Characterisation of Selected Materials in Medical Applications
Abstract
1. Introduction
2. Materials Used in Dentistry and Medicine
2.1. Metals
2.2. Polymers
Application of Polymeric Materials in Orthopaedics
2.3. Ceramic Materials
2.4. Composites
2.5. Manufacturing Techniques
3. 3D-Printing-Based Skull Reconstruction Surgery: Challenges and Material Solutions
3.1. Polymethylmethacrylate (PMMA)
3.2. Polyetheretherketone (PEEK)
3.3. Polypropylene–Polyester Knit (PPK)
3.4. Hydroxyapatite (HA)
3.5. General Considerations
4. Nanocomposite Transitional Implants for Guided Bone Regeneration
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alla, R.K.; Kn, R.S.; Vyas, R. Conventional and Contemporary Polymers for the Fabrication of Denture Prosthesis: Part I–Overview, Composition and Properties. Int. J. Appl. Dent. Sci. 2015, 1, 82–89. [Google Scholar]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric Materials and Films in Dentistry: An Overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Santarpia, P.; Lavender, S.; Gittins, E.; Liu, Z.; Anderson, M.H.; He, J.; Shi, W.; Eckert, R. Clinical Efficacy of a Specifically Targeted Antimicrobial Peptide Mouth Rinse: Targeted Elimination of Streptococcus Mutans and Prevention of Demineralization. Caries Res. 2011, 45, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ana, I.D. Bone Substituting Materials in Dental Implantology. In Bone Management in Dental Implantology; Budihardja, A.S., Mücke, T., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–141. ISBN 978-3-319-78951-4. [Google Scholar]
- Nampoothiri, D.P.; Subhash, A.K.; Aboobacker, F.; Mohan, A.; Keerthana, K.R. Biomimetic Materials in Implantology. Int. J. Oral Care Res. 2018, 6, 93–96. [Google Scholar]
- Peel, S.; Eggbeer, D.; Burton, H.; Hanson, H.; Evans, P.L. Additively Manufactured versus Conventionally Pressed Cranioplasty Implants: An Accuracy Comparison. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 949–961. [Google Scholar] [CrossRef]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J.N. Additive Technology: Update on Current Materials and Applications in Dentistry. J. Prosthodont. 2017, 26, 156–163. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Bilgin, M.S.; Erdem, A.; Aglarci, O.S.; Dilber, E. Fabricating Complete Dentures with CAD/CAM and RP Technologies. J. Prosthodont. 2015, 24, 576–579. [Google Scholar] [CrossRef]
- Alexakou, E.; Damanaki, M.; Zoidis, P.; Bakiri, E.; Mouzis, N.; Smidt, G.; Kourtis, S. PEEK High Performance Polymers: A Review of Properties and Clinical Applications in Prosthodontics and Restorative Dentistry. Eur. J. Prosthodont. Restor. Dent. 2019, 27, 113–121. [Google Scholar] [CrossRef]
- Hanawa, T. 1-Overview of Metals and Applications. In Metals for Biomedical Devices; Niinomi, M., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 3–29. ISBN 978-0-08-102666-3. [Google Scholar]
- Prasad, S.; Ehrensberger, M.; Gibson, M.P.; Kim, H.; Monaco, E.A. Biomaterial Properties of Titanium in Dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications—a Systematic Review. Biol. Trace Elem. Res. 2020, 197, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Slokar, L.; Pranjić, J.; Carek, A. Metallic Materials for Use in Dentistry. Holist. Approach Environ. 2017, 7, 39–58. [Google Scholar]
- Grünewald, T.A.; Rennhofer, H.; Hesse, B.; Burghammer, M.; Stanzl-Tschegg, S.E.; Cotte, M.; Löffler, J.F.; Weinberg, A.M.; Lichtenegger, H.C. Magnesium from Bioresorbable Implants: Distribution and Impact on the Nano- and Mineral Structure of Bone. Biomaterials 2016, 76, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. InVitro and inVivo Studies on the Degradation of High-Purity Mg (99.99wt.%) Screw with Femoral Intracondylar Fractured Rabbit Model. Biomaterials 2015, 64, 57–69. [Google Scholar] [CrossRef]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Progress and Challenge for Magnesium Alloys as Biomaterials-Zeng-2008-Advanced Engineering Materials-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/adem.200800035?casa_token=laCgr4TvImEAAAAA:MtlIR-Z4hvNz9jyGoLbMFAmBmtA-eRI01ADVzvP2r_jY1xMs-I3NYbyWEHXjJ9hAJGu8nk_bZ3QUqgkL (accessed on 6 March 2022).
- Rahim, M.I.; Eifler, R.; Rais, B.; Mueller, P.P. Alkalization Is Responsible for Antibacterial Effects of Corroding Magnesium. J. Biomed. Mater. Res. Part A 2015, 103, 3526–3532. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A Survey of Bio-Corrosion Rates of Magnesium Alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Ajib, F.A.; Childress, J.M. Magnesium Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the Corrosion of Biodegradable Magnesium Implants: A Critical Review of Current Methodologies and Their Limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Rahim, M.I.; Ullah, S.; Mueller, P.P. Advances and Challenges of Biodegradable Implant Materials with a Focus on Magnesium-Alloys and Bacterial Infections. Metals 2018, 8, 532. [Google Scholar] [CrossRef]
- Balog, M.; Snajdar, M.; Krizik, P.; Schauperl, Z.; Stanec, Z.; Catic, A. Titanium-Magnesium Composite for Dental Implants (BIACOM). In TMS 2017 146th Annual Meeting & Exhibition Supplemental Proceedings; TMS, T.M., Metals &. Materials Society, Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 271–284. [Google Scholar]
- Nowacki, J.; Dobrzanski, L.F.G. Implanty Śródszpikowe w Osteosyntezie Kości Długich. Open Access Libr. 2012, 11, 13–25. [Google Scholar]
- Ramazanzadeh, B.; Jahanbin, A.; Yaghoubi, M.; Shahtahmassbi, N.; Ghazvini, K.; Shakeri, M.; Shafaee, H. Comparison of Antibacterial Effects of ZnO and CuO Nanoparticles Coated Brackets against Streptococcus Mutans. J. Dent. 2015, 16, 200–205. [Google Scholar]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles Used in Dentistry: A Review. J. Oral Biol. Craniofacial Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, J. Biomaterialy; Wydawn, Politechniki Slaskiej: Gliwice, Poland, 2002; ISBN 978-83-7335-031-1. [Google Scholar]
- Świeczko-Żurek, B. Biomateriały; Wydawn, Politechniki Gdańskiej: Gdańsk, Poland, 2009. [Google Scholar]
- Kusz, D. Rys historyczny i uwarunkowania rozwoju endoprotezoplastyk stawu biodrowego. Inżynieria Mater. 1998, 19, 36–39. [Google Scholar]
- Zagadnienia Teoretyczne i Aplikacyjne w Implantach” Jan Łaskawiec. Available online: https://w.bibliotece.pl/57545/Zagadnienia+teoretyczne+i+aplikacyjne+w+implantach (accessed on 6 March 2022).
- Chatterjee, S.; Saxena, M.; Padmanabhan, D.; Jayachandra, M.; Pandya, H.J. Futuristic Medical Implants Using Bioresorbable Materials and Devices. Biosens. Bioelectron. 2019, 142, 111489. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- AL-Rabab’ah, M.; Hamadneh, W.; Alsalem, I.; Khraisat, A.; Abu Karaky, A. Use of High Performance Polymers as Dental Implant Abutments and Frameworks: A Case Series Report. J. Prosthodont. 2019, 28, 365–372. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Najeeb, S.; BDS, Z.K.; BDS, S.Z.; BDS, M.S.Z. Bioactivity and Osseointegration of PEEK Are Inferior to Those of Titanium: A Systematic Review. J. Oral Implantol. 2016, 42, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Wiesli, M.G.; Özcan, M. High-Performance Polymers and Their Potential Application as Medical and Oral Implant Materials: A Review. Implant. Dent. 2015, 24, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Durham III, J.W.; Allen, M.J.; Rabiei, A. Preparation, Characterization and in Vitro Response of Bioactive Coatings on Polyether Ether Ketone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Mahjoubi, H.; Buck, E.; Manimunda, P.; Farivar, R.; Chromik, R.; Murshed, M.; Cerruti, M. Surface Phosphonation Enhances Hydroxyapatite Coating Adhesion on Polyetheretherketone and Its Osseointegration Potential. Acta Biomater. 2017, 47, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.T.W.; Ling, J.M.; Dinesh, S.K. The Feasibility of Producing Patient-Specific Acrylic Cranioplasty Implants with a Low-Cost 3D Printer. J. Neurosurg. 2016, 124, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Kadambi, P.; Luniya, P.; Dhatrak, P. Current Advancements in Polymer/Polymer Matrix Composites for Dental Implants: A Systematic Review. Mater. Today Proc. 2021, 46, 740–745. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Burg, K.J.L.; Holder, W.D.; Culberson, C.R.; Beiler, R.J.; Greene, K.G.; Loebsack, A.B.; Roland, W.D.; Mooney, D.J.; Halberstadt, C.R. Parameters Affecting Cellular Adhesion to Polylactide Films. J. Biomater. Sci. Polym. Ed. 1999, 10, 147–161. [Google Scholar] [CrossRef]
- Russias, J.; Saiz, E.; Nalla, R.K.; Gryn, K.; Ritchie, R.O.; Tomsia, A.P. Fabrication and Mechanical Properties of PLA/HA Composites: A Study of in Vitro Degradation. Mater. Sci. Eng. C 2006, 26, 1289–1295. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- P Pawar, R.; U Tekale, S.; U Shisodia, S.; T Totre, J.; J Domb, A. Biomedical Applications of Poly(Lactic Acid). Recent Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Ranjbar, M.; Dehghan Noudeh, G.; Hashemipour, M.-A.; Mohamadzadeh, I. A Systematic Study and Effect of PLA/Al2O3 Nanoscaffolds as Dental Resins: Mechanochemical Properties. Artif. Cells Nanomed. Biotechnol. 2019, 47, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Battistella, E.; Varoni, E.; Cochis, A.; Palazzo, B.; Rimondini, L. Degradable Polymers May Improve Dental Practice. J. Appl. Biomater. Biomech. 2011, 9, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive Polymers and Their Biomedical Application in Tissue Engineering–a Review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-Responsive Polymers and Their Application as Smart Biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on Thermoresponsive Polymers: Phase Behaviour, Drug Delivery and Biomedical Applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Tham, D.Q.; Huynh, M.D.; Linh, N.T.D.; Van, D.T.C.; Cong, D.V.; Dung, N.T.K.; Trang, N.T.T.; Lam, P.V.; Hoang, T.; Lam, T.D. PMMA Bone Cements Modified with Silane-Treated and PMMA-Grafted Hydroxyapatite Nanocrystals: Preparation and Characterization. Polymers 2021, 13, 3860. [Google Scholar] [CrossRef]
- Webb, J.C.J.; Spencer, R.F. The Role of Polymethylmethacrylate Bone Cement in Modern Orthopaedic Surgery. J. Bone Jt. Surg. 2007, 89, 851–857. [Google Scholar] [CrossRef]
- Shirdar, M.R.; Taheri, M.M.; Qi, M.-L.; Gohari, S.; Farajpour, N.; Narayanan, S.; Foroozan, T.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Shokuhfar, T. Optimization of the Mechanical Properties and the Cytocompatibility for the PMMA Nanocomposites Reinforced with the Hydroxyapatite Nanofibers and the Magnesium Phosphate Nanosheets. Materials 2021, 14, 5893. [Google Scholar] [CrossRef]
- Bistolfi, A.; Ferracini, R.; Albanese, C.; Vernè, E.; Miola, M. PMMA-Based Bone Cements and the Problem of Joint Arthroplasty Infections: Status and New Perspectives. Materials 2019, 12, 4002. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Polymers as Biomaterials for Tissue Engineering and Controlled Drug Delivery. In Tissue Engineering I; Lee, K., Kaplan, D., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 47–90. ISBN 978-3-540-31948-1. [Google Scholar]
- Bistolfi, A.; Massazza, G.; Verné, E.; Massè, A.; Deledda, D.; Ferraris, S.; Miola, M.; Galetto, F.; Crova, M. Antibiotic-Loaded Cement in Orthopedic Surgery: A Review. ISRN Orthopedics 2011, 2011, 290851. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Chan, E.K.; Gupta, S.; Diwan, A.D. Polymethylmethacrylate Bone Cements and Additives: A Review of the Literature. World J. Orthop. 2013, 4, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Yiu, C.K.Y.; King, N.M.; Tay, F.R. Chlorhexidine Release and Antibacterial Properties of Chlorhexidine-Incorporated Polymethyl Methacrylate-Based Resin Cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Paz, E.; Forriol, F.; del Real, J.C.; Dunne, N. Graphene Oxide versus Graphene for Optimisation of PMMA Bone Cement for Orthopaedic Applications. Mater. Sci. Eng. C 2017, 77, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Valencia Zapata, M.E.; Mina Hernandez, J.H.; Grande Tovar, C.D.; Valencia Llano, C.H.; Diaz Escobar, J.A.; Vázquez-Lasa, B.; San Román, J.; Rojo, L. Novel Bioactive and Antibacterial Acrylic Bone Cement Nanocomposites Modified with Graphene Oxide and Chitosan. Int. J. Mol. Sci. 2019, 20, 2938. [Google Scholar] [CrossRef]
- Wright, Z.M.; Pandit, A.M.; Karpinsky, M.M.; Holt, B.D.; Zovinka, E.P.; Sydlik, S.A. Bioactive, Ion-Releasing PMMA Bone Cement Filled with Functional Graphenic Materials. Adv. Healthc. Mater. 2021, 10, 2001189. [Google Scholar] [CrossRef]
- Robu, A.; Antoniac, A.; Grosu, E.; Vasile, E.; Raiciu, A.D.; Iordache, F.; Antoniac, V.I.; Rau, J.V.; Yankova, V.G.; Ditu, L.M.; et al. Additives Imparting Antimicrobial Properties to Acrylic Bone Cements. Materials 2021, 14, 7031. [Google Scholar] [CrossRef]
- Shen, S.-C.; Letchmanan, K.; Chow, P.S.; Tan, R.B.H. Antibiotic Elution and Mechanical Property of TiO2 Nanotubes Functionalized PMMA-Based Bone Cements. J. Mech. Behav. Biomed. Mater. 2019, 91, 91–98. [Google Scholar] [CrossRef]
- Li, M.; Glassman, A. What’s New in Hip Replacement. JBJS 2019, 101, 1619–1627. [Google Scholar] [CrossRef]
- Cheng, C.-K.; Wang, X.-H.; Luan, Y.-C.; Zhang, N.-Z.; Liu, B.-L.; Ma, X.-Y.; Nie, M.-D. Challenges of Pre-Clinical Testing in Orthopedic Implant Development. Med. Eng. Phys. 2019, 72, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Manning, D.W.; Chiang, P.P.; Martell, J.M.; Galante, J.O.; Harris, W.H. In Vivo Comparative Wear Study of Traditional and Highly Cross-Linked Polyethylene in Total Hip Arthroplasty. J. Arthroplast. 2005, 20, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.K.; Henrichsen, J.L.; Siljander, M.; Moore, D.; Karadsheh, M. Polyethylene in Total Knee Arthroplasty: Where Are We Now? J. Orthop. Surg. 2018, 26, 2309499018808356. [Google Scholar] [CrossRef] [PubMed]
- Orita, K.; Minoda, Y.; Sugama, R.; Ohta, Y.; Ueyama, H.; Takemura, S.; Nakamura, H. Vitamin E-Infused Highly Cross-Linked Polyethylene Did Not Reduce the Number of in Vivo Wear Particles in Total Knee Arthroplasty. Bone Jt. J. 2020, 102, 1527–1534. [Google Scholar] [CrossRef]
- Min, B.-W.; Cho, C.-H.; Son, E.-S.; Lee, K.-J.; Lee, S.-W.; Song, K.-S. Highly Cross-Linked Polyethylene in Total Hip Arthroplasty in Patients Younger Than 50 Years With Osteonecrosis of the Femoral Head: A Minimum of 10 Years of Follow-Up. J. Arthroplast. 2020, 35, 805–810. [Google Scholar] [CrossRef]
- Lambert, B.; Neut, D.; van der Veen, H.C.; Bulstra, S.K. Effects of Vitamin E Incorporation in Polyethylene on Oxidative Degradation, Wear Rates, Immune Response, and Infections in Total Joint Arthroplasty: A Review of the Current Literature. Int. Orthop. 2019, 43, 1549–1557. [Google Scholar] [CrossRef]
- Rochcongar, G.; Remazeilles, M.; Bourroux, E.; Dunet, J.; Chapus, V.; Feron, M.; Praz, C.; Buia, G.; Hulet, C. Reduced Wear in Vitamin E-Infused Highly Cross-Linked Polyethylene Cups: 5-Year Results of a Randomized Controlled Trial. Acta Orthop. 2021, 92, 151–155. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for Medical Applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef]
- Barber, F.A.; Howard, M.S.; Ashraf, W.; Spenciner, D.B. The Biomechanical Performance of the Latest All-Inside Meniscal Repair Devices. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 3001–3007. [Google Scholar] [CrossRef]
- Liao, C.Z.; Li, K.; Wong, H.M.; Tong, W.Y.; Yeung, K.W.K.; Tjong, S.C. Novel Polypropylene Biocomposites Reinforced with Carbon Nanotubes and Hydroxyapatite Nanorods for Bone Replacements. Mater. Sci. Eng. C 2013, 33, 1380–1388. [Google Scholar] [CrossRef]
- Butterworth, M.L.; Ugrinich, M. First Metatarsophalangeal Joint Implant Options. Clin. Podiatr. Med. Surg. 2019, 36, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Kluin, O.S.; van der Mei, H.C.; Busscher, H.J.; Neut, D. Biodegradable vs Non-Biodegradable Antibiotic Delivery Devices in the Treatment of Osteomyelitis. Expert Opin. Drug Deliv. 2013, 10, 341–351. [Google Scholar] [CrossRef]
- Böstman, O.; Pihlajamäki, H. Clinical Biocompatibility of Biodegradable Orthopaedic Implants for Internal Fixation: A Review. Biomaterials 2000, 21, 2615–2621. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Eglin, D.; Alini, M. Degradable Polymeric Materials for Osteosynthesis: Tutorial. Eur. Cell Mater. 2008, 16, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Daghino, W.; Bistolfi, A.; Aprato, A.; Massè, A. Bioabsorbable Implants in Foot Trauma Surgery. Injury 2019, 50, S47–S55. [Google Scholar] [CrossRef]
- Ashby-Dobór Materiałów w Projektowaniu Inżynierskim-Pobierz pdf z Docer.pl. Available online: https://docer.pl/doc/n00811e (accessed on 7 March 2022).
