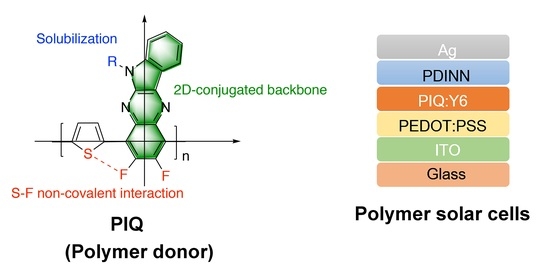
Synthesis of a Low-Cost Thiophene-Indoloquinoxaline Polymer Donor and Its Application to Polymer Solar Cells
Abstract
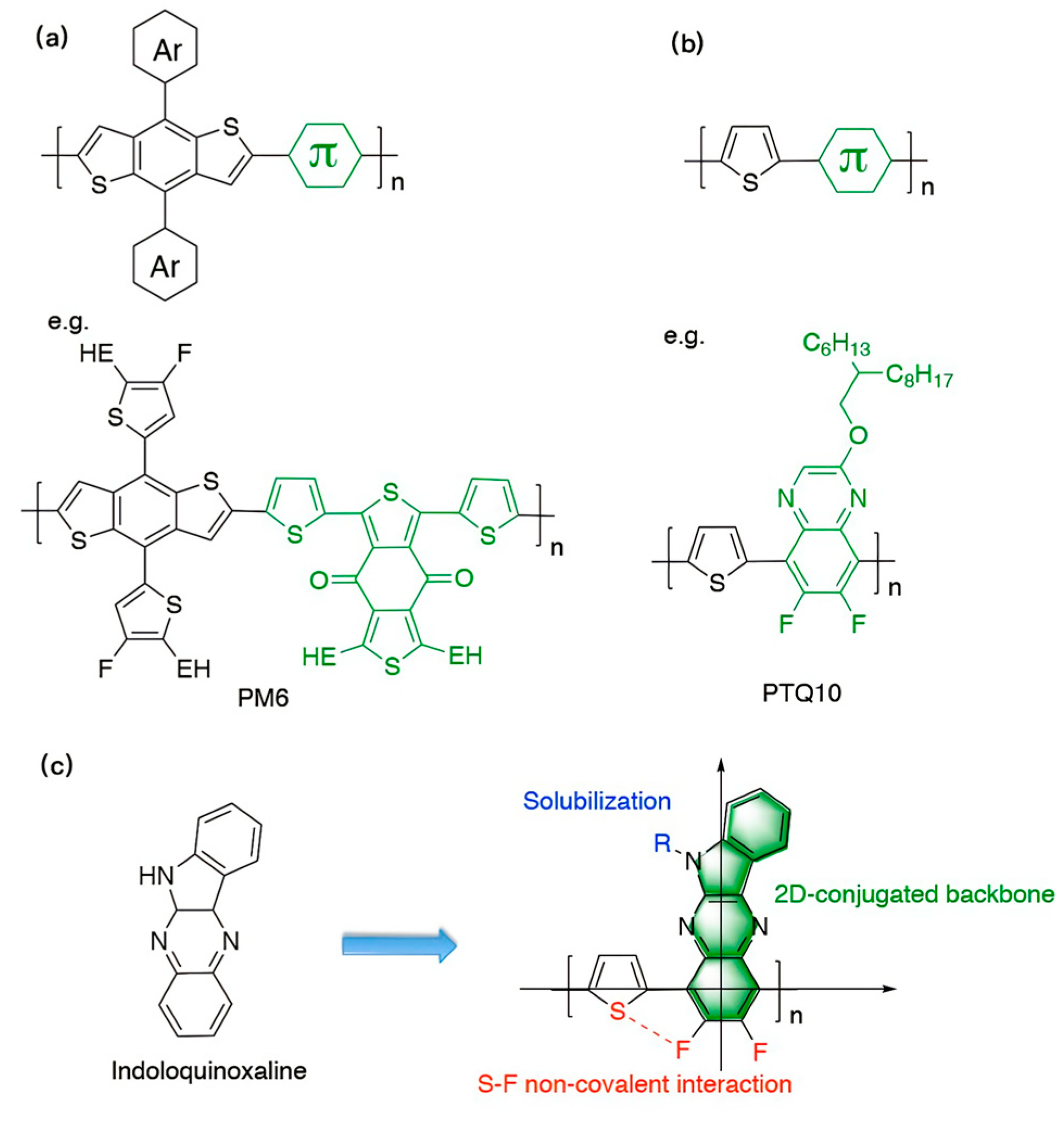
:1. Introduction
2. Materials and Methods
2.1. Materials
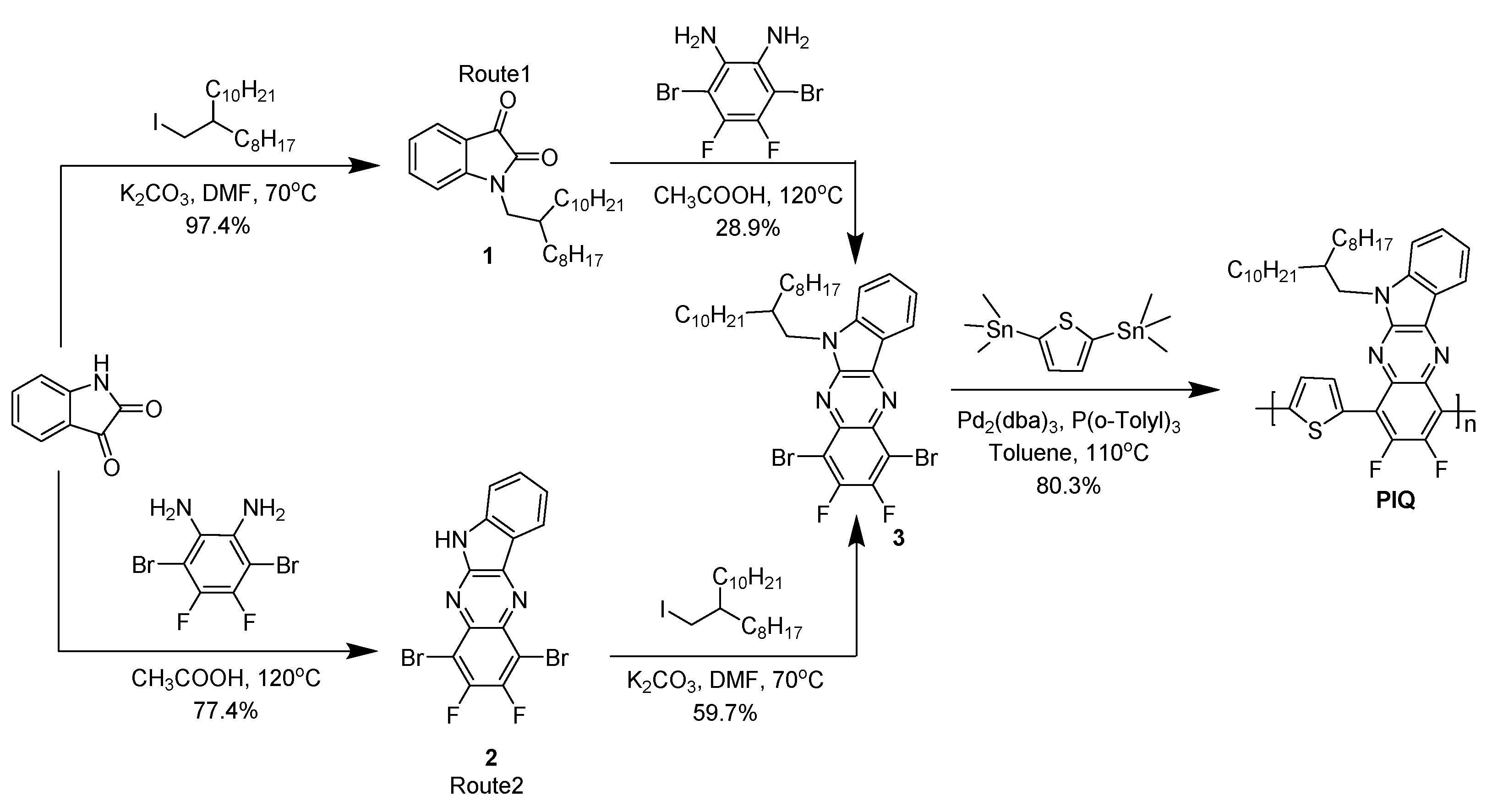
2.2. Synthesis of 1,4-Dibromo-2,3-difluoro-6-(2-octyldodecyl)-6H-indolo[2,3-b]quinoxaline
2.3. Synthesis of the Polymer PIQ
2.4. Device Fabrication and Characterization
3. Results and Discussion
3.1. Optical Properties
3.2. Electrochemical Properties
3.3. Thermal Properties and X-ray Diffraction Characterization
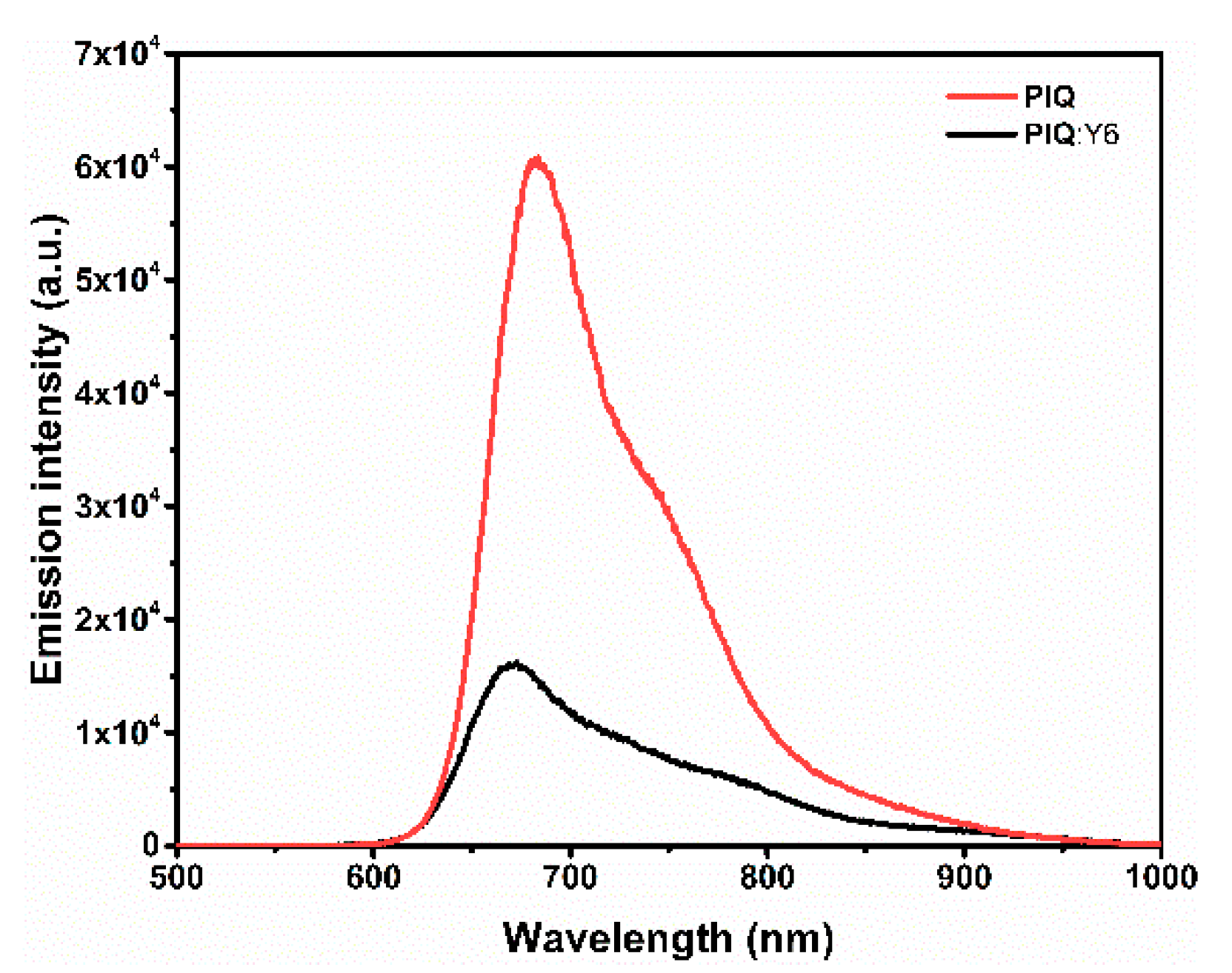
3.4. Photovoltaic Properties and Photoluminescence Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gopalakrishnan, V.; Balaji, D.; Dangate, M.S. Review-Conjugated Polymer Photovoltaic Materials: Performance and Applications of Organic Semiconductors in Photovoltaics. ECS J. Solid State Sci. Technol. 2022, 11, 035001. [Google Scholar] [CrossRef]
- Lim, I.; Hoa Thi, B.; Shrestha, N.K.; Lee, J.K.; Han, S.-H. Interfacial Engineering for Enhanced Light Absorption and Charge Transfer of a Solution-Processed Bulk Heterojunction Based on Heptazole as a Small Molecule Type of Donor. ACS Appl. Mater. Inter. 2016, 8, 8637–8643. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, P.; Jarząbek, B.; Vasylieva, M.; Godzierz, M.; Janeczek, H.; Musioł, M.; Domiński, A. The Effect of Alkyl Substitution of Novel Imines on Their Supramolecular Organization, Towards Photovoltaic Applications. Polymers 2021, 13, 1043. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Jang, W.; Babu, D.D.; Kim, M.S.; Wang, D.H.; Kyaw, A.K.K. Recent Progress in Organic Solar Cells Based on Non-Fullerene Acceptors: Materials to Devices. J. Mater. Chem. A 2022, 10, 3255–3295. