Comparison of Crosslinking Kinetics of UV-Transparent Ethylene-Vinyl Acetate Copolymer and Polyolefin Elastomer Encapsulants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Encapsulant Materials and Films
2.2. Infrared Spectroscopy
2.3. Dynamic Mechanical Analysis (DMA)
3. Results and Discussion
3.1. Structural Features of the Investigated Encapsulants
3.2. Temperature-Dependent Storage and Loss Modulus and Loss Factor
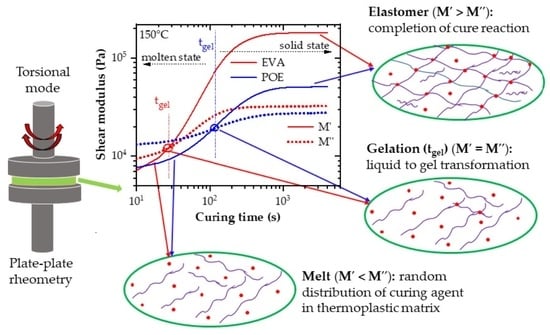
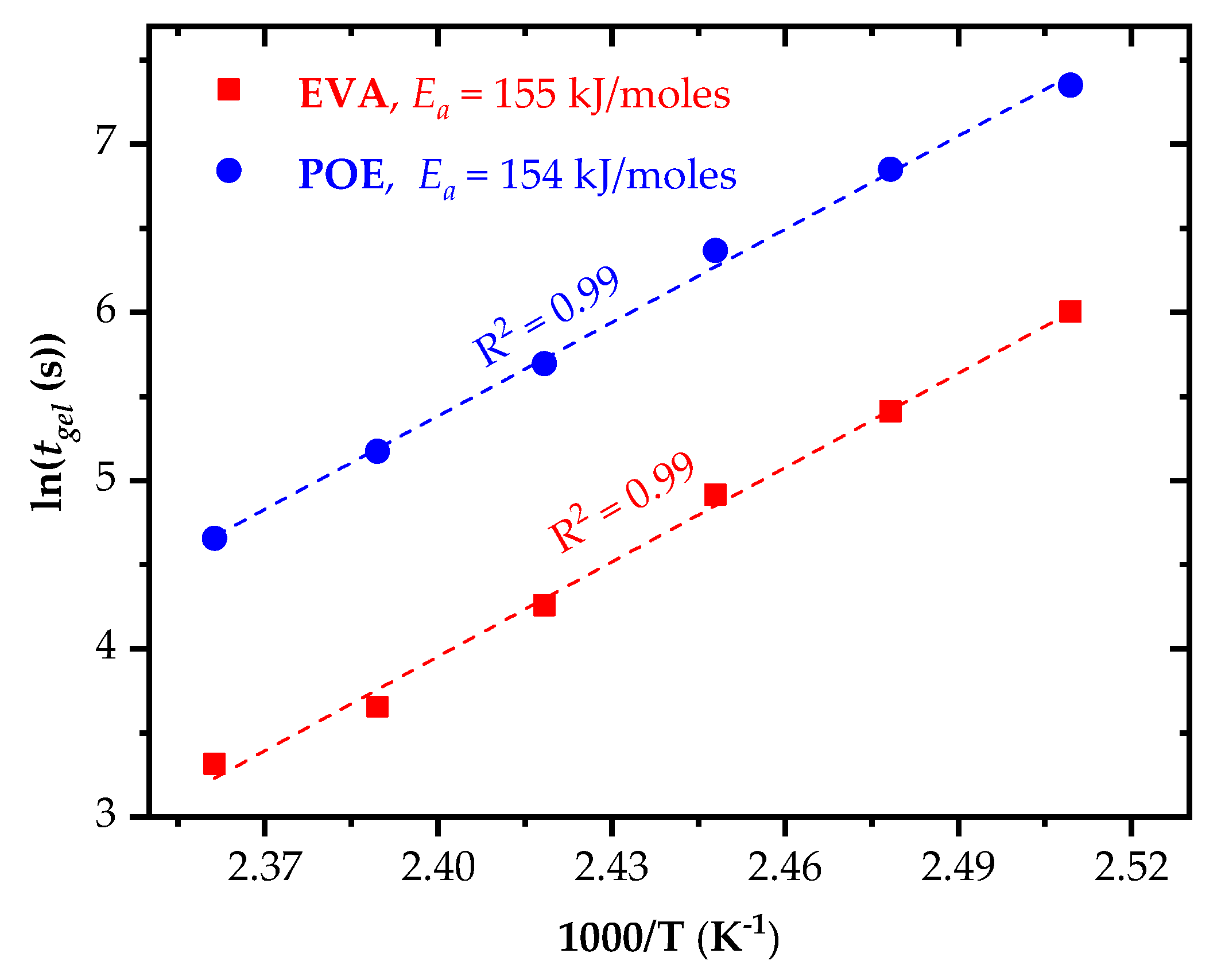
3.3. Curing Kinetics, Activation Energy and Progress of Crosslinking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czanderna, A.W.; Pern, F.J. Encapsulation of PV modules using an ethylene vinyl acetate copolymer as pottant: A critical review. Sol. Energy Mater. Sol. Cells 1996, 43, 101–181. [Google Scholar] [CrossRef]
- Blieske, U.; Stollwerck, G. Glass and other encapsulation materials. Semicond. Semimet. 2013, 28, 199–258. [Google Scholar]
- Peike, C.; Hädrich, I.; Weiß, K.-A.; Dürr, I. Overview of PV module encapsulation materials. Photovolt. Int. 2013, 19, 85–92. [Google Scholar]
- De Oliveira, M.C.C.; Cardoso, A.S.A.D.; Viana, M.M.; Lins, V.F.C. The causes and effects of degradation of encapsulant ethylene vinyl acetate copolymer (EVA) in crystalline silicon photovoltaic modules: A review. Renew. Sustain. Energy Rev. 2018, 81, 2299–2317. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Zhang, J. UV aging behaviour of ethylene-vinyl acetate copolymers (EVA) with different vinyl acetate contents. Polym. Degrad. Stab. 2010, 95, 725–732. [Google Scholar] [CrossRef]
- López-Escalante, M.; Caballero, L.J.; Martín, F.; Gabás, M.; Cuevas, A.; Ramos-Barrado, J. Polyolefin as PID-resistant encapsulant material in 5PV6 modules. Sol. Energy Mater. Sol. Cells 2016, 144, 691–699. [Google Scholar] [CrossRef]
- Lin, B.; Zheng, C.; Zhu, Q.; Xie, F. A polyolefin encapsulant material designed for photovoltaic modules: From perspectives of peel strength and transmittance. J. Therm. Anal. Calorim. 2019, 140, 2259–2265. [Google Scholar] [CrossRef]
- Oreski, G.; Omazic, A.; Eder, G.C.; Voronko, Y.; Neumaier, L.; Mühleisen, W.; Hirschl, C.; Ujvari, G.; Ebner, R.; Edler, M. Properties and degradation behaviour of polyolefin encapsulants for photovoltaic modules. Prog. Photovolt. Res. Appl. 2020, 28, 1277–1288. [Google Scholar] [CrossRef]