- Maw, J.L.; Quinn, J.V.; Wells, G.A.; Ducic, Y.; Odell, P.F.; Lamothe, A.; Brownrigg, P.J.; Sutcliffe, T. A Prospective Comparison of Octylcyanoacrylate Tissue Adhesive and Suture for the Closure of Head and Neck Incisions. J. Otolaryngol. 1997, 26, 26–30. [Google Scholar] [CrossRef]
- Osmond, M.H.; Klassen, T.P.; Quinn, J.V. Economic Comparison of a Tissue Adhesive and Suturing in the Repair of Pediatric Facial Lacerations. J. Pediatrics 1995, 126, 892–895. [Google Scholar] [CrossRef]
- Slosarczyk, A. Bioceramika Hydroksyapatytowa; Polskie Towarzystwo Ceramiczne: Krakow, Poland, 1997; ISBN 978-83-7108-015-9. [Google Scholar]
- BIOMATERIAŁY-Biocybernetyka I Inżynieria Biomedyczna 2000-Maciej Nałęcz. Available online: https://www.scribd.com/document/155700855/BIOMATERIA%C5%81Y-Biocybernetyka-i-in%C5%BCynieria-biomedyczna-2000-Maciej-Na%C5%82%C4%99cz (accessed on 7 March 2022).
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [PubMed]
- Tredwin, C.J.; Young, A.M.; Abou Neel, E.A.; Georgiou, G.; Knowles, J.C. Hydroxyapatite, Fluor-Hydroxyapatite and Fluorapatite Produced via the Sol–Gel Method: Dissolution Behaviour and Biological Properties after Crystallisation. J. Mater. Sci. Mater. Med. 2014, 25, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of Calcium Orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Ramazanoglu, M.; Oshida, Y. Osseointegration and Bioscience of Implant Surfaces-Current Concepts at Bone-Implant Interface; IntechOpen: London, UK, 2011; ISBN 978-953-307-658-4. [Google Scholar]
- Dorozhkin, S.V. Calcium Orthophosphate Cements for Biomedical Application. J. Mater. Sci. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-Hydroxyapatite and Its Applications in Preventive, Restorative and Regenerative Dentistry: A Review of Literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Arcos, D. Calcium Phosphate Bioceramics. In Bio-Ceramics with Clinical Applications; John Wiley & Sons, Ltd.: New York, NY, USA, 2014; pp. 23–71. ISBN 978-1-118-40674-8. [Google Scholar]
- Shin, H.; Ko, H.; Kim, M. Cytotoxicity and Biocompatibility of Zirconia (Y-TZP) Posts with Various Dental Cements. Restor. Dent. Endod. 2016, 41, 167–175. [Google Scholar] [CrossRef]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Zirconia: Established Facts and Perspectives for a Biomaterial in Dental Implantology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 519–529. [Google Scholar] [CrossRef]
- Galante, R.; Figueiredo-Pina, C.G.; Serro, A.P. Additive Manufacturing of Ceramics for Dental Applications: A Review. Dent. Mater. 2019, 35, 825–846. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J.R. Emerging Ceramic-Based Materials for Dentistry. J. Dent. Res. 2014, 93, 1235–1242. [Google Scholar] [CrossRef]
- Ghaemi, M.H.; Reichert, S.; Krupa, A.; Sawczak, M.; Zykova, A.; Lobach, K.; Sayenko, S.; Svitlychnyi, Y. Zirconia Ceramics with Additions of Alumina for Advanced Tribological and Biomedical Applications. Ceram. Int. 2017, 43, 9746–9752. [Google Scholar] [CrossRef]
- Hanif, M.; Jabbar, F.; Sharif, S.; Abbas, G.; Farooq, A.; Aziz, M. Halloysite Nanotubes as a New Drug-Delivery System: A Review. Clay Miner. 2016, 51, 469–477. [Google Scholar] [CrossRef]
- Gianni, E.; Avgoustakis, K.; Papoulis, D. Kaolinite Group Minerals: Applications in Cancer Diagnosis and Treatment. European J. Pharm. Biopharm. 2020, 154, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Gkouma, E.; Gianni, E.; Avgoustakis, K.; Papoulis, D. Applications of Halloysite in Tissue Engineering. Appl. Clay Sci. 2021, 214, 106291. [Google Scholar] [CrossRef]
- Fakhrullina, G.I.; Akhatova, F.S.; Lvov, Y.M.; Fakhrullin, R.F. Toxicity of Halloysite Clay Nanotubes in Vivo: A Caenorhabditis Elegans Study. Environ. Sci. Nano 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Cunha, D.A.; Rodrigues, N.S.; Souza, L.C.; Lomonaco, D.; Rodrigues, F.P.; Degrazia, F.W.; Collares, F.M.; Sauro, S.; Saboia, V.P.A. Physicochemical and Microbiological Assessment of an Experimental Composite Doped with Triclosan-Loaded Halloysite Nanotubes. Materials 2018, 11, 1080. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Genari, B.; Leitune, V.C.B.; Arthur, R.A.; Luxan, S.A.; Samuel, S.M.W.; Collares, F.M.; Sauro, S. Polymerisation, Antibacterial and Bioactivity Properties of Experimental Orthodontic Adhesives Containing Triclosan-Loaded Halloysite Nanotubes. J. Dent. 2018, 69, 77–82. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The Application of Halloysite Tubule Nanoclay in Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef]
- Chawla, K.K. Ceramic Matrix Composites. In Composite Materials: Science and Engineering; Chawla, K.K., Ed.; Springer: New York, NY, USA, 2012; pp. 249–292. ISBN 978-0-387-74365-3. [Google Scholar]
- Chawla, K.K. Metal Matrix Composites. In Composite Materials: Science and Engineering; Chawla, K.K., Ed.; Springer: New York, NY, USA, 2012; pp. 197–248. ISBN 978-0-387-74365-3. [Google Scholar]
- Freilich, M.A.; Meiers, J.C. Fiber-Reinforced Composite Prostheses. Dent. Clin. 2004, 48, 545–562. [Google Scholar] [CrossRef]
- Tezvergil, A.; Lassila, L.V.J.; Vallittu, P.K. The Effect of Fiber Orientation on the Thermal Expansion Coefficients of Fiber-Reinforced Composites. Dent. Mater. 2003, 19, 471–477. [Google Scholar] [CrossRef]
- Krishnakumar, S.; Senthilvelan, T. Polymer Composites in Dentistry and Orthopedic Applications-a Review. Mater. Today Proc. 2021, 46, 9707–9713. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.-W.; Seo, S.-J. Polymer-Ceramic Bionanocomposites for Dental Application. J. Nanomater. 2016, 2016, e3795976. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D.; Schmalz, G. Scaffolds for Dental Pulp Tissue Engineering. Adv. Dent. Res. 2011, 23, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Jewrajka, S.K. CHAPTER 3:Designing Multi-Component Biodegradable/Biocompatible Amphiphilic Polymer Co-Networks for Biomedical Applications. In Amphiphilic Polymer Co-Networks: Synthesis, Properties, Modelling and Applications; Patrickios, C.S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2020; pp. 47–76. [Google Scholar]
- Bera, A.; Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Degradable/Cytocompatible and PH Responsive Amphiphilic Conetwork Gels Based on Agarose-Graft Copolymers and Polycaprolactone. J. Mater. Chem. B 2015, 3, 8548–8557. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer Nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Zafar, M.S.; Alnazzawi, A.A.; Alrahabi, M.; Fareed, M.A.; Najeeb, S.; Khurshid, Z. 18-Nanotechnology and Nanomaterials in Dentistry. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 477–505. ISBN 978-0-08-102476-8. [Google Scholar]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health Concerns of Various Nanoparticles: A Review of Their in Vitro and in Vivo Toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Luo, S.-H.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Chen, G.-Y.; Peng, Q. Functional Nanomaterials and Their Potential Applications in Antibacterial Therapy. Pharm. Nanotechnol. 2019, 7, 129–146. [Google Scholar] [CrossRef]
- Chae, T.; Yang, H.; Ko, F.; Troczynski, T. Bio-Inspired Dicalcium Phosphate Anhydrate/Poly(Lactic Acid) Nanocomposite Fibrous Scaffolds for Hard Tissue Regeneration: In Situ Synthesis and Electrospinning. J. Biomed. Mater. Res. Part A 2014, 102, 514–522. [Google Scholar] [CrossRef]
- Bottino, M.C.; Yassen, G.H.; Platt, J.A.; Labban, N.; Windsor, L.J.; Spolnik, K.J.; Bressiani, A.H.A. A Novel Three-Dimensional Scaffold for Regenerative Endodontics: Materials and Biological Characterizations. J. Tissue Eng. Regen. Med. 2015, 9, E116–E123. [Google Scholar] [CrossRef]
- Fakhruddin, K.; Hassan, R.; Khan, M.U.A.; Allisha, S.N.; Razak, S.I.A.; Zreaqat, M.H.; Latip, H.F.M.; Jamaludin, M.N.; Hassan, A. Halloysite Nanotubes and Halloysite-Based Composites for Biomedical Applications. Arab. J. Chem. 2021, 14, 103294. [Google Scholar] [CrossRef]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative Toxicity of 24 Manufactured Nanoparticles in Human Alveolar Epithelial and Macrophage Cell Lines. Part. Fibre Toxicol. 2009, 6, 14. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Design, Fabrication and Drug Release Potential of Dual Stimuli-Responsive Composite Hydrogel Nanoparticle Interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance PH-Triggered Drug Release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-Assembly of Partially Alkylated Dextran-Graft-Poly[(2-Dimethylamino)Ethyl Methacrylate] Copolymer Facilitating Hydrophobic/Hydrophilic Drug Delivery and Improving Conetwork Hydrogel Properties. Biomacromolecules 2018, 19, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Effect of Polyethylene Glycol on Properties and Drug Encapsulation–Release Performance of Biodegradable/Cytocompatible Agarose–Polyethylene Glycol–Polycaprolactone Amphiphilic Co-Network Gels. ACS Appl. Mater. Interfaces 2016, 8, 3182–3192. [Google Scholar] [CrossRef]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and Evaluation of Forcespun Functionalized Carbon Nano-Onions Reinforced Poly (ε-Caprolactone) Composite Nanofibers for PH-Responsive Drug Release. Mater. Sci. Eng. C 2020, 112, 110928. [Google Scholar] [CrossRef]
- Mamidi, N.; Villela Castrejón, J.; González-Ortiz, A. Rational Design and Engineering of Carbon Nano-Onions Reinforced Natural Protein Nanocomposite Hydrogels for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103696. [Google Scholar] [CrossRef]
- Yahara, T.; Koga, T.; Yoshida, S.; Nakagawa, S.; Deguchi, H.; Shirouzu, K. Relationship Between Microvessel Density and Thermographic Hot Areas in Breast Cancer. Surg Today 2003, 33, 243–248. [Google Scholar] [CrossRef]
- Issels, R.D. Hyperthermia Adds to Chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; González-Ortiz, A. Engineering of Carbon Nano-Onion Bioconjugates for Biomedical Applications. Mater. Sci. Eng. C 2021, 120, 111698. [Google Scholar] [CrossRef]
- Mamidi, N.; González-Ortiz, A.; Lopez Romo, I.V.; Barrera, E. Development of Functionalized Carbon Nano-Onions Reinforced Zein Protein Hydrogel Interfaces for Controlled Drug Release. Pharmaceutics 2019, 11, 621. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Castrejón, J.V. Unconventional and Facile Production of a Stimuli-Responsive Multifunctional System for Simultaneous Drug Delivery and Environmental Remediation. Environ. Sci. Nano 2021, 8, 2081–2097. [Google Scholar] [CrossRef]
- Huang, S.H.; Liu, P.; Mokasdar, A.; Hou, L. Additive Manufacturing and Its Societal Impact: A Literature Review. Int. J. Adv. Manuf. Technol. 2013, 67, 1191–1203. [Google Scholar] [CrossRef]
- Braian, M.; Jimbo, R.; Wennerberg, A. Production Tolerance of Additive Manufactured Polymeric Objects for Clinical Applications. Dent. Mater. 2016, 32, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hickel, R.; Reymus, M. 3D Printing in Dentistry—State of the Art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D Printing of Polymer Nanocomposites via Stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous Liquid Interface Production of 3D Objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D Printing with Polymers: Challenges among Expanding Options and Opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Chen, L.; Wang, Y.; Sun, Y. Application of FDM Three-Dimensional Printing Technology in the Digital Manufacture of Custom Edentulous Mandible Trays. Sci. Rep. 2016, 6, 19207. [Google Scholar] [CrossRef]
- Standard Terminology for Additive Manufacturing—Coordinate Systems and Test Methodologies. Available online: https://www.astm.org/f2921-13r19.html (accessed on 18 March 2022).