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.; Zhao, D.; Wang, L.; Jiao, Z.; Huang, Q.; Wang, P.; Sun, M.; Yuan, G. Recent Progress in Organic Solar Cells: A Review on Materials from Acceptor to Donor. Molecules 2022, 27, 1800. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Bi, P.; Ren, J.; Wang, Y.; Yang, Y.; Liu, X.; Zhang, S.; Hou, J. Tandem Organic Solar Cell with 20.2% Efficiency. Joule 2022, 6, 171–184. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, J.; Li, Y.; Li, Y. Organic Solar Cell Materials toward Commercialization. Small 2018, 14, 1801793. [Google Scholar] [CrossRef]
- Li, S.; Yuan, X.; Zhang, Q.; Li, B.; Li, Y.; Sun, J.; Feng, Y.; Zhang, X.; Wu, Z.; Wei, H.; et al. Narrow-Bandgap Single-Component Polymer Solar Cells with Approaching 9% Efficiency. Adv. Mater. 2021, 33, 2101295. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, J.; He, D.; Wang, C.; Lin, Y. Low-Cost Materials for Organic Solar Cells. J. Mater. Chem. C 2021, 9, 15395–15406. [Google Scholar] [CrossRef]
- Jessop, I.A.; Chong, A.; Graffo, L.; Camarada, M.B.; Espinoza, C.; Angel, F.A.; Saldías, C.; Tundidor-Camba, A.; Terraza, C.A. Synthesis and Characterization of a 2,3-Dialkoxynaphthalene-Based Conjugated Copolymer Via Direct Arylation Polymerization for Organic Electronics. Polymers 2020, 12, 1377. [Google Scholar] [CrossRef]
