Abstract
The solar cell has been considered one of the safest modes for electricity generation. In a dye-sensitized solar cell, a commonly used iodide/triiodide redox mediator inhibits back-electron transfer reactions, regenerates dyes, and reduces triiodide into iodide. The use of iodide/triiodide redox, however, imposes several problems and hence needs to be replaced by alternative redox. This paper reports the first Co2+/Co3+ solid redox mediators, prepared using [(1−x)succinonitrile: xPEO] as a matrix and LiTFSI, Co(bpy)3(TFSI)2, and Co(bpy)3(TFSI)3 as sources of ions. The electrolytes are referred to as SN_E (x = 0), Blend 1_E (x = 0.5 with the ethereal oxygen of the PEO-to-lithium ion molar ratio (EO/Li+) of 113), Blend 2_E (x = 0.5; EO/Li+ = 226), and PEO_E (x = 1; EO/Li+ = 226), which achieved electrical conductivity of 2.1 × 10−3, 4.3 × 10−4, 7.2 × 10−4, and 9.7 × 10−7 S cm−1, respectively at 25 °C. Only the blend-based polymer electrolytes exhibited the Vogel-Tamman-Fulcher-type behavior (vitreous nature) with a required low pseudo-activation energy (0.05 eV), thermal stability up to 125 °C, and transparency in UV-A, visible, and near-infrared regions. FT-IR spectroscopy demonstrated the interaction between salt and matrix in the following order: SN_E < Blend 2_E < Blend 1_E << PEO_E. The results were compared with those of acetonitrile-based liquid electrolyte, ACN_E.
1. Introduction
Efficient utilization of fossil-fuel-based energy sources is one of the key factors of human social and economic development. However, this has led to an increase in the levels of greenhouse gases and pollution. The nuclear energy source is also not safe because of the hazards associated with it. Owing to the abundance of sunlight, the photovoltaic cell has emerged as an energy source, especially in regions near to and between the Tropics of Cancer and Capricorn (sunlight irradiance ~2 MWh m−2) [1].
One of the third generation photovoltaic cells, the dye-sensitized solar cell (DSSC), is highly attractive due to several advantages of DSSCs over other solar cells [2,3,4,5,6,7]. Some of the advantages are the simple cell structure, they are flexible and lightweight, the absence of toxic and less-available elements; energy payback time is less than a year, their all-direction-capturing of incident light; and their performance under real indoor and outdoor conditions. The first DSSC was reported by O’Regan and Gratzel in 1991 [8] with a power conversion efficiency (η) of 7.1% at 75 mW cm−2. They used a liquid electrolyte: tetrapropylammonium iodide, KI, and I2 in ethylene carbonate and acetonitrile (ACN). This electrolyte provided fast transport of the redox couple, (i) to regenerate dyes via the oxidation of I− into at the mesoporous and nanostructured TiO2 working electrode, (ii) to reduce into I− at the platinum counter electrode, and (iii) to inhibit back-electron transfer reactions. Since then, several redox mediators in a form of liquid, gel, or solid have been synthesized [9,10,11,12,13,14,15,16,17,18,19,20,21]. The DSSC has also been commercialized with the highest η-value of 11.9% at 100 mW cm−2 (1 sun), utilizing a liquid electrolyte: dimethyl-propyl imidazolium iodide (an ionic liquid), I2, LiI, and 4-tert-butylpyridine (TBP) in ACN [7,22]. The ionic liquid helped to reduce the organic solvent-related problems, thereby improving the stability of the device, though this required a hermetic sealing.
Owing to their corrosive nature, dissolving of many of the commonly used sealants and metal interconnects, sublimation, and partial absorption of visible light around 430 nm of the iodine-based redox-couple, the researchers started to think of replacing this by one using a molecular species of similar type such as Br−/Br2 redox (e.g., Br2 and LiBr in ACN), one using metal complex-based redox such as Co2+/Co3+, and one using organic radicals, such as TEMPO [2,6,9,10,23,24,25,26,27,28,29]. The Co2+/Co3+ redox electrolytes, in general, with ACN as an organic solvent showed η of more than 10% at 1 sun for several dyes. For example, 11.9% for YD2-o-C8 dye [23], 12.3% for YD2-o-C8+Y123 dyes [23], 10.3% for JF419 dye [30], 13% for SM315 dye [24], 10.6% for Y123 dye [31], 11.4% for YD2-o-C8 dye [32], 10.2% for C101 dye [33], 10.5% for LEG4+D35+Dyenamo Blue dyes [34], 10.42% for FW1+WS5 dyes [35], 12.8% for SM342+Y123 dyes [36], 11% for AQ310 dye [37], 13.6% for ZL003 dye [38], 10.3% for H2 dye [39], and 11.2% for YD2-o-C8 dye [40].
The researchers used ionic liquid to suppress the organic solvent-related problems. Xu et al. [41] synthesized a compound [Co{3,3′-(2,2′-bipyridine-4,4′-diyl-bis(methylene)) bis(1-methyl-1H-imidazol-3-ium) hexafluorophosphate}3]2+/3+ and mixed with 1-propyl-3-methylimidazolium iodine, 1-ethyl-3-methyl imidazolium thiocyanate, guanidinium thiocyanate (GuSCN), and TBP. They reported η~7.37% at 1 sun for N719 dye. Kakiage et al. reported η~12.5% at 1 sun for ADEKA-1 dye [42] and η~14.3% at 1 sun for ADEKA-1+LEG4 dyes [43], utilizing an electrolyte solution: [Co2+(phen)3](PF6)2, [Co3+(phen)3](PF6)3, LiClO4, NaClO4, tetrabutyl ammonium hexafluorophosphate, tetrabutylphosphonium hexafluorophosphate, 1-hexyl-3-methylimidazolium hexa fluorophosphate, TBP, 4-trimethylsilylpyridine, 4-methylpyridine, 4-cyano-4′-propyl biphenyl, 4-cyano-4′-pentylbiphenyl, 4-cyano-4′-octylbiphenyl in ACN. Wang et al. [44] reported η~8.1% at 1 sun for D205 dye with a redox mediator, bis(3-butyl-1-methylimidazolium) tetraisothiocyanato cobalt, 1-propyl-3-methyl-imidazolium iodine, nitrosyl tetrafluoroborate, LiClO4, and TBP in methoxy propionitrile.
The researchers synthesized the Co2+/Co3+ redox mediators in a gel (quasi-solid) form as well. The gel was prepared by incorporating a large amount of organic solvent mixed with a redox couple into an inorganic or organic frame. So far nanoparticles of SiO2 (η~2.58% at 1 sun for D35 dye) [45], TiO2 (η~5.1% at 1 sun for N719 dye) [46], and TiC (η~6.29% at 1 sun for N719 dye) [46] were used to form an inorganic frame. An organic frame was prepared using poly(ethylene glycol) with gelatin (η~4.1% at 1 sun for MK2 dye) [47], bisphenol A ethoxylate dimethacrylate with poly(ethylene glycol) methyl ether methacrylate (η~6.4% at 1 sun for LEG4 dye) [48], poly(ethylene glycol)/poly(methyl methacrylate) (η~1.9% at 1 sun for N719 dye) [49], poly(vinylidene fluoride-co-hexafluoropropylene) (η~8.7% at 1 sun for MK2 dye [50]; η~4.34% at 1 sun for Z907 dye [51], η~7.1% at 1 sun for MK2 dye [52]), poly(ethylene oxide-co-2-(2-methoxyethoxy) ethyl glycidyl ether-co-allyl glycidyl ether) (η~3.59% at 1 sun for MK2 dye and η~1.74% at 1 sun for Z907 dye) [53], poly(ethylene oxide-co-2-(2-methoxyethoxy) ethyl glycidyl ether (η < 0.1% at 1 sun for L0 dye) [54], poly(ethylene oxide) (η~21.1% at 200 lx for Y123 dye) [55], poly(ethylene oxide)-poly(methyl methacrylate) blend (η~18.7% at 200 lx for Y123 dye) [55], hydroxypropyl cellulose (η~9.1% at 0.7 sun for N719 dye) [56], and hydroxyethyl cellulose (η~4.5% at 1 sun for N3 dye) [57].
Unfortunately, the liquid nature of electrolytes creates internal pressure in the DSSCs at the ambient temperature range (50–80 °C), resulting in a leakage of solvent, thereby requiring hermetic sealing [9,10,11,12,13,14,15,16,17]. This also makes the manufacturing of DSSCs non-scale-up. A gel electrolyte exhibits problems similar to those of a liquid electrolyte, hence, it needs to be replaced by a solid one to sustain it in the hot weather of Gulf countries. However, until now no Co2+/Co3+ redox mediator in solid form has been reported.