- Specification for Additive Manufacturing File Format (AMF) Version 1.2. Available online: https://www.astm.org/f2915-20.html (accessed on 18 March 2022).
- 14:00–17:00 ISO/ASTM DIS 52931. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/46/74641.html (accessed on 18 March 2022).
- 14:00–17:00 ISO/ASTM PRF TR 52906. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/57/75716.html (accessed on 18 March 2022).
- Additive Manufacturing–Process Characteristics and Performance: Practice for Metal Powder Bed Fusion Process to Meet Critical Applications. Available online: https://www.astm.org/f3303-19.html (accessed on 18 March 2022).
- Additive Manufacturing—Feedstock Materials—Methods to Characterize Metallic Powders. Available online: https://www.astm.org/f3382-19.html (accessed on 18 March 2022).
- Marques, A.; Miranda, G.; Silva, F.; Pinto, P.; Carvalho, Ó. Review on Current Limits and Potentialities of Technologies for Biomedical Ceramic Scaffolds Production. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 377–393. [Google Scholar] [CrossRef]
- Yin, L.; Peng, H.X.; Yang, L.; Su, B. Fabrication of Three-Dimensional Inter-Connective Porous Ceramics via Ceramic Green Machining and Bonding. J. Eur. Ceram. Soc. 2008, 28, 531–537. [Google Scholar] [CrossRef]
- Jun, I.-K.; Koh, Y.-H.; Song, J.-H.; Kim, H.-E. Fabrication and Characterization of Dual-Channeled Zirconia Ceramic Scaffold. J. Am. Ceram. Soc. 2006, 89, 2021–2026. [Google Scholar] [CrossRef]
- Marques, A.; Miranda, G.; Faria, D.; Pinto, P.; Silva, F.; Carvalho, Ó. Novel Design of Low Modulus High Strength Zirconia Scaffolds for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2019, 97, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Lakshmi, T. Bioglass: A Novel Biocompatible Innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Vogt, S.; Weisser, J.; Grimm, G.; Rechtenbach, A.; Meyer, W.; Schnabelrauch, M. Development of a New Calcium Phosphate Powder-Binder System for the 3D Printing of Patient Specific Implants. J. Mater. Sci. Mater. Med. 2007, 18, 909–916. [Google Scholar] [CrossRef]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-Dimensional Printing of Porous Ceramic Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74, 782–788. [Google Scholar] [CrossRef]
- Stanciuc, A.-M.; Sprecher, C.M.; Adrien, J.; Roiban, L.I.; Alini, M.; Gremillard, L.; Peroglio, M. Robocast Zirconia-Toughened Alumina Scaffolds: Processing, Structural Characterisation and Interaction with Human Primary Osteoblasts. J. Eur. Ceram. Soc. 2018, 38, 845–853. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, C.-Y.; Liu, F.-H.; Chen, M.H.-C.; Lin, C.-P.; Ho, H.-N.; Liao, Y.-S. 3D Printing Bioceramic Porous Scaffolds with Good Mechanical Property and Cell Affinity. PLoS ONE 2015, 10, e0143713. [Google Scholar] [CrossRef]
- Russias, J.; Saiz, E.; Deville, S.; Gryn, K.; Liu, G.; Nalla, R.K.; Tomsia, A.P. Fabrication and in Vitro Characterization of Three-Dimensional Organic/Inorganic Scaffolds by Robocasting. J. Biomed. Mater. Res. Part A 2007, 83, 434–445. [Google Scholar] [CrossRef]
- Deckers, J.; Vleugels, J.; Kruth, J.-P. Additive Manufacturing of Ceramics: A Review. J. Ceram. Sci. Technol. 2014, 5, 245–260. [Google Scholar] [CrossRef]
- Shuai, C.; Li, P.; Liu, J.; Peng, S. Optimization of TCP/HAP Ratio for Better Properties of Calcium Phosphate Scaffold via Selective Laser Sintering. Mater. Charact. 2013, 77, 23–31. [Google Scholar] [CrossRef]
- Liu, F.-H.; Liao, Y.-S. Fabrication of Inner Complex Ceramic Parts by Selective Laser Gelling. J. Eur. Ceram. Soc. 2010, 30, 3283–3289. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-Dimensional Nanocomposite Scaffolds Fabricated via Selective Laser Sintering for Bone Tissue Engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Shen, Z. Laser Sintering of Ceramics. J. Asian Ceram. Soc. 2013, 1, 315–321. [Google Scholar] [CrossRef]
- Brniak, W.; Jachowicz, R.; Krupa, A.; Skorka, T.; Niwinski, K. Evaluation of Co-Processed Excipients Used for Direct Compression of Orally Disintegrating Tablets (ODT) Using Novel Disintegration Apparatus. Pharm. Dev. Technol. 2013, 18, 464–474. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications–Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Turek, P.; Budzik, G.; Oleksy, M.; Bulanda, K. Polymer Materials Used in Medicine Processed by Additive Techniques. Polimery 2020, 65, 510–515. [Google Scholar] [CrossRef]
- Abduo, J.; Lyons, K. Rationale for the Use of CAD/CAM Technology in Implant Prosthodontics. Int. J. Dent. 2013, 2013, e768121. [Google Scholar] [CrossRef]
- Takahashi, T.; Gunne, J. Fit of Implant Frameworks: An in Vitro Comparison between Two Fabrication Techniques. J. Prosthet. Dent. 2003, 89, 256–260. [Google Scholar] [CrossRef]
- Kuang, T.; Chen, F.; Chang, L.; Zhao, Y.; Fu, D.; Gong, X.; Peng, X. Facile Preparation of Open-Cellular Porous Poly (l-Lactic Acid) Scaffold by Supercritical Carbon Dioxide Foaming for Potential Tissue Engineering Applications. Chem. Eng. J. 2017, 307, 1017–1025. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Le, T.Y.L.; Daly, S.; Wang, Y.; Maitz, P.K.; Schindeler, A.; Dehghani, F. Formation of Porous Biodegradable Scaffolds Based on Poly(Propylene Carbonate) Using Gas Foaming Technology. Mater. Sci. Eng. C 2019, 96, 824–830. [Google Scholar] [CrossRef]
- Dickinson, E. Structuring of Colloidal Particles at Interfaces and the Relationship to Food Emulsion and Foam Stability. J. Colloid Interface Sci. 2015, 449, 38–45. [Google Scholar] [CrossRef]
- Daly, S.R.; Fathi, A.; Bahramian, B.; Manavitehrani, I.; McClure, D.D.; Valtchev, P.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. A Green Process for the Purification of Biodegradable Poly(β-Hydroxybutyrate). J. Supercrit. Fluids 2018, 135, 84–90. [Google Scholar] [CrossRef]
- Duarte, R.M.; Correia-Pinto, J.; Reis, R.L.; Duarte, A.R.C. Subcritical Carbon Dioxide Foaming of Polycaprolactone for Bone Tissue Regeneration. J. Supercrit. Fluids 2018, 140, 1–10. [Google Scholar] [CrossRef]
- Rana, D.; Arulkumar, S.; Vishwakarma, A.; Ramalingam, M. Chapter 10-Considerations on Designing Scaffold for Tissue Engineering. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 133–148. ISBN 978-0-12-397157-9. [Google Scholar]
- Miranda, G.; Buciumeanu, M.; Madeira, S.; Carvalho, O.; Soares, D.; Silva, F.S. Hybrid Composites–Metallic and Ceramic Reinforcements Influence on Mechanical and Wear Behavior. Compos. Part B Eng. 2015, 74, 153–165. [Google Scholar] [CrossRef]
- Miranda, G.; Araújo, A.; Bartolomeu, F.; Buciumeanu, M.; Carvalho, O.; Souza, J.C.M.; Silva, F.S.; Henriques, B. Design of Ti6Al4V-HA Composites Produced by Hot Pressing for Biomedical Applications. Mater. Des. 2016, 108, 488–493. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Carvalho, O.; Silva, F.S.; Miranda, G. 316L Stainless Steel Mechanical and Tribological Behavior—A Comparison between Selective Laser Melting, Hot Pressing and Conventional Casting. Addit. Manuf. 2017, 16, 81–89. [Google Scholar] [CrossRef]
- Bannon, R.; Parihar, S.; Skarparis, Y.; Varsou, O.; Cezayirli, E. 3D Printing the Pterygopalatine Fossa: A Negative Space Model of a Complex Structure. Surg. Radiol. Anat. 2018, 40, 185–191. [Google Scholar] [CrossRef]
- Bartikian, M.; Ferreira, A.; Gonçalves-Ferreira, A.; Neto, L.L. 3D Printing Anatomical Models of Head Bones. Surg. Radiol. Anat. 2019, 41, 1205–1209. [Google Scholar] [CrossRef]
- Chen, S.; Pan, Z.; Wu, Y.; Gu, Z.; Li, M.; Liang, Z.; Zhu, H.; Yao, Y.; Shui, W.; Shen, Z.; et al. The Role of Three-Dimensional Printed Models of Skull in Anatomy Education: A Randomized Controlled Trail. Sci. Rep. 2017, 7, 575. [Google Scholar] [CrossRef]
- Skrzat, J.; Zdilla, M.J.; Brzegowy, P.; Hołda, M. 3D printed replica of the human temporal bone intended for teaching gross anatomy. Folia Med. Crac. 2019, 59, 23–60. [Google Scholar] [CrossRef]
- Tanner, J.A.; Jethwa, B.; Jackson, J.; Bartanuszova, M.; King, T.S.; Bhattacharya, A.; Sharma, R. A Three-Dimensional Print Model of the Pterygopalatine Fossa Significantly Enhances the Learning Experience. Anat. Sci. Educ. 2020, 13, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Ugidos Lozano, M.T.; Blaya Haro, F.; Ruggiero, A.; Manzoor, S.; Nuere Menendez-Pidal, S.; Juanes Méndez, J.A. Different Digitalization Techniques for 3D Printing of Anatomical Pieces. J. Med. Syst. 2018, 42, 46. [Google Scholar] [CrossRef] [PubMed]
- Berli, J.U.; Thomaier, L.; Zhong, S.; Huang, J.; Quinones, A.; Lim, M.; Weingart, J.; Brem, H.; Gordon, C.R. Immediate Single-Stage Cranioplasty Following Calvarial Resection for Benign and Malignant Skull Neoplasms Using Customized Craniofacial Implants. J. Craniofacial Surg. 2015, 26, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Marzo, S.J.; Benscoter, B.; Leonetti, J.P. Contemporary Options for Lateral Skull Base Reconstruction Following Tumor Extirpation. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 330–334. [Google Scholar] [CrossRef]
- Young, C.C.; Hanak, B.W.; Patel, A.P.; Sekhar, L.N. Rapid Intraoperative in Situ Synthetic Cranioplasty. World Neurosurg. 2018, 112, 161–165. [Google Scholar] [CrossRef]
- Coulter, C.; Richards, R.; Peterson, D.; Collier, J. Parietal Skull Reconstruction Using Immediate Peek Cranioplasty Following Resection for Craniofacial Fibrous Dysplasia. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, e208–e209. [Google Scholar] [CrossRef]
- Müller, A.; Krishnan, K.G.; Uhl, E.; Mast, G. The Application of Rapid Prototyping Techniques in Cranial Reconstruction and Preoperative Planning in Neurosurgery. J. Craniofacial Surg. 2003, 14, 899–914. [Google Scholar] [CrossRef]
- Honeybul, S.; Morrison, D.A. Skull Vault Destruction After Rhinocerebral Mucormycosis. World Neurosurg. 2012, 78, 553.e1–553.e4. [Google Scholar] [CrossRef]
- Weiner, G.M.; Lacey, M.R.; Mackenzie, L.; Shah, D.P.; Frangos, S.G.; Grady, M.S.; Kofke, A.; Levine, J.; Schuster, J.; Le Roux, P.D. Decompressive Craniectomy for Elevated Intracranial Pressure and Its Effect on the Cumulative Ischemic Burden and Therapeutic Intensity Levels After Severe Traumatic Brain Injury. Neurosurgery 2010, 66, 1111–1119. [Google Scholar] [CrossRef]
- Honeybul, S.; Ho, K.M.; Lind, C.R.P.; Gillett, G.R. The Current Role of Decompressive Craniectomy for Severe Traumatic Brain Injury. J. Clin. Neurosci. 2017, 43, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Bor-Seng-Shu, E.; Hirsch, R.; Teixeira, M.J.; de Andrade, A.F.; Marino, R. Cerebral Hemodynamic Changes Gauged by Transcranial Doppler Ultrasonography in Patients with Posttraumatic Brain Swelling Treated by Surgical Decompression. J. Neurosurg. 2006, 104, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Balestreri, M.; Czosnyka, M.; Hutchinson, P.; Steiner, L.A.; Hiler, M.; Smielewski, P.; Pickard, J.D. Impact of Intracranial Pressure and Cerebral Perfusion Pressure on Severe Disability and Mortality after Head Injury. Neurocrit Care 2006, 4, 8–13. [Google Scholar] [CrossRef]
- Unterberg, A.W.; Stover, J.; Kress, B.; Kiening, K.L. Edema and Brain Trauma. Neuroscience 2004, 129, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.M.; Kochanek, P.M.; Simard, J.M. Pathophysiology and Treatment of Cerebral Edema in Traumatic Brain Injury. Neuropharmacology 2019, 145, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.; Timofeev, I.; Kirkpatrick, P. Surgery for Brain Edema. Neurosurg. Focus 2007, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kolias, A.G.; Kirkpatrick, P.J.; Hutchinson, P.J. Decompressive Craniectomy: Past, Present and Future. Nat. Rev. Neurol. 2013, 9, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Skoglund, T.S.; Eriksson-Ritzén, C.; Jensen, C.; Rydenhag, B. Aspects on Decompressive Craniectomy in Patients with Traumatic Head Injuries. J. Neurotrauma 2006, 23, 1502–1509. [Google Scholar] [CrossRef]
- Soustiel, J.F.; Sviri, G.E.; Mahamid, E.; Shik, V.; Abeshaus, S.; Zaaroor, M. Cerebral Blood Flow and Metabolism Following Decompressive Craniectomy for Control of Increased Intracranial Pressure. Neurosurgery 2010, 67, 65–72. [Google Scholar] [CrossRef]
