1. Introduction
Electrical steel laminates based on epoxy adhesive layers are of high relevance for the transformation of electric energy [
1,
2,
3,
4]. By the stacking and bonding of thin electrical steel sheets, eddy current losses are reduced and the efficiency of electric machines is raised [
3]. Epoxy plies act as multifunctional separation layers providing electrical isolation and full-surface bonding. To maintain the high power density of electric engines or generators, the adhesive layer thickness should be uniform and restricted to a minimum. Lamination and isolation of electrical steel with epoxy-based adhesives offers many advantages, but also a few drawbacks. Compared to classical joining methods like welding or interlocking, the high temperature performance of adhesively bonded laminates is limited [
3].
Epoxy adhesives and coatings provide remarkable properties such as good adherence to various substrates, thermal stability or electrical isolation. Waterborne epoxy varnishes, especially, allow for multifunctional coatings with a thickness in the µm range. Therefore, low viscosity varnishes are required. Both solvent-based and waterborne systems have been developed. Due to environmental and legislative restrictions, waterborne epoxy varnishes are preferred. Further advantages of waterborne epoxy varnishes are ease of applicability by coil coating processes, low volatile organic content (VOC) and odor or reduced flammability [
5,
6,
7]. In order to enhance interfacial adherence, hydroxyl groups, allowing for bonds to the oxidic passivation layer of the electrical steel substrate, are essential. While secondary bonds, such as hydrogen and van der Waals forces, provide good adherence at ambient conditions, chemical covalent or coordinative bonds are of special relevance for long-term behavior under hot-humid conditions [
8,
9,
10,
11].
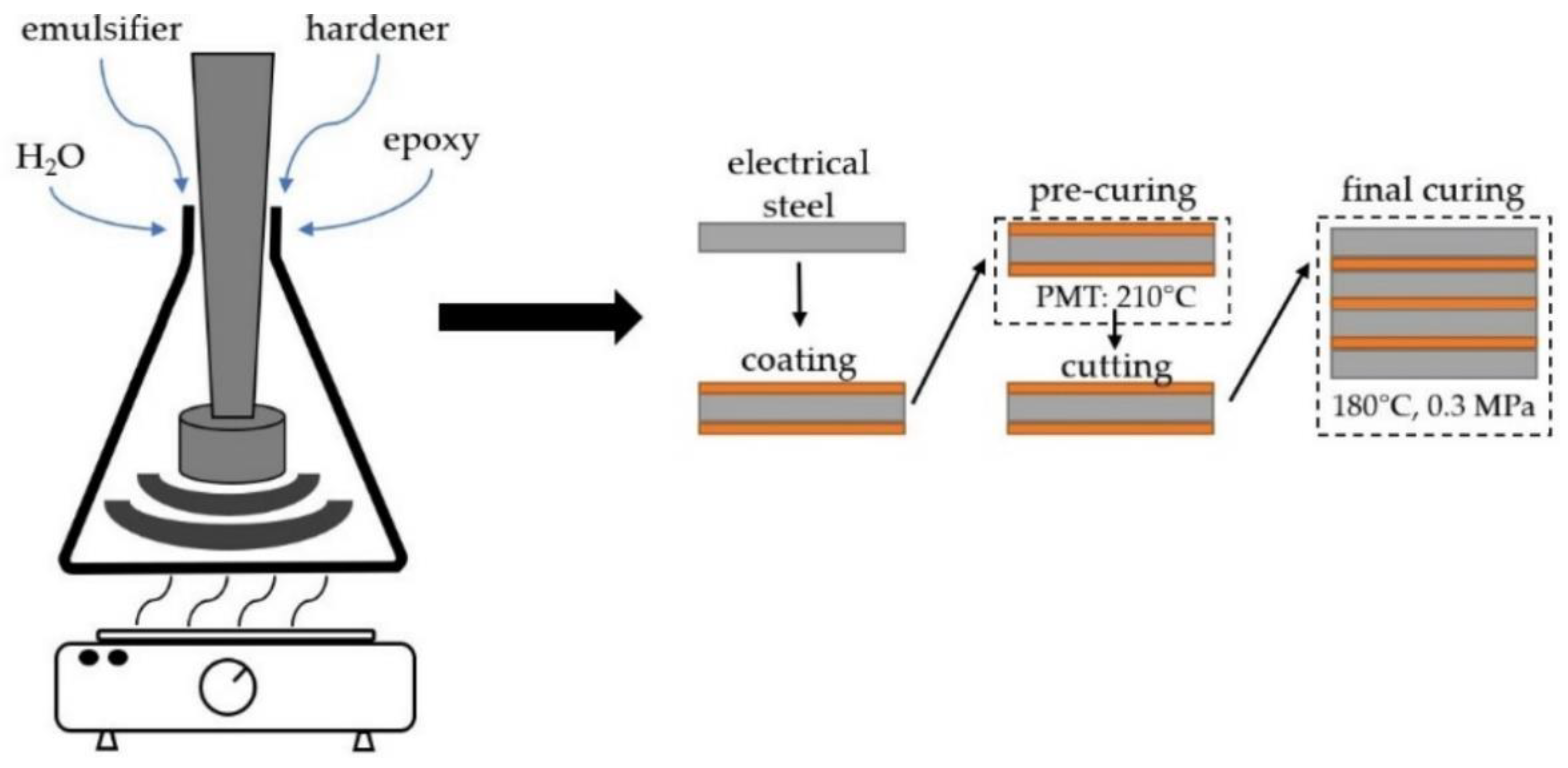
Liquid epoxy varnishes (A-stage) and coatings are applied in a two-stage process. First, the electrical steel substrate is coated with the waterborne epoxy varnish system, which is then dried and partly cured to the B-stage. The coated electrical steel substrates can be stored for up to several months. In a second step, the sheets are cut to the desired geometry and stacked. By heat press lamination, a high curing degree of the epoxy adhesive (in C-stage) is ensured. Processing temperature, time and pressure are dependent on the epoxy formulation but also on the geometry of the laminate [
2,
12,
13].