Earlier, a high-molecular-weight poly(ethylene oxide) (PEO) was used as a polymer matrix of the I−/I3− redox-based solid polymer electrolytes, PEO-PQ-MI-I2, where M represents an alkali metal cation [9,10,11,12,13,14,15,16,17]. The PEO offered its self-standing film-forming, thermal stability up to 200 °C, it was eco- and bio-benign, was of relatively low material cost, the dissociation/ complexation of salt due to its moderate dielectric constant (ε25°C)-value (5–8), Gutmann donor number of 22, had just the right spacing between coordinating ethereal oxygens for maximum solvation of the Li+ ions, and the segmental motion of polymeric chains for the ion transport through ethereal oxygen [58,59,60,61]. Ionic liquids, low molecular weight polymers, and copolymerization were used as plasticizers (PQs) to reduce PEO crystallinity (χ), thereby increasing the σ25 °C- and η-values.
Gupta et al. [62,63,64,65,66,67,68] showed that the equal weight proportion of succinonitrile (SN) can be used as a plasticizer without hampering the thin-film forming property of the PEO. This blending resulted in several beneficial properties, such as a higher σ25°C-value, ~10−8 S cm−1 than the PEO (σ25°C ~10−10 S cm−1), a lower χ-value, ~25% than the PEO (~82%), and higher thermal stability up to ~125 °C than the SN (~75 °C) [62]. The PEO-SN-MI-I2 solid polymer electrolytes achieved σ25°C-value 3–7 × 10−4 S cm−1, transparency more than 95% in visible and IR regions, χ~0%, thermal stability up to ~125 °C, and η-value between 2 and 3.7% at 1 sun with Ru-based N719 dye. The solid solvent/ plasticizing property of the plastic crystal, SN is due to its low molecular weight, high molecular diffusivity at the plastic phase between −35 °C (crystal-to-plastic-crystal phase transition temperature, Tpc) and 58 °C (melting temperature, Tm), low Tm-value, high ε-value ~55 at 25 °C and 62.6 at 58 °C, nitrile group for ion transport, and waxy nature [69,70,71,72,73].
In this work, we have extended the concept of blending for achieving the high electrical conductivity of the Co2+/Co3+ solid redox mediators. We reported electrical, structural, optical, and thermal properties of new [(1−x)SN: xPEO]-LiTFSI-Co(bpy)3(TFSI)2-Co(bpy)3(TFSI)3 solid redox mediators. The composition, x is 0, 0.5, and 1 in weight fraction. Other notations, bpy and TFSI stand for tris-(2,2′-bipyridine) and bis(trifluoromethyl) sulfonylimide, respectively. These solid redox mediators are based on a liquid electrolyte (0.1-M LiTFSI, 0.25-M Co(bpy)3(TFSI)2, and 0.06-M Co(bpy)3(TFSI)3 in acetonitrile), which resulted in η of 13% with SM315 dye [24]. This liquid electrolyte is hereafter referred to as ACN_E. We just replaced acetonitrile with succinonitrile for synthesizing SN_E (x = 0). Succinonitrile was then replaced by PEO for synthesizing PEO_E (x = 1 in weight fraction). This had the ethereal oxygen of the PEO-to-lithium ion mole ratio, abbreviated as EO/Li+ of 226. We also used a blend containing SN and PEO in an equal weight fraction for retaining the beneficial properties of SN and PEO, as discussed earlier. The value of EO/Li+ was kept at either 113 (Blend 1_E) or 226 (Blend 2_E) for understanding its effect on the electrical transport properties [65]. Figure 1a shows the chemical structure of the ingredients. The solid nature of SN_E, PEO_E, Blend 1_E, and Blend 2_E is shown in Figure 1b. Ionic salts with TFSI− anion were used because TFSI− offers a low value of lattice energy with delocalized electrons, making the salt highly dissociable in the solvent with a less anionic contribution to the total conductivity [58,59,60,61]. The lithium salt is thermally and electrochemically stable as well [58,59,60,61,74]. Owing to the small size, the Li+ ions get intercalated on the TiO2 nanoparticles of the DSSC, leading to faster electron injection from the excited dye molecules to the conduction band of the TiO2, thereby the higher photocurrent [67,75]. Contrary to this, the cobalt ions adsorb on the skirt of the TiO2 nanoparticles, resulting in a negative shift of the Fermi level of the TiO2 nanoparticles, thereby resulting in the higher open-circuit voltage. It is also known that an ion with a large size acts as a plasticizer in a polymer electrolyte, resulting in higher electrical conductivity [65,67,75].
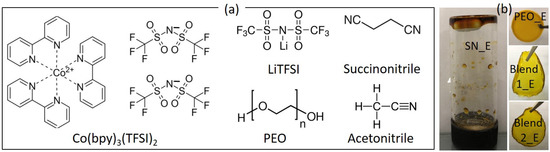
Figure 1.
(a) Chemical structure of ingredients. (b) Optical image of solid redox mediators.
We determined σ-value at different temperatures for knowing the nature of the electrolyte and determining the activation energy. The electrical transport properties were elucidated by X-ray diffractometry (XRD), Fourier-transform infrared (FT-IR) spectroscopy, UV-visible spectroscopy, polarized optical microscopy (POM), and differential scanning calorimetry (DSC). We used thermogravimetric analysis (TGA) for the thermal stability study.
2. Materials and Methods
2.1. Materials
Succinonitrile, PEO (1-M g mol−1), and LiTFSI were procured from Sigma Aldrich, Inc., St. Louis, MO, USA. Cobalt salts, Co(bpy)3(TFSI)2 (DN-C13) and Co(bpy)3(TFSI)3 (DN-C14) were procured from Dyenamo AB, Stockholm, Sweden. These chemicals were used without purification.
2.2. Synthesis
Table 1 shows the composition of ACN_E, SN_E, PEO_E, Blend 1_E, and Blend 2_E. As per the procedure of Mathew et al. [24], ACN_E was synthesized using LiTFSI (0.1 M), DN-C13 (0.25 M), and DN-C14 (0.06 M) in acetonitrile under stirring at 65 °C for 24 h. The SN_E was prepared identically by dissolving the salts in succinonitrile. The PEO_E and Blends 1_E & 2_E were prepared using the solution cast method. The ingredients were dissolved in 20-mL acetonitrile by vigorous stirring at 65 °C for 48 h. This resulted in a homogeneous polymeric solution which was poured on a Teflon Petri dish followed by drying at room temperature in a nitrogen gas atmosphere for two weeks and in a vacuum desiccator for a day. This produced a self-standing film of the solid polymer electrolyte.

Table 1.
Composition of liquid and solid redox mediators.
2.3. Characterizations
A specific sample holder [72] was used to measure the electrical conductivity of the ACN_E and SN_E electrolytes. The liquid electrolyte was poured on a space (area, A ~0.16 cm−2 and thickness, l~0.05 cm) created by a Teflon spacer between platinum plates (blocking electrode). For determining the electrical conductivity of the PEO_E and Blends 1_E & 2_E solid polymer electrolytes, another sample holder [64], having stainless steel plate as a blocking electrode, was used. The sandwiched electrolyte was subjected to 20 mV ac voltage and monitoring of real and imaginary impedances from 100 kHz to 1 Hz by a Palmsens4 impedance analyzer (PalmSens BV, Houten, the Netherlands). This resulted in a Nyquist curve, thereby a bulk resistance (Rb) and then the electrical conductivity (σ) using the formula, σ = l/(A Rb) [76].
For the XRD pattern of the solid electrolyte film, a D2 Phaser Bruker x-ray diffractometer (Karlsruhe, Germany) was used. The pattern was collected using the CuKα radiation (1.54184 Å) in a range of 10–40° with a step of 0.06°. The FT-IR spectrum of the electrolyte film on a potassium bromide pellet was recorded in a range of 400–4000 cm−1 and a resolution of 1 cm−1 using a Spectrum 100 Perkin Elmer FT-IR spectrometer (Waltham, MA, USA). The spectrum was analyzed using EZ-OMNIC software, ver. 7.2a (Thermo Scientific Inc., Waltham, MA, USA).