- Adothu, B.; Bhatt, P.; Zele, S.; Oderkerk, J.; Costa, F.R.; Mallick, S. Investigation of newly developed thermoplastic polyolefin encapsulant principle properties for the c-Si PV module application. Mater. Chem. Phys. 2020, 243, 122660. [Google Scholar] [CrossRef]
- Barretta, C.; Oreski, G.; Feldbacher, S.; Resch-Fauster, K.; Pantani, R. Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests. Polymers 2021, 13, 271. [Google Scholar] [CrossRef]
- Kempe, M. Overview of Scientific Issues Involved in Selection of polymers for PV applications. In Proceedings of the 2011 37th IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 19–24 June 2011; pp. 85–90. [Google Scholar]
- Chapuis, V.; Pélisset, S.; Raeis-Barnéoud, M.; Li, H.-Y.; Ballif, C.; Perret-Aebi, L.-E. Compressive-shear adhesion characterization of polyvinyl-butyral and ethylene-vinyl acetate at different curing times before and after exposure to damp-heat conditions. Prog. Photovolt. Res. Appl. 2014, 22, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.T.; Kum, C.K.; Lee, H.S.; Kim, J.S.; Yoon, H.G.; Kim, W.N. Effects of crystallinity and crosslinking on the thermal and rheological properties of ethylene vinyl acetate copolymer. Polymer 2005, 46, 11844–11848. [Google Scholar] [CrossRef]
- Zhu, J.; Montiel-Chicharro, D.; Betts, T.R.; Gottschalg, R. Correlation of Degree of EVA Crosslinking with Formation and Discharge of Acetic Acid in PV Modules. In Proceedings of the 33rd European Photovoltaic Solar Energy Conference and Exhibition (PVSEC 2017), Amsterdam, The Netherlands, 25–29 September 2017; pp. 1795–1798. [Google Scholar]
- Omazic, A.; Oreski, G.; Halwachs, M.; Eder, G.C.; Hirschl, C.; Neumaier, L.; Pinter, G.; Erceg, M. Relation between degradation of polymeric components in crystalline silicon PV module and climatic conditions: A literature review. Sol. Energy Mater. Sol. Cells 2019, 192, 123–133. [Google Scholar] [CrossRef]
- Adothu, B.; Singh, A.K.; Kumar, S.; Zele, S.; Mallick, S. Effect of curing temperature on properties of ethylene vinyl-acetate used for crystalline silicon solar module encapsulation. In Proceedings of the 36th European Photovoltaic Solar Energy Conference and Exhibition (EU PVSEC 2019), Marseille, France, 9–13 September 2019; pp. 1204–1207. [Google Scholar]