Maximum service temperatures of electrical steel laminates are around 180 °C [
14]. The glass transition temperature of commonly used epoxy adhesive systems is lower. Hence, the crosslinking density of epoxy networks, which is usually proportional to the mechanical properties, is of utmost importance [
15,
16,
17]. In addition to the bulk properties of adhesives, interfacial bonding to the passivation layer of the substrate is essential. In a few studies, structure–property correlations between the epoxy equivalent weight (or hydroxyl group concentration) of non-waterborne epoxies and adhesive strength at ambient temperatures have been established [
8,
10]. A higher EEW value, associated with increased monomer chain length, results in a reduced crosslinking density and enhanced hydroxyl group concentration [
18]. So far in the literature, no specific attention has been given to the structure–property correlations of waterborne epoxy varnishes allowing for micrometer thick adhesive layers in electrical steel laminates.
Hence, the main objective of this paper was to investigate the effect of structural parameters of waterborne epoxy varnishes on the thermomechanical and mechanical properties of electrical steel laminates. Varnish systems with varying EEW values were formulated and applied. Thermomechanical analysis was performed to determine the glass transition. The adherence strength of laminates was characterized by roll peel testing at ambient and elevated temperatures. Furthermore, fatigue crack growth kinetics were examined at ambient conditions.
3. Results and Discussion
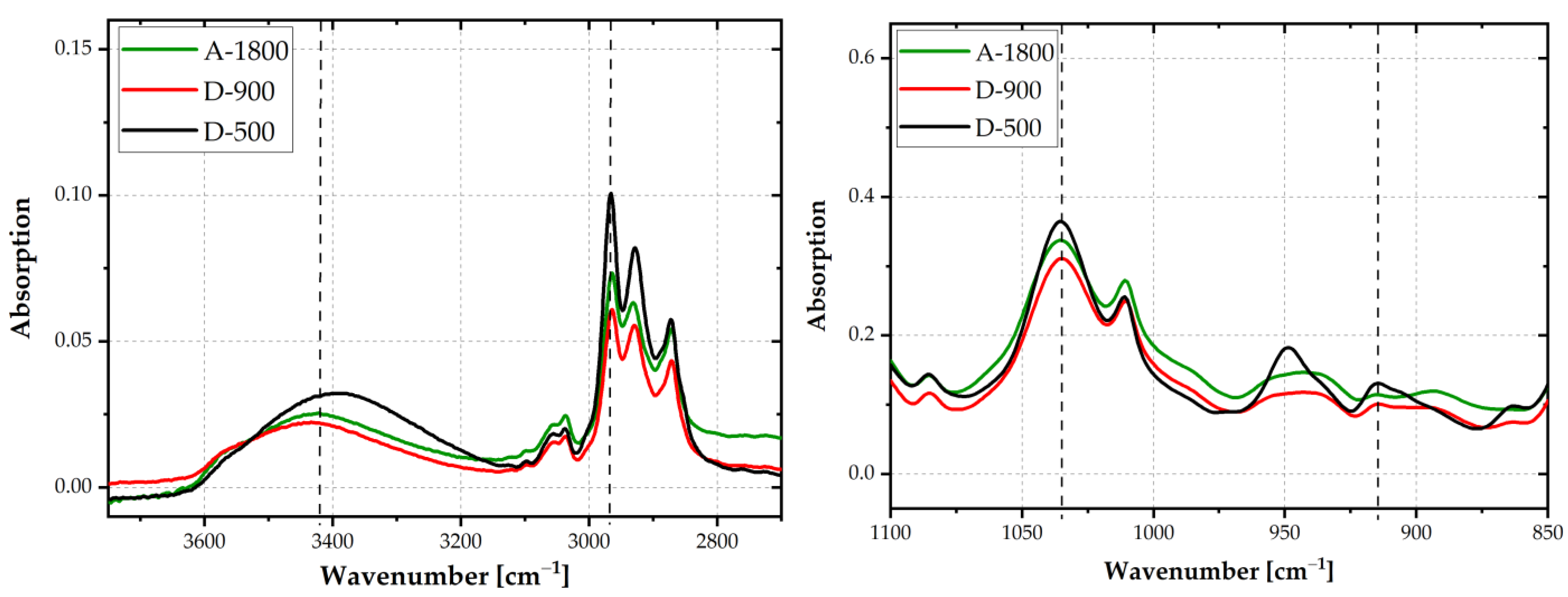
The chemical structure of the epoxy resins was evaluated by FTIR spectroscopy. IR-spectra are depicted in
Figure 4. Depending on the molar mass of the epoxides, the oxirane ring intensity at 915 cm
−1 varied. The lower the number of repeating units (n), the more pronounced was the oxirane ring peak. The oxirane ring functionality of a DGEBA molecule is equal to two. The intensity of this peak leveled off at EEW values of 900 and 1800 g/mol. Hence, the highest intensity of the oxirane ring was detected for epoxy D-500, with a decrease in intensity towards the highest EEW adhesive A-1800. By weighting the oxirane peak at 915 cm
−1 with the C-O-C ether stretching peak at 1035 cm
−1 [
21] (vertical dashed lines), this trend was confirmed. The ratio was 0.36 for D-500 and 0.33 for D-900 and A-1800.
Due to water uptake at the surface, no clear trend as to the OH-stretching peak at 3400 cm
−1 was discernible. With increasing n, the intensity of the OH-absorption peak should become more pronounced. By weighting the peak at 3400 cm
−1 with the CH
3 stretching peak at 2965 cm
−1 (vertical dashed lines), a clear indication for higher amounts of OH-groups was ascertained for A-1800 or D-900, compared to D-500. The peak ratios were 0.36 for A-1800 and D-900, and 0.32 for D-500. These findings are in good agreement with data in the literature [
22,
23].