Transmittance spectrum of the electrolyte film (thickness 2–3 µm) was collected using an Agilent UV-visible spectrometer (model 8453, Santa Clara, CA, USA). The POM image with a magnification of 100× for the polymer electrolyte film (thickness 2–3 µm) was obtained using a computer interfaced ZZCAT polarized optical microscope (Zhuzhou, Hunan, China).
The DSC curve of the electrolyte was measured using a DSC-60A differential scanning calorimeter (Shimadzu, Kyoto, Japan) under the purging of nitrogen gas with 10 °C min−1 heating rate and in the range of −50 to 90 °C. For the TGA curve, the weight loss of the electrolyte was monitored using a Shimadzu DTG-60H unit in the temperature range of room temperature to 550 °C with a heating rate of 10 °C min−1 under the purging of nitrogen gas.
3. Results
3.1. Electrical Transport Properties
Figure 2 shows the Nyquist curves for the liquid (ACN_E) and solid (SN_E, PEO_E, Blend 1_E, and Blend 2_E) redox mediators at 25 °C. These curves portrayed (i) the blocking electrode effect in the low-frequency domain, and (ii) the ionic diffusion effect in the high-frequency domain [76]. Being a liquid electrolyte, ACN_E depicted a perfect semi-circle in the high-frequency domain. SN_E and PEO_E also had a semi-circle; however, the semi-circle was slightly and largely depressed for the former and latter, respectively. This is most probably due to the existence of the plastic crystalline phase of succinonitrile and the semi-crystalline phase of PEO, respectively [72,76]. Contrary to this, Blends 1_E and 2_E had no semi-circle, indicating the existence of amorphous domains, the semi-random motion of short polymer chains, and the segmental motion, demonstrating the plasticizing effect of the succinonitrile [58,59,60,61,64,65,77,78]. The bulk resistance is marked by an arrow in the Nyquist curve and is used to calculate the σ25°C-value of the electrolyte.
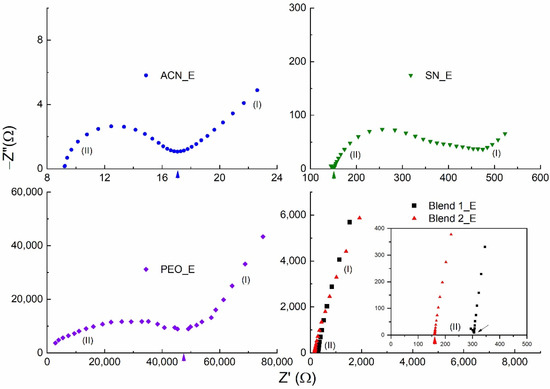
Figure 2.
Nyquist curves of the solid (SN_E, PEO_E, Blend 1_E, and Blend 2_E) and liquid (ACN_E) redox mediators at 25 °C. (I) and (II) represent low- and high-frequency domains, respectively. The inset shows the high-frequency domain of the Blends 1_E and 2_E.
Figure 3a shows electrical conductivity σ25°C) of solid electrolytes, SN_E, Blend 1_E, Blend 2_E, and PEO_E along with that of the liquid electrolyte, ACN_E. The ACN_E exhibited σ25°C~1.7 × 10−2 S cm−1, which is similar to those reported earlier for liquid electrolytes [60]. The high electrical conductivity is due to ε25°C of 36.6, donor number of 14.1 kcal mol−1, molar enthalpy of 40.6 kJ mol−1, and acceptor number of 18.9, helping to dissolve the salt completely and solvate the ions easily [79,80]. The replacement of ACN by SN resulted in SN_E with the σ25°C-value less than an order of magnitude to ~2.1 × 10−3 S cm−1. This conductivity value is similar to those obtained earlier for the SN-LiTFSI [69] and SN-LiI-I2 [72] electrolytes. As discussed earlier [69,72], this is due to the solid solvent property of the succinonitrile. The replacement of SN by PEO resulted in PEO_E with the σ25°C-value of ~9.7 × 10−7 S cm−1, which is 3-orders of magnitude less. This is legitimate too. The pure PEO-based solid polymer electrolytes are known to have high PEO crystallinity, hindering ion transport [58,59,60,61,64,65]. The blend-based solid polymer electrolytes, however, showed σ25°C-value less than that of SN_E and higher than that of PEO_E. Blend 1_E and Blend 2_E exhibited σ25°C of ~4.3 × 10−4 and ~7.2 × 10−4 S cm−1, respectively. As observed earlier [62,63,64,65,66,67,68], this is due to the plasticizing property of the succinonitrile. Also, a competition between the nitrile group of succinonitrile and the ethereal oxygen of PEO to bind metal ions leads to more free ions for transport [64,65]. Besides, the availability of a huge number of large-sized TFSI− ions is helpful to produce more amorphous regions in the Blends 1_E and 2_E for easy ion transport [74]. One can expect a similar scenario for Co2+/Co3+ ions too [65,75]. It is also notable that Blend 2_E had higher electrical conductivity than Blend 1_E. This is due to more amorphous regions for ion transport in the Blend 2_E as demonstrated by the FT-IR spectroscopy, UV-visible spectroscopy, and DSC studies, which will be discussed later.
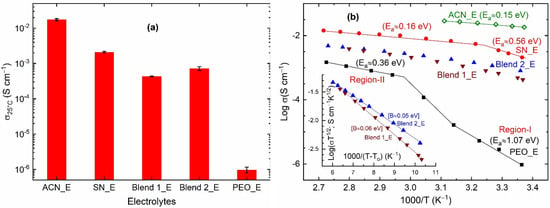
Figure 3.
(a) Electrical conductivity (σ25°C) and (b) log σ vs. T−1 plots of the solid redox mediators, SN_E, PEO_E, Blend 1_E, and Blend 2_E. Inset in (b) is VTF plots of Blends 1_E and 2_E. ACN_E, liquid electrolyte.
Figure 3b shows log σ vs. T−1 plots of the solid (SN_E, Blend 1_E, Blend 2_E, and PEO_E) and liquid (ACN_E) redox mediators. The ACN_E, SN_E, and PEO_E portrayed a linear curve, revealing the thermally activated Arrhenius-type behavior of molecules/ polymeric chains. Blends 1_E and 2_E depicted a slightly downward curve, indicating the existence of an amorphous phase, which follows the Vogel-Tamman-Fulcher (VTF)-type behavior. We have observed these trends for several redox mediators [64,65,68,72,73]. The Arrhenius behavior is expressed by the equation, σ = σo exp[−Ea/kBT], where σo is the pre-exponential factor, Ea is the activation energy, and kB is the Boltzmann constant. While the VTF behavior is represented by an expression, σ = AT−1/2 exp[−B/kB(T − To)], where A is the pre-exponential factor, B is the pseudo-activation energy, and To is the temperature at which the free volume vanishes. The Ea-value calculated from the slope of the Arrhenius plot is as follows: 0.56 eV (Region-I) and 0.16 eV (Region-II) for SN_E; and 1.07 eV (Region-I) and 0.36 eV (Region-II) for PEO_E. Region-I represents the solid-state region for SN_E and PEO_E, while Region-II corresponds to the liquid state for SN_E and the amorphous phase for PEO_E. The activation energy for SN_E in Region-II is similar to that observed (0.15 eV) for the liquid electrolyte, ACN_E. The pseudo-activation energy (B) calculated from the slope of the VTF plot is as follows: 0.06 eV and 0.05 eV for Blends 1_E and 2_E, respectively. The low activation energy values for the Blends 1_E and 2_E indicate easy ion transport, which is required for the DSSC application.
3.2. Structural Properties
Figure 4 shows XRD patterns of the solid redox mediators, SN_E, PEO_E, Blend 1_E, and Blend 2_E. The SN_E and PEO_E exhibited characteristic reflection peaks of succinonitrile and poly(ethylene oxide), respectively, though their peaks are broader and weaker than those of pure matrices, succinonitrile, and PEO. These indicate molecular disorder for SN_E and an increase in amorphicity for PEO_E [64,65,72], resulting in significantly enhanced electrical conductivity as compared to the pure matrices. The available cations and anions, having a large size, also acted as plasticizers and contributed to increasing the amorphicity [67,72,73,74,75]. Also, these electrolytes did not show any peak corresponding to cobalt and lithium salts, indicating complete salt dissociation/ complexation. The Blends 1_E and 2_E portrayed the absence of reflection peaks of ingredients, revealing the arrest of the glassy phase. As mentioned earlier, succinonitrile is a very good plasticizer to decrease the crystallinity of PEO [63,64,65]. This is also accompanied by the PEO-SN blend matrix-metal ions interaction, where SN molecules are more active [68,77,78]. These results are also supported by the findings of the FT-IR spectroscopy, which are discussed below.
