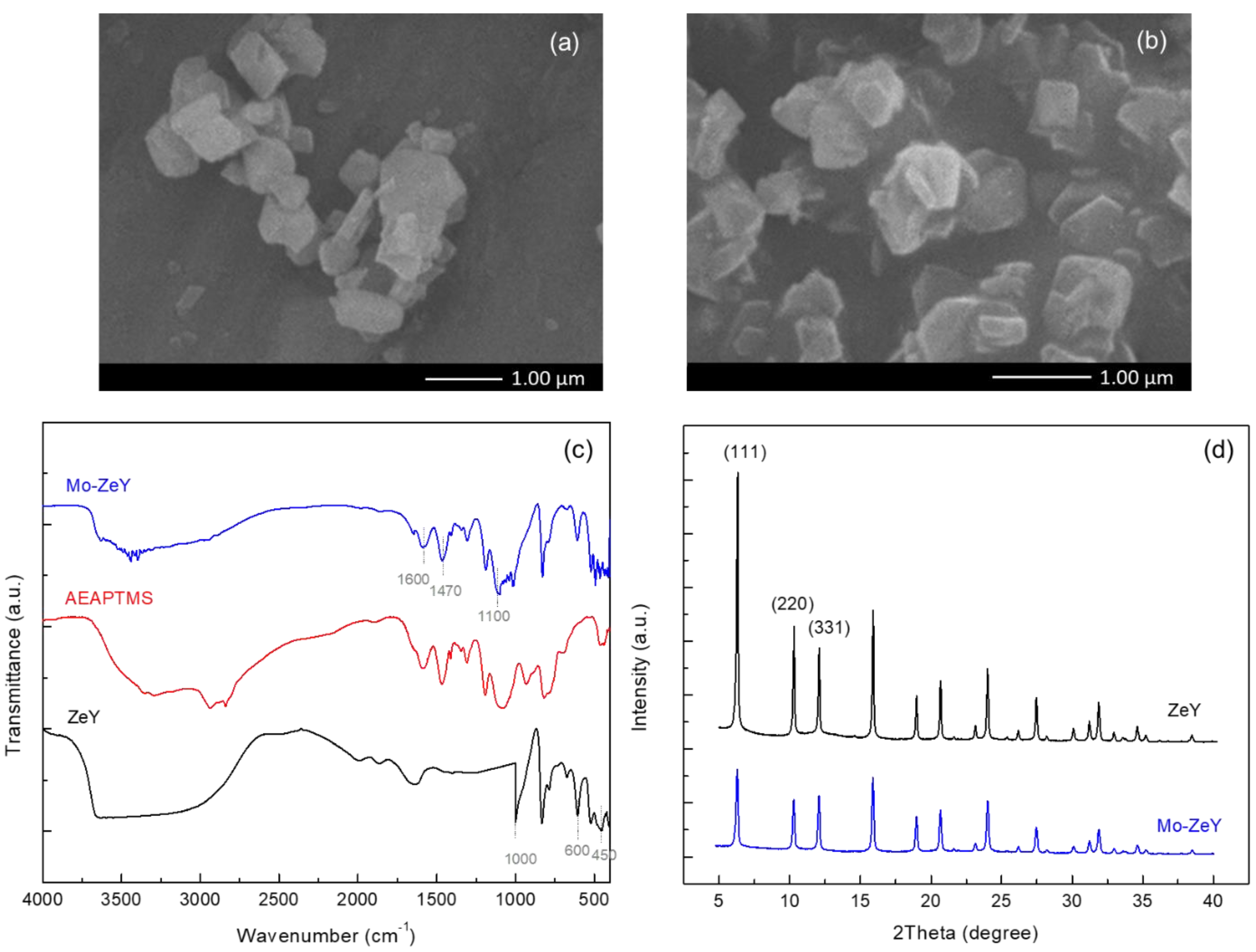


| Sample | Average Particle Size (nm) | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| ZeY | 506 | 750 | 0.40 | 2.32 |
| Mo-ZeY | 509 | 305 | 0.20 | 1.88 |
| Samples | Filler Loading (wt.%) | Tg (°C) | Crystallinity (%) |
|---|---|---|---|
| Pebax | 0 | −54.8 | 10.2 |
| ZeY/Pebax | 5 | −54.6 | 10.4 |
| 10 | −54.1 | 14.6 | |
| 15 | −53.6 | 15.3 | |
| 20 | −52.6 | 18.9 | |
| Mo-ZeY/Pebax | 5 | −54.5 | 10.9 |
| 10 | −54.0 | 14.9 | |
| 15 | −53.4 | 15.5 | |
| 20 | −51.8 | 19.0 |
| Membrane | Testing Conditions | Performance | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Filler | Membrane Type | Temperature (°C) | Pressure (bar) | Feed Gas Compositions (CO2/CH4) | Separation Factor | CO2 Permeability (Barrer) | |
| Modified zeolite Y | Composite hollow fiber | 30 | 5 | Mixed gas 50/50 | 31.4 | 37.7 | This work |
| Si-CHA zeolite | Flat-sheet | Ambient | 5 | Mixed gas 30/70 | 20.1 | 73.3 | [45] |
| ZSM-5 zeolite | Flat-sheet | 35 | 3 | Single gas | 25.1 | 251.5 | [46] |
| Modified SAPO-34 | Flat-sheet | 25 | 2 | Mixed gas 50/50 | 34.7 | 137.1 | [47] |
| COOH-functionalized NaX | Composite flat-sheet | 25 | 6 | Single gas | 57.4 | 187.76 | [48] |
| Zeolite NaX | Flat-sheet | Ambient | 8 | Single gas | 45 | 95 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.-C.; Wichidit, T.; Narkkun, T.; Tung, K.-L.; Faungnawakij, K.; Klaysom, C. Aminosilane-Functionalized Zeolite Y in Pebax Mixed Matrix Hollow Fiber Membranes for CO2/CH4 Separation. Polymers 2023, 15, 102. https://doi.org/10.3390/polym15010102
Lu S-C, Wichidit T, Narkkun T, Tung K-L, Faungnawakij K, Klaysom C. Aminosilane-Functionalized Zeolite Y in Pebax Mixed Matrix Hollow Fiber Membranes for CO2/CH4 Separation. Polymers. 2023; 15(1):102. https://doi.org/10.3390/polym15010102
Chicago/Turabian StyleLu, Soon-Chien, Thakorn Wichidit, Thanitporn Narkkun, Kuo-Lun Tung, Kajornsak Faungnawakij, and Chalida Klaysom. 2023. "Aminosilane-Functionalized Zeolite Y in Pebax Mixed Matrix Hollow Fiber Membranes for CO2/CH4 Separation" Polymers 15, no. 1: 102. https://doi.org/10.3390/polym15010102
APA StyleLu, S.-C., Wichidit, T., Narkkun, T., Tung, K.-L., Faungnawakij, K., & Klaysom, C. (2023). Aminosilane-Functionalized Zeolite Y in Pebax Mixed Matrix Hollow Fiber Membranes for CO2/CH4 Separation. Polymers, 15(1), 102. https://doi.org/10.3390/polym15010102