The formulated waterborne epoxy varnishes were applied to electrical steel, dried and pre-cured to coatings in B-stage in a convection oven at a peak metal temperature of 210 °C. The coating thickness in B-stage was 5 µm for all epoxy thermosets.
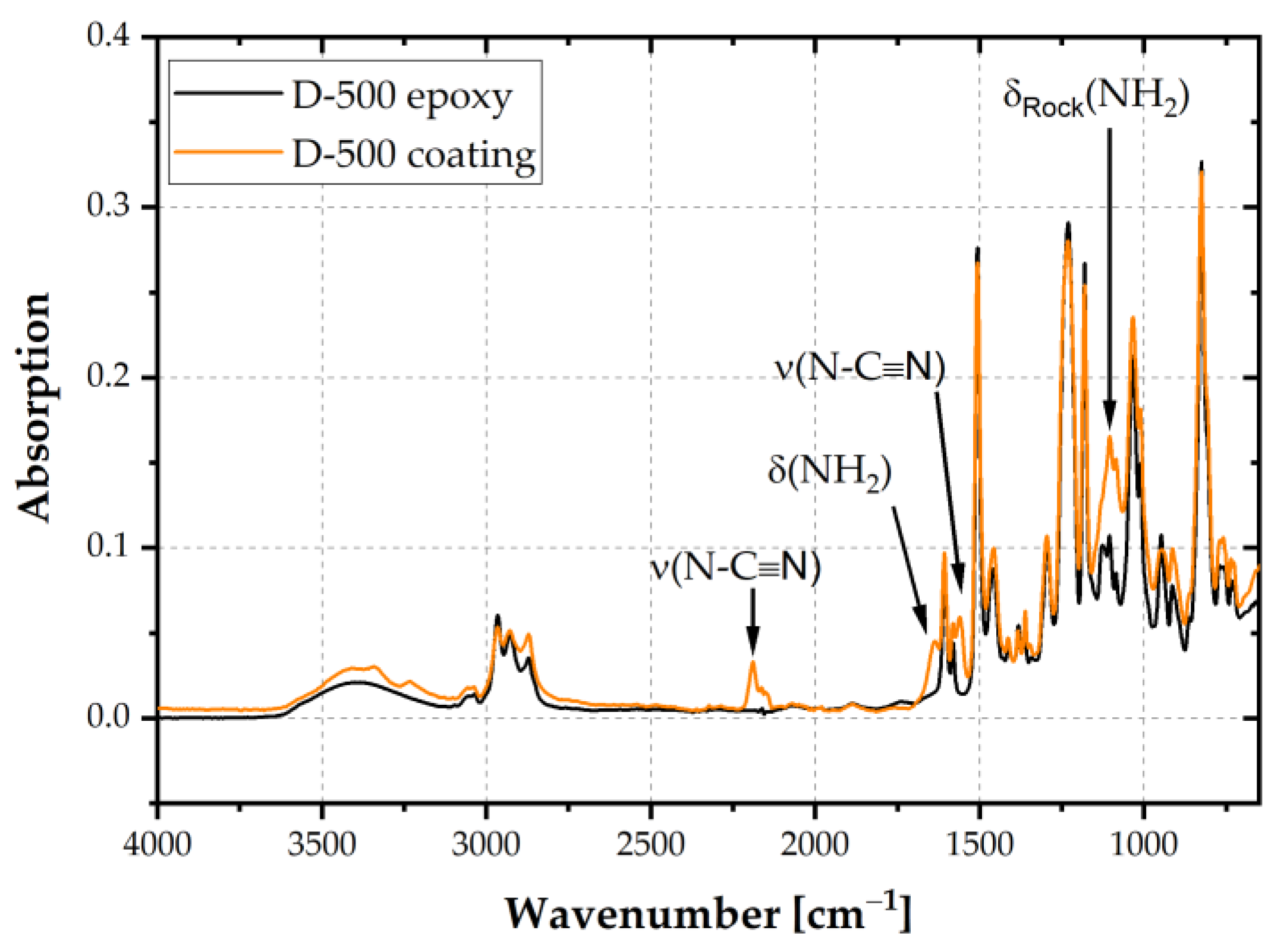
Figure 5 shows an exemplary comparison of the epoxy resin D-500 with the coating in B-stage. The spectra were normalized to the peak at 1509 cm
−1 as the intensity of the phenylene stretching vibration did not change during crosslinking. Peaks relevant to the curing agent DICY were found at 1638 cm
−1 and 1100 cm
−1 due to resonant states of the bending vibration of NH
2. The oligomer formation of DGEBA and DICY was proven by asymmetric stretching peaks of N-C≡N at 2187 cm
−1, which is characteristic for linear oligomers. Furthermore, the peak at 1560 cm
−1 (ν (N-C≡N)) was attributed to the guanidine unit resulting from oligomers [
23]. The peaks marked in
Figure 5 are characteristic for DICY and oligomers with DGEBA. Hence, partial crosslinking of the investigated coatings was confirmed.