- Timofeev, I.; Czosnyka, M.; Nortje, J.; Smielewski, P.; Kirkpatrick, P.; Gupta, A.; Hutchinson, P. Effect of Decompressive Craniectomy on Intracranial Pressure and Cerebrospinal Compensation Following Traumatic Brain Injury. J. Neurosurg. 2008, 108, 66–73. [Google Scholar] [CrossRef]
- Schmidt, J.H.; Reyes, B.J.; Fischer, R.; Flaherty, S.K. Use of Hinge Craniotomy for Cerebral Decompression: Technical Note. J. Neurosurg. 2007, 107, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Boström, S.; Bobinski, L.; Zsigmond, P.; Theodorsson, A. Improved Brain Protection at Decompressive Craniectomy–a New Method Using Palacos⌖ R-40 (Methylmethacrylate). Acta Neurochir 2005, 147, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Honeybul, S. Decompressive Craniectomy: A New Complication. J. Clin. Neurosci. 2009, 16, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Winkler, P.A.; Stummer, W.; Linke, R.; Krishnan, K.G.; Tatsch, K. The Influence of Cranioplasty on Postural Blood Flow Regulation, Cerebrovascular Reserve Capacity, and Cerebral Glucose Metabolism. Neurosurg. Focus 2000, 8, 1–9. [Google Scholar] [CrossRef]
- Akins, P.T.; Guppy, K.H. Sinking Skin Flaps, Paradoxical Herniation, and External Brain Tamponade: A Review of Decompressive Craniectomy Management. Neurocrit Care 2008, 9, 269–276. [Google Scholar] [CrossRef]
- Trnovec, S.; Halatsch, M.-E.; Behnke-Mursch, J.; Mursch, K. An Ultrasound Study of Brain Tissue Biomechanics in Patients Following Craniectomy. NeuroRehabilitation 2009, 24, 267–271. [Google Scholar] [CrossRef]
- Stiver, S.I.; Wintermark, M.; Manley, G.T. Reversible Monoparesis Following Decompressive Hemicraniectomy for Traumatic Brain Injury. J. Neurosurg. 2008, 109, 245–254. [Google Scholar] [CrossRef]
- Stiver, S.I. Complications of Decompressive Craniectomy for Traumatic Brain Injury. Neurosurg. Focus 2009, 26, E7. [Google Scholar] [CrossRef]
- Dujovny, M.; Agner, C.; Aviles, A. Syndrome of the Trephined: Theory and Facts. Crit. Rev. Neurosurg. 1999, 9, 271–278. [Google Scholar] [CrossRef]
- Honeybul, S.; Janzen, C.; Kruger, K.; Ho, K.M. The Incidence of Neurologic Susceptibility to a Skull Defect. World Neurosurg. 2016, 86, 147–152. [Google Scholar] [CrossRef]
- Ashayeri, K.; Jackson, E.M.; Huang, J.; Brem, H.; Gordon, C.R. Syndrome of the Trephined: A Systematic Review. Neurosurgery 2016, 79, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, E.A.; Cuello, J.F.; Purves, C.P. Coma due to syndrome of the trephined. Medicina 2018, 78, 282–285. [Google Scholar] [PubMed]
- Annan, M.; De Toffol, B.; Hommet, C.; Mondon, K. Sinking Skin Flap Syndrome (or Syndrome of the Trephined): A Review. Br. J. Neurosurg. 2015, 29, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Gadde, J.; Dross, P.; Spina, M. Syndrome of the Trephined (Sinking Skin Flap Syndrome) with and without Paradoxical Herniation: A Series of Case Reports and Review. Del. Med. J. 2012, 84, 213–218. [Google Scholar]
- Janzen, C.; Kruger, K.; Honeybul, S. Syndrome of the Trephined Following Bifrontal Decompressive Craniectomy: Implications for Rehabilitation. Brain Inj. 2012, 26, 101–105. [Google Scholar] [CrossRef]
- Láng, J.; Ganau, M.; Prisco, L.; Bozsik, K.; Banczerowski, P. Syndrome of Trephined-Underestimated and Poorly Understood Complication after Decompressive Craniectomy. Ideggyogy Sz 2016, 69, 227–232. [Google Scholar] [CrossRef]
- Bateman, E.A.; VanderEnde, J.; Sequeira, K.; MacKenzie, H.M. Postural Neurologic Deficits after Decompressive Craniectomy: A Case Series of Sinking Skin Flap Syndrome in Traumatic Brain Injury. NeuroRehabilitation 2021, 49, 663–672. [Google Scholar] [CrossRef]
- Lilja-Cyron, A.; Andresen, M.; Kelsen, J.; Andreasen, T.H.; Petersen, L.G.; Fugleholm, K.; Juhler, M. Intracranial Pressure before and after Cranioplasty: Insights into Intracranial Physiology. J. Neurosurg. 2019, 133, 1548–1558. [Google Scholar] [CrossRef]
- Ozoner, B. Cranioplasty Following Severe Traumatic Brain Injury: Role in Neurorecovery. Curr. Neurol. Neurosci. Rep. 2021, 21, 62. [Google Scholar] [CrossRef]
- Motherway, J.A.; Verschueren, P.; Van der Perre, G.; Vander Sloten, J.; Gilchrist, M.D. The Mechanical Properties of Cranial Bone: The Effect of Loading Rate and Cranial Sampling Position. J. Biomech. 2009, 42, 2129–2135. [Google Scholar] [CrossRef]
- Yoganandan, N.; Pintar, F.A. Biomechanics of Temporo-Parietal Skull Fracture. Clin. Biomech. 2004, 19, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, J.; Safavi, S.; Scholze, M.; Li, K.C.; Waddell, J.N.; Busse, B.; Ondruschka, B.; Hammer, N. Topographical Mapping of the Mechanical Characteristics of the Human Neurocranium Considering the Role of Individual Layers. Sci. Rep. 2021, 11, 3721. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Nauman, E.A.; Moryl, D.; Lycke, R.; Chen, W.W. The Effects of Loading-Direction and Strain-Rate on the Mechanical Behaviors of Human Frontal Skull Bone. J. Mech. Behav. Biomed. Mater. 2020, 103, 103597. [Google Scholar] [CrossRef] [PubMed]
- Autuori, B.; Bruyere-Garnier, K.; Morestin, F.; Brunet, M.; Verriest, J.-P. Finite Element Modeling of the Head Skeleton with a New Local Quantitative Assessment Approach. IEEE Trans. Biomed. Eng. 2006, 53, 1225–1232. [Google Scholar] [CrossRef]
- Johnson, W.C.; Ravindra, V.M.; Fielder, T.; Ishaque, M.; Patterson, T.T.; McGinity, M.J.; Lacci, J.V.; Grandhi, R. Surface Area of Decompressive Craniectomy Predicts Bone Flap Failure after Autologous Cranioplasty: A Radiographic Cohort Study. NeurotraumaRep. 2021, 2, 391–398. [Google Scholar] [CrossRef]
- Las, D.E.; Verwilghen, D.; Mommaerts, M.Y. A Systematic Review of Cranioplasty Material Toxicity in Human Subjects. J. Cranio-Maxillofac. Surg. 2021, 49, 34–46. [Google Scholar] [CrossRef]
- Morselli, C.; Zaed, I.; Tropeano, M.P.; Cataletti, G.; Iaccarino, C.; Rossini, Z.; Servadei, F. Comparison between the Different Types of Heterologous Materials Used in Cranioplasty: A Systematic Review of the Literature. J. Neurosurg. Sci. 2019, 63, 723–736. [Google Scholar] [CrossRef]
- Iaccarino, C.; Kolias, A.; Adelson, P.D.; Rubiano, A.M.; Viaroli, E.; Buki, A.; Cinalli, G.; Fountas, K.; Khan, T.; Signoretti, S.; et al. Consensus Statement from the International Consensus Meeting on Post-Traumatic Cranioplasty. Acta Neurochir. 2021, 163, 423–440. [Google Scholar] [CrossRef]
- Marcián, P.; Narra, N.; Borák, L.; Chamrad, J.; Wolff, J. Biomechanical Performance of Cranial Implants with Different Thicknesses and Material Properties: A Finite Element Study. Comput. Biol. Med. 2019, 109, 43–52. [Google Scholar] [CrossRef]
- Wallace, R.D.; Salt, C.; Konofaos, P. Comparison of Autogenous and Alloplastic Cranioplasty Materials Following Impact Testing. J. Craniofacial Surg. 2015, 26, 1551–1557. [Google Scholar] [CrossRef]
- Tsouknidas, A.; Maropoulos, S.; Savvakis, S.; Michailidis, N. FEM Assisted Evaluation of PMMA and Ti6Al4V as Materials for Cranioplasty Resulting Mechanical Behaviour and the Neurocranial Protection. Bio-Med. Mater. Eng. 2011, 21, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, D.S.; Cheong, J.H.; Kim, J.H.; Song, K.Y.; Kong, M.H. Comparison of Complications Following Cranioplasty Using a Sterilized Autologous Bone Flap or Polymethyl Methacrylate. Korean J. Neurotrauma 2017, 13, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Paliga, J.T.; Bartlett, S.P. Cranioplasty: Indications and Advances. Curr. Opin. Otolaryngol. Head Neck Surg. 2013, 21, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Manjila, S.; Selman, W.R.; Dean, D. The Recent Revolution in the Design and Manufacture of Cranial Implants: Modern Advancements and Future Directions. Neurosurgery 2015, 77, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Golz, T.; Graham, C.R.; Busch, L.C.; Wulf, J.; Winder, R.J. Temperature Elevation during Simulated Polymethylmethacrylate (PMMA) Cranioplasty in a Cadaver Model. J. Clin. Neurosci. 2010, 17, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Wu, C.-T.; Lee, S.-T.; Chen, P.-J. Cranioplasty Using Polymethyl Methacrylate Prostheses. J. Clin. Neurosci. 2009, 16, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Andereggen, L.; Erhardt, S.; Fathi, A.-R.; Fandino, J.; Raabe, A.; Beck, J. Intraoperative Template-Molded Bone Flap Reconstruction for Patient-Specific Cranioplasty. Neurosurg. Rev. 2012, 35, 527–535. [Google Scholar] [CrossRef]
- Guerrini, F.; Dallolio, V.; Grimod, G.; Cesana, C.; Vismara, D.; Franzin, A.B. It Is Time to Reduce Free-Hand Manipulation: Case Report of Our Proposal for an Innovative 1-Step Cranioplasty. World Neurosurg. 2017, 107, 1052.e7–1052.e10. [Google Scholar] [CrossRef]
- Schön, S.N.; Skalicky, N.; Sharma, N.; Zumofen, D.W.; Thieringer, F.M. 3D-Printer-Assisted Patient-Specific Polymethyl Methacrylate Cranioplasty: A Case Series of 16 Consecutive Patients. World Neurosurg. 2021, 148, e356–e362. [Google Scholar] [CrossRef]
- Abdel Hay, J.; Smayra, T.; Moussa, R. Customized Polymethylmethacrylate Cranioplasty Implants Using 3-Dimensional Printed Polylactic Acid Molds: Technical Note with 2 Illustrative Cases. World Neurosurg. 2017, 105, 971–979.e1. [Google Scholar] [CrossRef]
- Desai, J.B. Cost-Effective Technique of Fabrication of Polymethyl Methacrylate Based Cranial Implant Using Three-Dimensional Printed Moulds and Wax Elimination Technique. J. Craniofacial Surg. 2019, 30, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Pöppe, J.P.; Spendel, M.; Schwartz, C.; Winkler, P.A.; Wittig, J. The “Springform” Technique in Cranioplasty: Custom Made 3D-Printed Templates for Intraoperative Modelling of Polymethylmethacrylate Cranial Implants. Acta Neurochir. 2021, 164, 679–688. [Google Scholar] [CrossRef] [PubMed]
- da Silva Júnior, E.B.; de Aragão, A.H.; de Paula Loureiro, M.; Lobo, C.S.; Oliveti, A.F.; de Oliveira, R.M.; Ramina, R. Cranioplasty with Three-Dimensional Customised Mould for Polymethylmethacrylate Implant: A Series of 16 Consecutive Patients with Cost-Effectiveness Consideration. 3D Print. Med. 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Maricevich, J.P.B.R.; Cezar-Junior, A.B.; de Oliveira-Junior, E.X.; Veras e Silva, J.A.M.; da Silva, J.V.L.; Nunes, A.A.; Almeida, N.S.; Azevedo-Filho, H.R.C. Functional and Aesthetic Evaluation after Cranial Reconstruction with Polymethyl Methacrylate Prostheses Using Low-Cost 3D Printing Templates in Patients with Cranial Defects Secondary to Decompressive Craniectomies: A Prospective Study. Surg. Neurol. Int. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, H.-B.; Ha, S.-K.; Lim, D.-J.; Kim, S.-D. Predictive Factors of Surgical Site Infection Following Cranioplasty: A Study Including 3D Printed Implants. Front. Neurol. 2021, 12, 745575. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.R.; Madden, C.; Beshay, J.; White, J.; Gambrell, K.; Rickert, K. Autologous and Acrylic Cranioplasty: A Review of 10 Years and 258 Cases. World Neurosurg. 2014, 82, e525–e530. [Google Scholar] [CrossRef]
- Malcolm, J.G.; Mahmooth, Z.; Rindler, R.S.; Allen, J.W.; Grossberg, J.A.; Pradilla, G.; Ahmad, F.U. Autologous Cranioplasty Is Associated with Increased Reoperation Rate: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 116, 60–68. [Google Scholar] [CrossRef]
- Jaberi, J.; Gambrell, K.; Tiwana, P.; Madden, C.; Finn, R. Long-Term Clinical Outcome Analysis of Poly-Methyl-Methacrylate Cranioplasty for Large Skull Defects. J. Oral Maxillofac. Surg. 2013, 71, e81–e88. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Schlett, C.L.; Fournier, J.-Y.; Cadosch, D. Laboratory Confirmed Polymethyl-Methacrylate (Palacos®)-Hypersensitivity after Cranioplasty. Clin. Neurol. Neurosurg. 2010, 112, 915–916. [Google Scholar] [CrossRef]
- van de Vijfeijken, S.E.C.M.; Münker, T.J.A.G.; de Jager, N.; Vandertop, W.P.; Becking, A.G.; Kleverlaan, C.J.; Becking, A.G.; Dubois, L.; Karssemakers, L.H.E.; Milstein, D.M.J.; et al. Properties of an In Vivo Fractured Poly(Methyl Methacrylate) Cranioplasty After 15 Years. World Neurosurg. 2019, 123, e60–e68. [Google Scholar] [CrossRef]