- Molloy, B.M.; Johnson, K.-A.; Ross, R.J.; Parent, J.S. Functional group tolerance of AOTEMPO-mediated peroxide cure chemistry. Polymer 2016, 99, 598–604. [Google Scholar] [CrossRef]
- Twigg, C.; Ford, K.; Parent, J.S. Peroxide-initiated chemical modification of polyolefins: In search of a latent antioxidant. Polymer 2019, 176, 293–299. [Google Scholar] [CrossRef]
- Wallner, G.M.; Grabmayer, K.; Oreski, G. Physical characterization of EVA embedding materials for solar cells. In Proceedings of the 25th European Photovoltaic Solar Energy Conference and Exhibition/5th World Conference on Photovoltaic Energy Conversion, Valencia, Spain, 6–10 September 2010; pp. 4033–4035. [Google Scholar]
- Schulze, S.-H.; Ehrich, C.; Ebert, M.; Bagdahn, J. Mechanical Behavior and Lamination Issues of Solar Modules Containing Elastomeric and Amorphous Encapsulants. In Proceedings of the 25th European Photovoltaic Solar Energy Conference and Exhibition/5th World Conference on Photovoltaic Energy Conversion, Valencia, Spain, 6–10 September 2010; pp. 4064–4068. [Google Scholar]
- Stark, W.; Jaunich, M. Investigation of ethylene/vinyl acetate copolymer (EVA) by thermal analysis DSC and DMA. Polym. Test. 2011, 30, 236–242. [Google Scholar] [CrossRef]
- Stark, W.; Jaunich, M.; Bohmeyer, W.; Lange, K. Investigation of the crosslinking behaviour of ethylene vinyl acetate (EVA) for solar cell encapsulation by rheology and ultrasound. Polym. Test. 2012, 31, 904–908. [Google Scholar] [CrossRef]
- Hirschl, C.; Biebl-Rydlo, M.; Debiasio, M.; Mühleisen, W.; Neumaier, L.; Scherf, W.; Oreski, G.; Eder, G.; Chernev, B.; Schwab, W.; et al. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants—A comparative study. Sol. Energy Mater. Sol. Cells 2013, 116, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Schulze, S.H.; Apel, A.; Daßler, D.; Ehrich, C. Cure state assessment of EVA-copolymers for PV-applications comparing dynamic-mechanical, dielectric and calorimetric properties. Sol. Energy Mater. Sol. Cells 2015, 143, 411–417. [Google Scholar] [CrossRef]
- Hirschl, C.; Neumaier, L.; Puchberger, S.; Mühleisen, W.; Oreski, G.; Frank, M.K.R.; Tranitz, M.; Schoppa, M.; Wendt, M.; Bogdanski, N.; et al. Determination of the degree of ethylene vinyl acetate crosslinking via Soxhlet extraction: Gold standard or pitfall? Sol. Energy Mater. Sol. Cells 2015, 143, 494–502. [Google Scholar] [CrossRef]