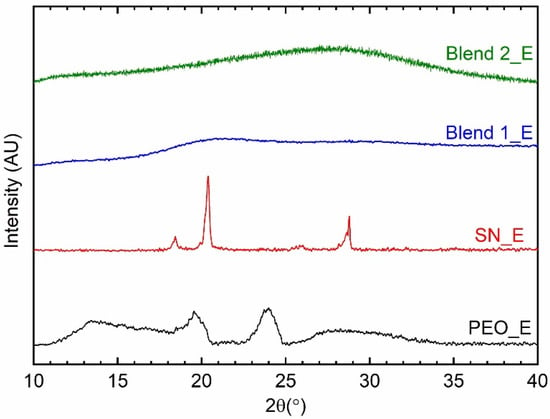
Figure 4.
XRD patterns of the solid redox mediators, SN_E, PEO_E, Blend 1_E, and Blend 2_E.
Figure 5 shows FT-IR spectra of the solid redox mediators, SN_E, PEO_E, Blend 1_E, and Blend 2_E. This figure also shows the spectra of liquid electrolyte, ACN_E and Co(bpy)3(TFSI)2 salt for comparison. The spectrum of the Co(bpy)3(TFSI)3 salt (data not shown) was similar to that of the Co(bpy)3(TFSI)2 salt. The observed vibrational frequencies of liquid and solid redox mediators along with those of constituents, ACN [81], SN [82], PEO [83], PEO-SN blend [62,64,65], LiTFSI [74,84,85], and Co(bpy)3(TFSI)2 [84,85,86], are listed in Table 2. This also shows the corresponding assignments. It is worth mentioning that the PEO-SN blending occurs via the interaction of the ethereal oxygen with the nitrogen of SN [62,64]. Wen et al. [84] asserted that the vibrational peaks at 1057, 1133, 1196, and 1351 cm−1 correspond to free and unpaired anions that are strongly solvated. Rey et al. [85] showed that the vibrational peaks at 1229 and 1331 cm−1 correspond to the ion-pairing peaks, though these peaks present free ions too if their position did not get changed with increasing salt concentration. The formation of an ion pair reduces the number of free ions. However, being uncharged and having nearly the same size as the cation, it has somewhat higher mobility in the polar polymer/solvent and lower solvent-salt interaction for increased amorphicity [84]. These have been discussed below.
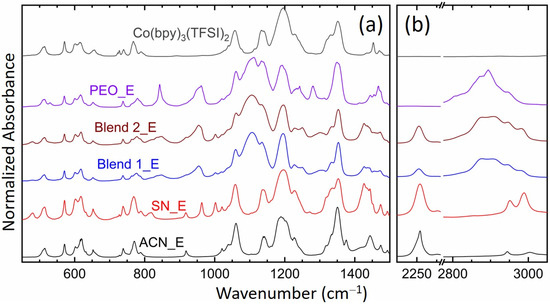
Figure 5.
FT-IR spectra of the solid (SN_E, PEO_E, Blend 1_E, and Blend 2_E) and liquid (ACN_E) redox mediators. The spectrum of the Co(II) salt is also included for direct comparison. (a) Fingerprint region. (b) νC≡N and νCH regions.

Table 2.
Observed vibrational frequencies (in cm−1) of the redox mediators, ACN_E, SN_E, PEO_E, and Blends 1_E & 2_E along with those of solvent/matrices and ionic salts.
The solute-solvent interaction in ACN_E is quite low as indicated by the comparatively unaltered position of several modes of ACN and ionic salts. We observed a change only at 739 cm−1 (νa,C-CN) and 2255 cm−1 (νs,C≡N) of the ACN_E relative to those of the ACN because of nitrile-metal ions coordination [87]. The ion-pairing peaks were present, however as weak shoulders only. This indicates the availability of a huge number of free ions for migration along with a negligible level of ion-pairing, resulting in a high value of σ25°C for the ACN_E. In this electrolyte, the metal cations migrate through the nitrile group of the ACN [69]. The SN_E showed a scenario similar to ACN_E except at 769 (δCH2, ring), 1228 (tCH2, i.p.ring), 1443 (δa,CH2, ring), and 1474 cm−1 (δa,CH2, ring), revealing the SN-ring interaction. Similar to ACN_E, SN_E portrayed a weak ion-pairing peak at 1228 cm−1. These indicate the availability of a large number of free ions for migration, however, with a higher level of ion-pairing, resulting in a lower σ25°C-value for SN_E than ACN_E. PEO_E experienced the PEO-salt interaction via shifts to 780 (767 cm−1, ring), 1113 (1109 cm−1, PEO; νa,COC), 1134 (1149 cm−1, PEO; νCC, νa,COC), and 1349 (1342 cm−1, PEO; ωa,CH2, νa,SO2). These were accompanied by a shift in νCH2 modes of the PEO from 2861 to 2872 cm−1 and from 2889 to 2894 cm−1, indicating a decrease in C-H bond length for ion solvation, and thereby increasing the amorphicity of the electrolyte [64,65,74]. However, the increase in amorphicity was inadequate to sufficiently increase the electrical conductivity as suggested by the absence of the ion-paring peaks. The Blend 1_E and Blend 2_E observed no significant change in the position of several modes, except at 777 cm−1 (δCH2, SN) and 1437 cm−1 (δCH2, ring) corresponding to the SN-bpy ligand interaction, and at 2253 cm−1 (νs,C≡N, SN) for the interaction of the nitrile-metal ions [87]. The blend-based electrolytes portrayed ion-pairing peaks at 1227 and 1334 cm−1 as well as a blue shift in the stretching C-H modes of PEO in the region, 2800–3050 cm−1, indicating a conformational change to form the amorphous phase. The blue shift was higher for the Blend 2_E. These findings suggest that succinonitrile and cobalt salts are crucial for improving the amorphicity of the blend-based electrolyte, thereby, enhancing the electrical conductivity.
Figure 6 shows relative intensities, ΔI1 (=I(1105 cm−1; νs,COC; PEO)/I(1196 cm−1; νa,CF3; TFSI−) and ΔI2 (=I(2255 cm−1; νs,C≡N; acetonitrile or succinonitrile)/I(1196 cm−1; νa,CF3; TFSI−) of the redox mediators. Intensity had the following order: ACN_E = SN_E (=0) << Blend 1_E = Blend 2_E = PEO_E (=1) at 1105 cm−1 and ACN_E < SN_E ≈ Blend 1_E > Blend 2_E < PEO_E at 1196 cm−1, resulting in ΔI1 as Blend 2_E > PEO_E > Blend 1_E >> SN_E = ACN_E (=0). This shows the effect of the PEO-salt interaction on the conformational change of PEO to form the amorphous phase, which is the highest for the Blend 2_E. A similar assertion can be made using ΔI2, which had the following order: SN_E > ACN_E > Blend 2_E > Blend 1_E >> PEO_E (=0). This demonstrates the effect of nitrile-salt interaction on the conformational change of ACN/SN to form the disordered/ amorphous structure, which is higher for SN_E than ACN_E, and more for Blend 2_E than Blend 1_E. These results are also supported by the UV-visible spectroscopy, POM, and DSC studies and are discussed in the later sections.
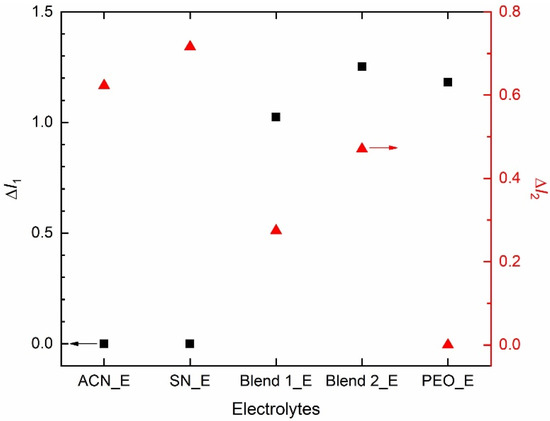
Figure 6.
Relative intensities, ΔI1 and ΔI2 of the electrolytes. For definition, please see the text.