- Punchak, M.; Chung, L.K.; Lagman, C.; Bui, T.T.; Lazareff, J.; Rezzadeh, K.; Jarrahy, R.; Yang, I. Outcomes Following Polyetheretherketone (PEEK) Cranioplasty: Systematic Review and Meta-Analysis. J. Clin. Neurosci. 2017, 41, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hanasono, M.M.; Goel, N.; DeMonte, F. Calvarial Reconstruction With Polyetheretherketone Implants. Ann. Plast. Surg. 2009, 62, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Aghlmandi, S.; Dalcanale, F.; Seiler, D.; Zeilhofer, H.-F.; Honigmann, P.; Thieringer, F.M. Quantitative Assessment of Point-of-Care 3D-Printed Patient-Specific Polyetheretherketone (PEEK) Cranial Implants. Int. J. Mol. Sci. 2021, 22, 8521. [Google Scholar] [CrossRef] [PubMed]
- Dodier, P.; Winter, F.; Auzinger, T.; Mistelbauer, G.; Frischer, J.M.; Wang, W.-T.; Mallouhi, A.; Marik, W.; Wolfsberger, S.; Reissig, L.; et al. Single-Stage Bone Resection and Cranioplastic Reconstruction: Comparison of a Novel Software-Derived PEEK Workflow with the Standard Reconstructive Method. Int. J. Oral Maxillofac. Surg. 2020, 49, 1007–1015. [Google Scholar] [CrossRef]
- Jonkergouw, J.; van de Vijfeijken, S.E.C.M.; Nout, E.; Theys, T.; Van de Casteele, E.; Folkersma, H.; Depauw, P.R.A.M.; Becking, A.G. Outcome in Patient-Specific PEEK Cranioplasty: A Two-Center Cohort Study of 40 Implants. J. Cranio-Maxillofac. Surg. 2016, 44, 1266–1272. [Google Scholar] [CrossRef]
- Thien, A.; King, N.K.K.; Ang, B.T.; Wang, E.; Ng, I. Comparison of Polyetheretherketone and Titanium Cranioplasty after Decompressive Craniectomy. World Neurosurg. 2015, 83, 176–180. [Google Scholar] [CrossRef]
- Rosenthal, G.; Ng, I.; Moscovici, S.; Lee, K.K.; Lay, T.; Martin, C.; Manley, G.T. Polyetheretherketone Implants for the Repair of Large Cranial Defects: A 3-Center Experience. Neurosurgery 2014, 75, 523–529. [Google Scholar] [CrossRef]
- Asaad, M.; Taslakian, E.N.; Banuelos, J.; Abu-Ghname, A.; Bite, U.; Mardini, S.; Van Gompel, J.J.; Sharaf, B. Surgical and Patient-Reported Outcomes in Patients With PEEK Versus Titanium Cranioplasty Reconstruction. J. Craniofacial Surg. 2021, 32, 193–197. [Google Scholar] [CrossRef]
- Pecoraro, F.; Shingaki, M.; Steuer, J.; Chaykovska, L.; Rancic, Z.; Weber, A.; Nguyen-Kim, T.D.L.; Bettex, D.; Veith, F.J.; Lachat, M. Treatment of Isolated Ascending Aortic Aneurysm by Off-Pump Epiaortic Wrapping Is Safe and Durable. Interact. Cardio Vascular Thorac. Surg. 2016, 23, 286–291. [Google Scholar] [CrossRef]
- Ruiz-Jasbon, F.; Norrby, J.; Ivarsson, M.-L.; Björck, S. Inguinal Hernia Repair Using a Synthetic Long-Term Resorbable Mesh: Results from a 3-Year Prospective Safety and Performance Study. Hernia 2014, 18, 723–730. [Google Scholar] [CrossRef]
- Andrzejak, S.; Fortuniak, J.; Wróbel-Wiśniewska, G.; Zawirski, M. Clinical Evaluation of the Polypropylene-Polyester Knit Used as a Cranioplasty Material. Acta Neurochir. 2005, 147, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, P.; Tomaszewski, G.; Kotwica, Z.; Kwinta, B.; Zwoliński, J. Reconstruction of Cranial Defects with Individually Formed Cranial Prostheses Made of Polypropylene Polyester Knitwear: An Analysis of 48 Consecutive Patients. J. Neurotrauma 2012, 29, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, P.; Tomaszewski, G.; Wróbel-Wiśniewska, G.; Zawirski, M. Polypropylene–Polyester Cranial Prostheses Prepared with CAD/CAM Technology. Report of First 15 Cases. Clin. Neurol. Neurosurg. 2011, 113, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, P.; Tomaszewski, G.; Sujka, W.; Kotwica, Z.; Stoma, F.; Kwinta, B.; Moskała, M.; Papierz, T.; Wójcik, R.; Zwoliński, J.; et al. Treatment of Very Large Cranial Defects with Individually Shaped Polypropylene Polyester Knitwear Prostheses: A Series of 11 Cases. J. Neurosurg. Sci. 2020, 64, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kotwica, Z.; Zawirski, M.; Andrzejak, S.; Papierz, W.; Chmielowski, M. Cranioplasty of an Extremely Large Cranial Defect Caused by Transitional Meningioma with a Knitted Polypropylen-Polyester Prothesis “Codubix”. Acta Neurochir. 1991, 112, 147–150. [Google Scholar] [CrossRef]
- Gawlik, J.D.; Balcerzak, R. The use of polypropylene-polyester prostheses in cranioplasty. Polim. Med. 1980, 10, 215–223. [Google Scholar]
- Staniszewska, J. Polypropylene-polyester prostheses. Experimental studies. Polim. Med. 1980, 10, 205–214. [Google Scholar]
- Chrzan, R.; Urbanik, A.; Karbowski, K.; Moskała, M.; Polak, J.; Pyrich, M. Cranioplasty Prosthesis Manufacturing Based on Reverse Engineering Technology. Med. Sci. Monit. 2012, 18, MT1–MT6. [Google Scholar] [CrossRef]
- Bagambisa, F.B.; Joos, U.; Schilli, W. Mechanisms and Structure of the Bond between Bone and Hydroxyapatite Ceramics. J. Biomed. Mater. Res. 1993, 27, 1047–1055. [Google Scholar] [CrossRef]
- Ono, H.; Sase, T.; Tanaka, Y.; Takasuna, H. Histological Assessment of Porous Custom-Made Hydroxyapatite Implants 6 Months and 2.5 Years after Cranioplasty. Surg. Neurol. Int. 2017, 8, 8. [Google Scholar] [CrossRef]
- Brie, J.; Chartier, T.; Chaput, C.; Delage, C.; Pradeau, B.; Caire, F.; Boncoeur, M.-P.; Moreau, J.-J. A New Custom Made Bioceramic Implant for the Repair of Large and Complex Craniofacial Bone Defects. J. Cranio-Maxillofac. Surg. 2013, 41, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Maenhoudt, W.; Hallaert, G.; Kalala, J.-P.; Baert, E.; Dewaele, F.; Bauters, W.; Van Roost, D. Hydroxyapatite Cranioplasty: A Retrospective Evaluation of Osteointegration in 17 Cases. Acta Neurochir. 2018, 160, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Stefini, R.; Esposito, G.; Zanotti, B.; Iaccarino, C.; Fontanella, M.M.; Servadei, F. Use of “Custom Made” Porous Hydroxyapatite Implants for Cranioplasty: Postoperative Analysis of Complications in 1549 Patients. Surg. Neurol. Int. 2013, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, B.; Foroutan, M.; Motavalli, S.; Dujovny, M.; Limaye, S. Computer-Aided Manufacturing of Implants for the Repair of Large Cranial Defects: An Improvement of the Stereolithography Technique. Neurol. Res. 1999, 21, 281–286. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J.; Wang, J. Clinical Outcomes of Digital Three-Dimensional Hydroxyapatite in Repairing Calvarial Defects. J. Craniofacial Surg. 2018, 29, 618–621. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Eichmiller, F.C.; Barndt, P.R. Effects of Fiber Length and Volume Fraction on the Reinforcement of Calcium Phosphate Cement. J. Mater. Sci. Mater. Med. 2001, 12, 57–65. [Google Scholar] [CrossRef]
- Khader, B.A.; Towler, M.R. Materials and Techniques Used in Cranioplasty Fixation: A Review. Mater. Sci. Eng. C 2016, 66, 315–322. [Google Scholar] [CrossRef]
- Lindner, D.; Schlothofer-Schumann, K.; Kern, B.-C.; Marx, O.; Müns, A.; Meixensberger, J. Cranioplasty Using Custom-Made Hydroxyapatite versus Titanium: A Randomized Clinical Trial. J. Neurosurg. 2017, 126, 175–183. [Google Scholar] [CrossRef]
- Iaccarino, C.; Viaroli, E.; Fricia, M.; Serchi, E.; Poli, T.; Servadei, F. Preliminary Results of a Prospective Study on Methods of Cranial Reconstruction. J. Oral Maxillofac. Surg. 2015, 73, 2375–2378. [Google Scholar] [CrossRef]
- Still, M.; Kane, A.; Roux, A.; Zanello, M.; Dezamis, E.; Parraga, E.; Sauvageon, X.; Meder, J.-F.; Pallud, J. Independent Factors Affecting Postoperative Complication Rates After Custom-Made Porous Hydroxyapatite Cranioplasty: A Single-Center Review of 109 Cases. World Neurosurg. 2018, 114, e1232–e1244. [Google Scholar] [CrossRef]
- Fricia, M.; Nicolosi, F.; Ganau, M.; Cebula, H.; Todeschi, J.; Santin, M. des N.; Nannavecchia, B.; Morselli, C.; Chibbaro, S. Cranioplasty with Porous Hydroxyapatite Custom-Made Bone Flap: Results from a Multicenter Study Enrolling 149 Patients Over 15 Years. World Neurosurg. 2019, 121, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.; Heudes, P.M.; Amelot, A.; Cristini, J.; Salaud, C.; Roualdes, V.; Riem, T.; Martin, S.A.; Raoul, S.; Terreaux, L.; et al. Long-Term Follow-Up Comparative Study of Hydroxyapatite and Autologous Cranioplasties: Complications, Cosmetic Results, Osseointegration. World Neurosurg. 2018, 111, e395–e402. [Google Scholar] [CrossRef] [PubMed]
- Staffa, G.; Nataloni, A.; Compagnone, C.; Servadei, F. Custom Made Cranioplasty Prostheses in Porous Hydroxy-Apatite Using 3D Design Techniques: 7 Years Experience in 25 Patients. Acta Neurochir. 2007, 149, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Le, B.Q.; Rai, B.; Hui Lim, Z.X.; Tan, T.C.; Lin, T.; Lin Lee, J.J.; Murali, S.; Teoh, S.H.; Nurcombe, V.; Cool, S.M. A Polycaprolactone-β-Tricalcium Phosphate–Heparan Sulphate Device for Cranioplasty. J. Cranio-Maxillofac. Surg. 2019, 47, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, W.; Wu, S.; Kuss, M.; Jiang, X.; Untrauer, J.B.; Reid, S.P.; Duan, B. 3D Printed Composite Scaffolds with Dual Small Molecule Delivery for Mandibular Bone Regeneration. Biofabrication 2020, 12, 035020. [Google Scholar] [CrossRef]
- Dai, Q.; Li, Q.; Gao, H.; Yao, L.; Lin, Z.; Li, D.; Zhu, S.; Liu, C.; Yang, Z.; Wang, G.; et al. 3D Printing of Cu-Doped Bioactive Glass Composite Scaffolds Promotes Bone Regeneration through Activating the HIF-1α and TNF-α Pathway of HUVECs. Biomater. Sci. 2021, 9, 5519–5532. [Google Scholar] [CrossRef]
- Tel, A.; Tuniz, F.; Fabbro, S.; Sembronio, S.; Costa, F.; Robiony, M. Computer-Guided In-House Cranioplasty: Establishing a Novel Standard for Cranial Reconstruction and Proposal of an Updated Protocol. J. Oral Maxillofac. Surg. 2020, 78, 2297.e1–2297.e16. [Google Scholar] [CrossRef]
- Morales-Gómez, J.A.; Garcia-Estrada, E.; Leos-Bortoni, J.E.; Delgado-Brito, M.; Flores-Huerta, L.E.; Cruz-Arriaga, A.A.D.L.; Torres-Díaz, L.J.; León, Á.R.M.-P. de Cranioplasty with a Low-Cost Customized Polymethylmethacrylate Implant Using a Desktop 3D Printer. J. Neurosurg. 2018, 130, 1721–1727. [Google Scholar] [CrossRef]
- Quereshy, F.A.; Dhaliwal, H.S.; El, S.A.; Horan, M.P.; Dhaliwal, S.S. Resorbable Screw Fixation for Cortical Onlay Bone Grafting: A Pilot Study With Preliminary Results. J. Oral Maxillofac. Surg. 2010, 68, 2497–2502. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kawai, H.; Nakano, K.; Kanno, T.; Takabatake, K.; Nagatsuka, H.; Furuki, Y. Feasible Advantage of Bioactive/Bioresorbable Devices Made of Forged Composites of Hydroxyapatite Particles and Poly-L-lactide in Alveolar Bone Augmentation: A Preliminary Study. Int. J. Med. Sci. 2019, 16, 311–317. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A Comprehensive Review of Biodegradable Synthetic Polymer-Ceramic Composites and Their Manufacture for Biomedical Applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Kim, M.; Kim, D.; Choi, E.-J.; Do, S.H.; Kim, G. 3D Macroporous Biocomposites with a Microfibrous Topographical Cue Enhance New Bone Formation through Activation of the MAPK Signaling Pathways. J. Ind. Eng. Chem. 2021, 104, 478–490. [Google Scholar] [CrossRef]
- Yu, J.; Lee, S.; Choi, S.; Kim, K.K.; Ryu, B.; Kim, C.-Y.; Jung, C.-R.; Min, B.-H.; Xin, Y.-Z.; Park, S.A.; et al. Fabrication of a Polycaprolactone/Alginate Bipartite Hybrid Scaffold for Osteochondral Tissue Using a Three-Dimensional Bioprinting System. Polymers 2020, 12, 2203. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, 2000395. [Google Scholar] [CrossRef]
- Yeon Kwon, D.; Seon Kwon, J.; Hun Park, S.; Hun Park, J.; Hee Jang, S.; Yun Yin, X.; Yun, J.-H.; Ho Kim, J.; Hyun Min, B.; Hee Lee, J.; et al. A Computer-Designed Scaffold for Bone Regeneration within Cranial Defect Using Human Dental Pulp Stem Cells. Sci. Rep. 2015, 5, 12721. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M. Impact Modified PLA-Hydroxyapatite Composites–Thermo-Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2018, 107, 326–333. [Google Scholar] [CrossRef]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Montagna, F.; Maiullaro, T.; Sannino, A.; Maffezzoli, A. 3D Printing of Hydroxyapatite Polymer-Based Composites for Bone Tissue Engineering. J. Polym. Eng. 2017, 37, 741–746. [Google Scholar] [CrossRef]