- Ramoroka, M.E.; Mdluli, S.B.; John-Denk, V.S.; Modibane, K.D.; Arendse, C.J.; Iwuoha, E.I. Synthesis and Photovoltaics of Novel 2,3,4,5-Tetrathienylthiophene-Co-Poly(3-Hexylthiophene-2,5-Diyl) Donor Polymer for Organic Solar Cell. Polymers 2020, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Pan, F.; Bin, H.; Zhang, J.; Xue, L.; Qiu, B.; Wei, Z.; Zhang, Z.; Li, Y. A Low Cost and High Performance Polymer Donor Material for Polymer Solar Cells. Nat. Commun. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, S.; Huo, L.; Zhang, M.; Hou, J. Molecular Design toward Highly Efficient Photovoltaic Polymers Based on Two-Dimensional Conjugated Benzodithiophene. Acc. Chem. Res. 2014, 47, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Q.; Liu, S.; Cao, Z.; Jiao, X.; Cai, Y.-P.; Huang, F. Bithieno [3,4-c]Pyrrole-4,6-Dione-Mediated Crystallinity in Large-Bandgap Polymer Donors Directs Charge Transportation and Recombination in Efficient Nonfullerene Polymer Solar Cells. ACS Energy Lett. 2020, 5, 367–375. [Google Scholar] [CrossRef]
- Li, H.; Yang, W.; Wang, W.; Wu, Y.; Wang, T.; Min, J. Wide Bandgap Donor Polymers Containing Carbonyl Groups for Efficient Non-Fullerene Polymer Solar Cells. Dye. Pigment. 2021, 186, 108987. [Google Scholar] [CrossRef]
- Sun, C.; Pan, F.; Chen, S.; Wang, R.; Sun, R.; Shang, Z.; Qiu, B.; Min, J.; Lv, M.; Meng, L.; et al. Achieving Fast Charge Separation and Low Nonradiative Recombination Loss by Rational Fluorination for High-Efficiency Polymer Solar Cells. Adv. Mater. 2019, 31, 1905480. [Google Scholar] [CrossRef]
- Qiu, B.; Chen, S.; Sun, C.; Yuan, J.; Zhang, X.; Zhu, C.; Qin, S.; Meng, L.; Zhang, Y.; Yang, C.; et al. Understanding the Effect of the Third Component PC71BM on Nanoscale Morphology and Photovoltaic Properties of Ternary Organic Solar Cells. Sol. RRL 2020, 4, 1900540. [Google Scholar] [CrossRef]