3.3. Optical Properties
Figure 7a shows the transmittance spectra of the solid redox mediators, SN_E, PEO_E, Blend 1_E, and Blend 2_E as well as liquid electrolytes, ACN_E and SN_E(L). In the UV-A region at 350 nm, the transmittance order was as follows: Blend 2_E (88.1%) > Blend 1_E (81.4%) >> PEO_E (49.6%) >> ACN-E (13.9%) >> SN_E(L) = SN_E(S) (=0%). In the visible region at 555 nm, transparency had the following order: ACN-E (99.9%) ≈ Blend 2_E (99.8%) > SN_E(L) (97%) > Blend 1_E (92.9%) > PEO_E (78.9%) >> SN_E(S) 21.6%. The Blend 2_E portrayed the transparency of all the UV-A, visible, and near-infrared regions; while ACN_E and SN_E(L) showed the transparency in the visible and near-infrared regions only. This shows the supremacy of Blend 2_E over ACN_E and SN_E(L) in terms of transparency. The high value of the transmittance for Blends 1_E and 2_E suggests a low level of the PEO crystallinity [62,65]. The transmittance of Blend 2_E is higher than Blend 1_E, revealing higher amorphicity for the Blend 2_E. These findings are also indicated by the POM study, which has been discussed below.
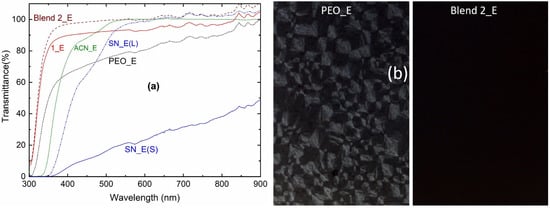
Figure 7.
(a) Transmittance spectra of the ACN_E, SN_E (L, liquid; S, solid), PEO_E, Blend 1_E, and Blend 2_E. (b) polarized optical micrographs of the PEO_E and Blend 2_E.
Figure 7b shows polarized optical micrographs of the PEO_E and Blend 2_E. The Blend 1_E had a micrograph similar to the Blend 2_E. The PEO_E micrograph depicted two parts: (i) several diamond-like spherulites due to the short and randomly oriented PEO chains; and (ii) a little dark region due to the amorphous domain [64,65]. This indicates the presence of a highly crystalline phase of the PEO, resulting in low electrical conductivity. Blend 2_E showed a complete dark region indicating arrest of the amorphous phase, which is responsible for the higher electrical conductivity. These findings are also supported by the DSC study, which has been described below.
3.4. Thermal Properties
Figure 8a shows DSC curves of the solid redox mediators, SN_E, PEO_E, Blend 1_E, and Blend 2_E. The DSC curves showed endothermic peaks corresponding to the melting temperature of the electrolytes, which are as follows: ~63.8 °C for PEO_E, ~47 °C for SN_E, ~6 °C for Blend 1_E, and ~4 °C for Blend 2_E. These values are less than those of pure matrices: ~65.7 °C for PEO [62], ~57.7 °C for SN [72], and ~30.1 °C for PEO-SN blend [62]. This indicates a decrease in the crystallinity of the PEO and SN, which are responsible for the conductivity enhancement of the electrolytes [62,63,64,65,72]. It is worth mentioning that the PEO, thereby the related compounds, do not lose the thin film-forming property of the PEO even after the Tm-value [58,59]. In fact, the electrolyte becomes amorphous, which provides highly conducting pathways for easy ion transport [62,63,64,65]. The TGA study discussed later showed that Blend-based electrolytes are thermally stable up to 125 °C. The SN_E depicted another endothermic peak at −37.8 °C, which is similar to that of pure SN (−38.4 °C [72]) and corresponds to the Tpc. The Blends 1_E and 2_E did not show the Tpc-peak. This is due to the matrix-salt interaction phenomenon [62,63,64,65]. It is also worth mentioning that the area under the melting point peak corresponds to the heat enthalpy of the electrolyte [62,63,64,65]. This area showed the following order for the PEO-based electrolytes: PEO_E ≫ Blend 1_E > Blend 2_E, indicating an extremely low level of crystallinity for the Blends 1_E and 2_E, which is one of the unique properties of the SN-PEO blend-based electrolytes [64,65,77,78].
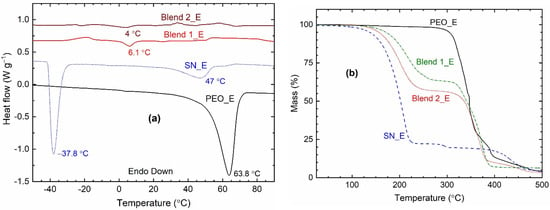
Figure 8.
(a) DSC and (b) TGA curves of the solid redox mediators.
Figure 8b shows TGA curves of the solid redox mediators, SN_E, PEO_E, Blend 1_E, and Blend 2_E. The thermal stability of the electrolyte is estimated by the initial plateau region for the mass, which is as follows: ~75 °C for SN_E, ~200 °C for PEO_E, and ~125 °C for Blends 1_E and 2_E. These values are similar to pure matrices reported earlier [62]. The SN_E and PEO_E exhibited a huge drop at ~125 °C and ~300 °C, respectively, due to single-stage decomposition. However, the Blends 1_E and 2_E portrayed two-stage degradation, first at ~125 °C and second at ~300 °C, corresponding to the decomposition of the ingredients, earlier SN, and later PEO.
4. Discussion
We synthesized solid redox mediators using [(1−x)SN: xPEO] as a solid matrix and LiTFSI, Co(bpy)3(TFSI)2, and Co(bpy)3(TFSI)3 ionic solids as sources of ions, following the procedure of Mathew et al. [24], which had acetonitrile as a solvent and 0.1-M LiTFSI, 0.25-M Co(bpy)3(TFSI)2, and 0.06-M Co(bpy)3(TFSI)3 as sources of ions. The acetonitrile-based liquid electrolyte, ACN_E exhibited σ25°C of ~1.7 × 10−2 S cm−1. The composition, x = 0 resulted in a pure plastic crystal-based electrolyte, SN_E with σ25°C of ~2.1 × 10−3 S cm−1. This value is similar to those of other succinonitrile-based electrolytes [69,72] and is attributed to the solid solvent property of the succinonitrile. The x = 1 yielded a pure PEO-based solid polymer electrolyte (EO/Li+ = 226), PEO_E with σ25°C of ~9.7 × 10−7 S cm−1. The PEO is a well-known polymer matrix for synthesizing a solid polymer electrolyte; however, the highly crystalline nature of the PEO results in poor electrical conductivity [58,59,60,61,64,65]. The blend-based solid polymer electrolytes (x = 0.5), Blend 1_E (EO/Li+ = 113) and Blend 2_E (EO/Li+ = 226) had σ25°C of ~4.3 × 10−4 and ~7.2 × 10−4 S cm−1, respectively, which were closer to that of the SN_E, disclosing the effect of plasticization property of succinonitrile [64,65].
The investigation of the temperature variation of electrical conductivity resulted in a log σ−T−1 plot, which was linear for ACN_E, SN_E, and PEO_E, and downward for Blend 1_E and Blend 2_E. The former corresponds to the thermally activated behavior of a homogeneous electrolyte. The latter corresponds to a mixed effect of amorphous domains, the semi-random motion of short polymer chains, and the segmental motion, which was produced by succinonitrile through the interaction with PEO [58,59,60,61,62,64,65,74,77,78]. The Arrhenius-type plot resulted in activation energy of 0.56 eV for SN_E and 1.07 eV for PEO_E in the solid-state region, more than the limiting condition (0.3 eV) for a device application [88]. The ACN_E had an activation energy of 0.15 eV. The VTF-type plot resulted in pseudo-activation energy of 0.06 eV for Blend 1_E and 0.05 eV for Blend 2_E, which are less than the limiting condition.
The XRD patterns of SN_E and PEO_E portrayed weak and broad characteristic peaks of succinonitrile and PEO, respectively, without the ionic salts’ peaks [62,64,65]. This indicated the molecular disorderedness of succinonitrile and a decrease in crystallinity of PEO along with a complete dissolution of ionic salts. On the contrary, Blends 1_E and 2_E had no characteristic peaks of ingredients, demonstrating the arrest of the glassy phase because of an interaction between PEO, succinonitrile, and ions [68,77,78]. These assertions can also be made using FT-IR spectroscopy. The FT-IR spectroscopy exhibited no significant change in modes of ionic salts and acetonitrile in ACN_E, revealing the least solvent-solute interaction. SN_E showed a similar scenario, however with an SN-bpy ligand interaction. PEO_E experienced a significant change in modes of ionic salts and PEO, revealing a conformational change of PEO by the large-sized ions. In contrast, the SN-PEO blend-based electrolytes, Blends 1_E and 2_E observed no significant change in modes, except at 777 and 1437 cm−1 for the SN-bpy ligand interaction and the νCH2 modes. The matrix/solvent-salts interaction can be put in an order as: ACN_E < SN_E < Blends 1_E & 2_E << PEO_E. It is also worth mentioning that the FT-IR spectra did not show the stability of the electrolytes via the hydrogen interaction with the nitrile group.