- Niaza, K.V.; Senatov, F.S.; Kaloshkin, S.D.; Maksimkin, A.V.; Chukov, D.I. 3D-Printed Scaffolds Based on PLA/HA Nanocomposites for Trabecular Bone Reconstruction. J. Phys. Conf. Ser. 2016, 741, 012068. [Google Scholar] [CrossRef]
- Persson, M.; Lorite, G.S.; Kokkonen, H.E.; Cho, S.-W.; Lehenkari, P.P.; Skrifvars, M.; Tuukkanen, J. Effect of Bioactive Extruded PLA/HA Composite Films on Focal Adhesion Formation of Preosteoblastic Cells. Colloids Surf. B Biointerfaces 2014, 121, 409–416. [Google Scholar] [CrossRef]
- Kutikov, A.B.; Song, J. An Amphiphilic Degradable Polymer/Hydroxyapatite Composite with Enhanced Handling Characteristics Promotes Osteogenic Gene Expression in Bone Marrow Stromal Cells. Acta Biomater. 2013, 9, 8354–8364. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Sheikh, F.A.; Lim, J.K. Air Jet Spinning of Hydroxyapatite/Poly(Lactic Acid) Hybrid Nanocomposite Membrane Mats for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2013, 102, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Tanodekaew, S.; Channasanon, S.; Kaewkong, P.; Uppanan, P. PLA-HA Scaffolds: Preparation and Bioactivity. Procedia Eng. 2013, 59, 144–149. [Google Scholar] [CrossRef]
- Chen, V.J.; Ma, P.X. Nano-Fibrous Poly(l-Lactic Acid) Scaffolds with Interconnected Spherical Macropores. Biomaterials 2004, 25, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Ito, Y.; Zhang, P.; Chen, X. A Comparative Study on the in Vivo Degradation of Poly(L-lactide) Based Composite Implants for Bone Fracture Fixation. Sci. Rep. 2016, 6, 20770. [Google Scholar] [CrossRef] [PubMed]
- Giulio Ghersi; Francesco Carfi Pavia; Gioacchino Conoscenti; Gianluca Antonio Mannella; Silvia Greco; Salvatrice Rigogliuso; Vincenzo La Carrubba; Valerio Brucato Plla Scaffold for Bone Tissue Engineering. Chem. Eng. Trans. 2016, 49, 301–306. [CrossRef]
- Albano, C.; González, G.; Palacios, J.; Karam, A.; Covis, M. PLLA-HA vs. PLGA-HA Characterization and Comparative Analysis. Polym. Compos. 2013, 34, 1433–1442. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.; Li, C. Preparation and Characterization of PLLA/NHA Nonwoven Mats via Laser Melt Electrospinning. Mater. Lett. 2012, 73, 103–106. [Google Scholar] [CrossRef]
- Delabarde, C.; Plummer, C.J.G.; Bourban, P.-E.; Månson, J.-A.E. Solidification Behavior of PLLA/NHA Nanocomposites. Compos. Sci. Technol. 2010, 70, 1813–1819. [Google Scholar] [CrossRef]
- Papenburg, B.J.; Bolhuis-Versteeg, L.A.M.; Grijpma, D.W.; Feijen, J.; Wessling, M.; Stamatialis, D. A Facile Method to Fabricate Poly(L-lactide) Nano-Fibrous Morphologies by Phase Inversion. Acta Biomater. 2010, 6, 2477–2483. [Google Scholar] [CrossRef]
- Deplaine, H.; Ribelles, J.L.G.; Ferrer, G.G. Effect of the Content of Hydroxyapatite Nanoparticles on the Properties and Bioactivity of Poly(L-lactide)–Hybrid Membranes. Compos. Sci. Technol. 2010, 70, 1805–1812. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Venugopal, J.; Ramakrishna, S. Electrospun Nanostructured Scaffolds for Bone Tissue Engineering. Acta Biomater. 2009, 5, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous Poly(Lactic Acid)/Hydroxyapatite Composite Scaffolds for Guided Tissue Regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Jiang, D.; Zhang, J.; Lin, Q. Fabrication of Bioactive Composite by Developing PLLA onto the Framework of Sintered HA Scaffold. Mater. Sci. Eng. C 2008, 28, 51–56. [Google Scholar] [CrossRef]
- Tan, K.H.; Chua, C.K.; Leong, K.F.; Cheah, C.M.; Gui, W.S.; Tan, W.S.; Wiria, F.E. Selective Laser Sintering of Biocompatible Polymers for Applications in Tissue Engineering. Bio-Med. Mater. Eng. 2005, 15, 113–124. [Google Scholar]
- Naik, A.; Shepherd, D.V.; Shepherd, J.H.; Best, S.M.; Cameron, R.E. The Effect of the Type of HA on the Degradation of PLGA/HA Composites. Mater. Sci. Eng. C 2017, 70, 824–831. [Google Scholar] [CrossRef]
- Naik, A.; Best, S.M.; Cameron, R.E. The Influence of Silanisation on the Mechanical and Degradation Behaviour of PLGA/HA Composites. Mater. Sci. Eng. C 2015, 48, 642–650. [Google Scholar] [CrossRef]
- Endogan Tanir, T.; Hasirci, V.; Hasirci, N. Preparation and Characterization of Chitosan and PLGA-Based Scaffolds for Tissue Engineering Applications. Polym. Compos. 2015, 36, 1917–1930. [Google Scholar] [CrossRef]
- Liuyun, J.; Chengdong, X.; Lixin, J.; Lijuan, X. Effect of HA with Different Grain Size Range on the Crystallization Behaviors and Mechanical Property of HA/PLGA Composite. Thermochim. Acta 2013, 565, 52–57. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, B.; Peng, S.; Li, Z. Development of Composite Porous Scaffolds Based on Poly(Lactide-Co-Glycolide)/Nano-Hydroxyapatite via Selective Laser Sintering. Int. J. Adv. Manuf. Technol. 2013, 69, 51–57. [Google Scholar] [CrossRef]
- Jiang, L.; Xiong, C.; Chen, D.; Jiang, L.; Pang, X. Effect of N-HA with Different Surface-Modified on the Properties of n-HA/PLGA Composite. Appl. Surf. Sci. 2012, 259, 72–78. [Google Scholar] [CrossRef]
- Mou, Z.-L.; Zhao, L.-J.; Zhang, Q.-A.; Zhang, J.; Zhang, Z.-Q. Preparation of Porous PLGA/HA/Collagen Scaffolds with Supercritical CO2 and Application in Osteoblast Cell Culture. J. Supercrit. Fluids 2011, 58, 398–406. [Google Scholar] [CrossRef]
- Lao, L.; Wang, Y.; Zhu, Y.; Zhang, Y.; Gao, C. Poly(Lactide-Co-Glycolide)/Hydroxyapatite Nanofibrous Scaffolds Fabricated by Electrospinning for Bone Tissue Engineering. J. Mater. Sci Mater. Med. 2011, 22, 1873–1884. [Google Scholar] [CrossRef]
- Kim, S.-S.; Sun Park, M.; Jeon, O.; Yong Choi, C.; Kim, B.-S. Poly(Lactide-Co-Glycolide)/Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.-Y.; Turng, L.-S. Comparison between PCL/Hydroxyapatite (HA) and PCL/Halloysite Nanotube (HNT) Composite Scaffolds Prepared by Co-Extrusion and Gas Foaming. Mater. Sci. Eng. C 2017, 72, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Koh, Y.-H. Synthesis of Aligned Porous Poly(ε-Caprolactone) (PCL)/Hydroxyapatite (HA) Composite Microspheres. Mater. Sci. Eng. C 2013, 33, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Zhang, E.; Xu, L.; Wei, S. Electrospun PCL/PLA/HA Based Nanofibers as Scaffold for Osteoblast-Like Cells. J. Nanosci. Nanotechnol. 2010, 10, 7747–7751. [Google Scholar] [CrossRef] [PubMed]
- Eosoly, S.; Brabazon, D.; Lohfeld, S.; Looney, L. Selective Laser Sintering of Hydroxyapatite/Poly-ε-Caprolactone Scaffolds. Acta Biomater. 2010, 6, 2511–2517. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, L.Y.; Yang, X.B.; Weng, J. Preparation of Bioactive Porous HA/PCL Composite Scaffolds. Appl. Surf. Sci. 2008, 255, 2942–2946. [Google Scholar] [CrossRef]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of Three-Dimensional Polycaprolactone/Hydroxyapatite Tissue Scaffolds and Osteoblast-Scaffold Interactions in Vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef]
- Baştan, F.E.; Atiq Ur Rehman, M.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic Co-Deposition of PEEK-Hydroxyapatite Composite Coatings for Biomedical Applications. Colloids Surf. B Biointerfaces 2018, 169, 176–182. [Google Scholar] [CrossRef]
- Ozeki, K.; Masuzawa, T.; Aoki, H. Fabrication of Hydroxyapatite Thin Films on Polyetheretherketone Substrates Using a Sputtering Technique. Mater. Sci. Eng. C 2017, 72, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.W.; Rabiei, A. Deposition, Heat Treatment and Characterization of Two Layer Bioactive Coatings on Cylindrical PEEK. Surf. Coat. Technol. 2016, 301, 106–113. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal Models for Bone Tissue Engineering and Modelling Disease. Dis. Models Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schütz, M.A.; Duda, G.N.; Schell, H.; van Griensven, M.; Redl, H.; Hutmacher, D.W. The Challenge of Establishing Preclinical Models for Segmental Bone Defect Research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef]
- Newman, E.; Turner, A.S.; Wark, J.D. The Potential of Sheep for the Study of Osteopenia: Current Status and Comparison with Other Animal Models. Bone 1995, 16, S277–S284. [Google Scholar] [CrossRef]
- Taylor, W.R.; Ehrig, R.M.; Heller, M.O.; Schell, H.; Seebeck, P.; Duda, G.N. Tibio-Femoral Joint Contact Forces in Sheep. J. Biomech. 2006, 39, 791–798. [Google Scholar] [CrossRef]
- den Boer, F.C.; Patka, P.; Bakker, F.C.; Wippermann, B.W.; van Lingen, A.; Vink, G.Q.M.; Boshuizen, K.; Haarman, H.J.T.M. New Segmental Long Bone Defect Model in Sheep: Quantitative Analysis of Healing with Dual Energy X-ray Absorptiometry. J. Orthop. Res. 1999, 17, 654–660. [Google Scholar] [CrossRef]
- Kon, E.; Muraglia, A.; Corsi, A.; Bianco, P.; Marcacci, M.; Martin, I.; Boyde, A.; Ruspantini, I.; Chistolini, P.; Rocca, M.; et al. Autologous Bone Marrow Stromal Cells Loaded onto Porous Hydroxyapatite Ceramic Accelerate Bone Repair in Critical-Size Defects of Sheep Long Bones. J. Biomed. Mater. Res. 2000, 49, 328–337. [Google Scholar] [CrossRef]
- Quarto, N.; Wan, D.C.; Kwan, M.D.; Panetta, N.J.; Li, S.; Longaker, M.T. Origin Matters: Differences in Embryonic Tissue Origin and Wnt Signaling Determine the Osteogenic Potential and Healing Capacity of Frontal and Parietal Calvarial Bones. J. Bone Miner. Res. 2010, 25, 1680–1694. [Google Scholar] [CrossRef]
- Reichert, J.C.; Gohlke, J.; Friis, T.E.; Quent, V.M.C.; Hutmacher, D.W. Mesodermal and Neural Crest Derived Ovine Tibial and Mandibular Osteoblasts Display Distinct Molecular Differences. Gene 2013, 525, 99–106. [Google Scholar] [CrossRef]
- Dadwal, U.; Falank, C.; Fairfield, H.; Linehan, S.; Rosen, C.J.; Kaplan, D.L.; Sterling, J.; Reagan, M.R. Tissue-Engineered 3D Cancer-in-Bone Modeling: Silk and PUR Protocols. Bonekey Rep. 2016, 5, 842. [Google Scholar] [CrossRef][Green Version]
- Toledano, M.; Gutierrez-Pérez, J.L.; Gutierrez-Corrales, A.; Serrera-Figallo, M.A.; Toledano-Osorio, M.; Rosales-Leal, J.I.; Aguilar, M.; Osorio, R.; Torres-Lagares, D. Novel Non-Resorbable Polymeric-Nanostructured Scaffolds for Guided Bone Regeneration. Clin. Oral. Investig. 2020, 24, 2037–2049. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, Y.; Kashif, M.; Jeong, D.; Kim, G. Evaluation of Bone Regeneration on Polyhydroxyethyl-Polymethyl Methacrylate Membrane in a Rabbit Calvarial Defect Model. In Vivo 2016, 30, 587–591. [Google Scholar] [PubMed]
- Reznikov, N.; Boughton, O.R.; Ghouse, S.; Weston, A.E.; Collinson, L.; Blunn, G.W.; Jeffers, J.R.T.; Cobb, J.P.; Stevens, M.M. Individual Response Variations in Scaffold-Guided Bone Regeneration Are Determined by Independent Strain- and Injury-Induced Mechanisms. Biomaterials 2019, 194, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.H.; Pires, L.D.N.; Beatrice, C.A.G.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Fabrication of Biocompatible Composites of Poly(Lactic Acid)/Hydroxyapatite Envisioning Medical Applications. Polym. Eng. Sci. 2020, 60, 636–644. [Google Scholar] [CrossRef]
- Si, J.; Lin, J.; Su, C.; Yu, S.; Cui, Z.; Wang, Q.; Chen, W.; Turng, L.-S. Ultrasonication-Induced Modification of Hydroxyapatite Nanoparticles onto a 3D Porous Poly(Lactic Acid) Scaffold with Improved Mechanical Properties and Biocompatibility. Macromol. Mater. Eng. 2019, 304, 1900081. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Jang, C.H.; Koo, Y.; Kim, G. ASC/Chondrocyte-Laden Alginate Hydrogel/PCL Hybrid Scaffold Fabricated Using 3D Printing for Auricle Regeneration. Carbohydr. Polym. 2020, 248, 116776. [Google Scholar] [CrossRef]
- Mohandesnezhad, S.; Alizadeh, E.; Pilehvar-Soltanahmadi, Y.; Davaran, S.; Goodarzi, A.; Khatamian, M.; Zarghami, N.; Samiei, M.; Aghazadeh, M.; Akbarzadeh, A. In Vitro Evaluation of Novel Zeolite-Hydroxyapatite Blended Scaffold for Dental Tissue Engineering. 2020. rs-11059/v1. Reseach Square. Available online: https://www.researchsquare.com/article/rs-11059/v1 (accessed on 18 December 2021).