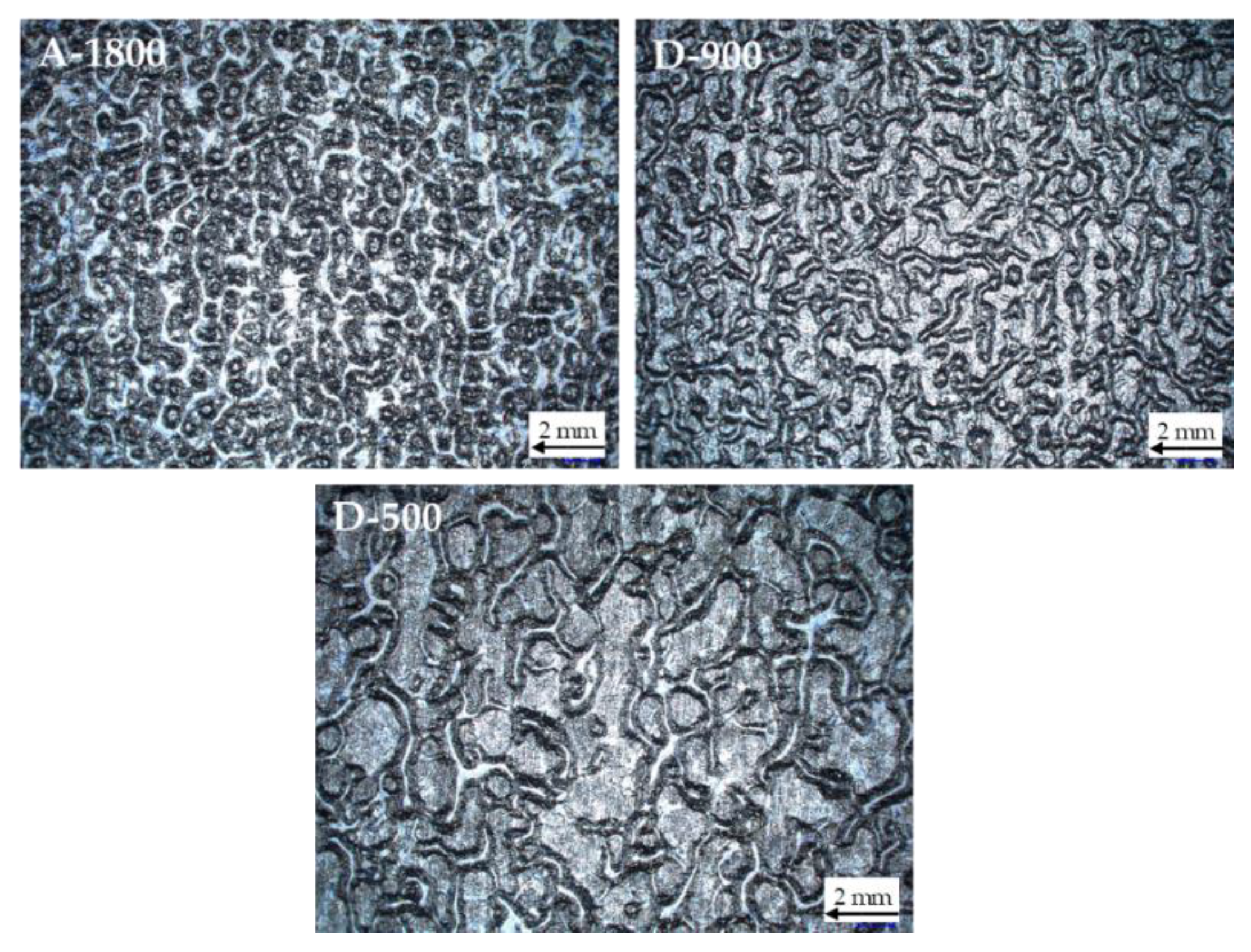
The topography of the applied coatings in B-stage is depicted in
Figure 6. Full surface wetting was ascertained optically and confirmed by FTIR-spectroscopy for all investigated varnish formulations. However, most of the coatings exhibited thickness variations and a wavelike topography. The wavelength of the pattern was finer for coatings based on epoxy adhesives of higher EEW. Most likely, the waviness is dependent on the viscosity of the varnish. This effect is described in the literature as orange peel. A main reason for orange peel effects is non-optimized solvent content of the varnish system [
24].
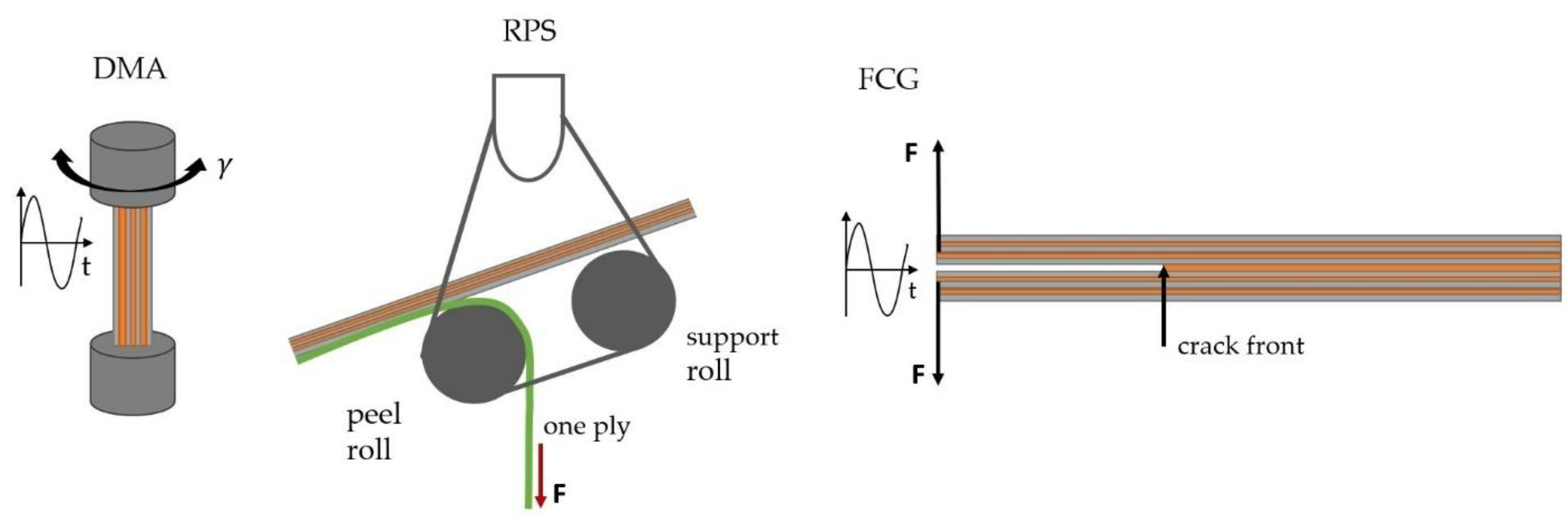
Storage modulus and loss factor thermograms of the investigated laminates are depicted in
Figure 7. While storage modulus of the laminate was about 70 GPa in the glassy state below glass transition temperature of the adhesive, it dropped by one magnitude to 7.3 GPa above T
G in the rubbery state of the adhesive. The T
G value was deduced from the maximum of the loss factor. The highest value of 102 °C was obtained for the laminate based on varnish D-500. In contrast, lower T
G values of 94 °C and 81 °C were detected for laminates with coatings based on higher molar mass epoxies D-900 and A-1800, respectively. Hence, in agreement with findings in the literature [
16], glass transition temperature was indirectly proportional to the EEW of the epoxy thermoset. This relationship was attributed to a lower crosslinking density of high EEW adhesives. The theoretical crosslinking density for adhesive A-1800 was a factor of 3 lower than that of D-500. The absolute values of the storage modulus in the glassy and rubbery plateaus did not reflect the pronounced effect of EEW. The storage modulus was mainly affected by the electrical steel substrate. Due to an adhesive layer thickness well below 10 µm, small fluctuations in thickness had a strong impact on scattering of modulus values, especially in the rubbery state. For laminates based on commercially available varnishes, higher glass transition temperatures, in the range from 110 to 117 °C, were reported in [
25], slightly dependent on the electrical steel grade. As described by Aufray and Roche [
26], chemical interactions of amine-based curing agents and metal oxide passive layers could also have an impact on glass transition temperature values and the macromolecular structure of the thermoset at the interphase.