The transmittance spectra had the following order: Blend 2_E > Blend 1_E >> PEO_E >> ACN-E >> SN_E (=0%) in the UV-A region and ACN-E ≈ Blend 2_E (~100%) > SN_E(L) > Blend 1_E > PEO_E >> SN_E(S) in the visible region. These electrolytes were transparent in the near-infrared region too. This showed the transparency of Blend 2_E in a wide wavelength range, which makes it superior to ACN_E and redox couple redox mediators. The high level of transparency makes the blend-based electrolyte suitable for various types of solar cells, such as the Gratzel cells, back-illuminated DSSCs, and tandem solar cells [2,3,4,5,6,89]. Also, nearly 100% of transparency for this electrolyte revealed its glassy nature. The same was observed by the polarized optical microscopy too.
The DSC curve showed Tm peak at ~63.8 °C for PEO_E (~65.7 °C for PEO), ~47 °C for SN_E (~57.7 °C for SN), ~6 °C for Blend 1_E, and ~4 °C for Blend 2_E (~30.1 °C for PEO-SN blend). The area under the Tm peak corresponds to the heat of enthalpy of electrolyte, which had the following order: PEO_E >> SN_E >> Blend 1_E ≈ Blend 2_E (≈0). A decrease in Tm-value and/ or the area indicated a decrease in crystallinity. Thus, PEO_E had a high level of crystallinity, and Blends 1_E and 2_E had a glassy nature. The TGA curves showed the thermal stability, up to ~75 °C for SN_E, ~200 °C for PEO_E, and ~125 °C for Blends 1_E and 2_E, which are similar to those of pure matrices [62].
5. Conclusions
We synthesized new Co2+/Co3+ solid redox mediators, [(1−x)SN: xPEO]-LiTFSI-Co(bpy)3(TFSI)2-Co(bpy)3(TFSI)3 with x equals to 0 (SN_E), 0.5 (Blends 1_E and 2_E), and 1 (PEO_E) in weight fraction. The electrolyte with SN was prepared identically just by replacing the ACN of the liquid redox mediator (ACN_E). The electrolytes exhibited σ25°C-value in the following order, ACN_E (1.7 × 10−2 S cm−1) > SN_E (2.1 × 10−3 S cm−1) > Blend 2_E (7.2 × 10−4 S cm−1) > Blend 1_E (4.3 × 10−4 S cm−1) >> PEO_E (9.7 × 10−7 S cm−1). The log σ−T−1 study showed Arrhenius behavior for SN_E and PEO_E similar to ACN_E, and VTF behavior for Blends 1_E and 2_E. Only Blend-based solid polymer electrolytes showed activation energy of less than 0.3 eV, a high level of transparency in UV-A, visible, and IR regions, and thermal stability up to 125 °C, which are the basic requirements for the DSSC application in the Gulf region. This electrolyte is also suitable for tandem solar cell application.
Author Contributions
Conceptualization, R.K.G. and I.B.; methodology, R.K.G. and I.B.; formal analysis, R.K.G. and H.S.; investigation, R.K.G., H.S., A.I. and A.F.A.; writing—original draft preparation, R.K.G.; writing—review and editing, R.K.G., I.B. and A.S.A.; supervision, R.K.G.; project administration, R.K.G.; funding acquisition, R.K.G. and I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (13-ENE886-02).
Data Availability Statement
Data is available up on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Luque, A.; Hegedus, S. Handbook of Photovoltaic Science and Engineering, 2nd ed.; John Wiley & Sons, Ltd.: London, UK, 2011. [Google Scholar]
- Hagfeldt, A.; Boschloo, G.; Sun, L.C.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.D.; Wen, X.R.; Wang, M.Y.; Iocozzia, J.; Zhang, N.; Lin, C.J.; Lin, Z.Q. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Gong, J.W.; Sumathy, K.; Qiao, Q.Q.; Zhou, Z.P. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Rondan-Gomez, V.; De Los Santos, I.; Seuret-Jimenez, D.; Ayala-Mato, F.; Zamudio-Lara, A.; Robles-Bonilla, T.; Courel, M. Recent advances in dye-sensitized solar cells. Appl. Phys. A-Mater. Sci. Process. 2019, 125, 836. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 59). Prog. Photovolt. Res. Appl. 2022, 30, 3–12. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Longo, C.; De Paoli, M.A. Polymers in dye sensitized solar cells: Overview and perspectives. Coord. Chem. Rev. 2004, 248, 1455–1468. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.D.; Kang, B.N.; Wang, P.; Qiu, Y. Review of recent progress in solid-state dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 549–573. [Google Scholar] [CrossRef]
- Singh, P.K.; Nagarale, R.K.; Pandey, S.P.; Rhee, H.W.; Bhattacharya, B. Present status of solid state photoelectrochemical solar cells and dye sensitized solar cells using PEO-based polymer electrolytes. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 023002. [Google Scholar] [CrossRef]
- Wu, J.H.; Lan, Z.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Fan, L.Q.; Luo, G.G. Electrolytes in dye-sensitized solar cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef] [PubMed]
- Su’ait, M.S.; Rahman, M.Y.A.; Ahmad, A. Review on polymer electrolyte in dye-sensitized solar cells (DSSCs). Sol. Energy 2015, 115, 452–470. [Google Scholar] [CrossRef]
- Singh, R.; Polu, A.R.; Bhattacharya, B.; Rhee, H.W.; Varlikli, C.; Singh, P.K. Perspectives for solid biopolymer electrolytes in dye sensitized solar cell and battery application. Renew. Sustain. Energy Rev. 2016, 65, 1098–1117. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, U.; Al-Ahmed, A.; Al-Sulaiman, F.A.; Malik, M.I.; Shehzad, F.; Khan, A.U.H. Effect of temperature on the photovoltaic performance and stability of solid-state dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 79, 946–959. [Google Scholar] [CrossRef]
- Venkatesan, S.; Lee, Y.L. Nanofillers in the electrolytes of dye-sensitized solar cells—A short review. Coord. Chem. Rev. 2017, 353, 58–112. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D. Progress on electrolytes development in dye-sensitized solar cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.M.; Islam, M.D.; Rashid, T.U. Biopolymer-based electrolytes for dye-sensitized solar cells: A critical review. Energy Fuels 2020, 34, 15634–15671. [Google Scholar] [CrossRef]
- Wang, N.; Hu, J.J.; Gao, L.G.; Ma, T.L. Current progress in solid-state electrolytes for dye-sensitized solar cells: A mini-review. J. Electron. Mater. 2020, 49, 7085–7097. [Google Scholar] [CrossRef]
- Abu Talip, R.A.; Yahya, W.Z.N.; Bustam, M.A. Ionic liquids roles and perspectives in electrolyte for dye-sensitized solar cells. Sustainability 2020, 12, 7598. [Google Scholar] [CrossRef]
- Teo, L.P.; Buraidah, M.H.; Arof, A.K. Polyacrylonitrile-based gel polymer electrolytes for dye-sensitized solar cells: A review. Ionics 2020, 26, 4215–4238. [Google Scholar] [CrossRef]
- Chiba, Y.; Islam, A.; Watanabe, Y.; Komiya, R.; Koide, N.; Han, L.Y. Dye-sensitized solar cells with conversion efficiency of 11.1%. Jpn. J. Appl. Phys. Part 2-Lett. Express Lett. 2006, 45, L638–L640. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.Y.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.G.; Yeh, C.Y.; Zakeeruddin, S.M.; Gratzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Gratzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giribabu, L.; Bolligarla, R.; Panigrahi, M. Recent advances of cobalt(II/III) redox couples for dye-sensitized solar cell applications. Chem. Rec. 2015, 15, 760–788. [Google Scholar] [CrossRef]