- Yang, J.; Geng, Y.F.; Li, J.F.; Zhao, B.M.; Guo, Q.; Zhou, E.J. A-DA’ D-A-Type Non-fullerene Acceptors Containing a Fused Heptacyclic Ring for Poly(3-hexylthiophene)-Based Polymer Solar Cells. J. Phys. Chem. C 2020, 124, 24616–24623. [Google Scholar] [CrossRef]
- He, K.; Kumar, P.; Abd-Ellah, M.; Liu, H.; Li, X.; Zhang, Z.; Wang, J.; Li, Y. Alkyloxime Side Chain Enabled Polythiophene Donors for Efficient Organic Solar Cells. Macromolecules 2020, 53, 8796–8808. [Google Scholar] [CrossRef]
- Ren, J.; Bi, P.; Zhang, J.; Liu, J.; Wang, J.; Xu, Y.; Wei, Z.; Zhang, S.; Hou, J. Molecular Design Revitalizes the Low-cost PTV-polymer for Highly Efficient Organic Solar Cells. Natl. Sci. Rev. 2021, 8, nwab031. [Google Scholar] [CrossRef]
- Xiao, J.; Jia, X.; Duan, C.; Huang, F.; Yip, H.-L.; Cao, Y. Surpassing 13% Efficiency for Polythiophene Organic Solar Cells Processed from Nonhalogenated Solvent. Adv. Mater. 2021, 33, 2008158. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, S.; Ren, J.; Bi, P.; Yuan, X.; Hou, J. Fluorination Strategy Enables Greatly Improved Performance for Organic Solar Cells Based on Polythiophene Derivatives. Chin. Chem. Lett. 2021, 32, 2274–2278. [Google Scholar] [CrossRef]
- Manna, K.; Agrawal, Y. Microwave Assisted Synthesis of New Indophenazine 1,3,5-Trisubstruted Pyrazoline Derivatives of Benzofuran and Their Antimicrobial Activity. Bioorg. Med. Chem. Lett. 2009, 19, 2688–2692. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Wang, X.; Shao, L.; Li, H.; Yan, R.; Hou, L. Molecular Engineering of D-D-pi A Type Organic Dyes Incorporating Indoloquinoxaline and Phenothiazine for Highly Efficient Dye Sensitized Solar Cells. J. Power Sour. 2016, 326, 129–136. [Google Scholar] [CrossRef]