The width of the loss factor peak at 50% of the maximum value was highest for laminates based on D-500, and lowest for D-900. In agreement with the findings of Misra [
16], the crosslinking network of coatings made from adhesives of lower EEW values was, presumably, less perfectly associated with wider loss factor peaks.
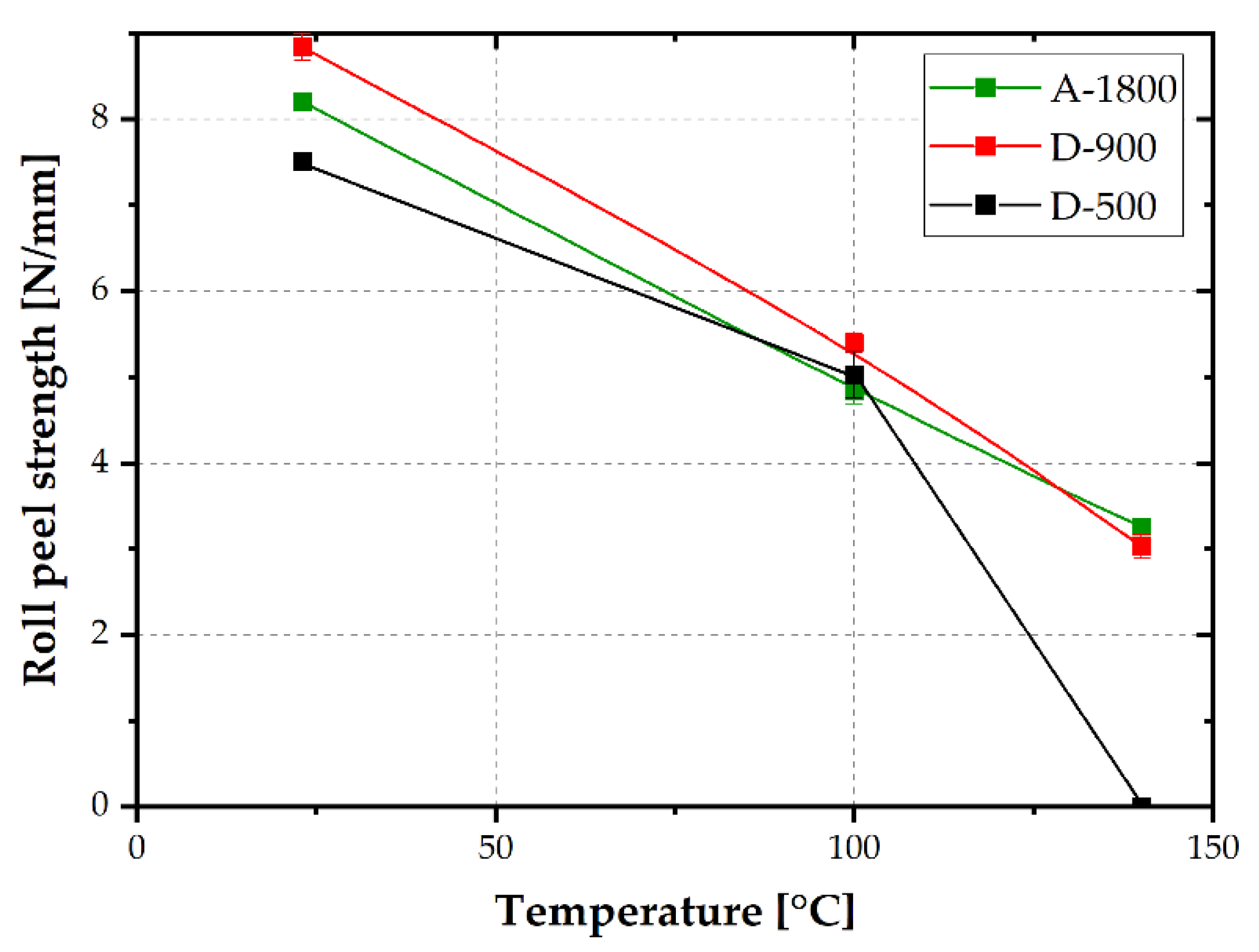
Adherence strength was determined by roll peel testing at 23, 100 and 140 °C. The temperature-dependent roll peel strength values of the investigated laminates with adhesives of differing EEW values are plotted in
Figure 8. At ambient temperature, the lowest roll peel strength, of 7.5 N/mm, was determined for D-500. Adhesives of higher EEW (A-1800 and D-900) exhibited increased roll peel strength, higher than 8 N/mm. Presumably, a lower crosslinking density is beneficial for roll peel strength at ambient conditions. Slightly lower crosslinking density would allow for less brittle fracture and, therefore, higher roll peel strength. A similar trend was proven by Pugstaller et al. [
25] for laminates made from a commercially available varnish. The roll peel strength values were in a similar range of about 8 N/mm.