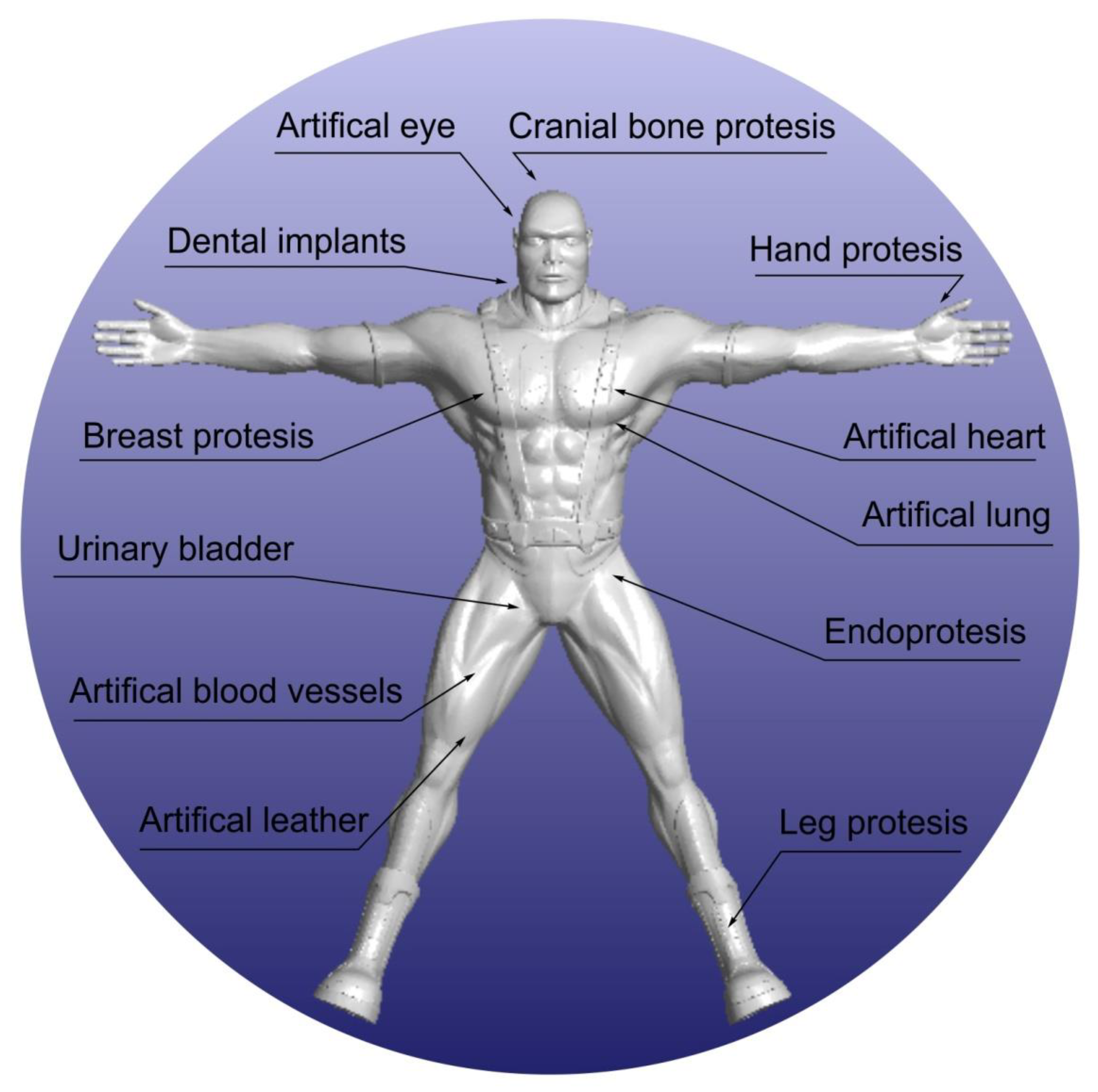
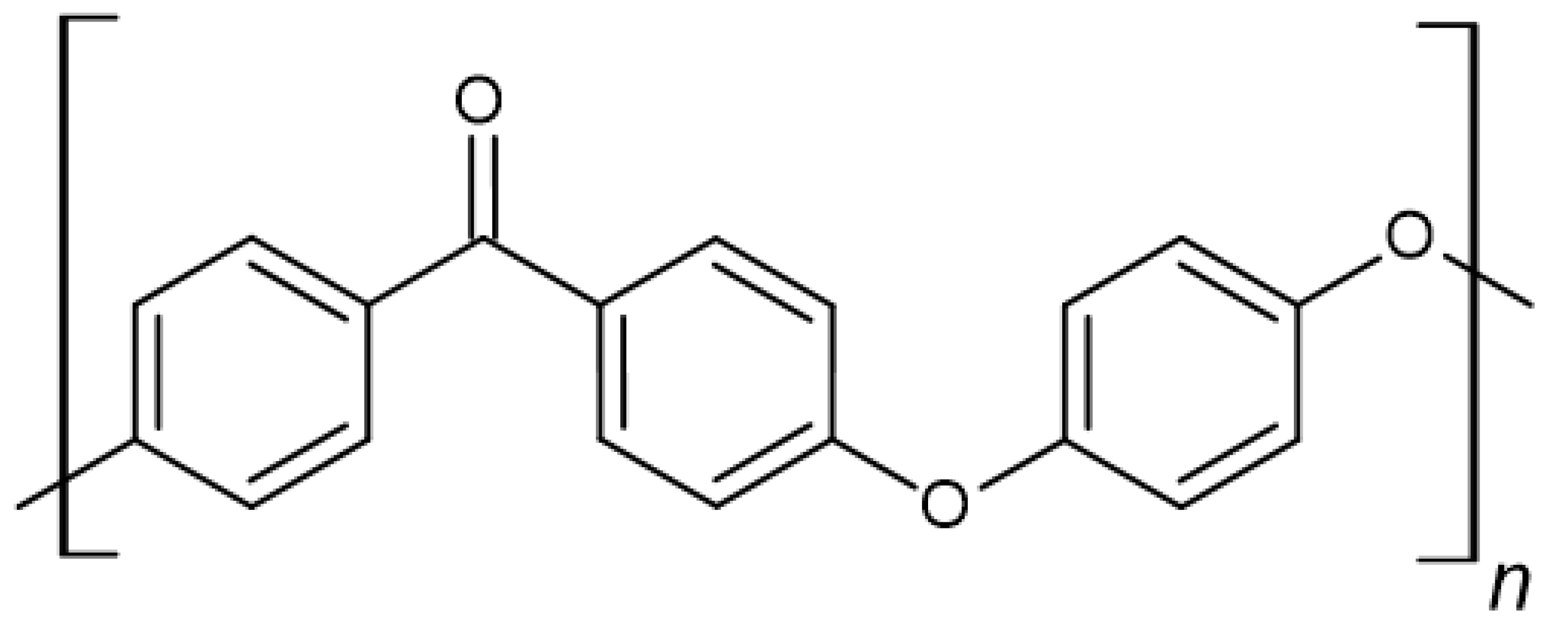
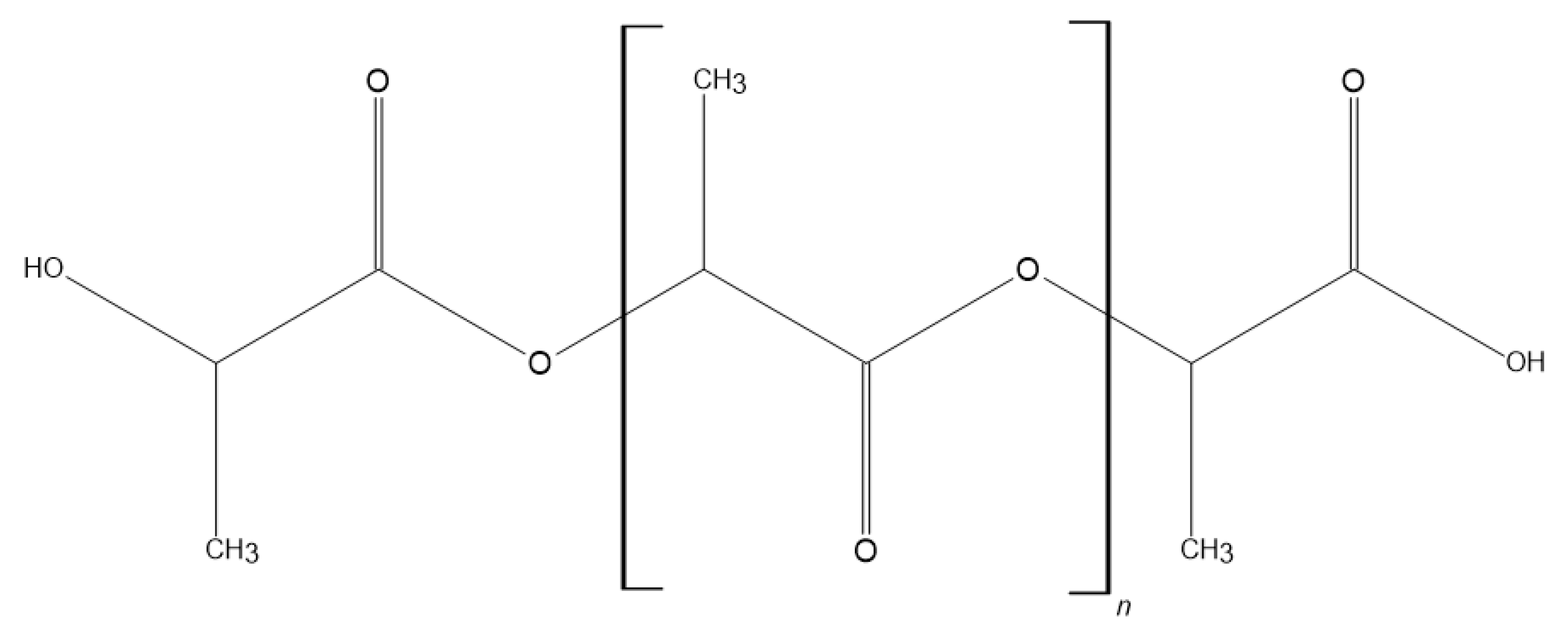
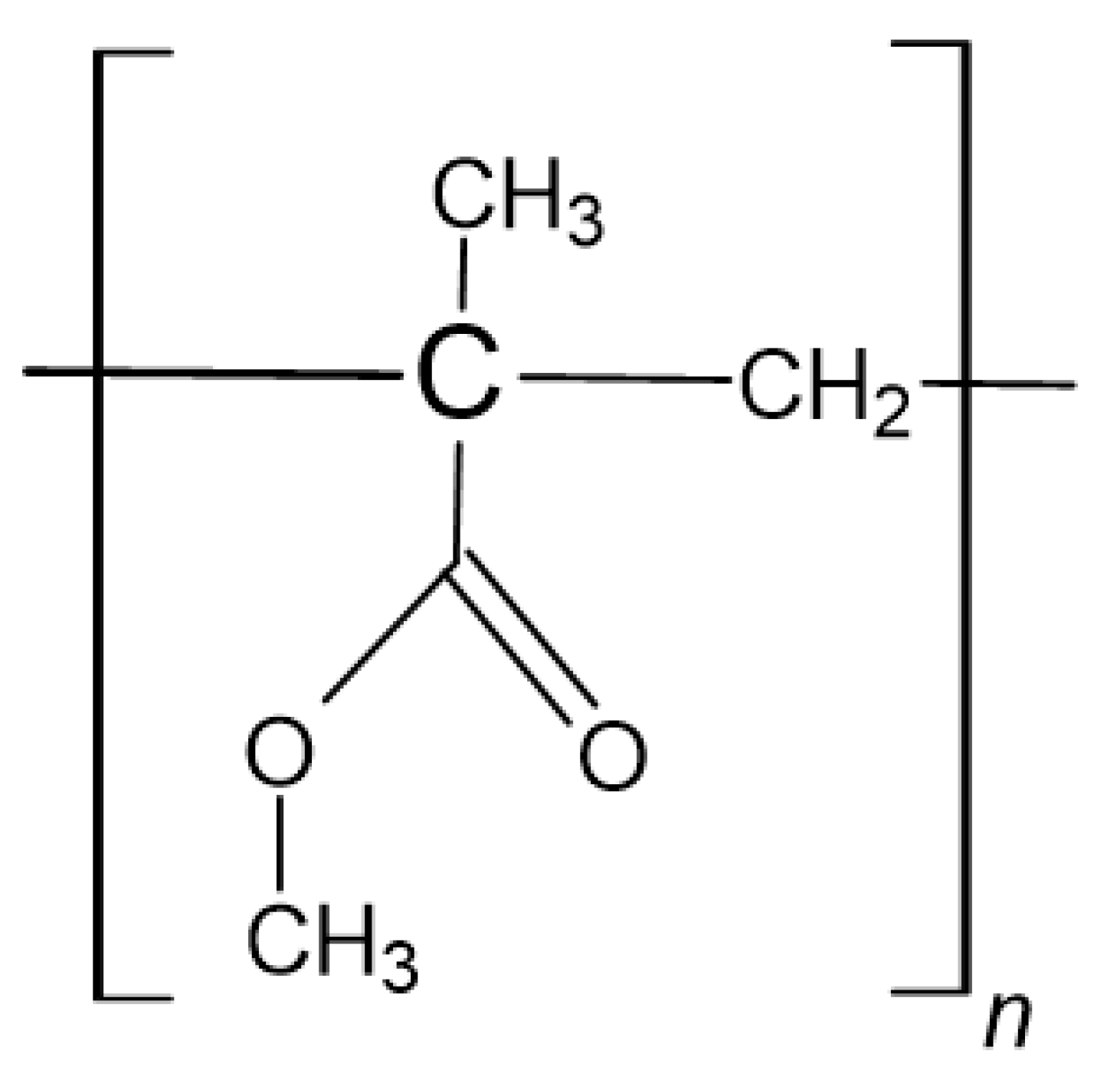
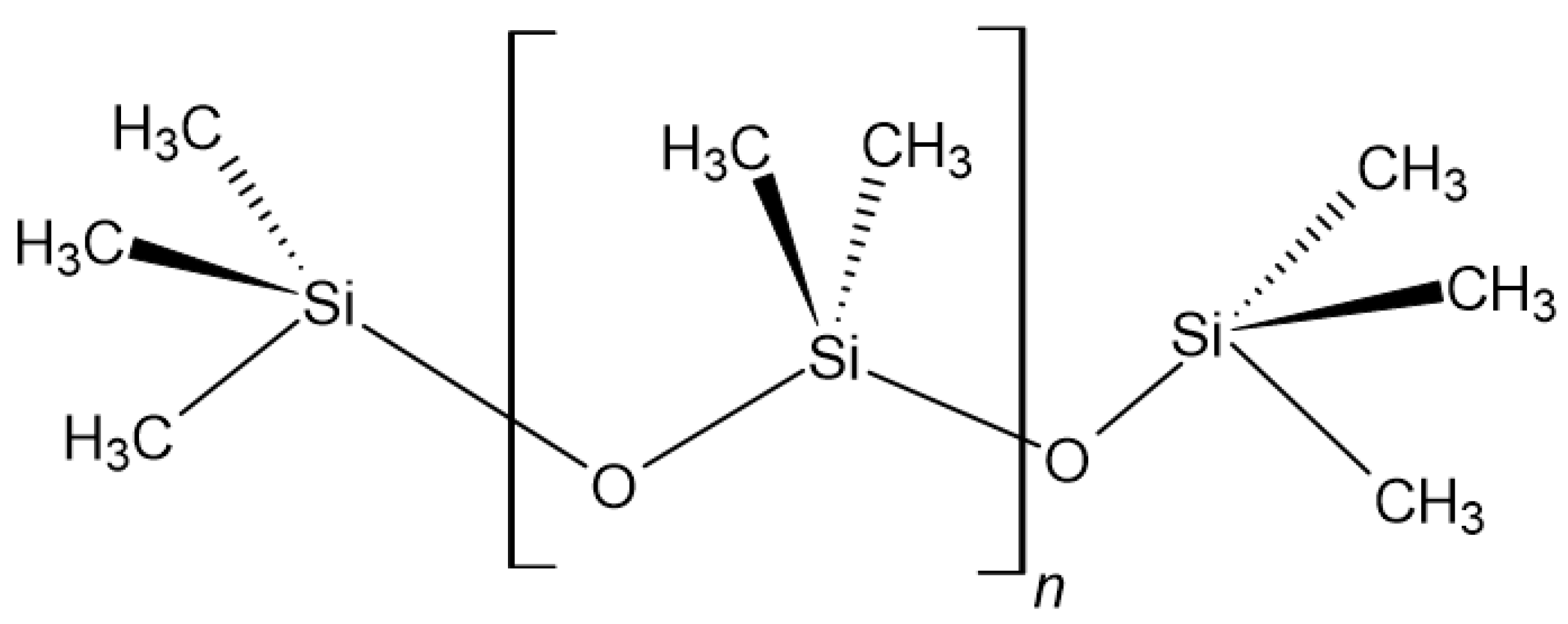
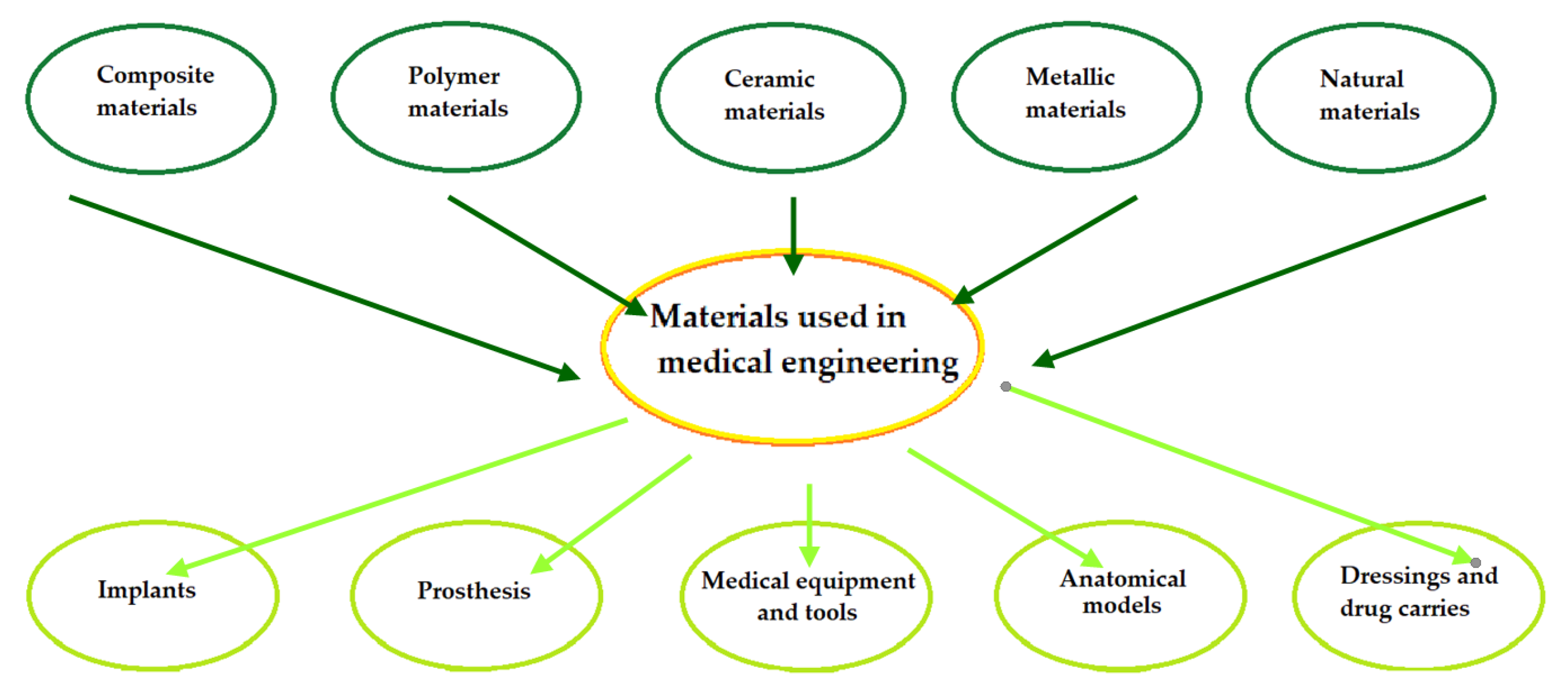
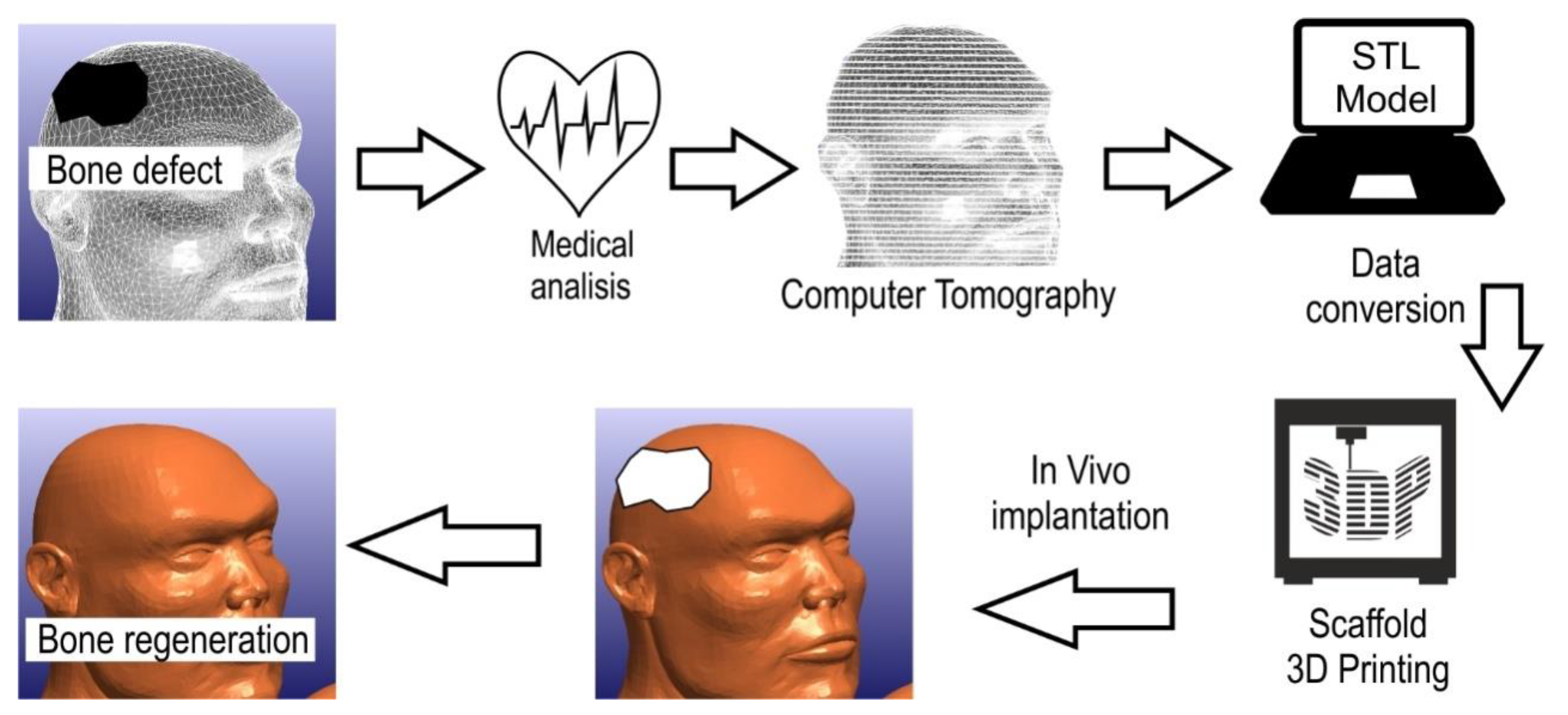
| Polymer | Purpose of Use | Biodegradation/ Bioresorbability | Biocompatibility | References |
|---|---|---|---|---|
| Poly-lactic acid (PLA) | Barrier membranes, drug delivery, guided tissue regeneration (in dental applications), orthopaedic applications, stents, staples, sutures, tissue engineering | + | + | [2,34,35] |
| Poly-glycolic acid (PGA) | Barrier membranes, drug delivery, guided tissue regeneration (in dental applications), orthopaedic applications, stents, staples, sutures, tissue engineering | + | + | [34,35] |
| Poly-caprolactone (PCL) | Long-term drug delivery, orthopaedic applications, staples, stents | + | + | [2,34,35] |
| poly(1,8 octanediol-co-citrate) (POC) | Mimics the mechanical properties of vessels, support the proliferation of human aortic endothelial cells while inhibiting the proliferation of human aortic smooth muscle cells in vitro, serve as therapeutic coatings to improve the long-term patency of transplants | + | + | [34] |
| Poly-lactic-co-glycolic acid (PLGA) | Barrier membranes, drug delivery, guided tissue regeneration (in dental applications), orthopaedic applications, stents, staples, sutures, tissue engineering | + | + | [34] |
| Polydimethylsiloxane (PMDS) | Uses for production of contact lenses | + | + | [36] |
| Polymethylmethacrylate (PMMA) | Masses for orthopaedics, surgery and dental prostheses, use in the production of intraocular lenses, gentamicin carrier in the treatment of infected joints | + | + | [1,2,36] |
| Polyethylene (PE) | Acts as a binder in prosthetics as an element of implants | − | + | [36] |
| Polyhydroxyalkanoates (PHAs) | Production of sutures, wound dressings, cardiovascular patches, orthopaedic pins, adhesive barriers, stents, guided tissue repair and regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, bone marrow scaffolds | + | n/a | [2] |
| poly-β-hydroxybutyrate (PHB) | Long-term drug delivery, orthopaedic applications, stapes stents | + | n/a | [35] |
| Poly-para-dioxanone (PPD) | Used in the field of medicine in the form of films, foams, laminates, adhesives and surface coating | + | + | [35] |
| Polyhydroxyvalerate (PHV) | Long-term drug delivery, orthopaedic applications, stapes stents | + | n/a | [35] |
| Polyether ether ketone (PEEK) | In the area of implantation, it is used for artificial skull plates, elements of finger and knee joints and spine implants, more and more often in dentistry as an element of abutments, fixed prosthetic skeletons and skeletons of partial skeletal dentures, including precise fixing | + | + | [10,37] |
| Polyphosphazenes (PPZs) | Blood contacting devices, drug delivery, skeletal reconstruction | n/a | n/a | [35] |
| Polydioxanone (PDS) | Fracture fixation in non-load-bearing bones, sutures, wound clip | + | n/a | [35] |
| Polycarbonate (PC) | Blood separators, surgical masks, high pressure syringes, disposable dental devices used in artificial kidney dialysis | − | + | [2] |
| Polyethylene glycol (PEG) | Component of hydrogel dressings successfully used in the treatment of open wounds, they are tested in terms of treatment of nervous system injuries of increasing the effectiveness of gene therapy | + | + | [2] |
| Polyurethane (PUR) | Artificial organs are created, the creation of breast prostheses, an artificial heart, transplants, membranes, catheters, artificial skin, oesophageal prosthesis, channels for nerve regeneration, they began to be used in heart valves and ventricles and in aortic transplants | + | + | [2,36] |
| Polypyrrole (PPy) | A potentially electrically addressable tissue or cell support medium, neuroprosthetics, biosensors and drug delivery | + | n/a | [2,34] |
| Nanoparticle | Purpose of Use | Advantages | Toxicity | References |
|---|---|---|---|---|
| Carbon nanotubes | Coating of the teeth surface, teeth filling | Large surface area, adheres easily to the tooth surfaces and to the surfaces of dentin and cementum, bring active agents to live cells | Blocks potassium channels, accumulation in the hippocampus which induces oxidative stress, increased ROS factors, increased apoptosis factor | [29,117,123] |
| Graphene | Teeth coating, biofilm reduction, suitable for implantation | Treat bacterial biofilm, cost effectiveness, low dentistry form a uniform crystal lattice, fracture resistant | Toxicity depends on purity, shape, size and oxidative state, | [29,117,124] |
| Hydroxyapatite | Reduce dental hypersensitivity, retard auxiliary demineralisation, act as cavity filler, repairment of enamel surface, promotion osteoconduction | HA particles can easily integrate into the dental tubules, similar composition with teeth and bone, adsorbed to the enamel of the teeth, biocompatible, protect the teeth by making a film of artificial enamel around the tooth, reform periodontal shortcomings | Particles travelled to and dispersed into lungs, spleen and liver by blood, the inflammatory response, signalling pathway, induce oxidative stress, | [29,124,125] |
| Halloysite | Drug delivery, promotion attachment and proliferation of human dental stem cells, tissue engineering, scaffolds | Significant adsorption and loading capacities, improving mechanical properties, antibacterial, biocompatible, haemocompatible, sustained release of therapeutic agents, increased water adsorption and degradation rate | No toxic effects in recent studies | [126,127] |
| Zirconia | Reduces bacterial adhesion to the tooth surface, effective polishing agent, provide protection against dental carries | Similar mechanical properties and colour to those of a tooth, sensible biocompatibility, have low cytotoxicity, high fracture resistance | Significant DNA damage in human T-cells, induce apoptosis, inhibition of cell proliferation, nanoparticles can stop the cell cycle | [29,128] |