- Su, R.; Ashraf, S.; Lyu, L.; El-Shafei, A. Tailoring Dual-Channel Anchorable Organic Sensitizers with Indolo [2,3-b]quinoxaline Moieties: Correlation Between Structure and DSSC Performance. Sol. Energy. 2020, 206, 443–454. [Google Scholar] [CrossRef]
- Venkateswararao, A.; Tyagi, P.; Thomas, K.; Chen, P.; Ho, K. Organic Dyes Containing Indolo [2,3-b]quinoxaline as a Donor: Synthesis, Optical and Photovoltaic Properties. Tetrahedron 2014, 70, 6318–6327. [Google Scholar] [CrossRef]
- Dong, D.; Fang, D.; Li, H.; Zhu, C.; Zhao, X.; Li, J.; Jin, L.; Xie, L.; Chen, L.; Zhao, J.; et al. Direct Arylated 6H-Indolo [2,3-b]quinoxaline Derivative as a Thickness-Dependent Hole-Injection Layer. Chem. Asian J. 2017, 12, 920–926. [Google Scholar] [CrossRef]
- Payne, A.; Li, S.; Dayneko, S.; Risko, C.; Welch, G. An Unsymmetrical Non-Fullerene Acceptor: Synthesis via Direct Heteroarylation, Self-Assembly, and Utility as A Low Energy Absorber in Organic Photovoltaic Cells. Chem. Commun. 2017, 53, 10168–10171. [Google Scholar] [CrossRef]
- Chao, P.; Wang, H.; Qu, S.; Mo, D.; Meng, H.; Chen, W.; He, F. From Semi- to Full-Two-Dimensional Conjugated Side-Chain Design: A Way toward Comprehensive Solar Energy Absorption. Macromolecules 2017, 50, 9617–9625. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Y.; Li, Y. Benzotriazole Based 2D-conjugated Polymer Donors for High Performance Polymer Solar Cells. Chinese J. Polym. Sci. 2021, 39, 1–13. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Heo, J.; Lim, B.; Kim, J. Highly Efficient Polymer Solar Cells with a Thienopyrroledione and Benzodithiophene Containing Planar Random Copolymer. Polym. Chem. 2018, 9, 1216–1222. [Google Scholar] [CrossRef]