At a test temperature of 100 °C, which was within the glass transition of the investigated adhesives, a pronounced decrease in roll peel strength was ascertained. This effect could be attributed to an enhanced segmental mobility of the polymeric adhesive at increasing temperatures [
27]. The drop was less pronounced for laminates based on D-500, with higher storage modulus values at 100 °C due to a slightly higher glass transition temperature. At 140 °C, well above T
G, a further drop of roll peel strength, to about 3 N/mm, was discernible. The laminate based on D-500, with the highest T
G, showed inferior adherence at 140 °C and could not be tested. Better adherence performance at 140 °C was obtained for thermosets with higher OH functionality (i.e., D-900 and A-1800). By comparing T
G or OH functionality with roll peel strength at 100 °C or 140 °C, a much higher impact of OH functionality was deduced. Hence, OH functionality is presumably much more important than crosslinking density. Due to hydroxyl groups, different bonds such as secondary or covalent bonds to the metal oxide surface are established [
8,
10,
28,
29].
As confirmed by IR spectroscopy in reflection mode using an Ulbricht sphere, the fractured surfaces of the investigated laminates, roll peel tested at 23, 100 and 140 °C, revealed, predominately, interfacial failure. This supports the hypothesis of high relevance of OH functionality for improvement of interfacial debonding performance.
In order to test adherence performance under more service-relevant conditions, fatigue fracture mechanics tests were performed. In roll peel testing, most of the peeling energy is attributable to plastic deformation of the substrate [
30]. This effect could be excluded by fracture mechanics delamination testing using a double cantilever beam specimen (DCB). In
Figure 9, crack growth kinetics curves of the laminates tested at ambient temperature are depicted. The curves can be divided into three different regions: the threshold, stable crack growth and unstable crack growth regime. In the threshold regime (ultra-low crack growth rates), the adhesive based on A-1800 showed lower strain energy release rate threshold values (G
th) than laminates prepared from D-900 or D-500 epoxies. Presumably, slow crack propagation in the threshold regime of laminates based on epoxies of low and intermediate EEW values was less pronounced due to better adherence to the passive layer of the substrate. Additionally, at low strain energy release rate values, the plastic zone size at the crack tip is less- or non-constrained by the substrate. If the plastic zone is constrained, the crack tends to propagate within the adhesive layer, associated with failure in cohesive manner [
13]. Therefore, at slow crack propagation rates, better cohesive strength of the epoxy layer would result in enhanced crack growth resistance.
In the stable crack growth regime (II), the lowest crack propagation rate was obtained for laminates based on D-900. Compared to fatigue crack growth data for laminates based on commercially available varnish systems [
13] (see grey area in
Figure 9), a similar fatigue crack growth resistance was confirmed in regime II. For monotonic fracture mechanic tests with a high crack tip radius, a trend of maximum fracture toughness at intermediate EEW values was also reported by Levita et al. [
31]. Furthermore, for amorphous polymeric materials, a higher crosslinking density was related to increased yield stress and reduced strain at break [
32]. These two contrary effects necessitate an optimization and a compromise between crosslinking density and ductility. A better stress distribution at the crack tip could be achieved by higher inner mobility (e.g., at higher temperatures). Nevertheless, a certain level of yield stress has to be exceeded. In addition to bulk adhesive properties, the plastic zone at the crack tip is constrained at higher strain energy release rates by the substrate. Subsequently, the crack tends to propagate in the substrate-adhesive interface. Under such conditions, substrate-adhesive interactions (eg., OH bonds or covalent bonds) are of utmost relevance to ensure sufficient fatigue crack growth resistance [
13].
The behavior of high EEW laminates (i.e., A-1800) was slightly better in the unstable crack growth regime. Interestingly, the ranking of roll peel strength values and crack growth resistance was equivalent in the unstable crack growth regime. However, contrary to roll peel testing, the DCB specimen failed, mainly in a cohesive manner. This difference is most likely related to excessive plastic deformation of the substrate during roll peel testing.