- Jaunich, M.; Bohning, M.; Braun, U.; Teteris, G.; Stark, W. Investigation of the curing state of ethylene/vinyl acetate copolymer (EVA) for photovoltaic applications by gel content determination, rheology, DSC and FTIR. Polym. Test. 2016, 52, 133–140. [Google Scholar] [CrossRef]
- Schlothauer, J.C.; Grabmayer, K.; Wallner, G.M.; Röder, B. Correlation of spatially resolved photoluminescence and viscoelastic mechanical properties of encapsulating EVA in differently aged PV modules. Prog. Photovolt. Res. Appl. 2016, 24, 855–870. [Google Scholar] [CrossRef]
- Oreski, G.; Rauschenbach, A.; Hirschl, C.; Kraft, M.; Eder, G.C.; Pinter, G. Crosslinking and post-crosslinking of ethylene vinyl acetate in photovoltaic modules. J. Appl. Polym. Sci. 2017, 134, 101–110. [Google Scholar] [CrossRef]
- Hintersteiner, I.; Sternbauer, L.; Beißmann, S.; Buchberger, W.W.; Wallner, G.M. Determination of stabilisers in polymeric materials used as encapsulants in photovoltaic modules. Polym. Test. 2014, 33, 172–178. [Google Scholar] [CrossRef]
- Beißmann, S.; Reisinger, M.; Grabmayer, K.; Wallner, G.M.; Nitsche, D.; Buchberger, W. Analytical evaluation of the performance of stabilization systems for polyolefinic materials. Part I: Interactions between hindered amine light stabilizers and phe-nolic antioxidants. Polym. Degrad. Stab. 2014, 110, 498–508. [Google Scholar] [CrossRef]
- Beißmann, S.; Grabmayer, K.; Wallner, G.; Nitsche, D.; Buchberger, W. Analytical evaluation of the performance of stabilization systems for polyolefinic materials. Part II: Interactions between hindered amine light stabilizers and thiosynergists. Polym. Degrad. Stab. 2014, 110, 509–517. [Google Scholar] [CrossRef]
- Wallner, G.M.; Oreski, G. Structure-infrared optical property-correlations of C,O,H-polymers for transparent insulation and greenhouse applications. Monatsh. Chem. 2006, 137, 899–910. [Google Scholar] [CrossRef]
- Oreski, G.; Wallner, G.M. Structure–infrared optical property-correlations of polar ethylene copolymer films for solar applications. Sol. Energy Mater. Sol. Cells 2006, 90, 1208–1219. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 7 March 2022).