- Lim, B.; Long, D.; Han, S.; Nah, Y.; Noh, Y. Well-defined Alternative Polymer Semiconductor Using Large Size Regioregular Building Blocks as Monomers: Electrical and Electrochemical Properties. J. Mater. Chem. C 2018, 6, 5662–5670. [Google Scholar] [CrossRef]
- Choi, E.; Eom, S.; Song, C.; Nam, S.; Lee, J.; Woo, H.; Jung, I.; Yoon, S.; Lee, C. Synthesis and Characterization of a Wide Bandgap Polymer Based on a Weak Donor-Weak Acceptor Structure for Dual Applications in Organic Solar Cells and Organic Photodetectors. Org. Electron. 2017, 46, 173–182. [Google Scholar] [CrossRef]
- Yang, J.; Uddin, M.; Tang, Y.; Wang, Y.; Wang, Y.; Su, H.; Gao, R.; Chen, Z.; Dai, J.; Woo, H.; et al. Quinoxaline-Based Wide Band Gap Polymers for Efficient Nonfullerene Organic Solar Cells with Large Open-Circuit Voltages. ACS Appl. Mater. Interfaces 2018, 10, 23235–23246. [Google Scholar] [CrossRef]
- He, K.; Li, X.; Liu, H.; Zhang, Z.; Kumar, P.; Ngai, J.; Wang, J.; Li, Y. D-A Polymer with a Donor Backbone-Acceptor-side-chain Structure for Organic Solar Cells. Asian J. Org. Chem. 2020, 9, 1301–1308. [Google Scholar] [CrossRef]
- Li, X.; Pan, F.; Sun, C.; Zhang, M.; Wang, Z.; Du, J.; Wang, J.; Xiao, M.; Xue, L.; Zhang, Z.-G.; et al. Simplified Synthetic Routes for Low Cost and High Photovoltaic Performance n-type Organic Semiconductor Acceptors. Nat. Commun. 2019, 10, 519. [Google Scholar] [CrossRef]
- Loewe, R.S.; Khersonsky, S.M.; McCullough, R.D. A Simple Method to Prepare Head-to-tail Coupled, Regioregular Poly(3-alkylthiophenes) Using Grignard Metathesis. Adv. Mater. 1999, 11, 250–253. [Google Scholar] [CrossRef]