- Bella, F.; Galliano, S.; Gerbaldi, C.; Viscardi, G. Cobalt-based electrolytes for dye-sensitized solar cells: Recent advances towards stable devices. Energies 2016, 9, 384. [Google Scholar] [CrossRef] [Green Version]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.E.; Gratzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Vlachopoulos, N.; Hagfeldt, A.; Benesperi, I.; Freitag, M.; Hashmi, G.; Jia, G.B.; Wahyuono, R.A.; Plentz, J.; Dietzek, B. New approaches in component design for dye-sensitized solar cells. Sustain. Energy Fuels 2021, 5, 367–383. [Google Scholar] [CrossRef]
- Srivishnu, K.S.; Prasanthkumar, S.; Giribabu, L. Cu(II/I) redox couples: Potential alternatives to traditional electrolytes for dye-sensitized solar cells. Mater. Adv. 2021, 2, 1229–1247. [Google Scholar] [CrossRef]
- Yella, A.; Humphry-Baker, R.; Curchod, B.F.E.; Astani, N.A.; Teuscher, J.; Polander, L.E.; Mathew, S.; Moser, J.E.; Tavernelli, I.; Rothlisberger, U.; et al. Molecular engineering of a fluorene donor for dye-sensitized solar cells. Chem. Mater. 2013, 25, 2733–2739. [Google Scholar] [CrossRef]
- Yum, J.H.; Moehl, T.; Yoon, J.; Chandiran, A.K.; Kessler, F.; Gratia, P.; Gratzel, M. Toward higher photovoltage: Effect of blocking layer on cobalt bipyridine pyrazole complexes as redox shuttle for dye-sensitized solar cells. J. Phys. Chem. C 2014, 118, 16799–16805. [Google Scholar] [CrossRef]
- Heiniger, L.P.; Giordano, F.; Moehl, T.; Gratzel, M. Mesoporous TiO2 beads offer improved mass transport for cobalt-based redox couples leading to high efficiency dye-sensitized solar cells. Adv. Energy Mater. 2014, 4, 1400168. [Google Scholar] [CrossRef]
- Liu, F.; Hu, S.L.; Ding, X.L.; Zhu, J.; Wen, J.; Pan, X.; Chen, S.H.; Nazeeruddin, M.K.; Dai, S.Y. Ligand-free nano-grain Cu2SnS3 as a potential cathode alternative for both cobalt and iodine redox electrolyte dye-sensitized solar cells. J. Mater. Chem. A 2016, 4, 14865–14876. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, W.X.; Zhang, L.; Jiang, R.; Mijangos, E.; Saygili, Y.; Hammarstrom, L.; Hagfeldt, A.; Boschloo, G. A small electron donor in cobalt complex electrolyte significantly improves efficiency in dye-sensitized solar cells. Nat. Commun. 2016, 7, 13934. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.D.; Fan, W.; Li, J.H.; Li, T.Y.; Robertson, N.; Song, X.R.; Wu, W.J.; Wang, Z.H.; Zhu, W.H.; Tian, H. High-performance porphyrin-based dye-sensitized solar cells with iodine and cobalt redox shuttles. Chemsuschem 2017, 10, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Mathew, S.; Aghazada, S.; Comte, P.; Gratzel, M.; Nazeeruddin, M.K. Dye-sensitized solar cells using cobalt electrolytes: The influence of porosity and pore size to achieve high-efficiency. J. Mater. Chem. C 2017, 5, 2833–2843. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, W.X.; Karlsson, M.; Cong, J.Y.; Wang, S.H.; Lo, X.; Xu, B.; Hua, J.L.; Kloo, L.; Boschloo, G. Efficient dye-sensitized solar cells with voltages exceeding 1 v through exploring tris(4-alkoxyphenyl)amine mediators in combination with the tris(bipyridine) cobalt redox system. ACS Energy Lett. 2018, 3, 1929–1937. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.C.; Wang, W.H.; Gurzadyan, G.G.; Li, J.J.; Li, X.X.; An, J.C.; Yu, Z.; Wang, H.X.; Cai, B.; et al. 13.6% efficient organic dye-sensitized solar cells by minimizing energy losses of the excited state. ACS Energy Lett. 2019, 4, 943–951. [Google Scholar] [CrossRef]
- Wu, H.; Xie, X.R.; Mei, Y.Y.; Ren, Y.T.; Shen, Z.C.; Li, S.N.; Wang, P. Phenalenothiophene-based organic dye for stable and efficient solar cells with a cobalt redox electrolyte. ACS Photonics 2019, 6, 1216–1225. [Google Scholar] [CrossRef]
- Peng, J.D.; Wu, Y.T.; Yeh, M.H.; Kuo, F.Y.; Vittal, R.; Ho, K.C. Transparent cobalt selenide/graphene counter electrode for efficient dye-sensitized solar cells with Co2+/(3+)-based redox couple. ACS Appl. Mater. Interfaces 2020, 12, 44597–44607. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.G.; Chen, X.J.; Yan, F. Imidazolium functionalized cobalt tris(bipyridyl) complex redox shuttles for high efficiency ionic liquid electrolyte dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 11933–11941. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Otsuka, T.; Kyomen, T.; Unno, M.; Hanaya, M. An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell. Chem. Commun. 2014, 50, 6379–6381. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Wang, L.; Zhang, Y.; Guo, J.N.; Li, H.; Yan, F. Dye-sensitized solar cells based on cobalt-containing room temperature ionic liquid redox shuttles. RSC Adv. 2017, 7, 13689–13695. [Google Scholar] [CrossRef] [Green Version]
- Stergiopoulos, T.; Bidikoudi, M.; Likodimos, V.; Falaras, P. Dye-sensitized solar cells incorporating novel Co(II/III) based-redox electrolytes solidified by silica nanoparticles. J. Mater. Chem. 2012, 22, 24430–24438. [Google Scholar] [CrossRef]
- Venkatesan, S.; Liu, I.P.; Chen, L.T.; Hou, Y.C.; Li, C.W.; Lee, Y.L. Effects of tio2 and tic nanofillers on the performance of dye sensitized solar cells based on the polymer gel electrolyte of a cobalt redox system. ACS Appl. Mater. Interfaces 2016, 8, 24559–24566. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.C.; Chen, D.H.; Caruso, R.A.; Cheng, Y.B.; Bach, U.; Spiccia, L. The effect of the scattering layer in dye-sensitized solar cells employing a cobalt-based aqueous gel electrolyte. Chemsuschem 2015, 8, 3704–3711. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Vlachopoulos, N.; Nonomura, K.; Zakeeruddin, S.M.; Gratzel, M.; Gerbaldi, C.; Hagfeldt, A. Direct light-induced polymerization of cobalt-based redox shuttles: An ultrafast way towards stable dye-sensitized solar cells. Chem. Commun. 2015, 51, 16308–16311. [Google Scholar] [CrossRef] [Green Version]
- Bendoni, R.; Barthelemy, A.L.; Sangiorgi, N.; Sangiorgi, A.; Sanson, A. Dye-sensitized solar cells based on N719 and cobalt gel electrolyte obtained through a room temperature process. J. Photochem. Photobiol. A-Chem. 2016, 330, 8–14. [Google Scholar] [CrossRef]
- Xiang, W.C.; Huang, W.C.; Bach, U.; Spiccia, L. Stable high efficiency dye-sensitized solar cells based on a cobalt polymer gel electrolyte. Chem. Commun. 2013, 49, 8997–8999. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Ahn, K.S.; Thogiti, S.; Kim, J.H. Mass transport effect on the photovoltaic performance of ruthenium-based quasi-solid dye sensitized solar cells using cobalt based redox couples. Dye. Pigment. 2015, 117, 83–91. [Google Scholar] [CrossRef]
- Zhang, X.L.; Huang, W.C.; Gu, A.N.; Xiang, W.C.; Huang, F.Z.; Guo, Z.X.; Cheng, Y.B.; Spiccia, L. High efficiency solid-state dye-sensitized solar cells using a cobalt(II/III) redox mediator. J. Mater. Chem. C 2017, 5, 4875–4883. [Google Scholar] [CrossRef]
- Sonai, G.G.; Tiihonen, A.; Miettunen, K.; Lund, P.D.; Nogueira, A.F. Long-term stability of dye-sensitized solar cells assembled with cobalt polymer gel electrolyte. J. Phys. Chem. C 2017, 121, 17577–17585. [Google Scholar] [CrossRef]
- dos Santos, G.A.; Nogueira, A.F. Thermal and electrochemical characterization of a new poly (ethylene oxide) copolymer-gel electrolyte containing polyvalent ion pair of cobalt (Co-II/III) or iron (Fe-II/III). J. Solid State Electrochem. 2018, 22, 1591–1605. [Google Scholar] [CrossRef]
- Venkatesan, S.; Liu, I.P.; Shan, C.M.T.; Teng, H.S.; Lee, Y.L. Highly efficient indoor light quasi -solid-state dye sensitized solar cells using cobalt polyethylene oxide -based printable electrolytes. Chem. Eng. J. 2020, 394, 124954. [Google Scholar] [CrossRef]