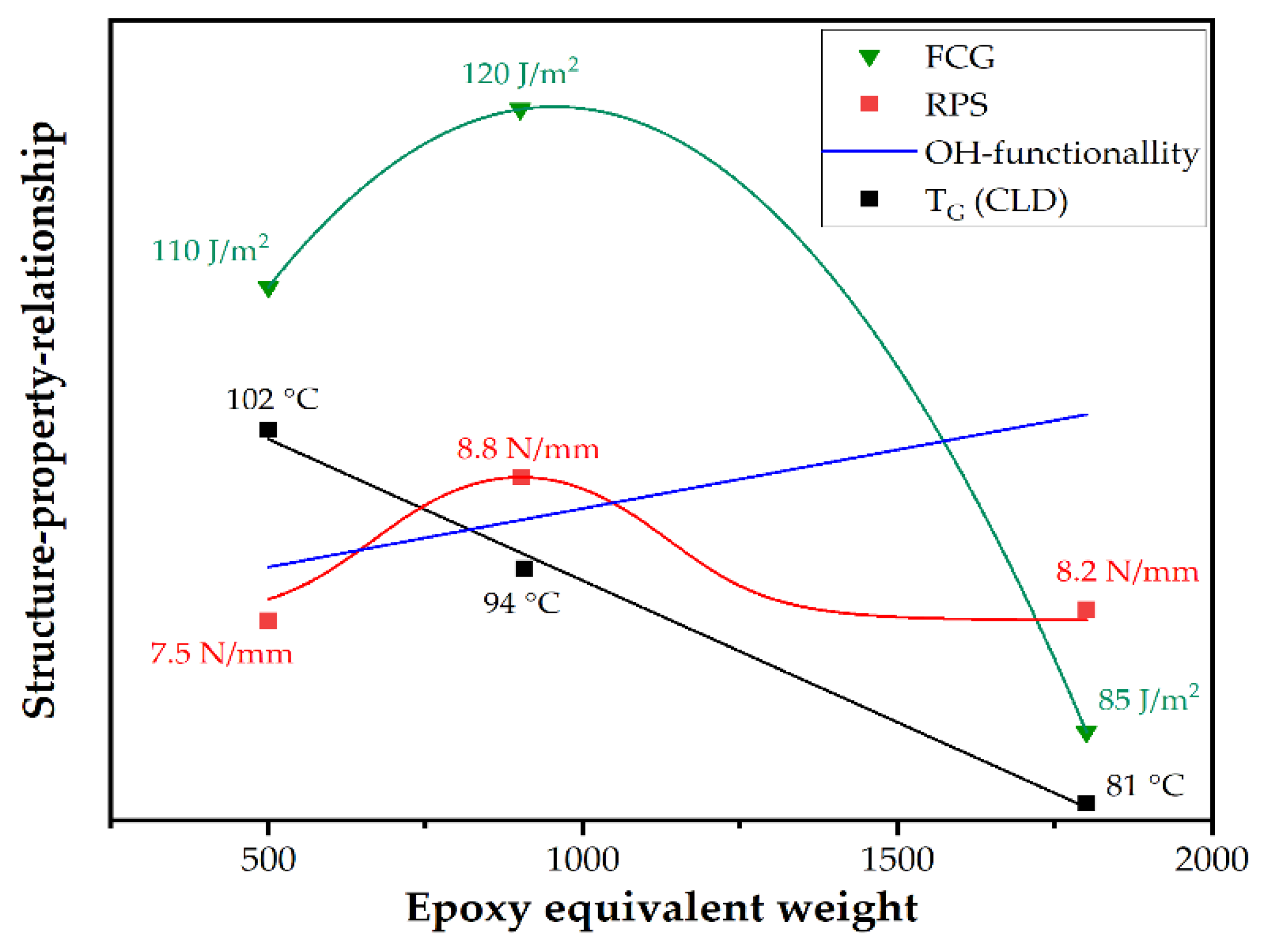
In
Figure 10, structure–property relationships, of molar mass or epoxy equivalent weight and the investigated properties, are depicted schematically. As confirmed by DMA, raising EEW causes a decrease in crosslinking density (CLD). In contrast, the hydroxyl functionality affecting the adherence to the substrate raises with higher molar mass of the epoxy resin. Both roll peel strength (RPS) and fatigue crack growth kinetics (FCG) at ambient temperature peak at intermediate EEW values of 900 g/mol. At low or high EEW values, roll peel strength and fatigue crack growth kinetics are lower, due to poor adherence (at low EEW) or less crosslinking density (at high EEW). However, the effect of hydroxyl groups on the epoxy-metal adherence is still under discussion [
33,
34,
35,
36]. The postulated schematic relationships do not account for chemical interactions of amine-based hardeners and metal substrates [
26], which have been investigated and ascertained in a recent study [
37]. Future research will focus on the potential effects of interfacial interactions and interphase formation in the coating and lamination process on the adherence performance of steel/epoxy laminates.
4. Conclusions
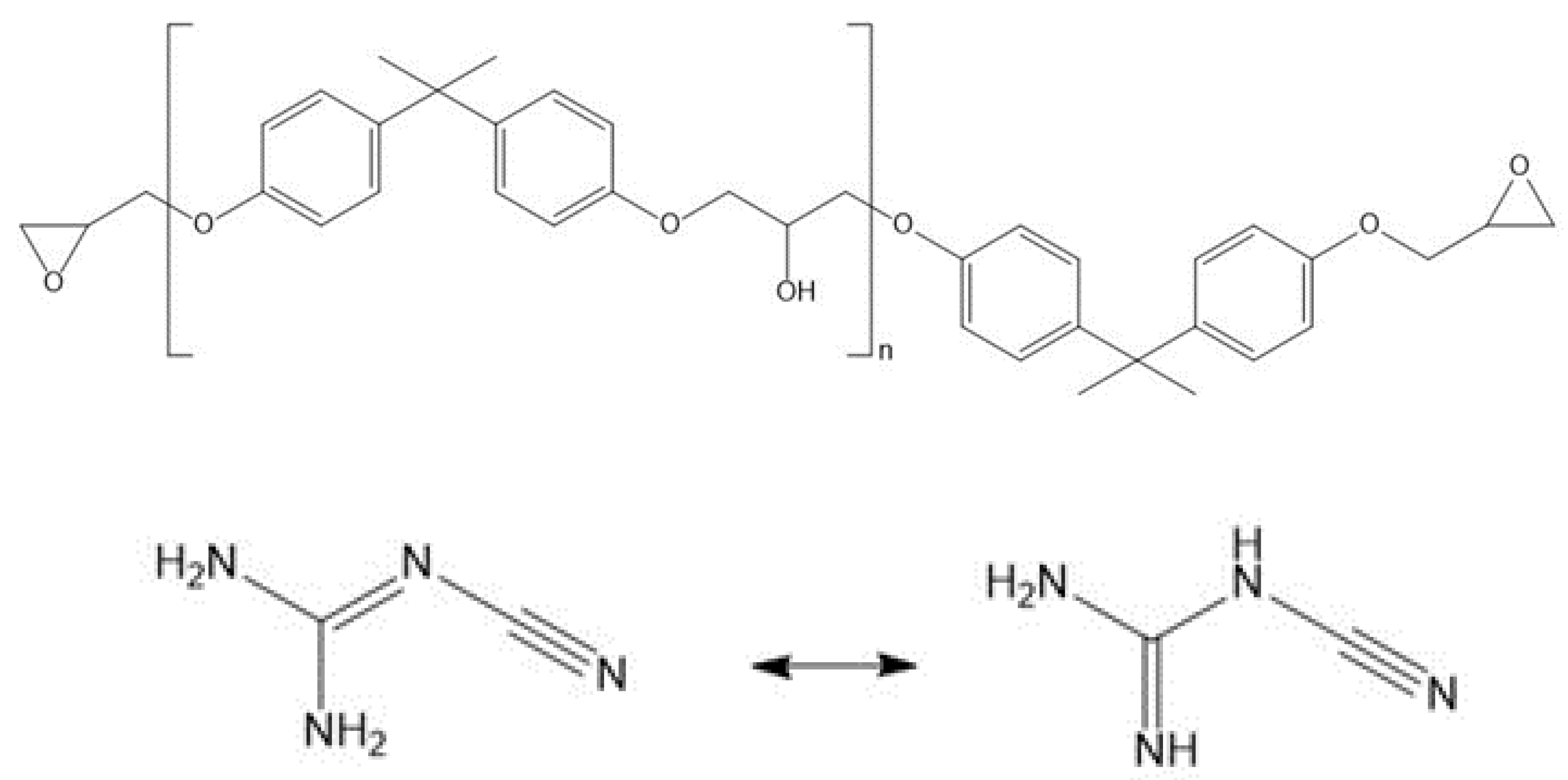
Waterborne varnishes for electrical steel laminates based on bisphenol-A-diglycidiyl ether (DGEBA) resins with varying epoxy equivalent weights (EEW) were prepared and investigated. The EEW values of the epoxy resins ranged from 500 to 1800 g/mol. 10 m% of poloxamer emulsifier was added to the formulation. As curing agent, dicyandiamide (DICY) was used in an over-stoichiometric amount. Electrical steel sheets were coated with the investigated varnish systems. After pre-curing, the coated steel sheets were stacked and hot press-cured. The investigated laminates consisted of 4 or 6 electrical steel plies (roll peel or fatigue crack growth tests) and epoxy adhesive layers with a thickness of about 5 µm.
By infrared spectroscopy, a higher amount of oxirane ring groups, but a lower OH functionality, was confirmed for lower molar mass epoxy. The orange peel effect of partly cured coatings on electrical steel substrates was also dependent on the epoxy equivalent weight (EEW) of the adhesives. For higher EEW values of 1800 g/mol, a much finer orange peel structure was discernible, most likely attributable to a higher viscosity in the coating process.
The thermomechanical properties of laminates with fully cured adhesive layers was examined by DMA. The lowest glass transition temperature was obtained at 81 °C for the highest EEW adhesive, with 1800 g/mol. With decreasing EEW value, the glass transition values rose up to 102 °C for the adhesive based on an EEW of 500 g/mol. Hence, crosslinking density was correlated in a negative manner with the EEW value of the adhesive.
The adhesive strength of the laminates was assessed by roll peel testing at 23, 100 and 140 °C. Laminates based on adhesives of higher EEW values exhibited better adherence performance below, and above, glass transition. A maximum strength of about 9 N/mm at 23 °C was obtained, which was in good agreement with data from the literature [