- Qian, D.; Ye, L.; Zhang, M.; Liang, Y.; Li, L.; Huang, Y.; Guo, X.; Zhang, S.; Tan, Z.; Hou, J. Design, Application, and Morphology Study of a New Photovoltaic Polymer with Strong Aggregation in Solution State. Macromolecules 2012, 45, 9611–9617. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X.; Ma, W.; Ade, H.; Hou, J. A Large-Bandgap Conjugated Polymer for Versatile Photovoltaic Applications with High Performance. Adv. Mater. 2015, 27, 4655–4660. [Google Scholar] [CrossRef]
- Xie, R.; Song, L.; Zhao, Z. Comparing Benzodithiophene Unit with Alkylthionaphthyl and Alkylthiobiphenyl Side-Chains in Constructing High-Performance Nonfullerene Solar Cells. Polymers 2020, 12, 1673. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A.; Saeed, S.R.; Abdulwahid, R.T. Fabrication of Alternating Copolymers Based on Cyclopentadithiophene-Benzothiadiazole Dicarboxylic Imide with Reduced Optical Band Gap: Synthesis, Optical, Electrochemical, Thermal, and Structural Properties. Polymers 2020, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Osw, P.; Nitti, A.; Abdullah, M.N.; Etkind, S.I.; Mwaura, J.; Galbiati, A.; Pasini, D. Synthesis and Evaluation of Scalable D-A-D π-Extended Oligomers as P-Type Organic Materials for Bulk-Heterojunction Solar Cells. Polymers 2020, 12, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.; Kumar, P.; Yuan, Y.; Zhang, Z.; Li, X.; Liu, H.; Wang, J.; Li, Y. A Wide Bandgap Polymer Donor Composed of Benzodi- thiophene and Oxime-Substituted Thiophene for High-Performance Organic Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 26441–26450. [Google Scholar] [CrossRef] [PubMed]