- Karthika, P.; Ganesan, S.; Thomas, A.; Rani, T.M.S.; Prakash, M. Influence of synthesized thiourea derivatives as a prolific additive with tris(1,10-phenanthroline)cobalt(II/III)bis/tris(hexafluorophosphate)/hydroxypropyl cellulose gel polymer electrolytes on dye-sensitized solar cells. Electrochim. Acta 2019, 298, 237–247. [Google Scholar] [CrossRef]
- Balamurugan, S.; Ganesan, S. Novel cobalt redox materials admitted in natrosol polymer with a thiophene based additive as a gel polymer electrolyte to tune up the efficiency of dye sensitized solar cells. Electrochim. Acta 2020, 329, 135169. [Google Scholar] [CrossRef]
- Bruce, P.G.; Gray, F.M. Polymer electrolytes II: Physical principles In Solid State Electrochemistry; Bruce, P.G., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 119–162. [Google Scholar]
- Agrawal, R.C.; Pandey, G.P. Solid polymer electrolytes: Materials designing and all-solid-state battery applications: An overview. J. Phys. D Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Electrolyte for energy storage/conversion (Li+, Na+, Mg2+) devices based on PVC and their associated polymer: A comprehensive review. J. Solid State Electrochem. 2019, 23, 997–1059. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. A glimpse on all-solid-state Li-ion battery (ASSLIB) performance based on novel solid polymer electrolytes: A topical review. J. Mater. Sci. 2020, 55, 6242–6304. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kim, H.M.; Rhee, H.W. Poly(ethylene oxide): Succinonitrile—A polymeric matrix for fast-ion conducting redox-couple solid electrolytes. J. Phys. D-Appl. Phys. 2011, 44, 205106. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rhee, H.W. Highly conductive redox-couple solid polymer electrolyte system: Blend-KI-I2 for dye-sensitized solar cells. Adv. OptoElectronics 2011, 2011, 102932. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Rhee, H.W. Effect of succinonitrile on electrical, structural, optical, and thermal properties of poly(ethylene oxide)-succinonitrile /LiI-I2 redox-couple solid polymer electrolyte. Electrochim. Acta 2012, 76, 159–164. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rhee, H.W. Plasticizing effect of k+ ions and succinonitrile on electrical conductivity of poly(ethylene oxide)-succinonitrile /KI-I2 redox-couple solid polymer electrolyte. J. Phys. Chem. B 2013, 117, 7465–7471. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Bedja, I.M. Improved cell efficiency of poly(ethylene oxide)-succinonitrile /LiI-I2 solid polymer electrolyte-based dye-sensitized solar cell. Phys. Status Solidi A Appl. Mater. Sci. 2014, 211, 1601–1604. [Google Scholar] [CrossRef]
- Gupta, R.K.; Bedja, I. Cationic effect on dye-sensitized solar cell properties using electrochemical impedance and transient absorption spectroscopy techniques. J. Phys. D Appl. Phys. 2017, 50, 245501. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rhee, H.W.; Bedja, I.; AlHazaa, A.N.; Khan, A. Effect of laponite (R) nanoclay dispersion on electrical, structural, and photovoltaic properties of dispersed poly(ethylene oxide)-succinonitrile -LiI-I2 solid polymer electrolyte. J. Power Sources 2021, 490, 229509. [Google Scholar] [CrossRef]
- Alarco, P.J.; Abu-Lebdeh, Y.; Abouimrane, A.; Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat. Mater. 2004, 3, 476–481. [Google Scholar] [CrossRef]
- Wang, P.; Dai, Q.; Zakeeruddin, S.M.; Forsyth, M.; MacFarlane, D.R.; Gratzel, M. Ambient temperature plastic crystal electrolyte for efficient, all-solid-state dye-sensitized solar cen. J. Am. Chem. Soc. 2004, 126, 13590–13591. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Yang, H.; Li, X.H.; Li, F.Y.; Yi, T.; Huang, C.H. Thermostable succinonitrile-based gel electrolyte for efficient, long-life dye-sensitized solar cells. J. Mater. Chem. 2007, 17, 1602–1607. [Google Scholar] [CrossRef]
- Gupta, R.K.; Bedja, I.; Islam, A.; Shaikh, H. Electrical, structural, and thermal properties of succinonitrile-LiI-I2 redox-mediator. Solid State Ion. 2018, 326, 166–172. [Google Scholar] [CrossRef]
- Gupta, R.K.; Shaikh, H.; Bedja, I. Understanding the electrical transport–structure relationship and photovoltaic properties of a [succinonitrile–ionic liquid]–LiI-I2 redox electrolyte. ACS Omega 2020, 5, 12346–12354. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Rhee, H.W. Detailed investigation into the electrical conductivity and structural properties of poly(ethylene oxide)-succinonitrile -Li(CF3SO2)2N solid polymer electrolytes. Bull. Korean Chem. Soc. 2017, 38, 356–363. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Lee, J.Y.; Geng, J.; Jung, H.T.; Park, J.K. Effect of cation size on solid polymer electrolyte based dye-sensitized solar cells. Langmuir 2009, 25, 3276–3281. [Google Scholar] [CrossRef] [PubMed]
- Haq, N.; Shakeel, F.; Alanazi, F.K.; Shaikh, H.; Bedja, I.; Gupta, R.K. Utilization of poly(ethylene terephthalate) waste for preparing disodium terephthalate and its application in a solid polymer electrolyte. J. Appl. Polym. Sci. 2019, 136, 47612. [Google Scholar] [CrossRef]
- Fan, L.Z.; Hu, Y.S.; Bhattacharyya, A.J.; Maier, J. Succinonitrile as a versatile additive for polymer electrolytes. Adv. Funct. Mater. 2007, 17, 2800–2807. [Google Scholar] [CrossRef]
- Patel, M.; Chandrappa, K.G.; Bhattacharyya, A.J. Increasing ionic conductivity and mechanical strength of a plastic electrolyte by inclusion of a polymer. Electrochim. Acta 2008, 54, 209–215. [Google Scholar] [CrossRef]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 89th ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Pace, E.L.; Noe, L.J. Infrared spectra of acetonitrile and acetonitrile-d3. J. Chem. Phys. 1968, 49, 5317–5325. [Google Scholar] [CrossRef]
- Fengler, O.I.; Ruoff, A. Vibrational spectra of succinonitrile and its 1,4-c-13(2)-, 2,2,3,3-h-2(4)- and 1,4-c-13(2)-2,2,3,3-h-2(4) -isotopomers and a force field of succinonitrile. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2001, 57, 105–117. [Google Scholar] [CrossRef]
- Yoshihara, T.; Tadokoro, H.; Murahashi, S. Normal vibrations of the polymer molecules of helical conformation. IV. Polyethylene oxide and polyethylene-d4 oxide. J. Chem. Phys. 1964, 41, 2902–2911. [Google Scholar] [CrossRef]
- Wen, S.J.; Richardson, T.J.; Ghantous, D.I.; Striebel, K.A.; Ross, P.N.; Cairns, E.J. Ftir characterization of PEO + LiN(CF3SO2)2 electrolytes. J. Electroanal. Chem. 1996, 408, 113–118. [Google Scholar] [CrossRef]
- Rey, I.; Lassègues, J.C.; Grondin, J.; Servant, L. Infrared and Raman study of the PEO-LITFSI polymer electrolyte. Electrochim. Acta 1998, 43, 1505–1510. [Google Scholar] [CrossRef]
- Castellucci, E.; Angeloni, L.; Neto, N.; Sbrana, G. IR and Raman spectra of a 2,2′-bipyridine single crystal: Internal modes. Chem. Phys. 1979, 43, 365–373. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Agrawal, R.C.; Gupta, R.K. Superionic solids: Composite electrolyte phase—An overview. J. Mater. Sci. 1999, 34, 1131–1162. [Google Scholar] [CrossRef]
- Liska, P.; Thampi, K.R.; Gratzel, M.; Bremaud, D.; Rudmann, D.; Upadhyaya, H.M.; Tiwari, A.N. Nanocrystalline dye-sensitized solar cell/copper indium gallium selenide thin-film tandem showing greater than 15% conversion efficiency. Appl. Phys. Lett. 2006, 88, 203103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).