3]. At 100 and 140 °C, roll peel strength dropped by 50 and 75%, respectively. This drop was attributed to enhanced main chain mobility within or above the glass transition regime of the epoxy adhesives. Especially at 140 °C, the laminates based on the adhesive of lowest EEW failed prior to testing. In contrast, laminates with coatings of intermediate or high EEW value revealed roll peel strength values of 3 N/mm. Hence, it was clearly deduced that higher EEW, associated with more OH functional groups, is of utmost importance to mechanical strength at temperatures around glass transition or higher. Most likely, the OH functionality and associated interfacial bonds are of higher relevance than crosslinking density. Due to mainly interfacial failure of the investigated laminates at testing temperatures below and above glass transition, there is significant potential for further improvement of the varnish formulations. Special attention should be given to adhesion promoters or adequate surface treatment procedures.
Fatigue crack growth resistance was characterized at ambient conditions. The best performance in the threshold and stable crack growth regime was deduced for laminates based on the adhesive of intermediate EEW of 900 g/mol. At slow crack propagation rates, the plastic zone size was less constrained by the substrate. Therefore, higher cohesive strength of the epoxy layer resulted in increased crack growth resistance. In the stable crack growth regime, the higher crosslinking density was related to enhanced yield stress and reduced strain at break. Overall, it was deduced that a well-balanced ratio of crosslinking density and OH functionality is of high relevance to ensure good performance under near-service conditions.
Finally, structure–property correlations were established and deduced for waterborne epoxy varnish systems with systematically varied EEW values and electrical steel laminates. It was clearly shown that enhancement of glass transition temperature and associated crosslinking density did not necessarily lead to better mechanical performance of the laminates. Especially at elevated temperatures and under fatigue loading conditions, the adherence was dependent on both cohesive strength of the epoxy layer and interfacial bonding to the metal oxide passivation layer of the substrate. Hence, a well-balanced compromise of inner mobility and ductility of the epoxy layer and interfacial bonding to the substrate is essential for the optimization of monotonic and fatigue delamination resistance. The model varnish-based approach allowed for a clear separation of opposing or reinforcing effects. The investigations confirmed that the energy-based fatigue testing approach was much more sensitive for elucidation of material structure effects than conventional force-based, monotonic testing.
To also assess the effect of ageing on the adherence of electrical steel laminates, future research will focus on fatigue testing under superimposed mechanical stresses and environmental influences. Furthermore, potential interactions of the micrometer-thin epoxy coating with the passive layer of electrical steel substrates, and their consequences on the thermoset network structure, will be investigated.