- Leenaers, P.J.; Maufort, A.J.L.A.; Wienk, M.M.; Janssen, R.A.J. Impact of π-Conjugated Linkers on the Effective Exciton Binding Energy of Diketopyrrolopyrrole–Dithienopyrrole Copolymers. J. Phys. Chem. C 2020, 124, 27403–27412. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, J.; Ding, Z.; Kan, B.; Lin, B.; Wan, X.; Ma, W.; Chen, Y.; Long, X.; Dou, C.; et al. Efficient and thermally stable organic solar cells based on small molecule donor and polymer acceptor. Nat. Commun. 2019, 10, 3271. [Google Scholar] [CrossRef] [Green Version]

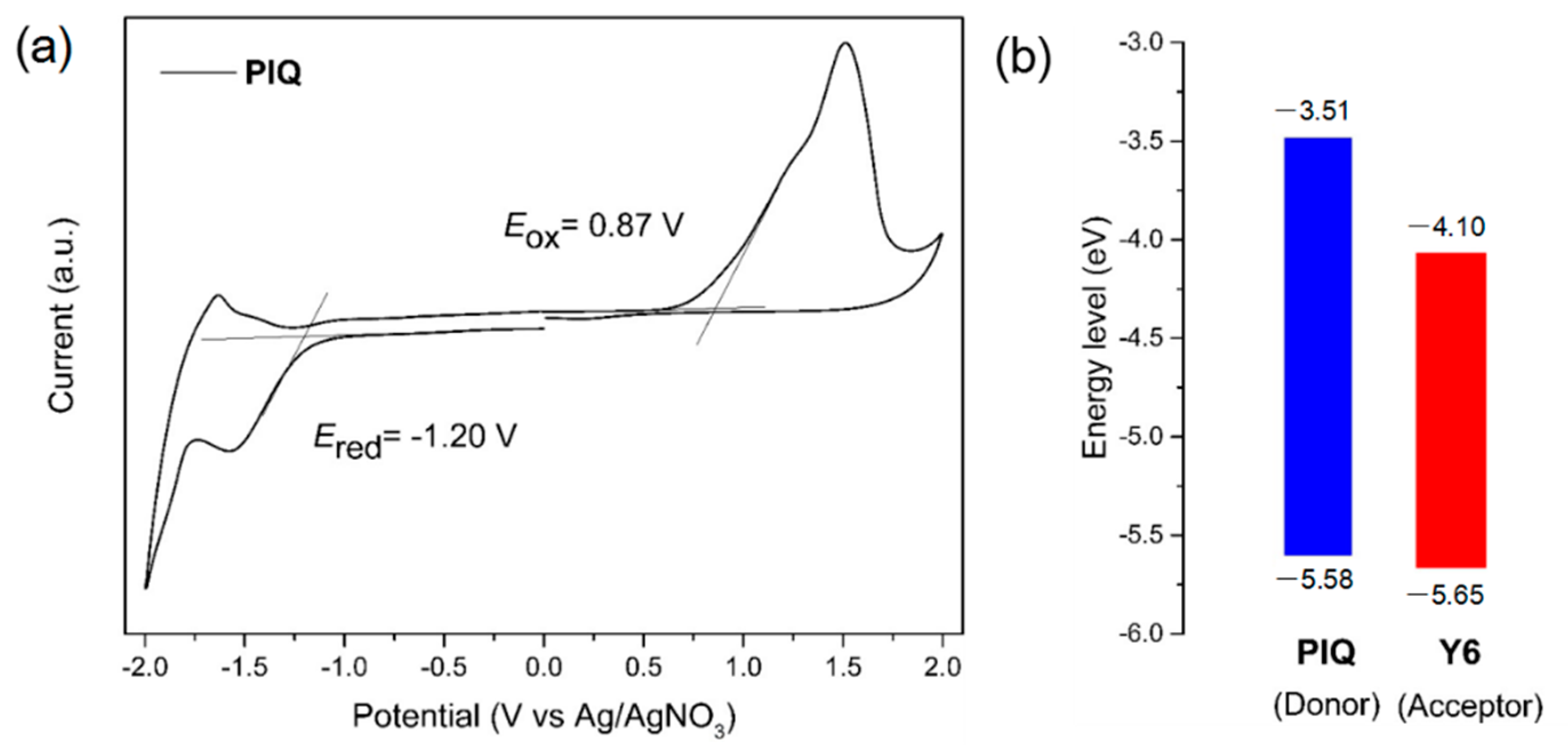

| Polymer | λmaxsol (nm) | λmaxfilm (nm) | λonsetfilm (nm) | Egopt1 (eV) | Ered/ELUMO2 (V/eV) | Eox/EHOMO3 (V/eV) |
|---|---|---|---|---|---|---|
| PIQ | 564 | 570 | 687 | 1.80 | −1.20/−3.51 | 0.87/−5.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Li, Z.; Sha, M.; Deng, P.; Lin, X.; Li, J.; Zhang, L.; Yin, H.; Zhan, H. Synthesis of a Low-Cost Thiophene-Indoloquinoxaline Polymer Donor and Its Application to Polymer Solar Cells. Polymers 2022, 14, 1554. https://doi.org/10.3390/polym14081554
Guo Y, Li Z, Sha M, Deng P, Lin X, Li J, Zhang L, Yin H, Zhan H. Synthesis of a Low-Cost Thiophene-Indoloquinoxaline Polymer Donor and Its Application to Polymer Solar Cells. Polymers. 2022; 14(8):1554. https://doi.org/10.3390/polym14081554
Chicago/Turabian StyleGuo, Yiping, Zeyang Li, Mengzhen Sha, Ping Deng, Xinyu Lin, Jun Li, Liang Zhang, Hang Yin, and Hongbing Zhan. 2022. "Synthesis of a Low-Cost Thiophene-Indoloquinoxaline Polymer Donor and Its Application to Polymer Solar Cells" Polymers 14, no. 8: 1554. https://doi.org/10.3390/polym14081554
APA StyleGuo, Y., Li, Z., Sha, M., Deng, P., Lin, X., Li, J., Zhang, L., Yin, H., & Zhan, H. (2022). Synthesis of a Low-Cost Thiophene-Indoloquinoxaline Polymer Donor and Its Application to Polymer Solar Cells. Polymers, 14(8), 1554. https://doi.org/10.3390/polym14081554