- Jentsch, A.; Eichhorn, K.-J.; Voit, B. Influence of typical stabilizers on the aging behavior of EVA foils for photovoltaic applications during artificial UV-weathering. Polym. Test. 2015, 44, 242–247. [Google Scholar] [CrossRef]
- Pacansky, J.; England, C.; Waltman, R.J. Complex Refractive Indexes for Polymers over the Infrared Spectral Region: Specular Reflection IR Spectra of Polymers. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 901–933. [Google Scholar] [CrossRef]
- Vacque, V.; Sombret, B.; Huvenne, J.P.; Legrand, P.; Suc, S. Characterisation of the O-O peroxide bond by vibrational spectroscopy. Spectrochim. Acta Part A Mol. Spectrosc. 1997, 53, 55–66. [Google Scholar] [CrossRef]
- Fujii, A.; Iwasaki, A.; Yoshida, K.; Ebata, T.; Mikami, N. Infrared spectroscopy of (phenol)n+ (n = 2-4) and (phenol-benzene) + cluster ions. J. Phys. Chem. A 1997, 101, 1798–1803. [Google Scholar] [CrossRef]
- Duh, Y.S.; Kao, C.S.; Hwang, H.H.; Lee, W.W.L. Thermal decomposition kinetics of cumene hydroperoxide. Process. Saf. Environ. Prot. 1998, 76, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Zuga, N. Crosslinking and grafting ethylene vinyl acetate copolymer with accelerated electrons in the presence of polyfunctional monomers. Polym. Bull. 2012, 68, 263–285. [Google Scholar] [CrossRef]
- NIST. Available online: https://webbook.nist.gov/cgi/cbook.cgi?Spec=C3006-82-4&Index=0&Type=IR&Large=on (accessed on 29 March 2022).
- Sipaut, C.S.; Dayou, J. In situ FTIR analysis in determining possible chemical reactions for peroxide crosslinked LDPE in the presence of triallylcyanurate. Funct. Compos. Struct. 2019, 1, 025003. [Google Scholar] [CrossRef] [Green Version]
- Smedberg, A.; Hjertberg, T.; Gustafsson, B. Crosslinking reactions in an unsaturated low density polyethylene. Polymer 1997, 38, 4127–4138. [Google Scholar] [CrossRef]





| Encapsulant | Ton, °C | Tgel, °C | Toff, °C | Cure Time, min |
|---|---|---|---|---|
| EVA | 125 | 130 | 164 | 13 |
| POE | 135 | 140 | 162 | 9 |
| Temperature, °C | Gelation Time tgel, s | Progress of Crosslinking X at tgel, % | Cure Time for X = 95%, s | |||
|---|---|---|---|---|---|---|
| EVA | POE | EVA | POE | EVA | POE | |
| 125 | 405 | 1559 | 6 | 36 | 5370 | 6320 |
| 130 | 224 | 945 | 6 | 35 | 2995 | 4120 |
| 135 | 136 | 582 | 5 | 34 | 2275 | 2975 |
| 140 | 71 | 297 | 5 | 29 | 1325 | 2040 |
| 145 | 39 | 177 | 5 | 26 | 900 | 1240 |
| 150 | 28 | 111 | 7 | 25 | 535 | 875 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallner, G.M.; Adothu, B.; Pugstaller, R.; Costa, F.R.; Mallick, S. Comparison of Crosslinking Kinetics of UV-Transparent Ethylene-Vinyl Acetate Copolymer and Polyolefin Elastomer Encapsulants. Polymers 2022, 14, 1441. https://doi.org/10.3390/polym14071441
Wallner GM, Adothu B, Pugstaller R, Costa FR, Mallick S. Comparison of Crosslinking Kinetics of UV-Transparent Ethylene-Vinyl Acetate Copolymer and Polyolefin Elastomer Encapsulants. Polymers. 2022; 14(7):1441. https://doi.org/10.3390/polym14071441
Chicago/Turabian StyleWallner, Gernot M., Baloji Adothu, Robert Pugstaller, Francis R. Costa, and Sudhanshu Mallick. 2022. "Comparison of Crosslinking Kinetics of UV-Transparent Ethylene-Vinyl Acetate Copolymer and Polyolefin Elastomer Encapsulants" Polymers 14, no. 7: 1441. https://doi.org/10.3390/polym14071441
APA StyleWallner, G. M., Adothu, B., Pugstaller, R., Costa, F. R., & Mallick, S. (2022). Comparison of Crosslinking Kinetics of UV-Transparent Ethylene-Vinyl Acetate Copolymer and Polyolefin Elastomer Encapsulants. Polymers, 14(7), 1441. https://doi.org/10.3390/polym14071441