| Silica | Tooth polishing, an antibacterial agent, dental filling agent, prevents dental caries, to teat dental hypersensitivity | Biocompatible, low density, significant adsorption ability, low toxic effect, effective cost, reduces roughness of teeth surface (polishing agent) | Ability to induce silicosis, cytotoxicity, genotoxicity, possibility to induce oxidative stress, mediate apoptosis, g | [29] |
| Titania | Dental implants | Long-term effect on dental implants, surface modification—less bacterial adhesion, improved hardness, enhance the bone grow, protein adsorption and cell adhesion | Increased ROS factors, increased oxidative stress, genotoxicity, induce cellular apoptosis, increased inflammatory responses | [13,29,123,124,128] |
| Silver | Antimicrobial agent, dental implants, dental prosthetics, dental restorative material | Decrease bacterial colonisation, increases oral health, long-term antibacterial activity | Reduces mitochondrial viability, increased LDH release, increased ROS, up-regulated Bax protein expression, changes in astrocyte morphology, acute calcium response, induce apoptosis of lung cellular, increased cardiocyte deformity and lipid peroxidation, decreased levels of GSH, SOD and CAT, increased oxidative stress, increased release of inflammatory mediators in liver, inhibits mitochondrial ATP-ase in spleen | [13,29,117,123,124,128] |
| 3D Printing Technique | Type of Material | Name of Commercial Polymer | Area of Application |
|---|---|---|---|
| MEX Fused Deposition Modelling (FDM) | Polylactide (PLA) | Resorb x | Used to receive surgical sutures and dental implants |
| Polycarbonate (PC) | PC-ISO | Pharmaceutical industry, biomedical engineering, food packaging | |
| Acrylonitrile-butadiene-styrene (ABS) | ABS-M30i | Products in contact with skin, food and medicines | |
| Polyetheretherketone (PEEK) | LUVOCOM 3F PEEK 9581 | Production of surgical and dental instruments | |
| Polyetherimide (PEI) | ULTEM 1010 | Production of surgical and dental instruments | |
| MJ Material Jetting (PolyJet) | Acrylic resins | MED610 | Dentistry, orthodontic laboratories in the production of, among other things, crowns and dental bridges |
| VeroGlaze (MED620) | |||
| VeroDent MED670 | |||
| VeroDentPlus MED690 | |||
| PBF Selective Laser Sintering (SLS) | Polyamide (PA) | PA 2105 | Production of dental instruments |
| VPP Stereolithography (SLA) | Acrylic resins | dental SG | Production of precise surgical measures and dental models |
| dental LT clear | |||
| Epoxy resin | Accura® ClearVue™ | ||
| VPP Digital Light Processing (DLP) | Acrylic resins | 3Delta Model 320 3Delta Model Ortho | Production of prosthetic models, gingival masks, surgical templates, orthodontic models |
| Polymer–HA Composite | Manufacturing Technique | References |
|---|---|---|
| PLA/HA | Vacuum-assisted solvent casting | [187] (2019) |
| Extrusion and injection moulding | [301] (2018) | |
| Fused deposition melting | [302] (2017) | |
| 3D printing | [303] (2016) | |
| Extrusion process | [304] (2014) | |
| Electrospinning | [305] (2013) | |
| Air jet spinning | [306] (2013) | |
| Stereolithography | [307] (2013) | |
| Hot pressing | [48] (2006) | |
| Solvent casting | [308] (2005) | |
| PLLA/HA | Precipitation | [309] (2016) |
| Thermally induced phase separation | [310] (2016) | |
| Solvent casting | [311] (2013) | |
| Laser melt electrospinning | [312] (2012) | |
| Melt extrusion | [313] (2010) | |
| Phase inversion | [314] (2010) | |
| Freeze extraction | [315] (2010) | |
| Hot pressing | [316] (2009) | |
| Electrospinning | [317] (2009) | |
| Two-step immersing replication | [318] (2008) | |
| Selective laser sintering | [319] (2005) | |
| PLGA/HA | Solvent casting and injection moulding | [320] (2017) |
| Injection moulding | [321] (2015) | |
| Freeze drying | [322] (2014) | |
| Solution mixing | [323] (2013) | |
| Selective laser sintering | [324] (2013) | |
| Co-solution | [325] (2012) | |
| Supercritical fluid extractor | [326] (2011) | |
| Electrospinning | [327] (2011) | |
| Gas foaming and particulate leaching | [328] (2006) | |
| PCL/HA | Co-extrusion | [329] (2017) |
| Freezing of emulsions | [330] (2013) | |
| Electrospinning | [331] (2010) | |
| Selective laser sintering | [332] (2010) | |
| Polymer impregnating | [333] (2008) | |
| Fuse deposition melting | [334] (2007) | |
| PEEK/HA | Electrophoretic deposition and suspension | [335] (2018) |
| Sputtering | [336] (2017) | |
| Post-deposition heat treatment | [337] (2016) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kroczek, K.; Turek, P.; Mazur, D.; Szczygielski, J.; Filip, D.; Brodowski, R.; Balawender, K.; Przeszłowski, Ł.; Lewandowski, B.; Orkisz, S.; et al. Characterisation of Selected Materials in Medical Applications. Polymers 2022, 14, 1526. https://doi.org/10.3390/polym14081526
Kroczek K, Turek P, Mazur D, Szczygielski J, Filip D, Brodowski R, Balawender K, Przeszłowski Ł, Lewandowski B, Orkisz S, et al. Characterisation of Selected Materials in Medical Applications. Polymers. 2022; 14(8):1526. https://doi.org/10.3390/polym14081526
Chicago/Turabian StyleKroczek, Kacper, Paweł Turek, Damian Mazur, Jacek Szczygielski, Damian Filip, Robert Brodowski, Krzysztof Balawender, Łukasz Przeszłowski, Bogumił Lewandowski, Stanisław Orkisz, and et al. 2022. "Characterisation of Selected Materials in Medical Applications" Polymers 14, no. 8: 1526. https://doi.org/10.3390/polym14081526
APA StyleKroczek, K., Turek, P., Mazur, D., Szczygielski, J., Filip, D., Brodowski, R., Balawender, K., Przeszłowski, Ł., Lewandowski, B., Orkisz, S., Mazur, A., Budzik, G., Cebulski, J., & Oleksy, M. (2022). Characterisation of Selected Materials in Medical Applications. Polymers, 14(8), 1526. https://doi.org/10.3390/polym14081526












